Light-Driven Charge Transport and Optical Sensing in Molecular Junctions
Abstract
:1. Introduction
2. Light-Driven Charge Transport in MJs
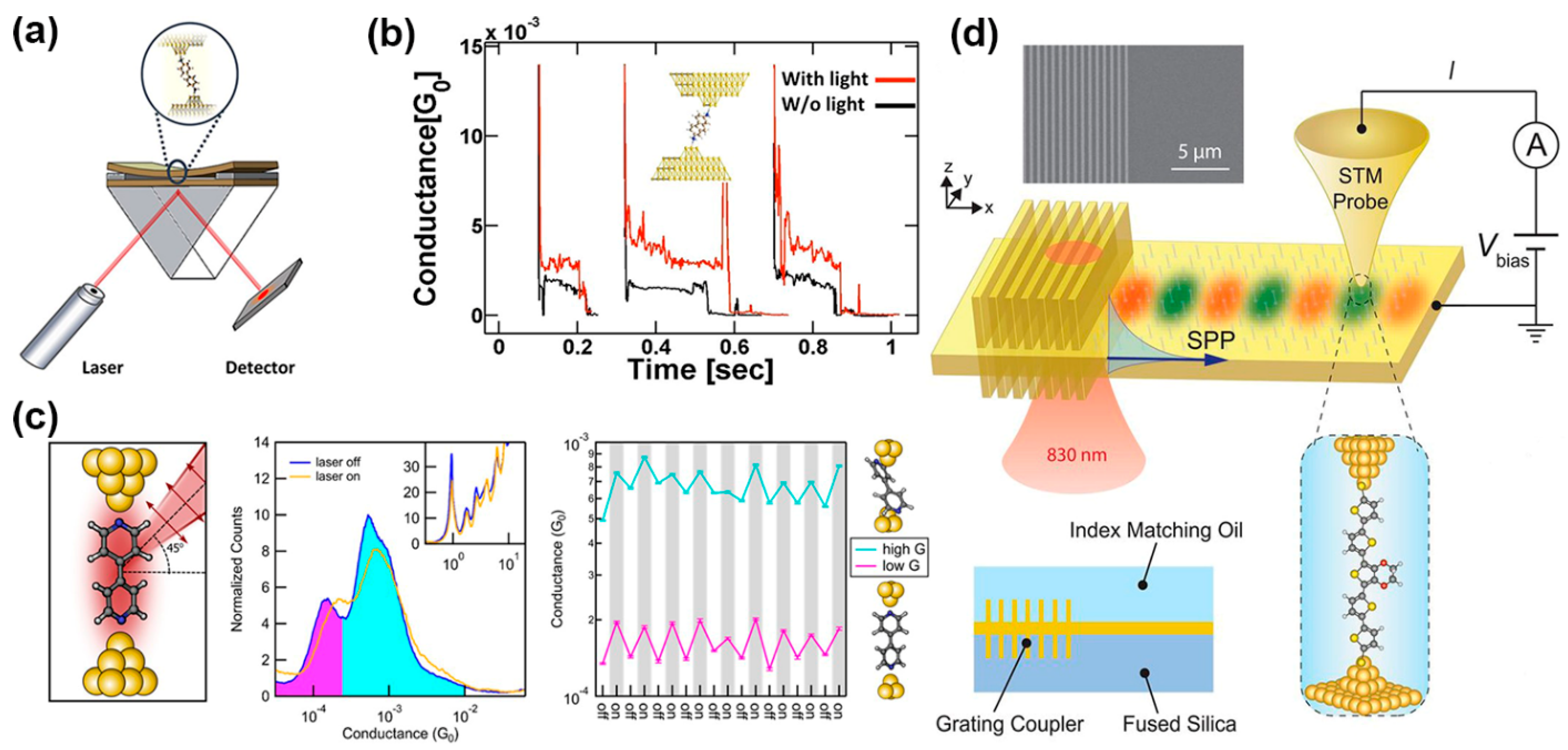
2.1. Conductance Enhancement in Illuminated Single-MJs
2.1.1. Photo-Induced Conductance Mechanisms in Single MJs
2.1.2. Illuminated Metal-Molecule-Semiconductor MJs
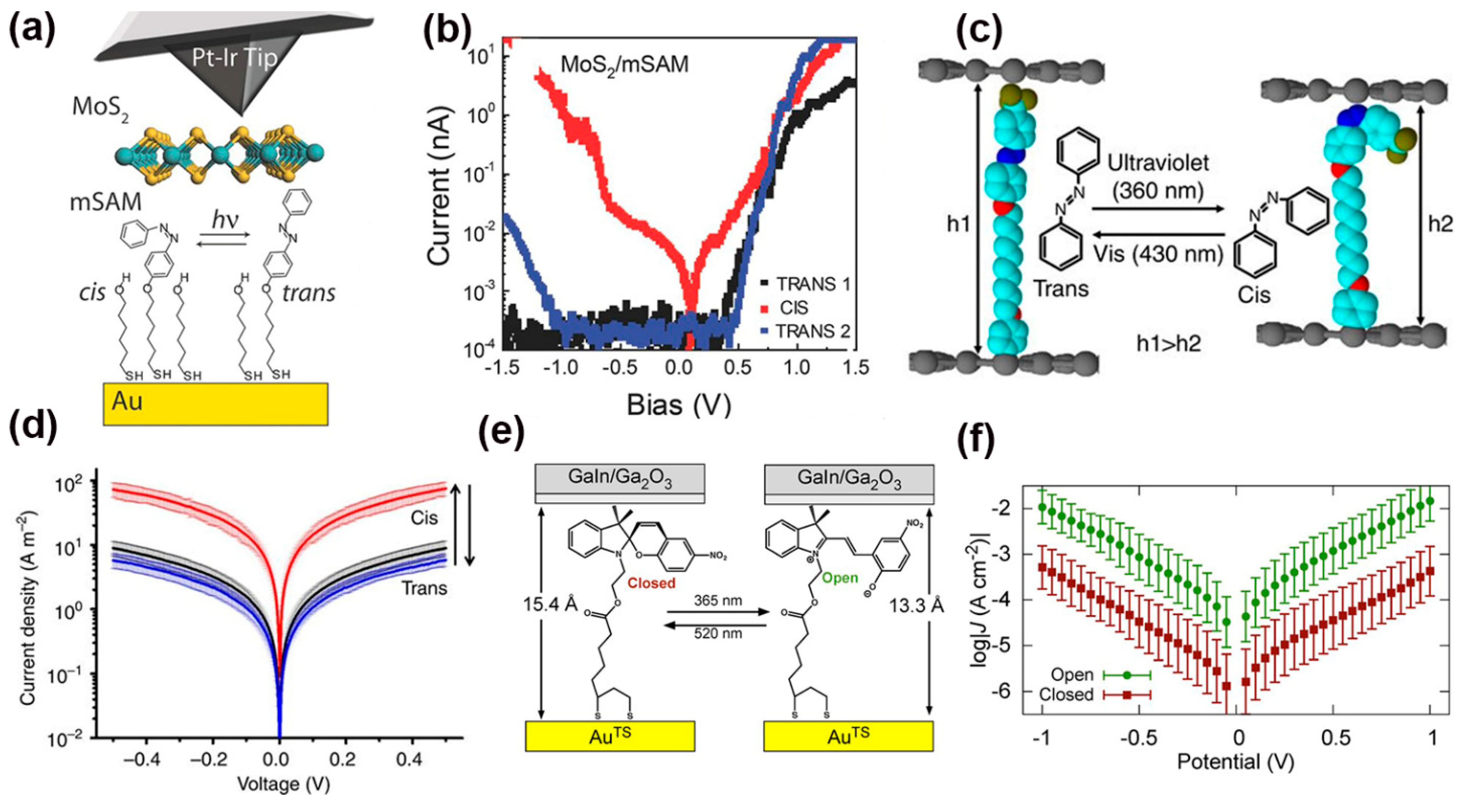
2.2. Photo-Induced Conductance Switching in Single MJs
2.2.1. Photoswitches Based on Diarylethene Molecules
2.2.2. Photoswitches Based on Azobenzene Molecules
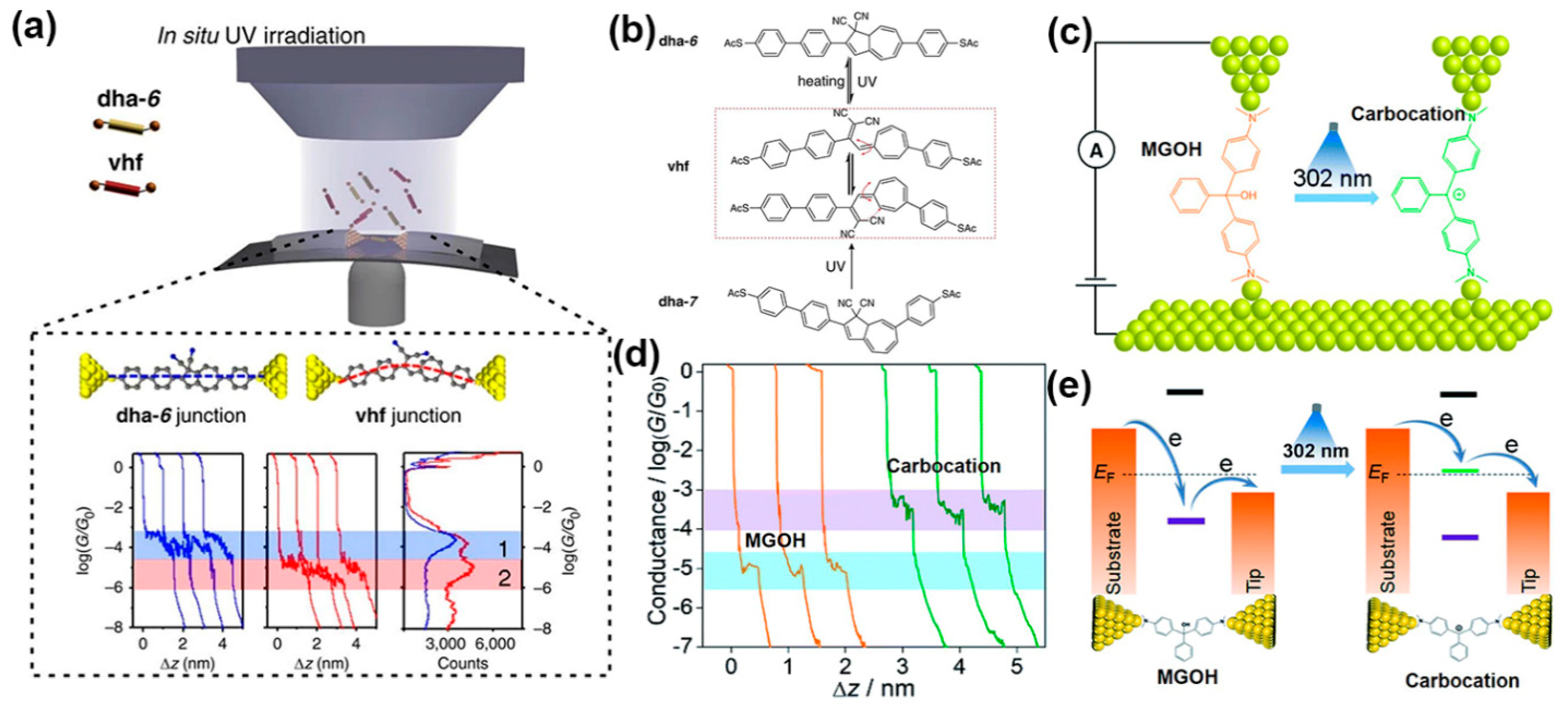
2.2.3. Photoswitches Based on Dihydropyrene Molecules

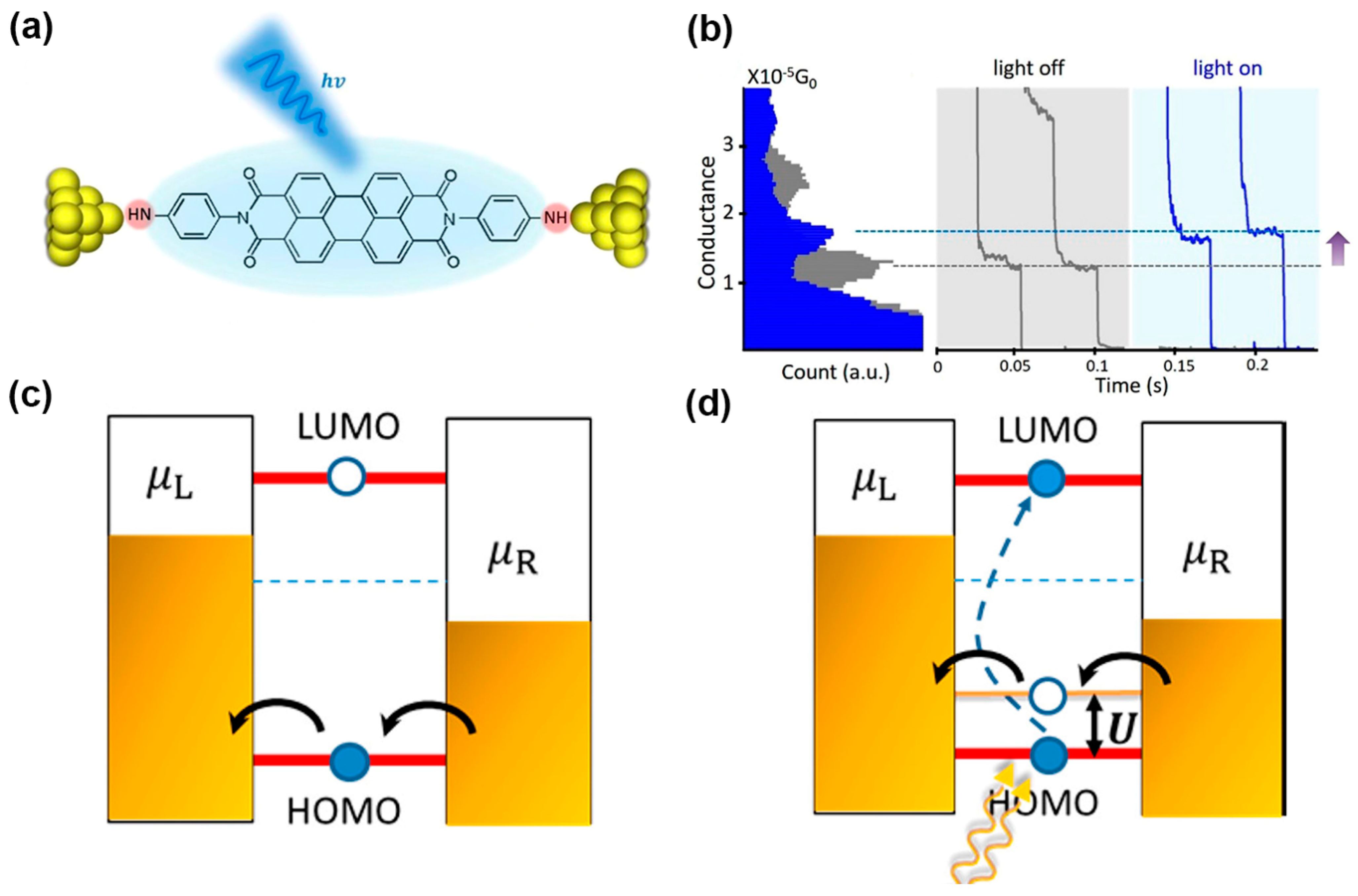
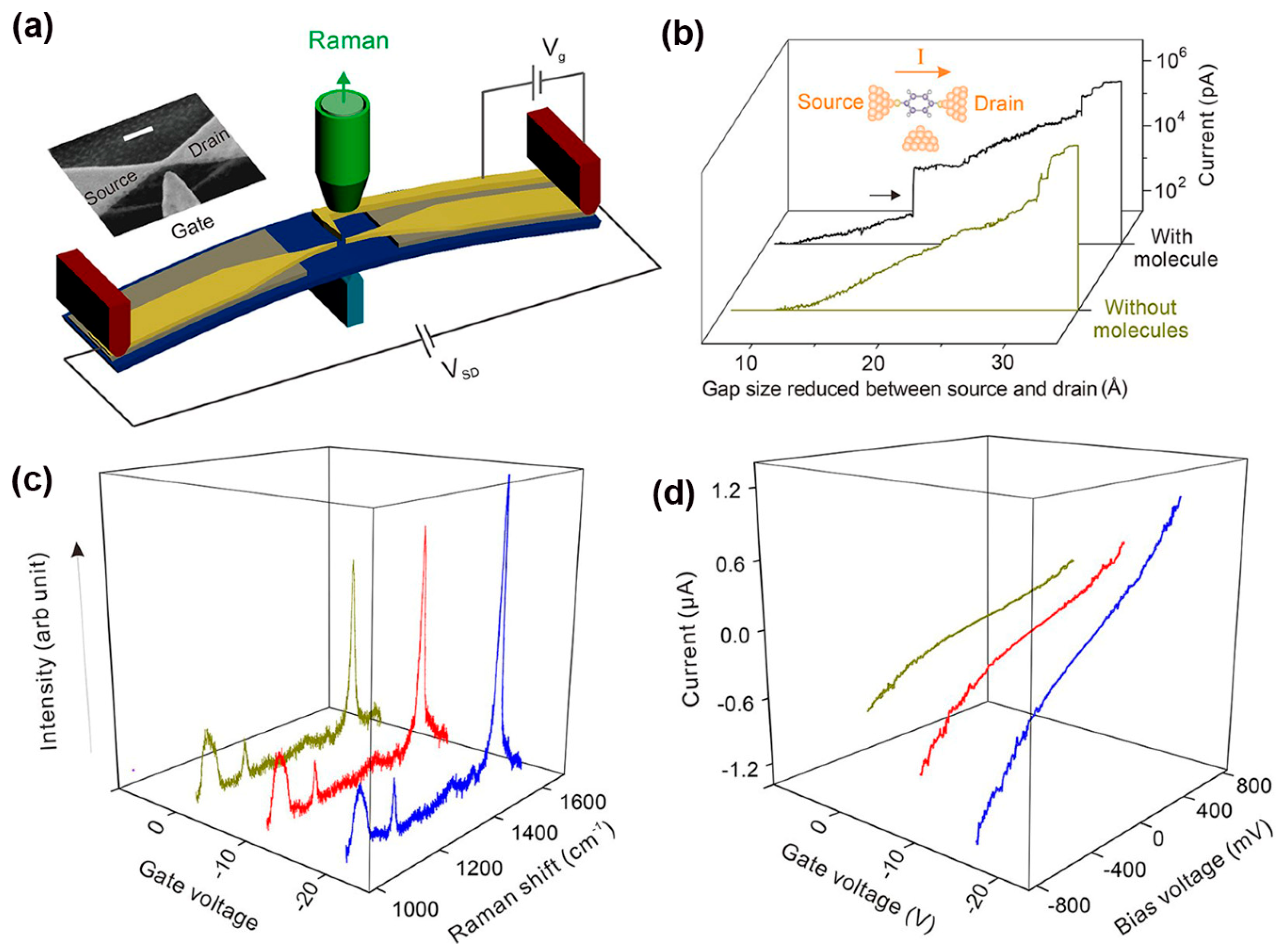
2.3. Photoemission in MJs
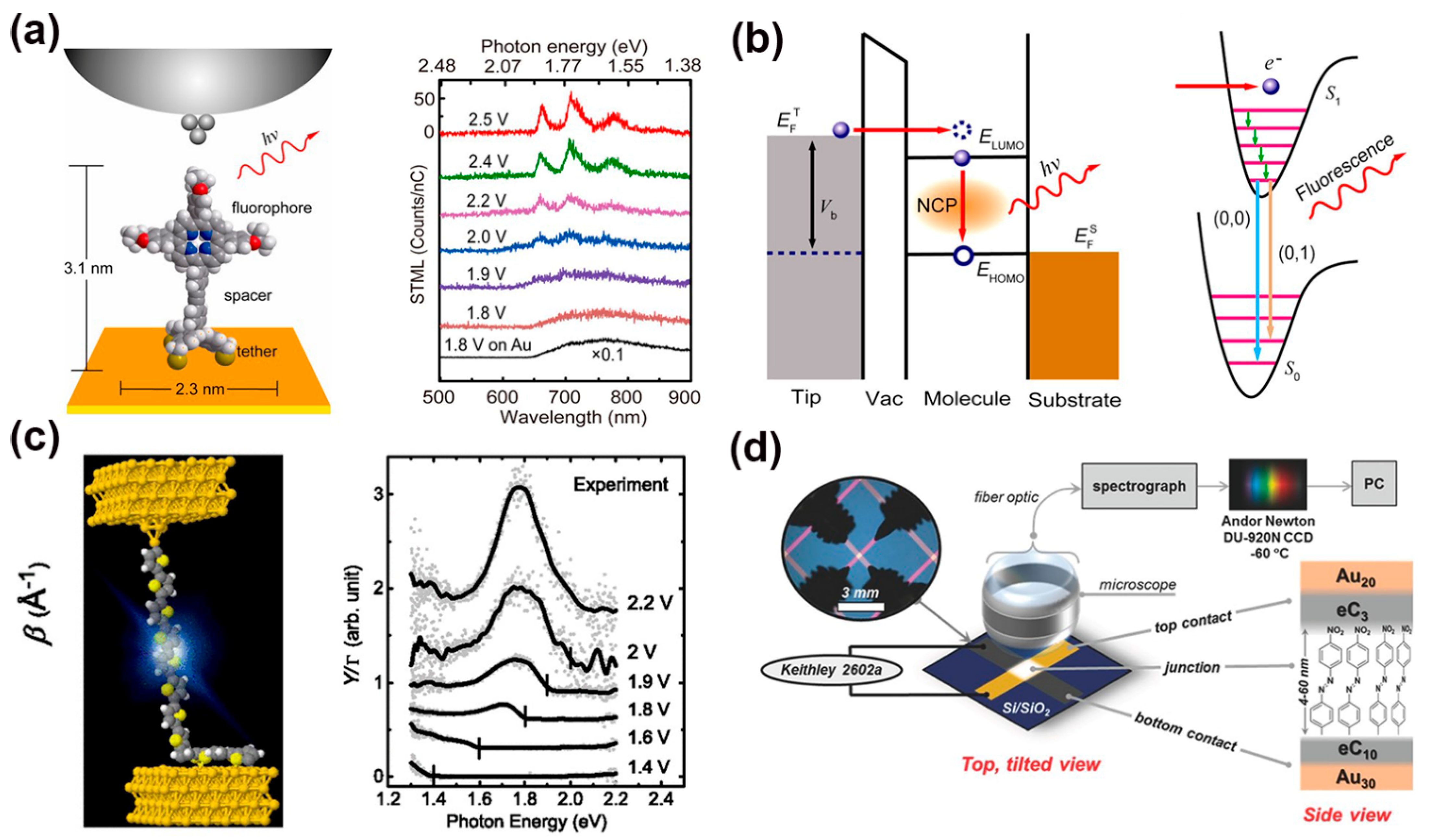
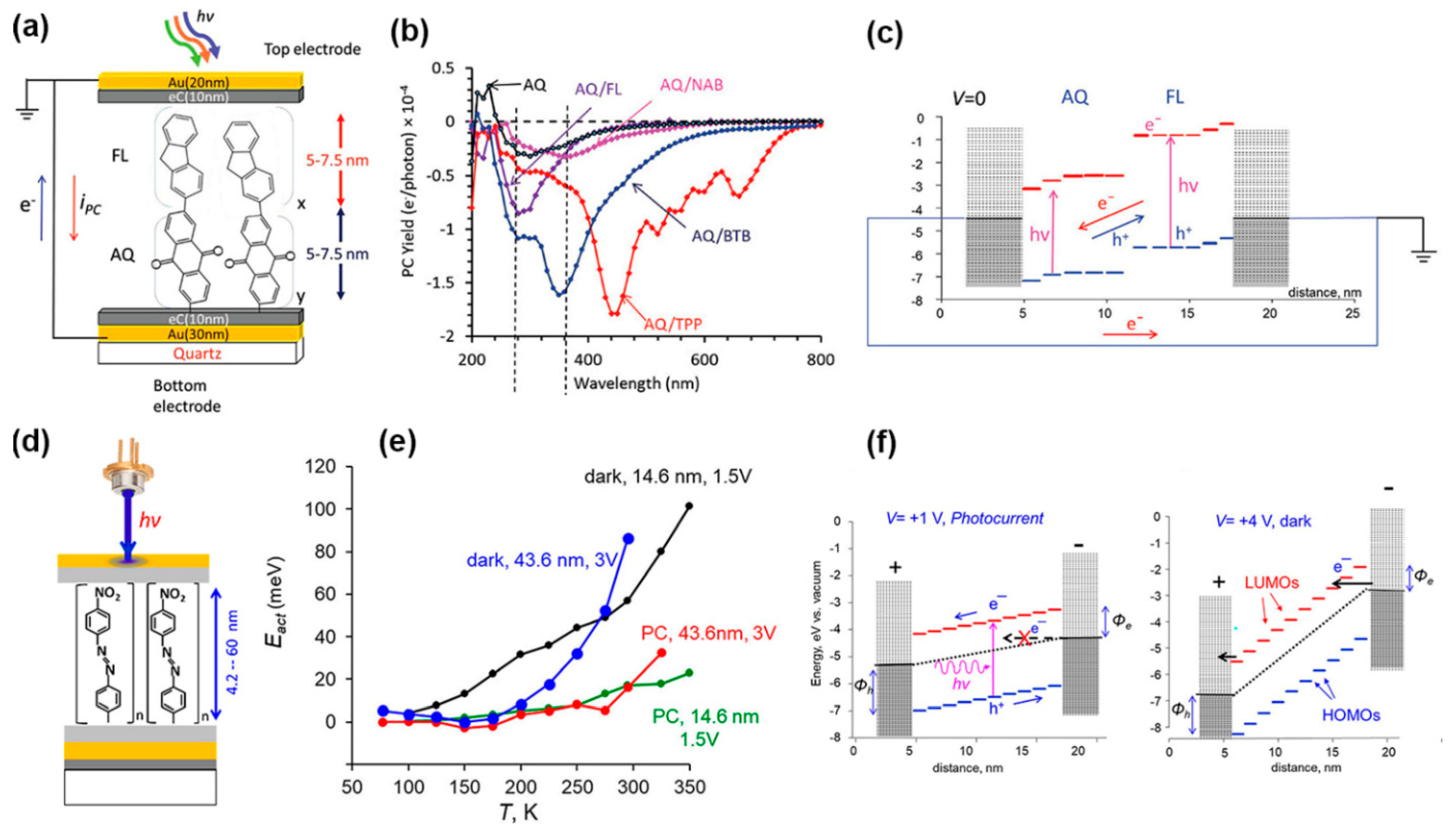
2.4. Optoelectronic in SAM Junctions
2.4.1. Photo-Induced Transport in SAM Junctions
2.4.2. Photoswitches in SAM Junctions
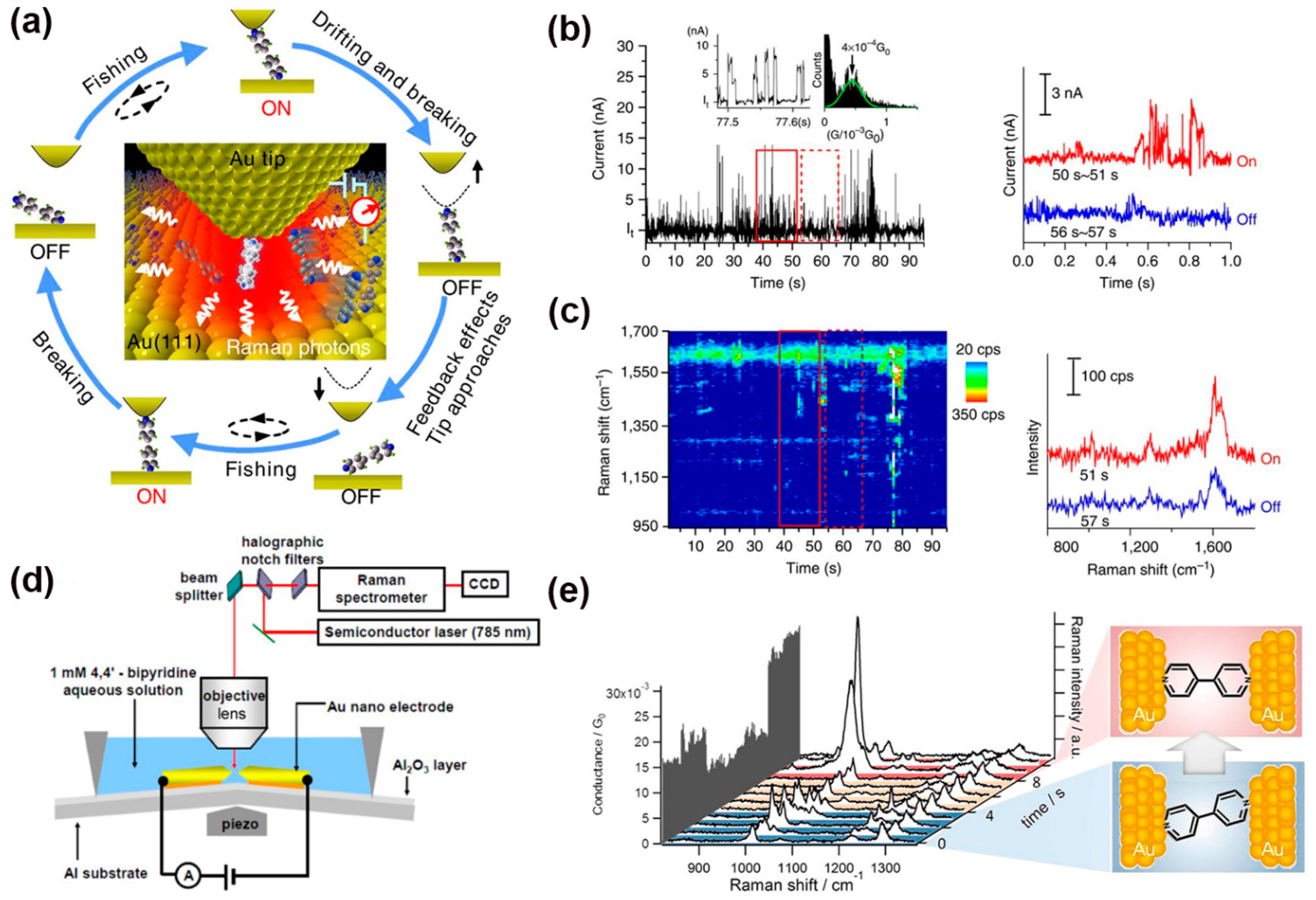
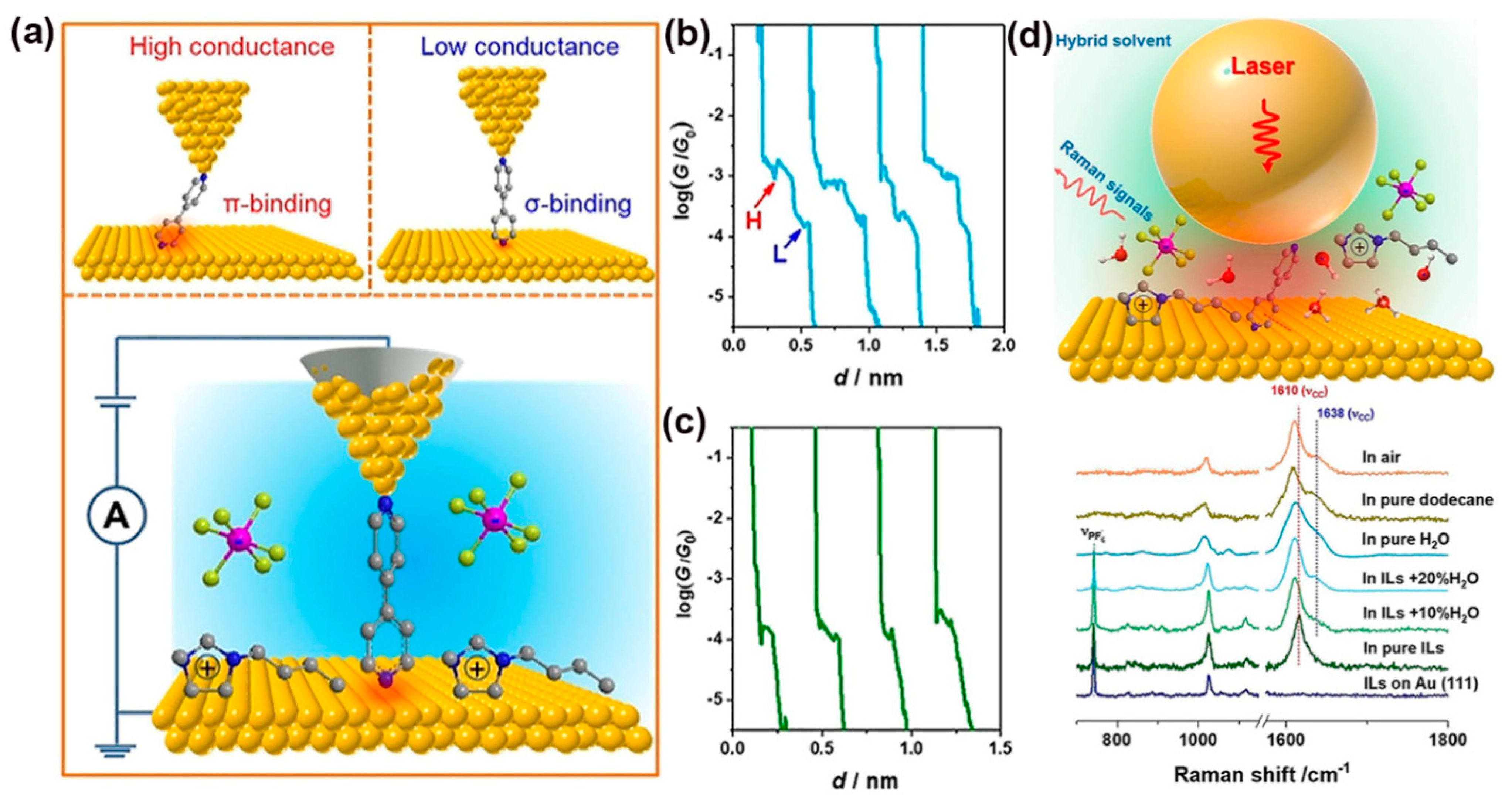
3. Raman Sensing in MJs
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aviram, A.; Ratner, M.A. Molecular rectifiers. Chem. Phys. Lett. 1974, 29, 277–283. [Google Scholar] [CrossRef]
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a Molecular Junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef] [Green Version]
- Di Ventra, M.; Pantelides, S.T.; Lang, N.D. First-Principles Calculation of Transport Properties of a Molecular Device. Phys. Rev. Lett. 2000, 84, 979–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porath, D.; Bezryadin, A.; de Vries, S.; Dekker, C. Direct measurement of electrical transport through DNA molecules. Nature 2000, 403, 635–638. [Google Scholar] [CrossRef]
- Reichert, J.; Ochs, R.; Beckmann, D.; Weber, H.B.; Mayor, M.; Löhneysen, H.v. Driving Current through Single Organic Molecules. Phys. Rev. Lett. 2002, 88, 176804. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Datta, S.; Ratner, M.A. First-principles based matrix Green’s function approach to molecular electronic devices: General formalism. Chem. Phys. 2002, 281, 151–170. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Tao, N.J. Measurement of Single-Molecule Resistance by Repeated Formation of Molecular Junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, L.; Klare, J.E.; Nuckolls, C.; Hybertsen, M.S.; Steigerwald, M.L. Dependence of single-molecule junction conductance on molecular conformation. Nature 2006, 442, 904–907. [Google Scholar] [CrossRef] [Green Version]
- Ratner, M. A brief history of molecular electronics. Nat. Nanotechnol. 2013, 8, 378–381. [Google Scholar] [CrossRef]
- Guo, C.; Wang, K.; Zerah-Harush, E.; Hamill, J.; Wang, B.; Dubi, Y.; Xu, B. Molecular rectifier composed of DNA with high rectification ratio enabled by intercalation. Nat. Chem. 2016, 8, 484–490. [Google Scholar] [CrossRef]
- Jia, C.; Migliore, A.; Xin, N.; Huang, S.; Wang, J.; Yang, Q.; Wang, S.; Chen, H.; Wang, D.; Feng, B.; et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 2016, 352, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Roemer, M.; Yuan, L.; Du, W.; Thompson, D.; del Barco, E.; Nijhuis, C.A. Molecular diodes with rectification ratios exceeding 105 driven by electrostatic interactions. Nat. Nanotechnol. 2017, 12, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Martin, P.; Frath, D.; Della Rocca, M.L.; Lafolet, F.; Barraud, C.; Lafarge, P.; Mukundan, V.; James, D.; McCreery, R.L.; et al. Control of Rectification in Molecular Junctions: Contact Effects and Molecular Signature. J. Am. Chem. Soc. 2017, 139, 11913–11922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Nijhuis, C.A. Functional Redox-Active Molecular Tunnel Junctions. Chem. Asian J. 2020, 15, 3752–3770. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blanco, J.; Nacci, C.; Erwin, S.C.; Kanisawa, K.; Locane, E.; Thomas, M.; von Oppen, F.; Brouwer, P.W.; Fölsch, S. Gating a single-molecule transistor with individual atoms. Nat. Phys. 2015, 11, 640–644. [Google Scholar] [CrossRef]
