New Epoxy Thermosets Organic-Inorganic Hybrid Nanomaterials Derived from Imidazolium Ionic Liquid Monomers and POSS®Ph
Abstract
:1. Introduction
2. Materials and Characterization Methods
2.1. Materials
2.2. Epoxy Network Preparation
2.3. Determination of the Soluble Fraction Obtained from Hybrid O/I Networks
2.4. Characterization Methods
3. Results and Discussion
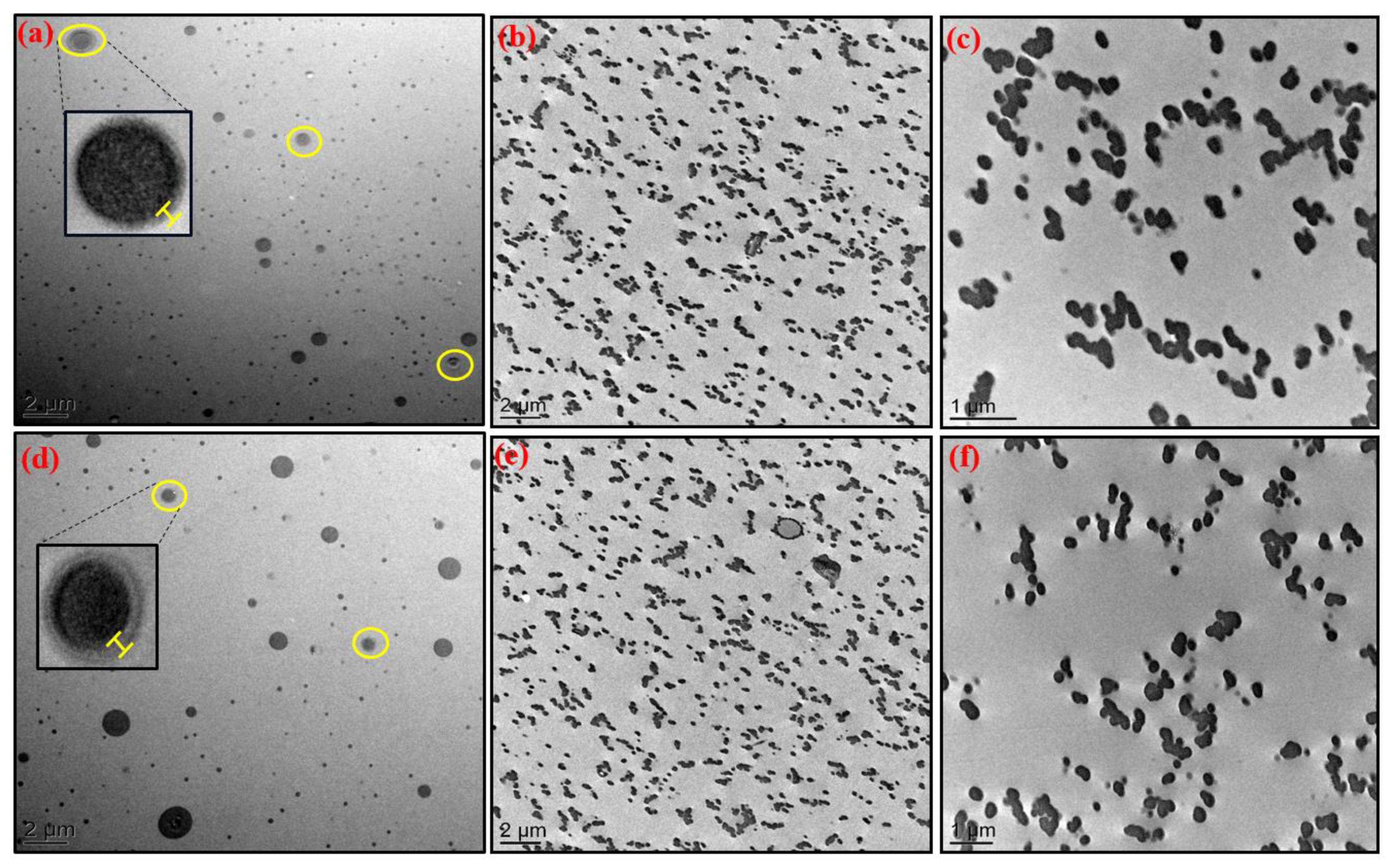
3.1. Morphology of Hybrid O/I Networks
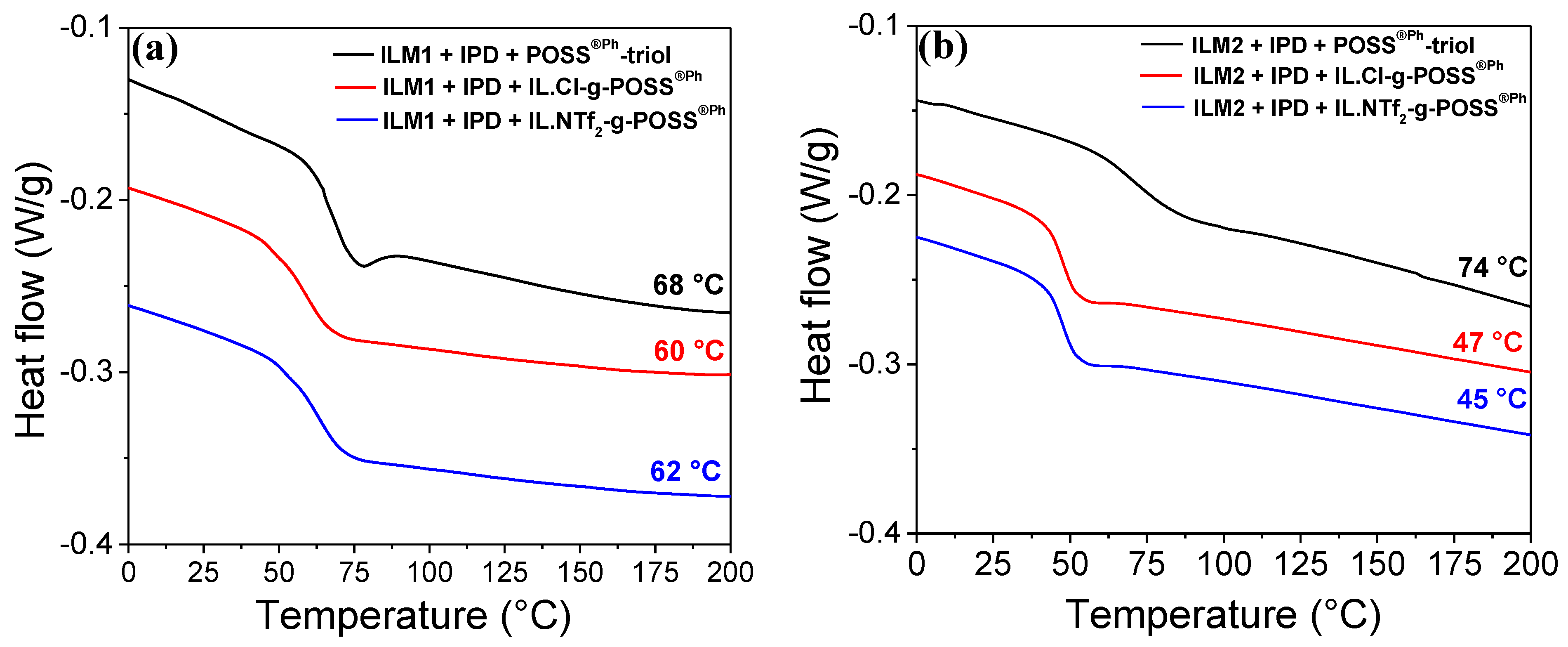
3.2. Epoxy Conversion and Glass Transition Temperatures
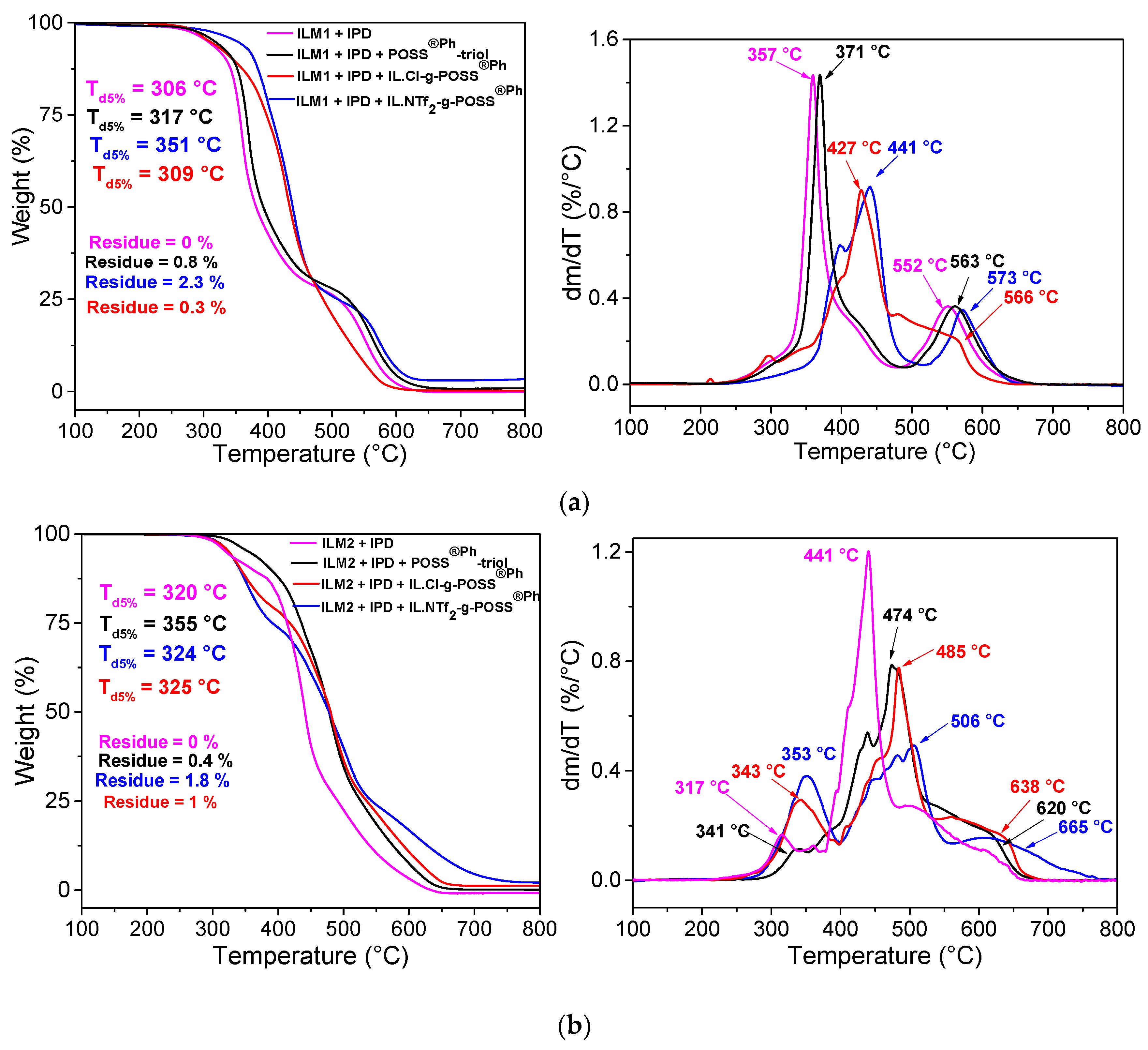
3.3. Thermal Stability and Oxidative Resistance of Hybrid O/I Networks
3.4. Surface Properties of Hybrid O/I Networks
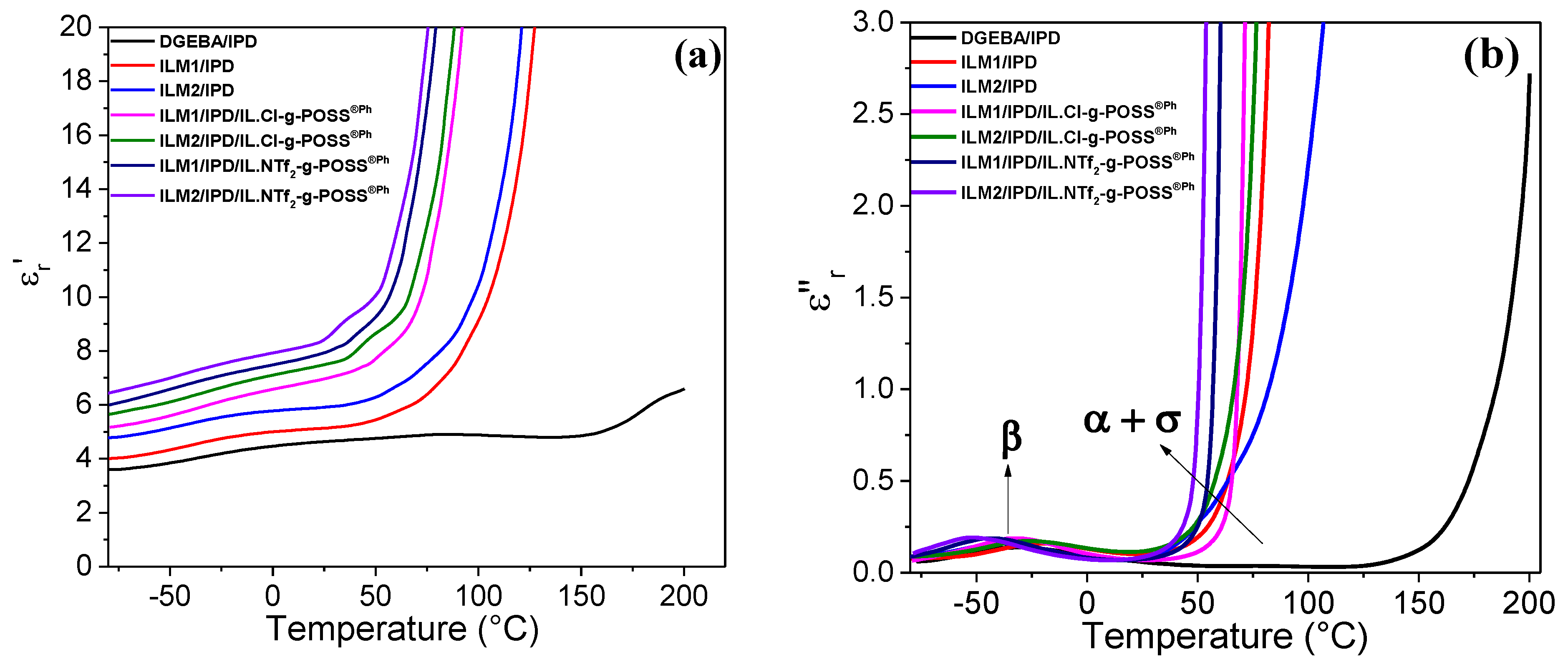
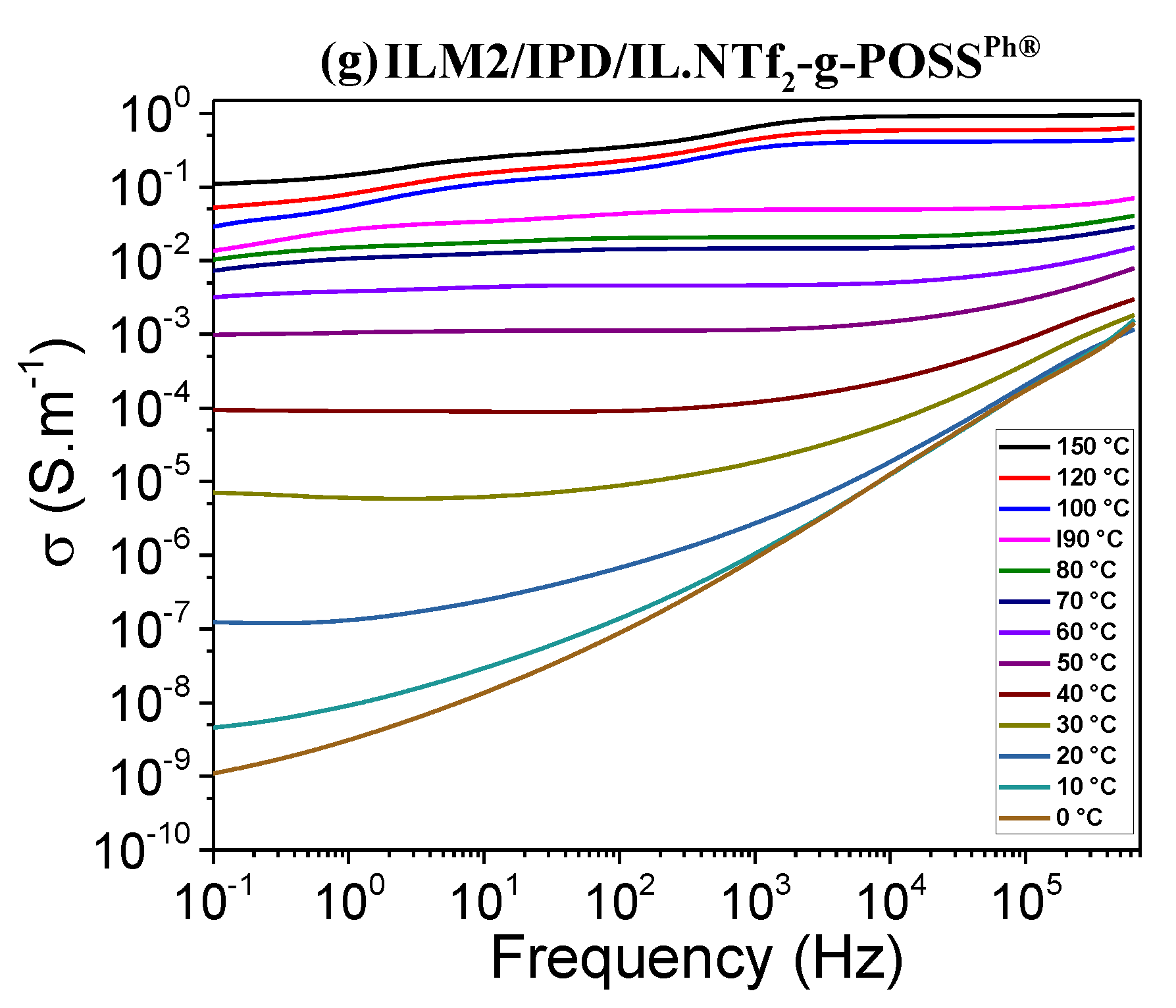
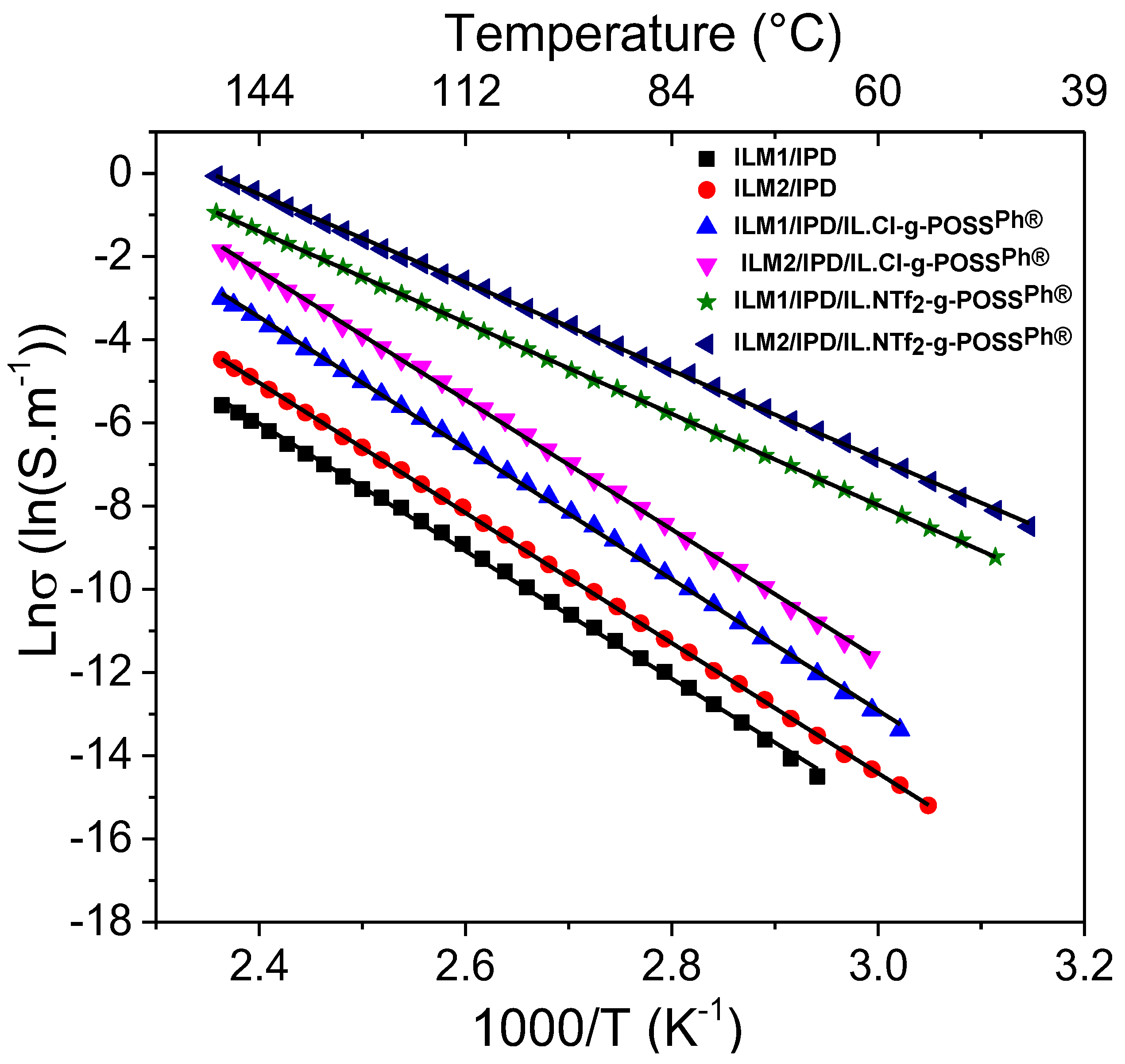
3.5. Ionic Conductivity of Hybrid O/I Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Sun, W.; Zheng, C.; Xie, K. Flame retardant and stable Li1.5Al0.5Ge1.5 (PO4)3-supported ionic liquid gel polymer electrolytes for high safety rechargeable solid-state lithium metal batteries. J. Phys. Chem. C 2018, 122, 10334–10342. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.B.; Kang, F.; Li, B. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Pandian, A.S.; Chen, X.C.; Chen, J.; Lokitz, B.S.; Ruther, R.E.; Yang, G.; Lou, K.; Nanda, J.; Delnick, F.M.; Dudney, N.J. Facile and scalable fabrication of polymer-ceramic composite electrolyte with high ceramic loadings. J. Power Sources 2018, 390, 153–164. [Google Scholar] [CrossRef]
- Wang, A.; Liu, X.; Wang, S.; Chen, J.; Xu, H.; Xing, Q.; Zhang, L. Polymeric ionic liquid enhanced all-solid-state electrolyte membrane for high-performance lithium-ion batteries. Electrochim. Acta 2018, 276, 184–193. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Investigation of ionic conductivity and relaxation in plasticized PMMA-LiClO4 solid polymer electrolytes. Solid State Ion. 2018, 319, 117–124. [Google Scholar] [CrossRef]
- Ban, X.; Zhang, W.; Chen, N.; Sun, C. A high-performance and durable poly (ethylene oxide)-based composite solid electrolyte for all solid-state lithium battery. J. Phys. Chem. C 2018, 122, 9852–9858. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, J.T.N.; Bertin, D.; Gigmes, D.; Devaux, D. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Devaux, D.; Bouchet, R.; Glé, D.; Denoyel, R. Mechanism of ion transport in PEO/LiTFSI complexes: Effect of temperature, molecular weight and end groups. Solid State Ion. 2012, 227, 119–127. [Google Scholar] [CrossRef]
