Coronene and Phthalocyanine Trapping Efficiency of a Two-Dimensional Kagomé Host-Nanoarchitecture
Abstract
:1. Introduction
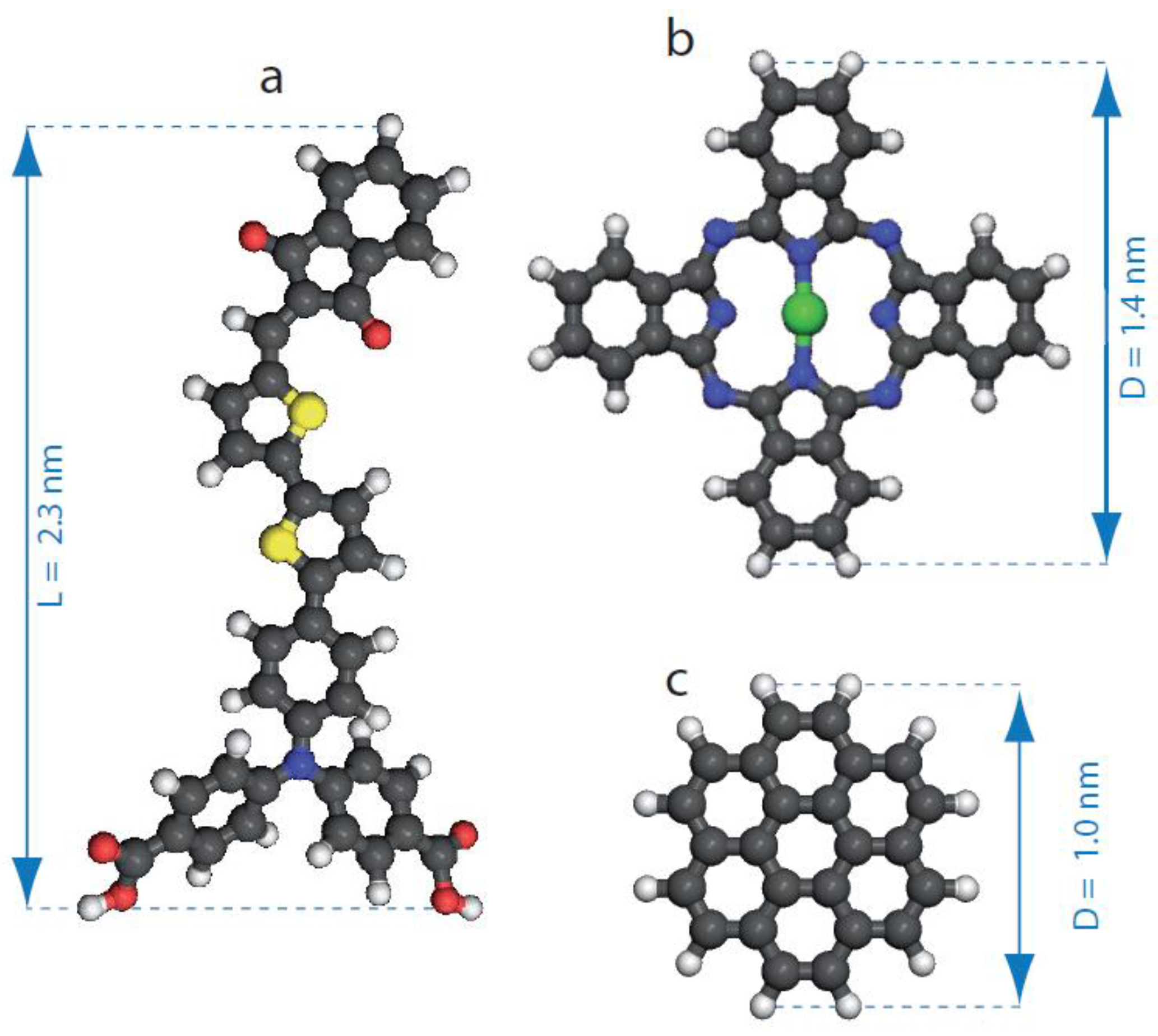
2. Materials and Methods
3. Results
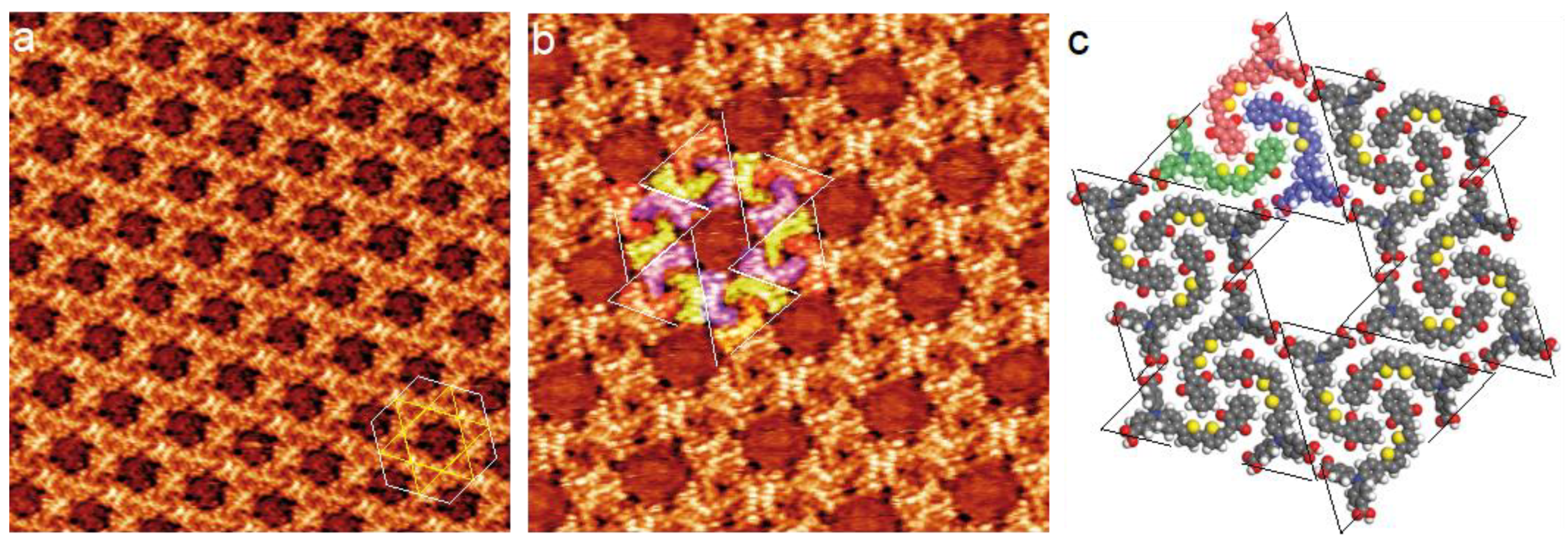
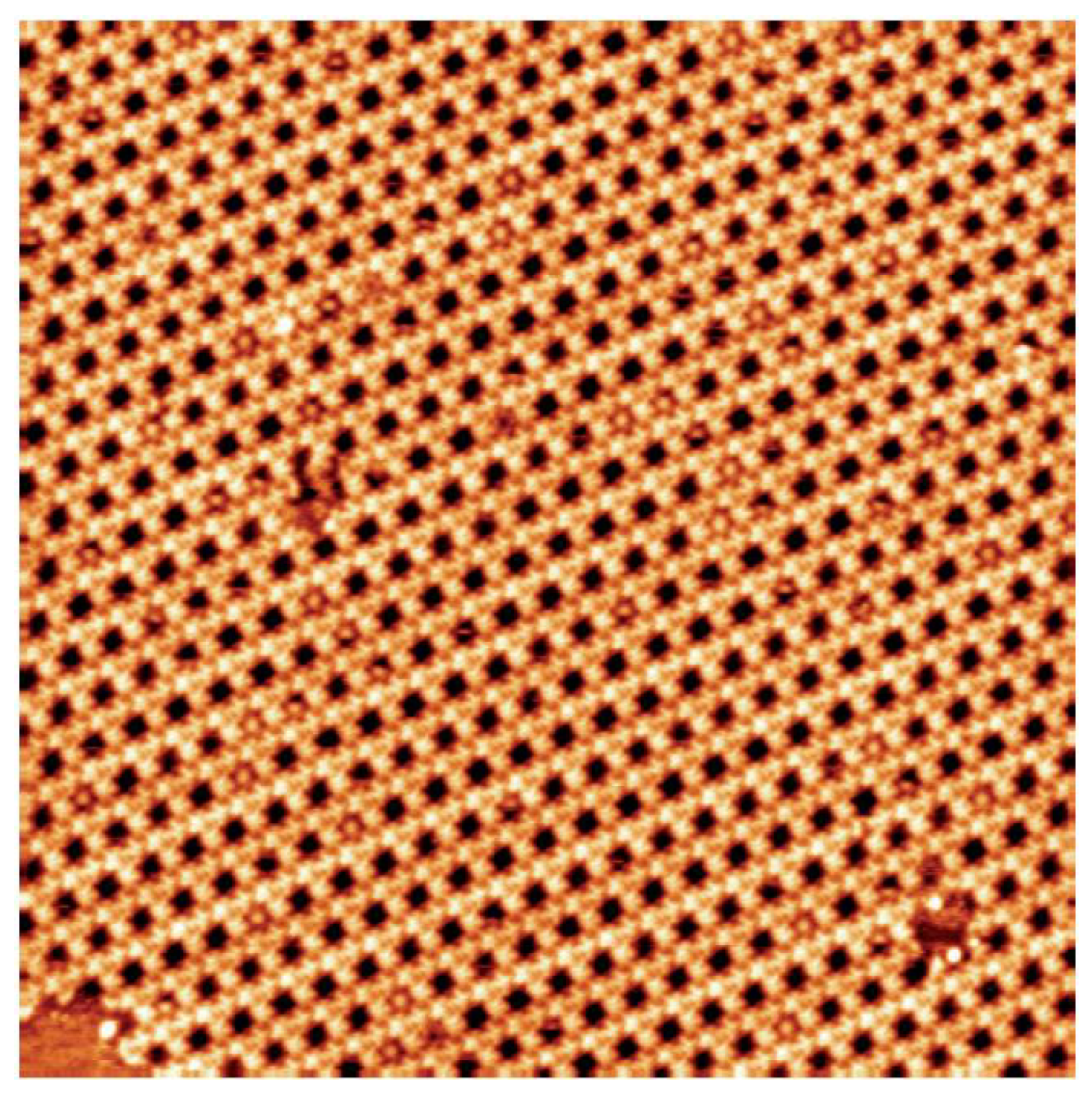
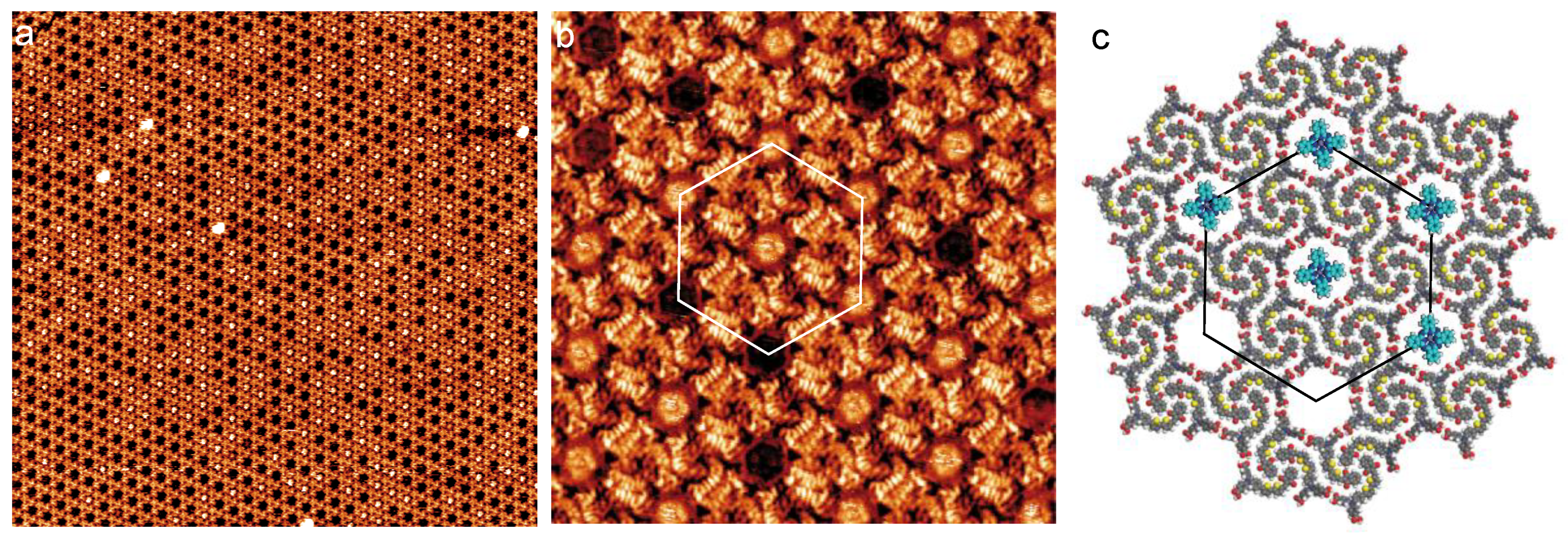
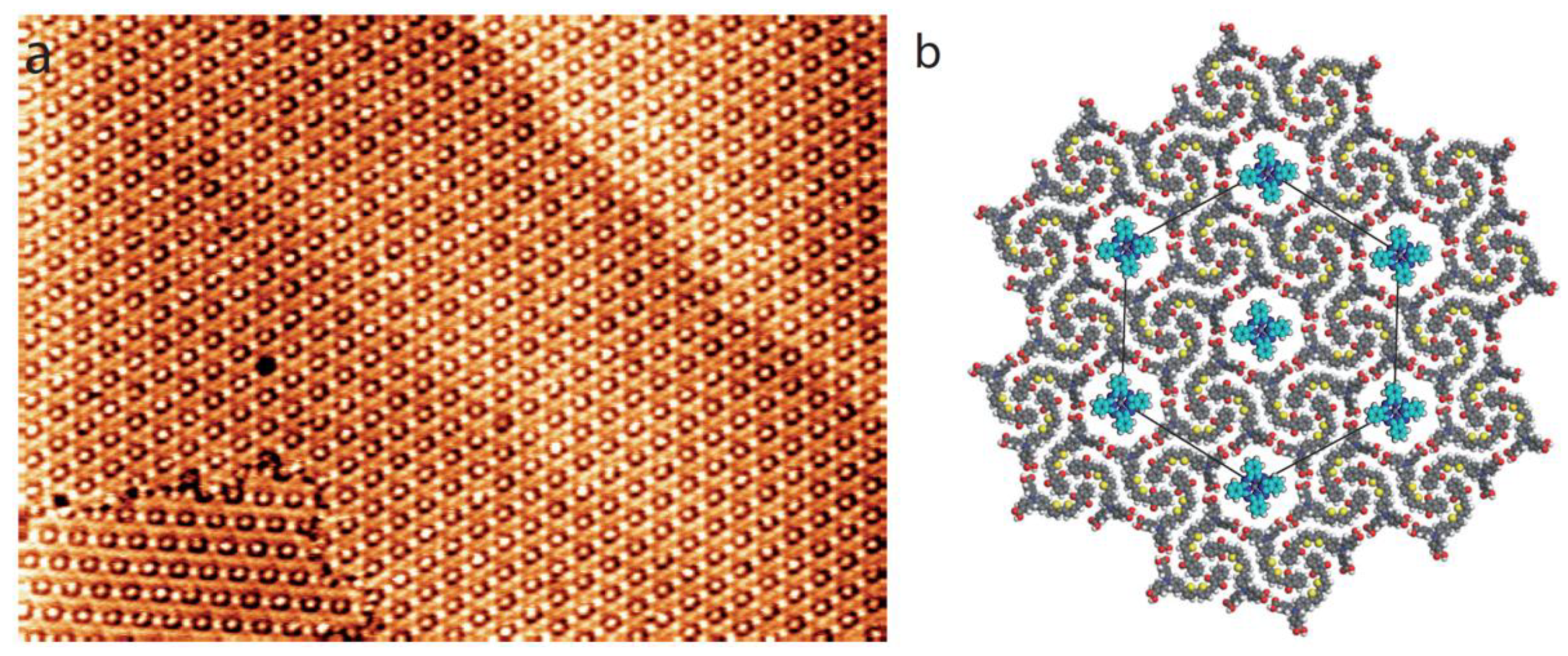
3.1. Dye Porous Kagomé Nanoarchitecture
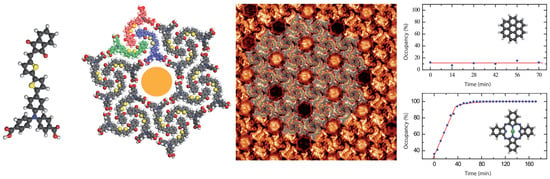
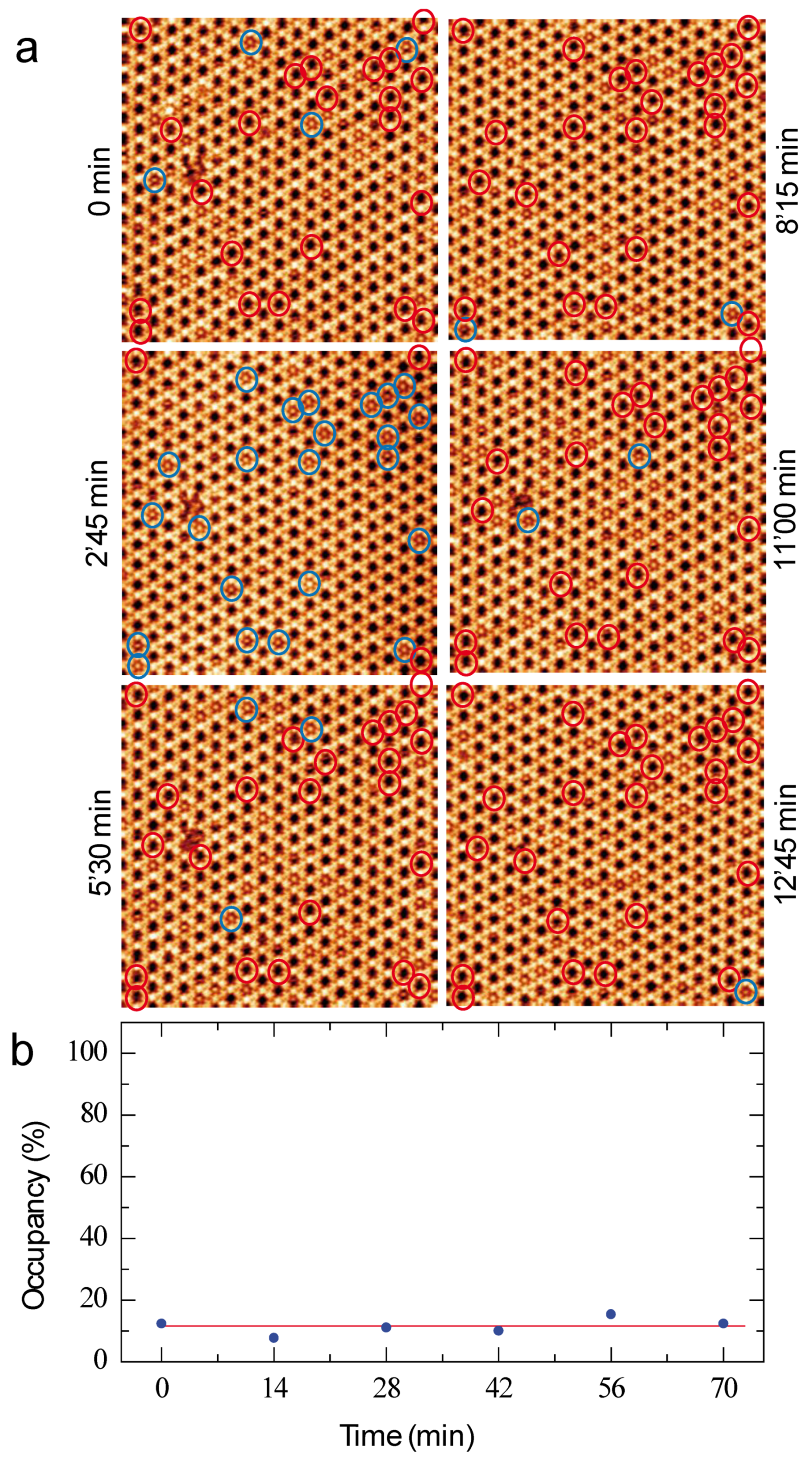
3.2. Coronene-Dye Guest–Host Nanoarchitecture
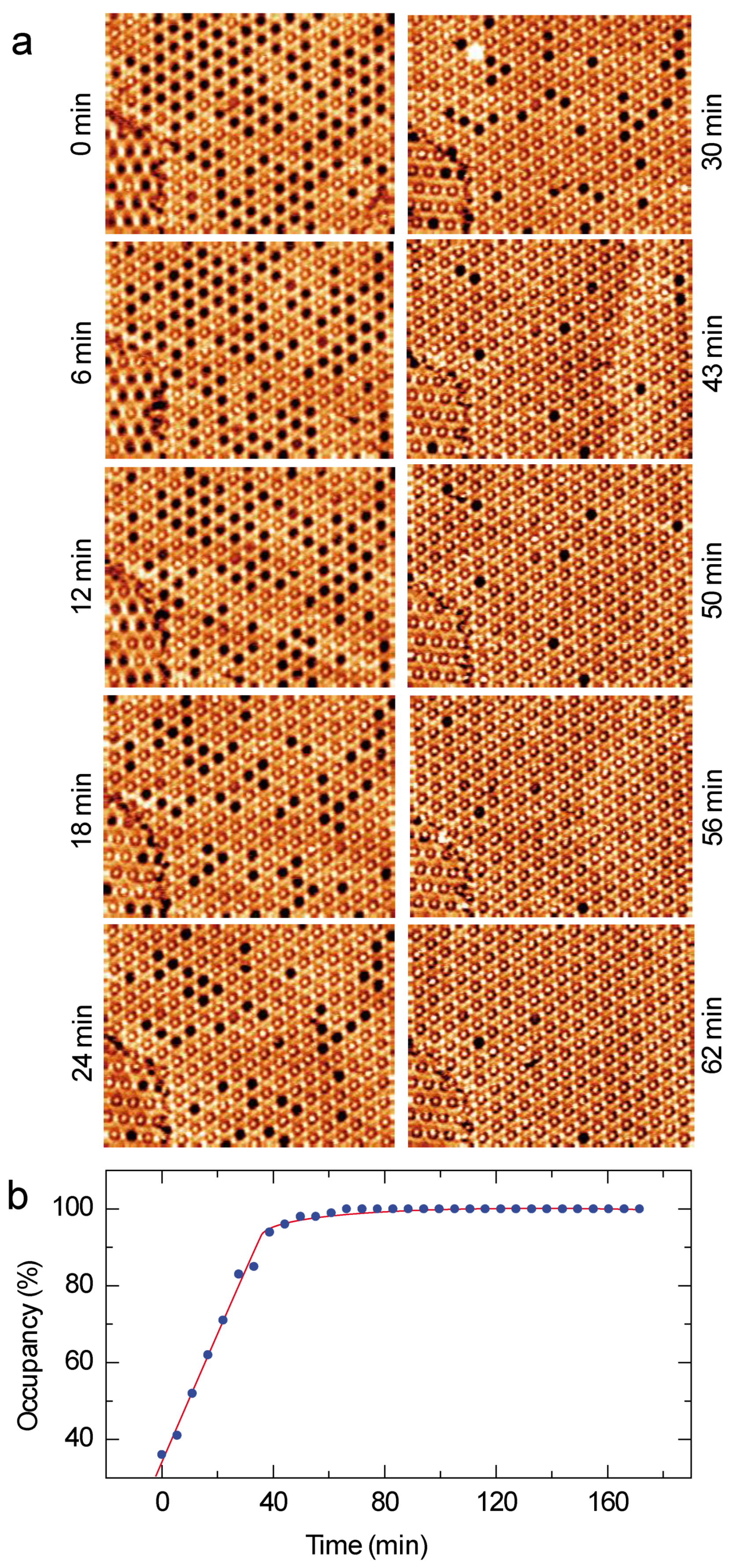
3.3. ZnPc-Dye Guest–Host Nanoarchitecture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Desai, A.V.; Lei, S.; Ghosh, S.K. Advanced porous materials for sensing, capture and detoxification of organic pollutants toward water remediation. ACS Sustain. Chem. Eng. 2019, 7, 7456–7478. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Barth, J.V. Molecular Architectonic on Metal Surfaces. Annu. Rev. Phys. Chem. 2007, 58, 375–407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cheng, Z.; Huan, Q.; He, X.; Lin, X.; Gao, L.; Deng, Z.; Jiang, N.; Liu, Q.; Du, S.; et al. Site- and Configuration-Selective Anchoring of Iron–Phthalocyanine on the Step Edges of Au(111) Surface. J. Phys. Chem. C 2011, 115, 10791–10796. [Google Scholar] [CrossRef]
- Zhang, J.L.; Niu, T.C.; Wee, A.T.S.; Chen, W. Self-assembly of binary molecular nanostructure arrays on graphite. Phys. Chem. Chem. Phys. 2013, 15, 12414–12427. [Google Scholar] [CrossRef]