- Bai, J.; Daaoub, A.; Sangtarash, S.; Li, X.; Tang, Y.; Zou, Q.; Sadeghi, H.; Liu, S.; Huang, X.; Tan, Z.; et al. Anti-resonance features of destructive quantum interference in single-molecule thiophene junctions achieved by electrochemical gating. Nat. Mater. 2019, 18, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zheng, H.; Hu, C.; Cai, K.; Jiao, Y.; Zhang, L.; Jiang, F.; Roy, I.; Qiu, Y.; Shen, D.; et al. Giant Conductance Enhancement of Intramolecular Circuits through Interchannel Gating. Matter 2020, 2, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Huang, L.; Sangtarash, S.; Noori, M.; Sadeghi, H.; Xia, H.; Hong, W. Reversible Switching between Destructive and Constructive Quantum Interference Using Atomically Precise Chemical Gating of Single-Molecule Junctions. J. Am. Chem. Soc. 2021, 143, 9385–9392. [Google Scholar] [CrossRef]
- Quek, S.Y.; Kamenetska, M.; Steigerwald, M.L.; Choi, H.J.; Louie, S.G.; Hybertsen, M.S.; Neaton, J.B.; Venkataraman, L. Mechanically controlled binary conductance switching of a single-molecule junction. Nat. Nanotechnol. 2009, 4, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Su, T.A.; Li, H.; Steigerwald, M.L.; Venkataraman, L.; Nuckolls, C. Stereoelectronic switching in single-molecule junctions. Nat. Chem. 2015, 7, 215–220. [Google Scholar] [CrossRef]
- Ismael, A.K.; Wang, K.; Vezzoli, A.; Al-Khaykanee, M.K.; Gallagher, H.E.; Grace, I.M.; Lambert, C.J.; Xu, B.; Nichols, R.J.; Higgins, S.J. Side-Group-Mediated Mechanical Conductance Switching in Molecular Junctions. Angew. Chem. Int. Ed. 2017, 56, 15378–15382. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hur, S.; Akbar, Z.A.; Klöckner, J.C.; Jeong, W.; Pauly, F.; Jang, S.-Y.; Reddy, P.; Meyhofer, E. Thermal conductance of single-molecule junctions. Nature 2019, 572, 628–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosso, N.; Sadeghi, H.; Gemma, A.; Sangtarash, S.; Drechsler, U.; Lambert, C.; Gotsmann, B. Thermal Transport through Single-Molecule Junctions. Nano Lett. 2019, 19, 7614–7622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.; Jang, S.-Y.; Segalman, R.A.; Majumdar, A. Thermoelectricity in Molecular Junctions. Science 2007, 315, 1568–1571. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, W.; Kim, K.; Lee, W.; Reddy, P. Electrostatic control of thermoelectricity in molecular junctions. Nat. Nanotechnol. 2014, 9, 881–885. [Google Scholar] [CrossRef]
- Kim, Y. Photoswitching Molecular Junctions: Platforms and Electrical Properties. ChemPhysChem 2020, 21, 2368–2383. [Google Scholar] [CrossRef]
- Huang, X.; Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Diez Cabanes, V.; Van Dyck, C.; Osella, S.; Cornil, D.; Cornil, J. Challenges for Incorporating Optical Switchability in Organic-Based Electronic Devices. ACS Appl. Mater. Interfaces 2021, 13, 27737–27748. [Google Scholar] [CrossRef]
- Schwöbel, J.; Fu, Y.; Brede, J.; Dilullo, A.; Hoffmann, G.; Klyatskaya, S.; Ruben, M.; Wiesendanger, R. Real-space observation of spin-split molecular orbitals of adsorbed single-molecule magnets. Nat. Commun. 2012, 3, 953. [Google Scholar] [CrossRef] [Green Version]
- Aragonès, A.C.; Aravena, D.; Cerdá, J.I.; Acís-Castillo, Z.; Li, H.; Real, J.A.; Sanz, F.; Hihath, J.; Ruiz, E.; Díez-Pérez, I. Large Conductance Switching in a Single-Molecule Device through Room Temperature Spin-Dependent Transport. Nano Lett. 2016, 16, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Chen, H.; Pope, T.; Hu, Y.; Liu, L.; Wang, D.; Tao, L.; Xiao, W.; Fei, X.; Zhang, Y.-Y.; et al. Tunable giant magnetoresistance in a single-molecule junction. Nat. Commun. 2019, 10, 3599. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.N.; Li, D.; Sarkar, S.; Chakrabarti, S.; Vilan, A.; Kronik, L.; Smogunov, A.; Tal, O. Nonmagnetic single-molecule spin-filter based on quantum interference. Nat. Commun. 2019, 10, 5565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willke, P.; Bilgeri, T.; Zhang, X.; Wang, Y.; Wolf, C.; Aubin, H.; Heinrich, A.; Choi, T. Coherent Spin Control of Single Molecules on a Surface. ACS Nano 2021, 15, 17959–17965. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, X.; Li, P.; Li, M.; Yang, L.; Jia, C.; Guo, X. Recent progress in single-molecule transistors: Their designs, mechanisms and applications. J. Mater. Chem. C 2022, 10, 2375–2389. [Google Scholar] [CrossRef]
