3. Results and Discussion
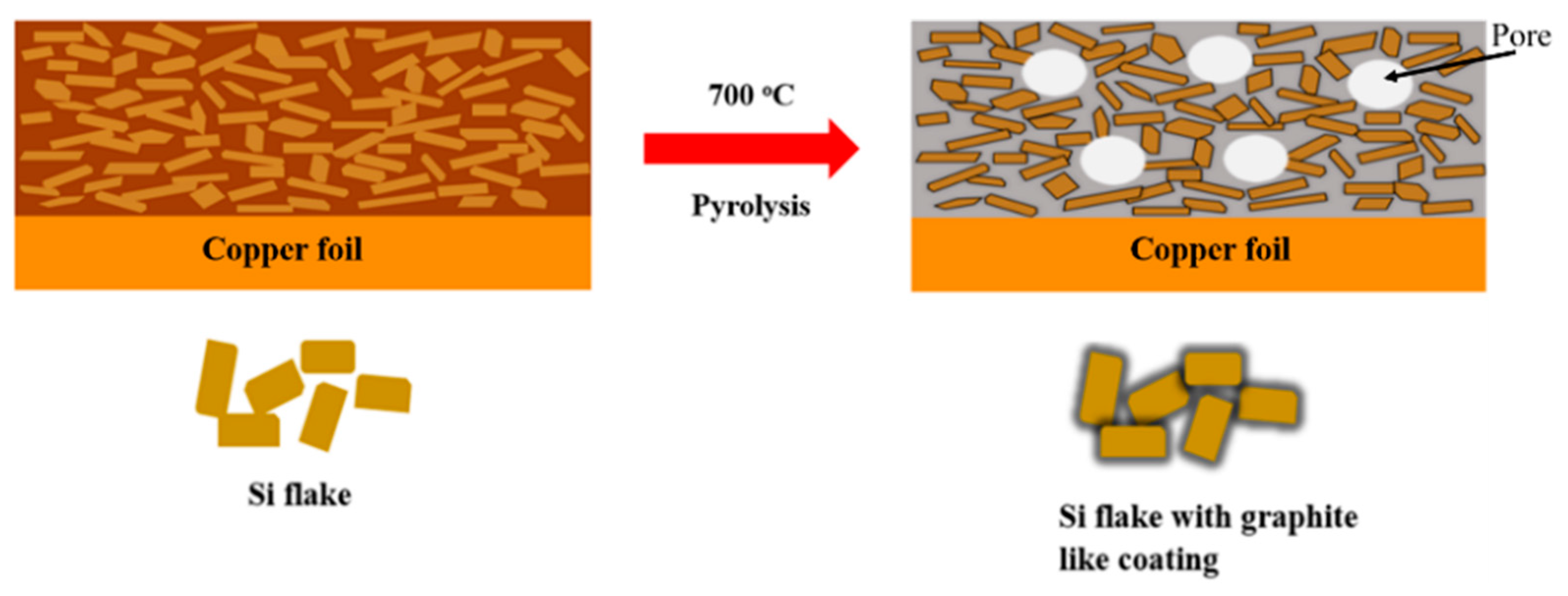
Figure 1 shows schematically an anode made of silicon flakes and binder on a copper foil before and after a pyrolysis of the anode in an inert argon environment at 700 °C. The pyrolysis converted the binder of a mixture of CMC and SBR to graphitic carbon coatings on individual silicon flakes which are embedded in a porous carbon structure. Pores are shown as white circles.
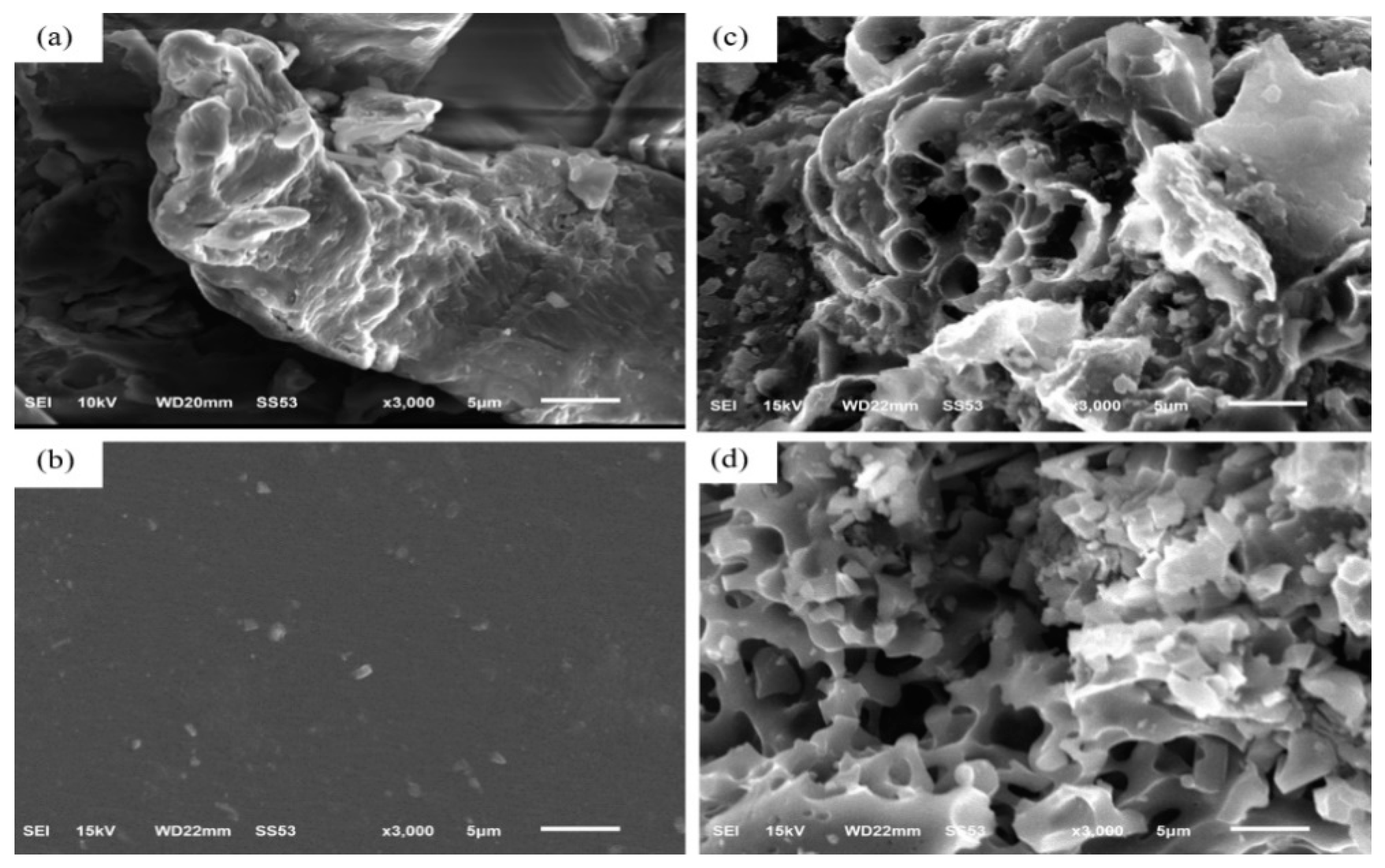
Figure 2a shows a SEM image of CMC after heating at 60 °C in vacuum to remove moisture.