- Appetecchi, G.; Hassoun, J.; Scrosati, B.; Croce, F.; Cassel, F.; Salomon, M. Hot-pressed, solvent-free, nanocomposite, PEO-based electrolyte membranes: II. All solid-state Li/LiFePO4 polymer batteries. J. Power Sources 2003, 124, 246–253. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A high-performance graphene oxide-doped ion gel as gel polymer electrolyte for all-solid-state supercapacitor applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Zhang, Z.; Sherlock, D.; West, R.; West, R.; Amine, K.; Lyons, L.J. Cross-linked network polymer electrolytes based on a polysiloxane backbone with oligo (oxyethylene) side chains: Synthesis and conductivity. Macromolecules 2003, 36, 9176–9180. [Google Scholar] [CrossRef]
- MacGlashan, G.S.; Andreev, Y.G.; Bruce, P.G. Structure of the polymer electrolyte poly (ethylene oxide) 6: LiAsF6. Nature 1999, 398, 792–794. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly (ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.R.; Kulandainathan, M.A.; Kumar, R.S.; Ferrara, C.; Mustarelli, P.; Stephan, A.M. Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 9948–9954. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, K.; Chen, R.; Liu, T.; Zhang, Y.; Nan, C.W. Oxide electrolytes for lithium batteries. J. Am. Ceram. Soc. 2015, 98, 3603–3623. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly (ionic liquid) s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Marcilla, R.; Alcaide, F.; Sardon, H.; Pomposo, J.A.; Pozo-Gonzalo, C.; Mecerreyes, D. Tailor-made polymer electrolytes based upon ionic liquids and their application in all-plastic electrochromic devices. Electrochem. Commun. 2006, 8, 482–488. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly (ionic liquid) s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and recycling of highly cross-linked ion-conducting networks through transalkylation exchanges of C–N bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Phan, T.N.; Gigmes, D.; Drockenmuller, E. Enhancing properties of anionic poly (ionic liquid) s with 1, 2, 3-triazolium counter cations. ACS Macro Lett. 2014, 3, 658–662. [Google Scholar] [CrossRef]
- Porcarelli, L.; Vlasov, P.S.; Ponkratov, D.O.; Lozinskaya, E.I.; Antonov, D.Y.; Nair, J.R.; Gerbaldi, C.; Mecerreyes, D.; Shaplov, A.S. Design of ionic liquid like monomers towards easy-accessible single-ion conducting polymer electrolytes. Eur. Polym. J. 2018, 107, 218–228. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly (ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarland, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Ohno, H. Design of Ion Conductive Polymers Based on Ionic Liquids. Macromol. Symp. 2007, 249–250, 551–556. [Google Scholar] [CrossRef]
- Livi, S.; Chardin, C.; Lins, L.C.; Halawani, N.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J.F.; Baudoux, J. From Ionic Liquid Epoxy Monomer to Tunable Epoxy-Amine Network: Reaction Mechanism and Final Properties. ACS Sustain. Chem. Eng. 2019, 7, 3602–3613. [Google Scholar] [CrossRef]