- Wang, K.; Meyhofer, E.; Reddy, P. Thermal and Thermoelectric Properties of Molecular Junctions. Adv. Funct. Mater. 2020, 30, 1904534. [Google Scholar] [CrossRef]
- Ke, G.; Duan, C.; Huang, F.; Guo, X. Electrical and spin switches in single-molecule junctions. InfoMat 2020, 2, 92–112. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef]
- Reecht, G.; Scheurer, F.; Speisser, V.; Dappe, Y.J.; Mathevet, F.; Schull, G. Electroluminescence of a Polythiophene Molecular Wire Suspended between a Metallic Surface and the Tip of a Scanning Tunneling Microscope. Phys. Rev. Lett. 2014, 112, 047403. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; Miwa, K.; Imada, H.; Imai-Imada, M.; Kawahara, S.; Takeya, J.; Kawai, M.; Galperin, M.; Kim, Y. Selective triplet exciton formation in a single molecule. Nature 2019, 570, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, G.; Ghafoor, A.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Yang, J.; Sandoghdar, V.; Aizpurua, J.; et al. Sub-nanometre resolution in single-molecule photoluminescence imaging. Nat. Photonics 2020, 14, 693–699. [Google Scholar] [CrossRef]
- Sorgenfrei, S.; Chiu, C.-y.; Gonzalez, R.L.; Yu, Y.-J.; Kim, P.; Nuckolls, C.; Shepard, K.L. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat. Nanotechnol. 2011, 6, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X. Single-Molecule Electrical Biosensors Based on Single-Walled Carbon Nanotubes. Adv. Mater. 2013, 25, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Li, W.; Chen, J.; He, G.; Li, L.; Olson, M.A.; Sue, A.C.-H.; Stoddart, J.F.; Guo, X. Complex formation dynamics in a single-molecule electronic device. Sci. Adv. 2016, 2, e1601113. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Jia, C.; Li, Y.; Liu, Z.; Wang, J.; Yang, Z.; Gu, C.; Su, D.; Houk, K.N.; Zhang, D.; et al. Direct single-molecule dynamic detection of chemical reactions. Sci. Adv. 2018, 4, eaar2177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhen, Y.-R.; Neumann, O.; Day, J.K.; Nordlander, P.; Halas, N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance. Nat. Commun. 2014, 5, 4424. [Google Scholar] [CrossRef]
- Chikkaraddy, R.; de Nijs, B.; Benz, F.; Barrow, S.J.; Scherman, O.A.; Rosta, E.; Demetriadou, A.; Fox, P.; Hess, O.; Baumberg, J.J. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 2016, 535, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Jaculbia, R.B.; Imada, H.; Miwa, K.; Iwasa, T.; Takenaka, M.; Yang, B.; Kazuma, E.; Hayazawa, N.; Taketsugu, T.; Kim, Y. Single-molecule resonance Raman effect in a plasmonic nanocavity. Nat. Nanotechnol. 2020, 15, 105–110. [Google Scholar] [CrossRef]
- Huang, C.; Jevric, M.; Borges, A.; Olsen, S.T.; Hamill, J.M.; Zheng, J.-T.; Yang, Y.; Rudnev, A.; Baghernejad, M.; Broekmann, P.; et al. Single-molecule detection of dihydroazulene photo-thermal reaction using break junction technique. Nat. Commun. 2017, 8, 15436. [Google Scholar] [CrossRef] [Green Version]
- Sendler, T.; Luka-Guth, K.; Wieser, M.; Lokamani; Wolf, J.; Helm, M.; Gemming, S.; Kerbusch, J.; Scheer, E.; Huhn, T.; et al. Light-Induced Switching of Tunable Single-Molecule Junctions. Adv. Sci. 2015, 2, 1500017. [Google Scholar] [CrossRef] [Green Version]