- Liang, H.; He, Y.; Ye, Y.; Xu, X.; Cheng, F.; Sun, W.; Shao, X.; Wang, Y.; Li, J.; Wu, K. Two-dimensional molecular porous networks constructed by surface assembling. Coord. Chem. Rev. 2009, 253, 2959–2979. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C. Hierarchical construction of self-assembled low-dimensional molecular architectures observed by using scanning tunneling microscopy. Chem. Soc. Rev. 2009, 38, 2576–2589. [Google Scholar] [CrossRef]
- Gao, H.J.; Li, G. Scanning tunneling microscopy of functional nanostructures on solid surfaces: Manipulation, self-assembly, and applications. Prog. Surf. Sci. 2010, 85, 28–91. [Google Scholar] [CrossRef]
- Shang, J.; Wang, Y.; Chen, M.; Dai, J.; Zhou, X.; Kuttner, J.; Hilt, G.; Shao, X.; Gottfried, J.M.; Wu, K. Assembling molecular Sierpiński triangle fractals. Nat. Chem. 2015, 7, 389–393. [Google Scholar] [CrossRef]
- Gao, H.Y.; Shen, Q.J.; Zhao, X.R.; Yan, X.Q.; Pang, X.; Jin, W.J. Phosphorescent co-crystal assembled by 1,4-diiodotetrafluorobenzene with carbazole based on C−I···π halogen bonding. J. Mater. Chem. 2012, 22, 5336–5343. [Google Scholar] [CrossRef]
- Silly, F. Selecting Two-dimensional halogen−halogen bonded self-assembled 1,3,5-tris(4-iodophenyl)benzene porous nano-architectures at the solid−liquid interface. J. Phys. Chem. C 2013, 117, 20244–20249. [Google Scholar] [CrossRef]
- Getmanenko, Y.A.; Fonari, M.; Risko, C.; Sandhu, B.; Galán, E.; Zhu, L.; Tongwa, P.; Hwang, D.K.; Singh, S.; Tiwari, S.P.; et al. Benzo[1,2-b:6,5-b′]dithiophene(dithiazole)-4,5-dione derivatives: Synthesis, electronic properties, crystal packing and charge transport. J. Mater. Chem. C 2013, 1, 1467–1481. [Google Scholar] [CrossRef]
- Peyrot, D.; Silly, F. On-Surface Synthesis of Two-Dimensional Covalent Organic Structures versus Halogen-Bonded Self-Assembly: Competing Formation of Organic Nanoarchitectures. ACS Nano 2016, 10, 5490–5498. [Google Scholar] [CrossRef]