- Jung, G.Y.; Choi, J.H.; Lee, J.K. Thermal behavior and ion conductivity of polyethylene oxide/polyhedral oligomeric silsesquioxane nanocomposite electrolytes. Adv. Polym. Tech. 2015, 34, 21499. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, L.; Chen, L.; Fu, J.; Shi, L.; Li, M.; Zeng, X.; Lei, H.; Zheng, F. Inorganic-organic gel electrolytes with 3D cross-linking star-shaped structured networks for lithium ion batteries. Chem. Eng. J. 2020, 393, 124708. [Google Scholar] [CrossRef]
- Laik, S.; Galy, J.; Gérard, J.F.; Monti, M.; Camino, G. Fire behaviour and morphology of epoxy matrices designed for composite materials processed by infusion. Polym. Degrad. Stab. 2016, 127, 44–55. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Liang, R.; Wang, B. Combustion and thermal properties of epoxy/phenyltrisilanol polyhedral oligomeric silsesquioxane nanocomposites. J. Therm. Anal. Calorim. 2010, 100, 1009–1015. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS®) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S. Surface morphology and dewettability of self-organized thermosets involving epoxy and POSS®-capped poly (ethylene oxide) telechelics. Mater. Chem. Phys. 2012, 136, 744–754. [Google Scholar] [CrossRef]
- Zeng, K.; Zheng, S. Nanostructures and surface dewettability of epoxy thermosets containing hepta (3, 3, 3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly (ethylene oxide). J. Phys. Chem. B 2007, 111, 13919–13928. [Google Scholar] [CrossRef]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS®) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Peng, W.; Xu, S.; Li, L.; Zhang, C.; Zheng, S. Organic-inorganic nanocomposites via self-assembly of an amphiphilic triblock copolymer bearing a Poly (butadiene-g-POSS®) subchain in epoxy thermosets: Morphologies, surface hydrophobicity, and dielectric properties. J. Phys. Chem. B 2016, 120, 12003–12014. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Wunder, S.L. Polyoctahedral silsesquioxane-nanoparticle electrolytes for lithium batteries: POSS®-lithium salts and POSS®-PEGs. Chem. Mater. 2011, 23, 5111–5121. [Google Scholar] [CrossRef]
- Devaux, D.; Villaluenga, I.; Bhatt, M.; Shah, D.; Chen, X.C.; Thelen, J.L.; DeSimone, J.M.; Balsara, N.P. Crosslinked perfluoropolyether solid electrolytes for lithium ion transport. Solid State Ion. 2017, 310, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Yang, G.; Chanthad, C.; Oh, H.; Ayhan, I.A.; Wang, Q. Organic-inorganic hybrid electrolytes from ionic liquid-functionalized octasilsesquioxane for lithium metal batteries. J. Mater. Chem. A 2017, 5, 18012–18019. [Google Scholar] [CrossRef]
- Yang, G.; Oh, H.; Chanthad, C.; Wang, Q. Dumbbell-shaped octasilsesquioxanes functionalized with ionic liquids as hybrid electrolytes for lithium metal batteries. Chem. Mater. 2017, 29, 9275–9283. [Google Scholar] [CrossRef]