- Reddy, H.; Wang, K.; Kudyshev, Z.; Zhu, L.; Yan, S.; Vezzoli, A.; Higgins, S.J.; Gavini, V.; Boltasseva, A.; Reddy, P.; et al. Determining plasmonic hot-carrier energy distributions via single-molecule transport measurements. Science 2020, 369, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Doppagne, B.; Chong, M.C.; Bulou, H.; Boeglin, A.; Scheurer, F.; Schull, G. Electrofluorochromism at the single-molecule level. Science 2018, 361, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Nijs, B.; Benz, F.; Barrow, S.J.; Sigle, D.O.; Chikkaraddy, R.; Palma, A.; Carnegie, C.; Kamp, M.; Sundararaman, R.; Narang, P.; et al. Plasmonic tunnel junctions for single-molecule redox chemistry. Nat. Commun. 2017, 8, 994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessel, J. Surface-enhanced optical microscopy. J. Opt. Soc. Am. B 1985, 2, 1538–1541. [Google Scholar] [CrossRef]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Hayazawa, N.; Inouye, Y.; Sekkat, Z.; Kawata, S. Metallized tip amplification of near-field Raman scattering. Opt. Commun. 2000, 183, 333–336. [Google Scholar] [CrossRef]
- Anderson, M.S. Locally enhanced Raman spectroscopy with an atomic force microscope. Appl. Phys. Lett. 2000, 76, 3130–3132. [Google Scholar] [CrossRef]
- Tian, J.-H.; Liu, B.; Li, X.; Yang, Z.-L.; Ren, B.; Wu, S.-T.; Tao, N.; Tian, Z.-Q. Study of Molecular Junctions with a Combined Surface-Enhanced Raman and Mechanically Controllable Break Junction Method. J. Am. Chem. Soc. 2006, 128, 14748–14749. [Google Scholar] [CrossRef]
- Gehring, P.; Thijssen, J.M.; van der Zant, H.S.J. Single-molecule quantum-transport phenomena in break junctions. Nat. Rev. Phys. 2019, 1, 381–396. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-Scale Electronics: From Concept to Function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef]
- Daub, J.; Knöchel, T.; Mannschreck, A. Photosensitive Dihydroazulenes with Chromogenic Properties. Angew. Chem. Int. Ed. Engl. 1984, 23, 960–961. [Google Scholar] [CrossRef]
- Broman, S.L.; Nielsen, M.B. Dihydroazulene: From controlling photochromism to molecular electronics devices. Phys. Chem. Chem. Phys. 2014, 16, 21172–21182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irie, M. Light-induced reversible pH change. J. Am. Chem. Soc. 1983, 105, 2078–2079. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Li, F.; Yang, Y.; Wang, W.; Song, Y.; Liu, D. Light-Driven Conformational Switch of i-Motif DNA. Angew. Chem. Int. Ed. 2007, 46, 2515–2517. [Google Scholar] [CrossRef] [PubMed]
- Bei, Z.; Huang, Y.; Chen, Y.; Cao, Y.; Li, J. Photo-induced carbocation-enhanced charge transport in single-molecule junctions. Chem. Sci. 2020, 11, 6026–6030. [Google Scholar] [CrossRef]
- Kuciauskas, D.; Lin, S.; Seely, G.R.; Moore, A.L.; Moore, T.A.; Gust, D.; Drovetskaya, T.; Reed, C.A.; Boyd, P.D.W. Energy and Photoinduced Electron Transfer in Porphyrin−Fullerene Dyads. J. Phys. Chem. 1996, 100, 15926–15932. [Google Scholar] [CrossRef]
- Battacharyya, S.; Kibel, A.; Kodis, G.; Liddell, P.A.; Gervaldo, M.; Gust, D.; Lindsay, S. Optical Modulation of Molecular Conductance. Nano Lett. 2011, 11, 2709–2714. [Google Scholar] [CrossRef]
- Vadai, M.; Nachman, N.; Ben-Zion, M.; Bürkle, M.; Pauly, F.; Cuevas, J.C.; Selzer, Y. Plasmon-Induced Conductance Enhancement in Single-Molecule Junctions. J. Phys. Chem. Lett. 2013, 4, 2811–2816. [Google Scholar] [CrossRef]
- Fung, E.D.; Adak, O.; Lovat, G.; Scarabelli, D.; Venkataraman, L. Too Hot for Photon-Assisted Transport: Hot-Electrons Dominate Conductance Enhancement in Illuminated Single-Molecule Junctions. Nano Lett. 2017, 17, 1255–1261. [Google Scholar] [CrossRef]
- Viljas, J.K.; Cuevas, J.C. Role of electronic structure in photoassisted transport through atomic-sized contacts. Phys. Rev. B 2007, 75, 075406. [Google Scholar] [CrossRef] [Green Version]