- Baris, B.; Luzet, V.; Duverger, E.; Sonnet, P.; Palmino, F.; Cherioux, F. Robust and Open Tailored Supramolecular Networks Controlled by the Template Effect of a Silicon Surface. Angew. Chem. Int. Ed. 2011, 50, 4094–4098. [Google Scholar] [CrossRef]
- Zha, B.; Dong, M.; Miao, X.; Peng, S.; Wu, Y.; Miao, K.; Hu, Y.; Deng, W. Cooperation and competition between halogen bonding and van der Waals forces in supramolecular engineering at the aliphatic hydrocarbon/graphite interface: Position and number of bromine group effects. Nanoscale 2017, 9, 237–250. [Google Scholar] [CrossRef]
- Yagai, S.; Goto, Y.; Lin, X.; Karatsu, T.; Kitamura, A.; Kuzuhara, D.; Yamada, H.; Kikkawa, Y.; Saeki, A.; Seki, S. Self-Organization of Hydrogen-Bonding Naphthalene Chromophores into J-type Nanorings and H-type Nanorods: Impact of Regioisomerism. Angew. Chem. Int. Ed. 2012, 51, 6643–6647. [Google Scholar] [CrossRef]
- Barth, J.V.; Weckesser, J.; Cai, C.; Günter, P.; Bürgi, L.; Jeandupeux, O.; Kern, K. Building Supramolecular Nanostructures at Surfaces by Hydrogen Bonding. Angew. Chem. Int. Ed. 2000, 39, 1230–1234. [Google Scholar] [CrossRef]
- Silly, F.; Shaw, A.Q.; Castell, M.R.; Briggs, G.A.D.; Mura, M.; Martsinovich, N.; Kantorovich, L. Melamine Structures on the Au(111) Surface. J. Phys. Chem. C 2008, 112, 11476–11480. [Google Scholar] [CrossRef]
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, D.; Mohnani, S.; Llanes-Pallas, A. Supramolecular Chemistry at Interfaces: Molecular Recognition on Nanopatterned Porous Surfaces. Chem. A Eur. J. 2009, 15, 7004–7025. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dong, M.; Gersen, H.; Rauls, E.; Vázquez-Campos, S.; Crego-Calama, M.; Reinhoudt, D.N.; Laegsgaard, E.; Stensgaard, I.; Linderoth, T.R.; et al. Influence of Alkyl Side Chains on Hydrogen-Bonded Molecular Surface Nanostructures. Small 2008, 4, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Miao, X.R.; Cui, L.H.; Liu, P.; Deng, W.L. Chiral transition of the supramolecular assembly by concentration modu-lation at the liquid/solid interface. J. Phys. Chem. C 2015, 119, 17920–17929. [Google Scholar] [CrossRef]
- Xu, L.; Miao, X.R.; Cui, L.H.; Liu, P.; Chen, X.F.; Deng, W.L. Concentration-dependent structure and structural transition from chirality to nonchirality at the liquid-solid interface by co-assembly. Nanoscale 2015, 7, 11734–11745. [Google Scholar] [CrossRef]
- Madueno, R.; Raisanen, M.T.; Silien, C.; Buck, M. Functionalizing hydrogen-bonded surface networks with self-assembled monolayers. Nature 2008, 454, 618–621. [Google Scholar] [CrossRef]
- Marta, E.; Cañas, V.; Kamel, A.M.; Pascal, R.; Ralph, R.; Klaus, M.; Harald, B.; Roman, F. Complex interplay and hierarchy of interactions in two-dimensional supramolecular assemblies. ACS Nano 2011, 5, 457–469. [Google Scholar]
- Silien, C.; Räisänen, M.T.; Buck, M. A Supramolecular Network as Sacrificial Mask for the Generation of a Nanopatterned Binary Self-Assembled Monolayer. Small 2010, 6, 391–394. [Google Scholar] [CrossRef]
- Lu, J.; Lei, S.B.; Zeng, Q.D.; Kang, S.Z.; Wang, C.; Wan, L.J. Template-induced inclusion structures with copper(II) phthal-ocyanine and coronene as guests in two-dimensional hydrogen-bonded host networks. J. Phys. Chem. B 2004, 108, 5161–5165. [Google Scholar] [CrossRef]
- Griessl, S.; Lackinger, M.; Edelwirth, M.; Hietschold, M.; Heckl, W.M. Self-assembled two-dimensional molecular host-guest architectures from trimesic acid. Single Mol. 2002, 3, 25–31. [Google Scholar] [CrossRef]