- Na, W.; Lee, A.S.; Lee, J.H.; Hong, S.M.; Kim, E.; Koo, C.M. Hybrid ionogel electrolytes with POSS® epoxy networks for high temperature lithium ion capacitors. Solid State Ion. 2017, 309, 27–32. [Google Scholar] [CrossRef]
- Chabane, H.; Livi, S.; Benes, H.; Ladavière, C.; Ecorchard, P.; Duchet-Rumeau, J.; Gérard, J.F. Polyhedral oligomeric silsesquioxane-supported ionic liquid for designing nanostructured hybrid organic-inorganic networks. Eur. Polym. J. 2019, 114, 332–337. [Google Scholar] [CrossRef]
- Chabane, H.; Livi, S.; Morelle, X.P.; Sonnier, R.; Dumazert, L.; Duchet-Rumeau, J.; Gerard, J.F. Synthesis of New Ionic Liquid-Grafted Metal-Oxo Nanoclusters—Design of Nanostructured Hybrid Organic-Inorganic Polymer Networks. Polymer 2021, 224, 123721. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Chabane, H.; Demir, B.; Searles, D.J.; Duchet-Rumeau, J.; Gerard, J.F.; Baudoux, J.; Livi, S. New Epoxy Thermosets Derived from Bisimidazolium Ionic Liquid Monomer: An Experimental and Modelling Investigation. ACS Sustain. Chem. Eng. 2020, 8, 12208–12221. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Duchet-Rumeau, J.; Gérard, J.F.; Baudoux, J.; Livi, S. Cycloaliphatic epoxidized ionic liquids as new versatile monomers for the development of shape memory PIL networks by 3D printing. Polym. Chem. 2020, 11, 5475–5483. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S. Epoxy resin containing octamaleimidophenyl polyhedral oligomeric silsesquioxane. Macromol. Chem. Phys. 2005, 206, 2075–2083. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S.; Nie, K. Morphology and thermal properties of inorganic-organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Ababsa, H.S.; Safidine, Z.; Mekki, A.; Grohens, Y.; Ouadah, A.; Chabane, H. Fire behavior of flame-retardant polyurethane semi-rigid foam in presence of nickel (II) oxide and graphene nanoplatelets additives. J. Polym. Res. 2021, 28, 1–14. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/ionic liquid systems: The influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Ueno, K.; Watanabe, M. From colloidal stability in ionic liquids to advanced soft materials using unique media. Langmuir 2011, 27, 9105–9115. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.S.; Machado, G.; Teixeira, S.R.; Fecher, G.H.; Morais, J.; Alves, M.C.; Dupont, J. Synthesis and characterization of catalytic iridium nanoparticles in imidazolium ionic liquids. J. Colloid Interface Sci. 2006, 301, 193–204. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Synthesis of very small TiO2 nanocrystals in a room-temperature ionic liquid and their self-assembly toward mesoporous spherical aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef]