- Viljas, J.K.; Pauly, F.; Cuevas, J.C. Photoconductance of organic single-molecule contacts. Phys. Rev. B 2007, 76, 033403. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, K.; Xu, B.; Dubi, Y. Photoconductance from Exciton Binding in Molecular Junctions. J. Am. Chem. Soc. 2018, 140, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Whalley, A.C.; Kamenetska, M.; Steigerwald, M.L.; Hybertsen, M.S.; Nuckolls, C.; Venkataraman, L. Contact Chemistry and Single-Molecule Conductance: A Comparison of Phosphines, Methyl Sulfides, and Amines. J. Am. Chem. Soc. 2007, 129, 15768–15769. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, P.; Gulcur, M.; Manrique, D.Z.; Pope, T.; Hong, W.; Kaliginedi, V.; Huang, C.; Batsanov, A.S.; Bryce, M.R.; Lambert, C.; et al. Single-Molecule Conductance of Functionalized Oligoynes: Length Dependence and Junction Evolution. J. Am. Chem. Soc. 2013, 135, 12228–12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leary, E.; La Rosa, A.; González, M.T.; Rubio-Bollinger, G.; Agraït, N.; Martín, N. Incorporating single molecules into electrical circuits. The role of the chemical anchoring group. Chem. Soc. Rev. 2015, 44, 920–942. [Google Scholar] [CrossRef]
- Vezzoli, A.; Brooke, R.J.; Higgins, S.J.; Schwarzacher, W.; Nichols, R.J. Single-Molecule Photocurrent at a Metal-Molecule-Semiconductor Junction. Nano Lett. 2017, 17, 6702–6707. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, L.; Edelmann, K.; Homberg, J.; Valášek, M.; Bahoosh, S.G.; Lukas, M.; Pauly, F.; Mayor, M.; Wulfhekel, W. An electrically actuated molecular toggle switch. Nat. Commun. 2017, 8, 14672. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Nickle, C.; Zhang, Z.; Astier, H.P.A.G.; Duffin, T.J.; Qi, D.; Wang, Z.; del Barco, E.; Thompson, D.; Nijhuis, C.A. Electric-field-driven dual-functional molecular switches in tunnel junctions. Nat. Mater. 2020, 19, 843–848. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, J.; Ye, Y.; Liu, J.; Chen, L.; Yan, Z.; Chen, Z.; Chen, L.; Huang, X.; Bai, J.; et al. Electric-Field-Induced Connectivity Switching in Single-Molecule Junctions. IScience 2020, 23, 100770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, H.; Guo, X. Charge Transport: Precise Control of Interfacial Charge Transport for Building Functional Optoelectronic Devices. Adv. Mater. Technol. 2019, 4, 1970025. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Wang, J.; Yao, C.; Cao, Y.; Zhong, Y.; Liu, Z.; Liu, Z.; Guo, X. Conductance Switching and Mechanisms in Single-Molecule Junctions. Angew. Chem. Int. Ed. 2013, 52, 8666–8670. [Google Scholar] [CrossRef] [PubMed]
- Taherinia, D.; Frisbie, C.D. Photoswitchable Hopping Transport in Molecular Wires 4 nm in Length. J. Phys. Chem. C 2016, 120, 6442–6449. [Google Scholar] [CrossRef]
- Reecht, G.; Lotze, C.; Sysoiev, D.; Huhn, T.; Franke, K.J. Visualizing the Role of Molecular Orbitals in Charge Transport through Individual Diarylethene Isomers. ACS Nano 2016, 10, 10555–10562. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Jia, C.; Wang, J.; Wang, S.; Li, M.; Gong, Y.; Zhang, G.; Zhu, D.; Guo, X. Thermally Activated Tunneling Transition in a Photoswitchable Single-Molecule Electrical Junction. J. Phys. Chem. Lett. 2017, 8, 2849–2854. [Google Scholar] [CrossRef]
- Xin, N.; Hu, C.; Al Sabea, H.; Zhang, M.; Zhou, C.; Meng, L.; Jia, C.; Gong, Y.; Li, Y.; Ke, G.; et al. Tunable Symmetry-Breaking-Induced Dual Functions in Stable and Photoswitched Single-Molecule Junctions. J. Am. Chem. Soc. 2021, 143, 20811–20817. [Google Scholar] [CrossRef]
- Weston, C.E.; Richardson, R.D.; Haycock, P.R.; White, A.J.P.; Fuchter, M.J. Arylazopyrazoles: Azoheteroarene Photoswitches Offering Quantitative Isomerization and Long Thermal Half-Lives. J. Am. Chem. Soc. 2014, 136, 11878–11881. [Google Scholar] [CrossRef]
- Tallarida, N.; Rios, L.; Apkarian, V.A.; Lee, J. Isomerization of One Molecule Observed through Tip-Enhanced Raman Spectroscopy. Nano Lett. 2015, 15, 6386–6394. [Google Scholar] [CrossRef]
- Chen, X.; Yeoh, Y.Q.; He, Y.; Zhou, C.; Horsley, J.R.; Abell, A.D.; Yu, J.; Guo, X. Unravelling Structural Dynamics within a Photoswitchable Single Peptide: A Step Towards Multimodal Bioinspired Nanodevices. Angew. Chem. Int. Ed. 2020, 59, 22554–22562. [Google Scholar] [CrossRef]
- Meng, L.; Xin, N.; Hu, C.; Wang, J.; Gui, B.; Shi, J.; Wang, C.; Shen, C.; Zhang, G.; Guo, H.; et al. Side-group chemical gating via reversible optical and electric control in a single molecule transistor. Nat. Commun. 2019, 10, 1450. [Google Scholar] [CrossRef]