- Park, K.-W.; Adisoejoso, J.; Plas, J.; Hong, J.; Müllen, K.; De Feyter, S. Self-Assembly Behavior of Alkylated Isophthalic Acids Revisited: Concentration in Control and Guest-Induced Phase Transformation. Langmuir 2014, 30, 15206–15211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velpula, G.; Takeda, T.; Adisoejoso, J.; Inukai, K.; Tahara, K.; Mali, K.S.; Tobe, Y.; De Feyter, S. On the formation of concentric 2D multicomponent assemblies at the solution–solid interface. Chem. Commun. 2017, 53, 1108–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griessl, S.J.H.; Lackinger, M.; Jamitzky, F.; Markert, T.; Hietschold, M.; Heckl, W.M. Room-Temperature Scanning Tunneling Microscopy Manipulation of Single C60 Molecules at the Liquid−Solid Interface: Playing Nanosoccer. J. Phys. Chem. B 2004, 108, 11556–11560. [Google Scholar] [CrossRef]
- Dong, M.; Miao, X.; Brisse, R.; Deng, W.; Jousselme, B.; Silly, F. Molecular trapping in two-dimensional chiral organic Kagomé nanoarchitectures composed of Baravelle spiral triangle enantiomers. NPG Asia Mater. 2020, 12, 20. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, C.; Yin, S.; Zeng, Q.; Xu, A.B.; Bai, C. Self-Assembly and Immobilization of Metallophthalocyanines by Alkyl Substituents Observed with Scanning Tunneling Microscopy. J. Phys. Chem. B 2000, 104, 3570–3574. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Lei, S. Host–guest architectures with a surface confined imine covalent organic framework as two-dimensional host networks. Chem. Commun. 2016, 52, 8691–8694. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Temperature dependent 2D self-assembled motif transition of cop-per−phthalocyanine derivates at air/HOPG interface: An STM study. RSC Adv. 2014, 4, 20256–20261. [Google Scholar] [CrossRef]