- Shim, Y.; Kim, H.J. Solvation of carbon nanotubes in a room-temperature ionic liquid. ACS Nano 2009, 3, 1693–1702. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Donato, R.; Perchacz, M.; Ponyrko, S.; Donato, K.; Schrekker, H.; Beneš, H.; Matějka, L. Epoxy-silica nanocomposite interphase control using task-specific ionic liquids via hydrolytic and non-hydrolytic sol-gel processes. RSC Adv. 2015, 5, 91330–91339. [Google Scholar] [CrossRef]
- Perchacz, M.; Donato, R.K.; Seixas, L.; Zhigunov, A.; Konefał, R.; Serkis-Rodzeń, M.; Beneš, H. Ionic Liquid-Silica Precursors via Solvent-Free Sol-Gel Process and Their Application in Epoxy-Amine Network: A Theoretical/Experimental Study. ACS Appl. Mate. Interfaces 2017, 9, 16474–16487. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.; Toghiani, H.; Daulton, T.L.; Koyama, K.; Pittman, C.U. Viscoelastic and mechanical properties of epoxy/multifunctional polyhedral oligomeric silsesquioxane nanocomposites and epoxy/ladderlike polyphenylsilsesquioxane blends. Macromolecules 2001, 34, 8686–8693. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; Nie, K. Morphology and thermomechanical properties of organic-inorganic hybrid composites involving epoxy resin and an incompletely condensed polyhedral oligomeric silsesquioxane. Macromolecules 2005, 38, 5088–5097. [Google Scholar] [CrossRef]
- Deng, Y.; Bernard, J.; Alcouffe, P.; Galy, J.; Dai, L.; Gérard, J.F. Nanostructured hybrid polymer networks from in situ self-assembly of RAFT-synthesized POSS®-based block copolymers. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 4343–4352. [Google Scholar] [CrossRef]
- Abad, M.J.; Barral, L.; Fasce, D.P.; Williams, R.J. Epoxy networks containing large mass fractions of a monofunctional polyhedral oligomeric silsesquioxane (POSS®). Macromolecules 2003, 36, 3128–3135. [Google Scholar] [CrossRef]
- Zucchi, I.A.; Galante, M.J.; Williams, R.J.; Franchini, E.; Galy, J.; Gérard, J.F. Monofunctional epoxy-POSS® dispersed in epoxy-amine networks: Effect of a prereaction on the morphology and crystallinity of POSS® domains. Macromolecules 2007, 40, 1274–1282. [Google Scholar] [CrossRef]
- Cartier, H.; Chopin, A.; Perego, C. Radiation Crosslinking of Halogen-Free Flame Retardant Polymer. International Patent Application No. WO2007106074A2. U.S. Patent 7,423,080, 3 March 2007. [Google Scholar]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H. Thermal degradation behaviors of epoxy resin/POSS® hybrids and phosphorus-silicon synergism of flame retardancy. J. Polym. Sci. Part B: Polym. Phys. 2010, 48, 693–705. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Canavese, D.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire retardant halogen- antimony-clay synergism in polypropylene layered silicate nanocomposites. Chem. Mater. 2002, 14, 189–193. [Google Scholar] [CrossRef]