- Bakkar, A.; Lafolet, F.; Boggio-Pasqua, M.; Jouvenot, D.; Saint-Aman, E.; Cobo, S. Electrochemical control of the switching process of photochromic dimethyldihydropyrene derivatives. Chem. Commun. 2017, 53, 9360–9363. [Google Scholar] [CrossRef]
- Muratsugu, S.; Kishida, M.-a.; Sakamoto, R.; Nishihara, H. Comparative Study of Photochromic Ferrocene-Conjugated Dimethyldihydropyrene Derivatives. Chem. A Eur. J. 2013, 19, 17314–17327. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, G.-P.; Duan, S.; Fu, Q.; Luo, Y. Molecular Design to Enhance the Thermal Stability of a Photo Switchable Molecular Junction Based on Dimethyldihydropyrene and Cyclophanediene Isomerization. J. Phys. Chem. C 2015, 119, 11468–11474. [Google Scholar] [CrossRef]
- Roldan, D.; Kaliginedi, V.; Cobo, S.; Kolivoska, V.; Bucher, C.; Hong, W.; Royal, G.; Wandlowski, T. Charge Transport in Photoswitchable Dimethyldihydropyrene-Type Single-Molecule Junctions. J. Am. Chem. Soc. 2013, 135, 5974–5977. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.; Aragonès, A.C.; Darwish, T.; Ciampi, S.; Díez-Pérez, I. Multi-Responsive Photo- and Chemo-Electrical Single-Molecule Switches. Nano Lett. 2014, 14, 7064–7070. [Google Scholar] [CrossRef]
- Tebikachew, B.E.; Li, H.B.; Pirrotta, A.; Börjesson, K.; Solomon, G.C.; Hihath, J.; Moth-Poulsen, K. Effect of Ring Strain on the Charge Transport of a Robust Norbornadiene-Quadricyclane-Based Molecular Photoswitch. J. Phys. Chem. C 2017, 121, 7094–7100. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Zhou, Y.; Shi, J.; Li, G.; Song, H.; Yang, Y.; Shi, J.; Hong, W. Single Dynamic Covalent Bond Tailored Responsive Molecular Junctions. Angew. Chem. Int. Ed. 2021, 60, 20872–20878. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, K.; Gerhard, L.; Winkler, M.; Wilmes, L.; Rai, V.; Schumann, M.; Kern, C.; Meyer, M.; Wegener, M.; Wulfhekel, W. Light collection from a low-temperature scanning tunneling microscope using integrated mirror tips fabricated by direct laser writing. Rev. Sci. Instrum. 2018, 89, 123107. [Google Scholar] [CrossRef]
- Balzer, N.; Lukášek, J.; Valášek, M.; Rai, V.; Sun, Q.; Gerhard, L.; Wulfhekel, W.; Mayor, M. Synthesis and Surface Behaviour of NDI Chromophores Mounted on a Tripodal Scaffold: Towards Self-Decoupled Chromophores for Single-Molecule Electroluminescence. Chem. A Eur. J. 2021, 27, 12144–12155. [Google Scholar] [CrossRef]
- Tefashe, U.M.; Van Dyck, C.; Saxena, S.K.; Lacroix, J.-C.; McCreery, R.L. Unipolar Injection and Bipolar Transport in Electroluminescent Ru-Centered Molecular Electronic Junctions. J. Phys. Chem. C 2019, 123, 29162–29172. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Kuang, Y.-M.; Geng, F.; Zhu, J.-Z.; Wang, C.-Z.; Yu, Y.-J.; Luo, Y.; Xiao, Y.; Liu, K.-Q.; Meng, Q.-S.; et al. Self-Decoupled Porphyrin with a Tripodal Anchor for Molecular-Scale Electroluminescence. J. Am. Chem. Soc. 2013, 135, 15794–15800. [Google Scholar] [CrossRef]
- Dong, Z.C.; Zhang, X.L.; Gao, H.Y.; Luo, Y.; Zhang, C.; Chen, L.G.; Zhang, R.; Tao, X.; Zhang, Y.; Yang, J.L.; et al. Generation of molecular hot electroluminescence by resonant nanocavity plasmons. Nat. Photonics 2010, 4, 50–54. [Google Scholar] [CrossRef]
- Fereiro, J.A.; Kondratenko, M.; Bergren, A.J.; McCreery, R.L. Internal Photoemission in Molecular Junctions: Parameters for Interfacial Barrier Determinations. J. Am. Chem. Soc. 2015, 137, 1296–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, E.D.; Venkataraman, L. Too Cool for Blackbody Radiation: Overbias Photon Emission in Ambient STM Due to Multielectron Processes. Nano Lett. 2020, 20, 8912–8918. [Google Scholar] [CrossRef] [PubMed]
- Tefashe, U.M.; Nguyen, Q.V.; Lafolet, F.; Lacroix, J.-C.; McCreery, R.L. Robust Bipolar Light Emission and Charge Transport in Symmetric Molecular Junctions. J. Am. Chem. Soc. 2017, 139, 7436–7439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashenko, O.; Bergren, A.J.; McCreery, R.L. Light Emission as a Probe of Energy Losses in Molecular Junctions. J. Am. Chem. Soc. 2016, 138, 722–725. [Google Scholar] [CrossRef]