- Melville, O.A.; Grant, T.M.; Lochhead, K.; King, B.; Ambrose, R.; Rice, N.A.; Boileau, N.T.; Peltekoff, A.J.; Tousignant, M.N.; Hill, I.G.; et al. Contact Engineering Using Manganese, Chromium, and Bathocuproine in Group 14 Phthalocyanine Organic Thin-Film Transistors. ACS Appl. Electron. Mater. 2020, 2, 1313–1322. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.L.; Xu, J.J.; Lei, H.W.; Chen, C.; Shan, H.Q.; Liu, X.Y.; Xu, Z.X.; Fang, G.J. A facile molecularly engineered copper(II) phthalocyanine as hole transport material for planar perovskite solar cells with enhanced performance and stability. Nano Energy 2017, 31, 322–330. [Google Scholar] [CrossRef]
- Lai, Q.; Quadir, M.Z.; Aguey-Zinsou, K.F. LiBH4 electronic destabilization with nickel(II) phthalocyanine-leading to a reversible hydrogen storage system. ACS Appl. Energy Mater. 2018, 1, 6824–6832. [Google Scholar] [CrossRef]
- Zentner, C.A.; Lai, H.W.H.; Greenfield, J.T.; Wiscons, R.A.; Zeller, M.; Campana, C.F.; Talu, O.; FitzGerald, S.A.; Rowsell, J.L.C. High surface area and Z′ in a thermally stable 8-fold polycatenated hydrogen-bonded framework. Chem. Commun. 2015, 51, 11642–11645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zeng, Q.; Wang, C. Molecular templates and nano-reactors: Two-dimensional hydrogen bonded supramolecular networks on solid/liquid interfaces. RSC Adv. 2013, 3, 11351–11366. [Google Scholar] [CrossRef]
- Zhou, H.; Wuest, J.D. Crankshafts: Using Simple, Flat C2h-Symmetric Molecules to Direct the Assembly of Chiral 2D Nanopatterns. Langmuir 2012, 29, 7229–7238. [Google Scholar] [CrossRef]