- Bizet, S.; Galy, J.; Gérard, J.F. Structure-property relationships in organic-inorganic nanomaterials based on methacryl-POSS® and dimethacrylate networks. Macromolecules 2006, 39, 2574–2583. [Google Scholar] [CrossRef]
- Hirata, T.; Kashiwagi, T.; Brown, J.E. Thermal and oxidative degradation of poly (methyl methacrylate): Weight loss. Macromolecules 1985, 18, 1410–1418. [Google Scholar] [CrossRef]
- Mantz, R.; Jones, P.; Chaffee, K.; Lichtenhan, J.; Gilman, J.; Ismail, I.; Burmeister, M. Thermolysis of polyhedral oligomeric silsesquioxane (POSS®) macromers and POSS®-siloxane copolymers. Chem. Mater. 1996, 8, 1250–1259. [Google Scholar] [CrossRef]
- Tomczak, S.J.; Marchant, D.; Svejda, S.; Minton, T.K.; Brunsvold, A.L.; Gouzman, I.; Grossman, E.; Schatz, G.C.; Troya, D.; Sun, L. Properties and improved space survivability of POSS® (polyhedral oligomeric silsesquioxane) polyimides. MRS Online Proc. Libr. (OPL) 2004, 851, 395–406. [Google Scholar] [CrossRef]
- Badey, J.; Espuche, E.; Sage, D.; Chabert, B.; Jugnet, Y.; Batier, C.; Duc, T.M. A comparative study of the effects of ammonia and hydrogen plasma downstream treatment on the surface modification of polytetrafluoroethylene. Polymer 1996, 37, 1377–1386. [Google Scholar] [CrossRef]
- Włoch, J.; Terzyk, A.P.; Wiśniewski, M.; Kowalczyk, P. Nanoscale water contact angle on Polytetrafluoroethylene surfaces characterized by molecular Dynamics-Atomic force microscopy imaging. Langmuir 2018, 34, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lichtenhan, J.D.; Otaigbe, J.U. Facile route to nature inspired hydrophobic surface modification of phosphate glass using polyhedral oligomeric silsesquioxane with improved properties. Appl. Surf. Sci. 2019, 470, 733–743. [Google Scholar] [CrossRef]
- Almeida, H.F.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A. Surface tension and refractive index of pure and water-saturated tetradecyltrihexylphosphonium-based ionic liquids. J. Chem. Thermodyn. 2013, 57, 372–379. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Neves, C.M.; Coutinho, J.A. Surface tensions of bis (trifluoromethylsulfonyl) imide anion-based ionic liquids. J. Chem. Eng. Data 2010, 55, 3807–3812. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, G.; Kumar, S.; Kang, T.S. Thermally stable microemulsions comprising imidazolium based surface active ionic liquids, non-polar ionic liquid and ethylene glycol as polar phase. J. Colloid Interface Sci. 2018, 511, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Heux, L.; Halary, J.; Lauprêtre, F.; Monnerie, L. Dynamic mechanical and 13C NMR investigations of molecular motions involved in the β relaxation of epoxy networks based on DGEBA and aliphatic amines. Polymer 1997, 38, 1767–1778. [Google Scholar] [CrossRef]