Figure 2b shows a SEM image of a SBR solution after having been placed in a vacuum oven at 80 °C for 8 h. These samples were subjected to pyrolysis in argon at 700 °C for 1 h; porous carbon materials are shown in
Figure 2c,d, respectively. Pyrolysis process produced CO
2, CO, C
2H
4O
2, and CH
4 gases [
18]. The weight loss by the pyrolysis of these CMC and SBR binders were 85% and 87%, respectively.
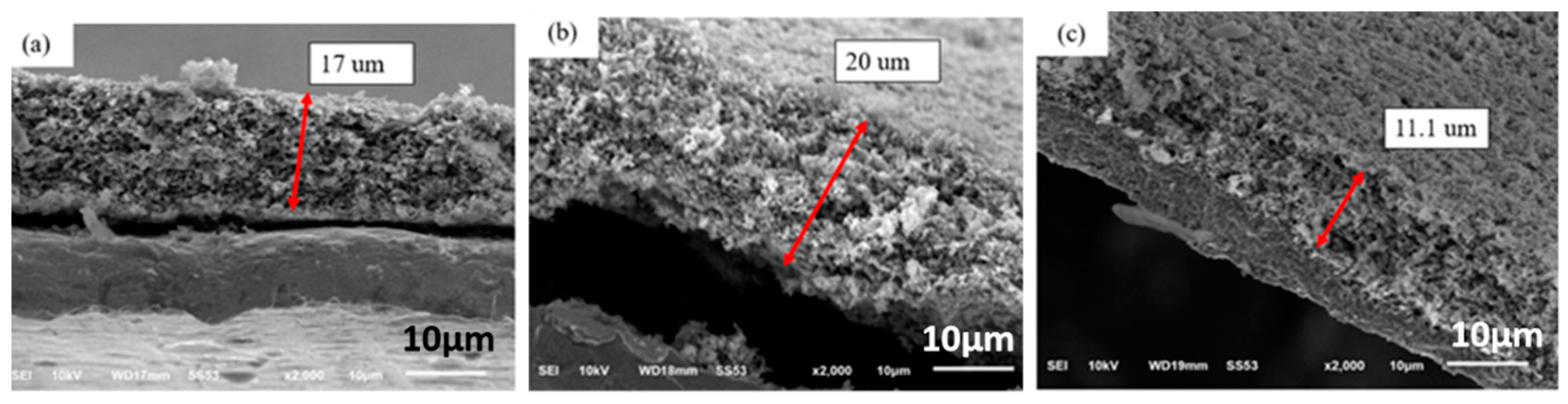
Figure 3 shows cross-sectional SEM images of electrodes made of silicon flake and binder of the weight ratio of 70%:30%.
Figure 3a shows an as-fabricated anode.