- Hu, F.-Y.; Zhang, X.-M.; Wang, X.-C.; Wang, S.; Wang, H.-Q.; Duan, W.-B.; Zeng, Q.-D.; Wang, C. In Situ STM Investigation of Two-Dimensional Chiral Assemblies through Schiff-Base Condensation at a Liquid/Solid Interface. ACS Appl. Mater. Interfaces 2013, 5, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Cheon, Y.E.; Lee, E.Y. Syntheses and functions of porous metallosupramolecular networks. Coord. Chem. Rev. 2008, 252, 1007–1026. [Google Scholar] [CrossRef]
- Silly, F. Two-dimensional 1,3,5-tris(4-carboxyphenyl)benzene self-assembly at the 1-phenyloctane/graphite interface revisited. J. Phys. Chem. C 2012, 116, 10029–10032. [Google Scholar] [CrossRef] [Green Version]
- Miao, K.; Hu, Y.; Zha, B.; Xu, L.; Miao, X.R.; Deng, W.L. Hydroxyl vs. carboxyl substituent: Effects of competitive and coop-erative multiple hydrogen bonds on concentration-controlled self-assembly. J. Phys. Chem. C 2016, 120, 14187–14197. [Google Scholar] [CrossRef]
- Brisse, R.; Praveen, C.; Maffeis, V.; Bourgeteau, T.; Tondelier, D.; Berthelot, T.; Geffroy, B.; Gustavsson, T.; Raimundo, J.M.; Jousselme, B. A red to blue series of push–pull dyes for NiO based p-DSSCs. Sustain. Energy Fuels 2018, 2, 648–654. [Google Scholar] [CrossRef]
- Silly, F. A robust method for processing scanning probe microscopy images and determining nanoobject position and dimensions. J. Microsc-Oxford 2010, 236, 211–218. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F. Handbook of Thiophene-Based Materials 2v Set: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2009. [Google Scholar]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Ouchi, H.; Kizaki, T.; Yamato, M.; Lin, X.; Hoshi, N.; Silly, F.; Kajitani, T.; Fukushima, T.; Nakayama, K.