- Ivashenko, O.; Bergren, A.J.; McCreery, R.L. Monitoring of Energy Conservation and Losses in Molecular Junctions through Characterization of Light Emission. Adv. Electron. Mater. 2016, 2, 1600351. [Google Scholar] [CrossRef]
- Morteza Najarian, A.; Bayat, A.; McCreery, R.L. Orbital Control of Photocurrents in Large Area All-Carbon Molecular Junctions. J. Am. Chem. Soc. 2018, 140, 1900–1909. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.K.; Tefashe, U.M.; Supur, M.; McCreery, R.L. Evaluation of Carbon Based Molecular Junctions as Practical Photosensors. ACS Sens. 2021, 6, 513–522. [Google Scholar] [CrossRef]
- Saxena, S.K.; Smith, S.R.; Supur, M.; McCreery, R.L. Light-Stimulated Charge Transport in Bilayer Molecular Junctions for Photodetection. Adv. Opt. Mater. 2019, 7, 1901053. [Google Scholar] [CrossRef]
- Saxena, S.K.; Tefashe, U.M.; McCreery, R.L. Photostimulated Near-Resonant Charge Transport over 60 nm in Carbon-Based Molecular Junctions. J. Am. Chem. Soc. 2020, 142, 15420–15430. [Google Scholar] [CrossRef]
- Koo, J.; Jang, Y.; Martin, L.; Kim, D.; Jeong, H.; Kang, K.; Lee, W.; Kim, J.; Hwang, W.-T.; Xiang, D.; et al. Unidirectional Real-Time Photoswitching of Diarylethene Molecular Monolayer Junctions with Multilayer Graphene Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 11645–11653. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, H.; Hwang, W.-T.; Jang, Y.; Sysoiev, D.; Scheer, E.; Huhn, T.; Min, M.; Lee, H.; Lee, T. Reversible Switching Phenomenon in Diarylethene Molecular Devices with Reduced Graphene Oxide Electrodes on Flexible Substrates. Adv. Funct. Mater. 2015, 25, 5918–5923. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Wang, G.; Choe, M.; Kim, J.; Lee, S.; Park, S.; Kim, D.-Y.; Lee, B.H.; Lee, T. Electronic properties associated with conformational changes in azobenzene-derivative molecular junctions. Org. Electron. 2011, 12, 2144–2150. [Google Scholar] [CrossRef]
- Cho, D.; Yang, M.; Shin, N.; Hong, S. Mapping reversible photoswitching of molecular resistance fluctuations during the conformational transformation of azobenzene-terminated molecular switches. Nanotechnology 2018, 29, 365704. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Tian, Z.; Ma, J. Light- and Electric-Field-Induced Switching of Thiolated Azobenzene Self-Assembled Monolayer. J. Phys. Chem. C 2013, 117, 19934–19944. [Google Scholar] [CrossRef]
- Gobbi, M.; Bonacchi, S.; Lian, J.X.; Vercouter, A.; Bertolazzi, S.; Zyska, B.; Timpel, M.; Tatti, R.; Olivier, Y.; Hecht, S.; et al. Collective molecular switching in hybrid superlattices for light-modulated two-dimensional electronics. Nat. Commun. 2018, 9, 2661. [Google Scholar] [CrossRef]
- Kumar, S.; Merelli, M.; Danowski, W.; Rudolf, P.; Feringa, B.L.; Chiechi, R.C. Chemical Locking in Molecular Tunneling Junctions Enables Nonvolatile Memory with Large On-Off Ratios. Adv. Mater. 2019, 31, 1807831. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Guérin, D.; Quinard, B.; Jacquet, E.; Mattana, R.; Seneor, P.; Vuillaume, D.; Mélin, T.; Lenfant, S. Conductance switching at the nanoscale of diarylethene derivative self-assembled monolayers on La0.7Sr0.3MnO3. Nanoscale 2020, 12, 8268–8276. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Arbouch, I.; Guérin, D.; Wallart, X.; van Dyck, C.; Mélin, T.; Cornil, J.; Vuillaume, D.; Lenfant, S. Conductance switching of azobenzene-based self-assembled monolayers on cobalt probed by UHV conductive-AFM. Nanoscale 2021, 13, 6977–6990. [Google Scholar] [CrossRef]
- Hnid, I.; Grempka, A.; Khettabi, A.; Sun, X.; Lacroix, J.C.; Lafolet, F.; Cobo, S. Combining Photomodulation and Rectification in Coordination Molecular Wires Based on Dithienylethene Molecular Junctions. J. Phys. Chem. C 2020, 124, 26304–26309. [Google Scholar] [CrossRef]