- Halawani, N.; Augé, J.L.; Morel, H.; Gain, O.; Pruvost, S. Electrical, thermal and mechanical properties of poly-etherimide epoxy-diamine blend. Compo. B. Eng. 2017, 110, 530–541. [Google Scholar] [CrossRef]
- Dyre, J.C.; Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 2000, 72, 873. [Google Scholar] [CrossRef] [Green Version]
- Psarras, G. Hopping conductivity in polymer matrix-metal particles composites. Compos. Part A: Appl. Sci. Manuf. 2006, 37, 1545–1553. [Google Scholar] [CrossRef]
- Emmert, S.; Wolf, M.; Gulich, R.; Krohns, S.; Kastner, S.; Lunkenheimer, P.; Loidl, A. Electrode polarization effects in broadband dielectric spectroscopy. Eur. Phys. J. B. 2011, 83, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Choi, J.H.; Salas-de la Cruz, D.; Winey, K.I.; Elabd, Y.A. Polymerized ionic liquids: The effect of random copolymer composition on ion conduction. Macromolecules 2009, 42, 4809–4816. [Google Scholar] [CrossRef]
- Hemp, S.T.; Zhang, M.; Allen, M.H., Jr.; Cheng, S.; Moore, R.B.; Long, T.E. Comparing ammonium and phosphonium polymerized ionic liquids: Thermal analysis, conductivity, and morphology. Macromol. Chem. Phys. 2013, 214, 2099–2107. [Google Scholar] [CrossRef]
- Ye, Y.; Elabd, Y.A. Anion exchanged polymerized ionic liquids: High free volume single ion conductors. Polymer 2011, 52, 1309–1317. [Google Scholar] [CrossRef]

| Name | Chemical Formula | Characteristics |
|---|---|---|
| Diglycidyl ether of bisphenol A (DGEBA) | 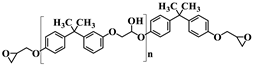 n = 0.15 | 377 g.mol−1, eew: 185–192 g/eq a, Tg = −13 °C b, Td = 330 °C b |
| 3-[2-(oxiran-2-yl)ethyl]-1-[6,7]imidazolium 1,1,1-trifluoroN-[(trifluoromethyl)sulfonyl]methanesulfonamide ILM1 |  | Prepared following published procedure [28] Td = 489 °C b |
| 3,3′-(1,4-butanediyl)bis [1-(4-(2-(oxiran-2-yl)ethyl)phenyl)]imidazolium 1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]methanesulfonamide ILM2 | 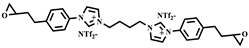 | Prepared following published procedure [46] Tm = 71 °C a Td = 460 °C b |
| Isophoronediamine (IPD) | 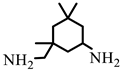 | M = 170.3 g.mol−1 |
| Heptaphenyl-trisilanol POSS® (POSSPh-triol) | 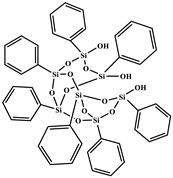 | M = 931.34 g mol−1, Tm1 = 217 °C a, Tm2 = 230 °C a, Td = 632 °C b |
| 1-methyl-3-propyl heptaphenyl octasilesquioxane imidazolium chloride (IL.Cl-g-POSS®Ph) | 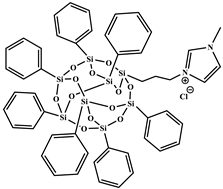 | Prepared following the procedure reported in [45] M = 1116.04 g mol−1, Tm1 = 127 °C a, Tm2 = 168 °C a, Td = 632 °C b |
| 1-methyl-3-propyl heptaphenyl octasilesquioxane imidazolium bis(trifluoromethane)sulfonimide (IL.NTf2-g-POSS®Ph) | 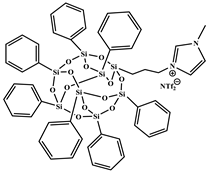 | Prepared following the procedure reported in [45] M = 1360.73 g mol−1, Tm1 = 135 °C a Tm2 = 158 °C a, Td = 534 °C b |