-I.; Yagai, S. Impact of helical organization on the photovoltaic properties of oligothiophene supramolecular polymers. Chem. Sci. 2018, 9, 3638–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieulle, J.; Silly, F. Localized intermolecular electronic coupling in two-dimensional self-assembled 3,4,9,10-perylenetetracarboxylic diimide nanoarchitectures. J. Mater. Chem. C 2013, 1, 4536–4539. [Google Scholar] [CrossRef] [Green Version]
- Imada, H.; Miwa, K.; Imai-Imada, M.; Kawahara, S.; Kimura, K.; Kim, Y. Real-space investigation of energy transfer in het-erogeneous molecular dimers. Nature 2016, 538, 364–367. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Miao, X.; Deng, W.; Brisse, R.; Jousselme, B.; Silly, F. Coronene and Phthalocyanine Trapping Efficiency of a Two-Dimensional Kagomé Host-Nanoarchitecture. Nanomaterials 2022, 12, 775. https://doi.org/10.3390/nano12050775
Wang Y, Miao X, Deng W, Brisse R, Jousselme B, Silly F. Coronene and Phthalocyanine Trapping Efficiency of a Two-Dimensional Kagomé Host-Nanoarchitecture. Nanomaterials. 2022; 12(5):775. https://doi.org/10.3390/nano12050775
Chicago/Turabian StyleWang, Yi, Xinrui Miao, Wenli Deng, Romain Brisse, Bruno Jousselme, and Fabien Silly. 2022. "Coronene and Phthalocyanine Trapping Efficiency of a Two-Dimensional Kagomé Host-Nanoarchitecture" Nanomaterials 12, no. 5: 775. https://doi.org/10.3390/nano12050775
APA StyleWang, Y., Miao, X., Deng, W., Brisse, R., Jousselme, B., & Silly, F. (2022). Coronene and Phthalocyanine Trapping Efficiency of a Two-Dimensional Kagomé Host-Nanoarchitecture. Nanomaterials, 12(5), 775. https://doi.org/10.3390/nano12050775