- Margapoti, E.; Li, J.; Ceylan, Ö.; Seifert, M.; Nisic, F.; Anh, T.L.; Meggendorfer, F.; Dragonetti, C.; Palma, C.-A.; Barth, J.V.; et al. A 2D Semiconductor-Self-Assembled Monolayer Photoswitchable Diode. Adv. Mater. 2015, 27, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Min, M.; Lee, S.M.; Lee, H. Photo-switchable molecular monolayer anchored between highly transparent and flexible graphene electrodes. Nat. Commun. 2013, 4, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; van Herpt, J.T.; Gengler, R.Y.N.; Feringa, B.L.; Rudolf, P.; Chiechi, R.C. Mixed Monolayers of Spiropyrans Maximize Tunneling Conductance Switching by Photoisomerization at the Molecule-Electrode Interface in EGaIn Junctions. J. Am. Chem. Soc. 2016, 138, 12519–12526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hnid, I.; Frath, D.; Lafolet, F.; Sun, X.; Lacroix, J.-C. Highly Efficient Photoswitch in Diarylethene-Based Molecular Junctions. J. Am. Chem. Soc. 2020, 142, 7732–7736. [Google Scholar] [CrossRef] [PubMed]
- Hnid, I.; Liu, M.; Frath, D.; Bellynck, S.; Lafolet, F.; Sun, X.; Lacroix, J.-C. Unprecedented ON/OFF Ratios in Photoactive Diarylethene-Bisthienylbenzene Molecular Junctions. Nano Lett. 2021, 21, 7555–7560. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, S.-Y.; Chen, Z.-B.; Wang, X.; Tian, J.-H.; Anema, J.R.; Zhou, X.-S.; Wu, D.-Y.; Mao, B.-W.; Xu, X.; et al. Revealing the molecular structure of single-molecule junctions in different conductance states by fishing-mode tip-enhanced Raman spectroscopy. Nat. Commun. 2011, 2, 305. [Google Scholar] [CrossRef] [Green Version]
- Konishi, T.; Kiguchi, M.; Takase, M.; Nagasawa, F.; Nabika, H.; Ikeda, K.; Uosaki, K.; Ueno, K.; Misawa, H.; Murakoshi, K. Single Molecule Dynamics at a Mechanically Controllable Break Junction in Solution at Room Temperature. J. Am. Chem. Soc. 2013, 135, 1009–1014. [Google Scholar] [CrossRef]
- Jeong, H.; Domulevicz, L.K.; Hihath, J. Design and Fabrication of a MEMS-Based Break Junction Device for Mechanical Strain-Correlated Optical Characterization of a Single-Molecule. J. Microelectromech. Syst. 2021, 30, 126–136. [Google Scholar] [CrossRef]
- Jeong, H.; Li, H.B.; Domulevicz, L.; Hihath, J. An On-Chip Break Junction System for Combined Single-Molecule Conductance and Raman Spectroscopies. Adv. Funct. Mater. 2020, 30, 2000615. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.-X.; Su, J.-Q.; Radjenovic, P.M.; Wang, Y.-H.; Zheng, J.-F.; Teng, B.; Shao, Y.; Zhou, X.-S.; Li, J.-F. Probing Interfacial Electronic Effects on Single-Molecule Adsorption Geometry and Electron Transport at Atomically Flat Surfaces. Angew. Chem. Int. Ed. 2021, 60, 15452–15458. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Lin, J.-S.; Lin, R.-K.; Radjenovic, P.M.; Yang, W.-M.; Xu, J.; Dong, J.-C.; Yang, Z.-L.; Hang, W.; Tian, Z.-Q.; et al. In situ Raman study of the photoinduced behavior of dye molecules on TiO2(hkl) single crystal surfaces. Chem. Sci. 2020, 11, 6431–6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Chen, X.; Ding, S.-Y.; Mayer, D.; Wang, Q.; Zhao, Z.; Ni, L.; Liu, H.; Lee, T.; Xu, B.; et al. Molecular Orbital Gating Surface-Enhanced Raman Scattering. ACS Nano 2018, 12, 11229–11235. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Shiri, M.; Zhang, H.; Ayinla, R.T.; Wang, K. Light-Driven Charge Transport and Optical Sensing in Molecular Junctions. Nanomaterials 2022, 12, 698. https://doi.org/10.3390/nano12040698
Tang C, Shiri M, Zhang H, Ayinla RT, Wang K. Light-Driven Charge Transport and Optical Sensing in Molecular Junctions. Nanomaterials. 2022; 12(4):698. https://doi.org/10.3390/nano12040698
Chicago/Turabian StyleTang, Chaolong, Mehrdad Shiri, Haixin Zhang, Ridwan Tobi Ayinla, and Kun Wang. 2022. "Light-Driven Charge Transport and Optical Sensing in Molecular Junctions" Nanomaterials 12, no. 4: 698. https://doi.org/10.3390/nano12040698
APA StyleTang, C., Shiri, M., Zhang, H., Ayinla, R. T., & Wang, K. (2022). Light-Driven Charge Transport and Optical Sensing in Molecular Junctions. Nanomaterials, 12(4), 698. https://doi.org/10.3390/nano12040698