| Samples | ΘWater (°) | ΘCH2I2 (°) | γnon-dispersive (mJ.m−2) | γdispersive (mJ.m−2) | γtotal (mJ.m−2) |
|---|---|---|---|---|---|
| DGEBA/IPD | 79 | 49 | 4.8 | 34.8 | 39.6 |
| ILM1/IPD | 102 | 62 | 0.8 | 24.6 | 25.4 |
| ILM1-IPD/POSS®Ph-triol | 108 | 75 | 0.3 | 20.4 | 20.7 |
| ILM1-IPD/IL.Cl-g-POSS®Ph | 110 | 78 | 0.3 | 19.9 | 20.2 |
| ILM1-IPD/IL.NTf2-g-POSS®Ph | 112 | 82 | 0.2 | 19.6 | 19.8 |
| ILM2/IPD | 106 | 72 | 0.6 | 21.0 | 21.6 |
| ILM2-IPD/POSS®Ph-triol | 115 | 86 | 0.2 | 19.4 | 19.6 |
| ILM2-IPD/IL.Cl-g-POSS®Ph | 116 | 88 | 0.2 | 18.8 | 19.0 |
| ILM2-IPD/IL.NTf2-g-POSS®Ph | 120 | 92 | 0.2 | 18.0 | 18.2 |
| Samples | σAC @ 40 °C | σAC @ 50 °C | σAC @ 70 °C | σAC @ 100 °C |
|---|---|---|---|---|
| ILM1-IPD | 3.40 × 10−8 | 1.15 × 10−7 | 3.91 × 10−6 | 3.12 × 10−4 |
| ILM1-IPD/IL.Cl-g-POSS®Ph | 5.74 × 10−6 | 3.46 × 10−5 | 3.05 × 10−4 | 1.63 × 10−2 |
| ILM1-IPD/IL.NTf2-g-POSS®Ph | 2.11 × 10−5 | 6.12 × 10−5 | 6.28 × 10−4 | 2.15 × 10−2 |
| ILM2-IPD | 5.42 × 10−8 | 3.80 × 10−6 | 2.08 × 10−4 | 1.28 × 10−3 |
| ILM2-IPD/IL.Cl-g-POSS®Ph | 2.54 × 10−5 | 8.39 × 10−5 | 1.19 × 10−3 | 4.38 × 10−2 |
| ILM2-IPD/IL.NTf2-g-POSS®Ph | 9.37 × 10−5 | 9.81 × 10−4 | 7.27 × 10−3 | 6.84 × 10−2 |
| Samples | σ0 (S.m−1) | Ea (eV) | R-Square |
|---|---|---|---|
| ILM1-IPD | 4.87 × 1013 | 1.36 | 0.99856 |
| ILM1-IPD/IL.Cl-g-POSS®Ph | 2.71 × 1015 | 1.14 | 0.99612 |
| ILM1-IPD/IL.NTf2-g-POSS®Ph | 1.14 × 1015 | 1.06 | 0.99712 |
| ILM2-IPD | 6.15 × 1013 | 1.35 | 0.99875 |
| ILM2-IPD/IL.Cl-g-POSS®Ph | 7.72 × 1010 | 0.92 | 0.99436 |
| ILM2-IPD/IL.NTf2-g-POSS®Ph | 3.28 × 1010 | 0.86 | 0.99236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabane, H.; Livi, S.; Duchet-Rumeau, J.; Gérard, J.-F. New Epoxy Thermosets Organic-Inorganic Hybrid Nanomaterials Derived from Imidazolium Ionic Liquid Monomers and POSS®Ph. Nanomaterials 2022, 12, 550. https://doi.org/10.3390/nano12030550
Chabane H, Livi S, Duchet-Rumeau J, Gérard J-F. New Epoxy Thermosets Organic-Inorganic Hybrid Nanomaterials Derived from Imidazolium Ionic Liquid Monomers and POSS®Ph. Nanomaterials. 2022; 12(3):550. https://doi.org/10.3390/nano12030550
Chicago/Turabian StyleChabane, Houssém, Sébastien Livi, Jannick Duchet-Rumeau, and Jean-François Gérard. 2022. "New Epoxy Thermosets Organic-Inorganic Hybrid Nanomaterials Derived from Imidazolium Ionic Liquid Monomers and POSS®Ph" Nanomaterials 12, no. 3: 550. https://doi.org/10.3390/nano12030550
APA StyleChabane, H., Livi, S., Duchet-Rumeau, J., & Gérard, J.-F. (2022). New Epoxy Thermosets Organic-Inorganic Hybrid Nanomaterials Derived from Imidazolium Ionic Liquid Monomers and POSS®Ph. Nanomaterials, 12(3), 550. https://doi.org/10.3390/nano12030550