Figure 3b shows the cross-sectional morphology of anodes after pyrolysis at 700 °C in argon for 1hr. The anode shown in
Figure 3b was subjected to an oxidation process in air at 600 °C for 2 hr in order to remove amorphous carbon produced by pyrolysis in argon at 700 °C.
Figure 3c shows the SEM cross-sectional image of the oxidized anode.
Figure 3a shows that the thickness of the as-fabricated electrode is about 17 µm. After the pyrolysis process, the electrode lost about 25.8 wt%, but the thickness of the electrode did not decrease, but, instead increased from 17 µm to about 20 µm as shown in
Figure 3b. Gaseous by-products generated by the pyrolysis of the binders created an internal pressure for the slight volume expansion and the formation of pores in the electrode.
The oxidation of the electrode at 600 °C in air atmosphere for 2 h removed amorphous carbon from the binder which had been subjected to pyrolysis in argon. The thickness of the electrode decreased significantly from 20 µ to 11.4 µm as shown in
Figure 3c. The dark black surface of the anode changed to light coffee color that is similar to that of silicon flakes.
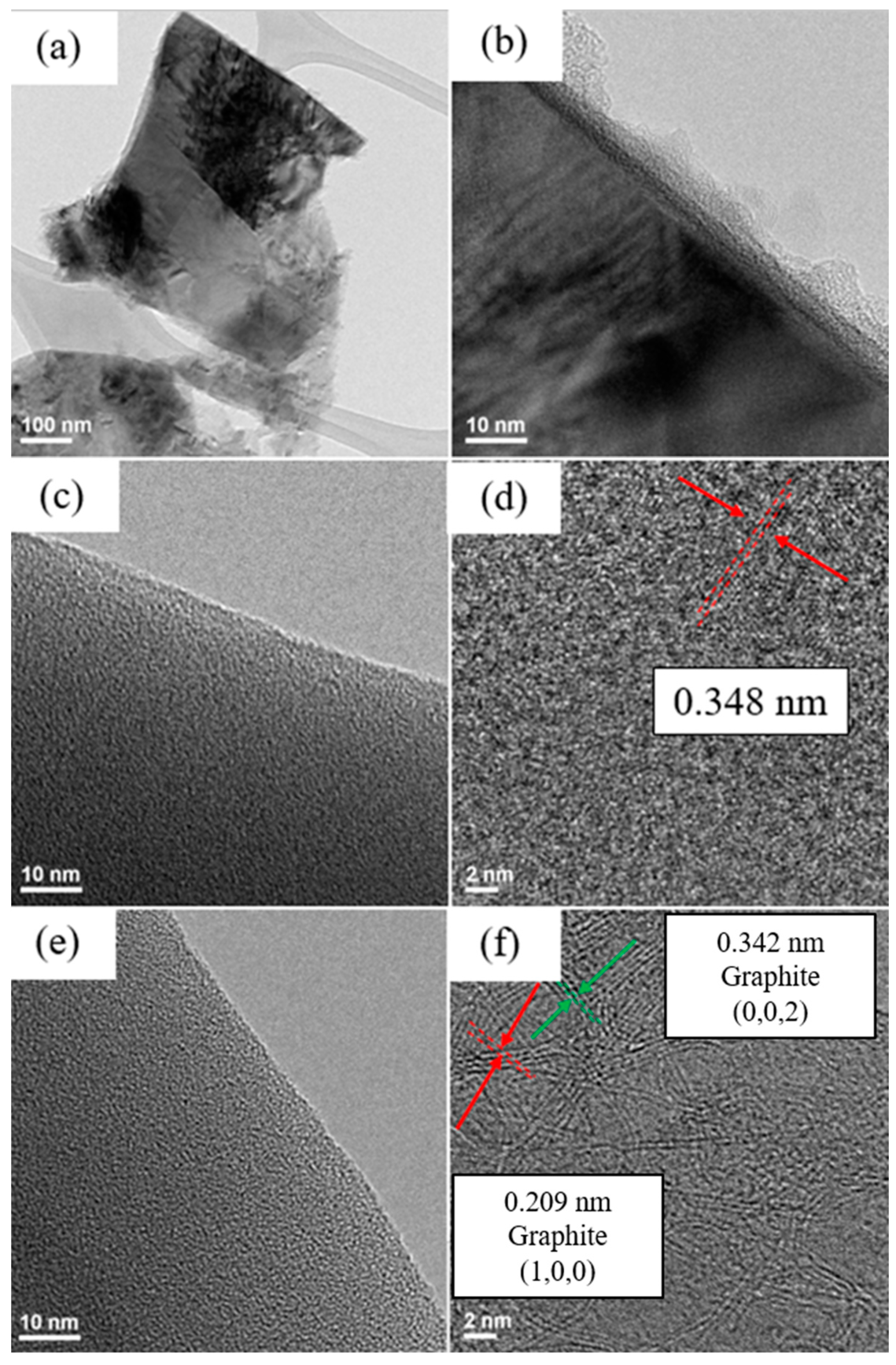
Figure 4 and
Figure 5 show TEM images of silicon flakes and binders before and after pyrolysis.
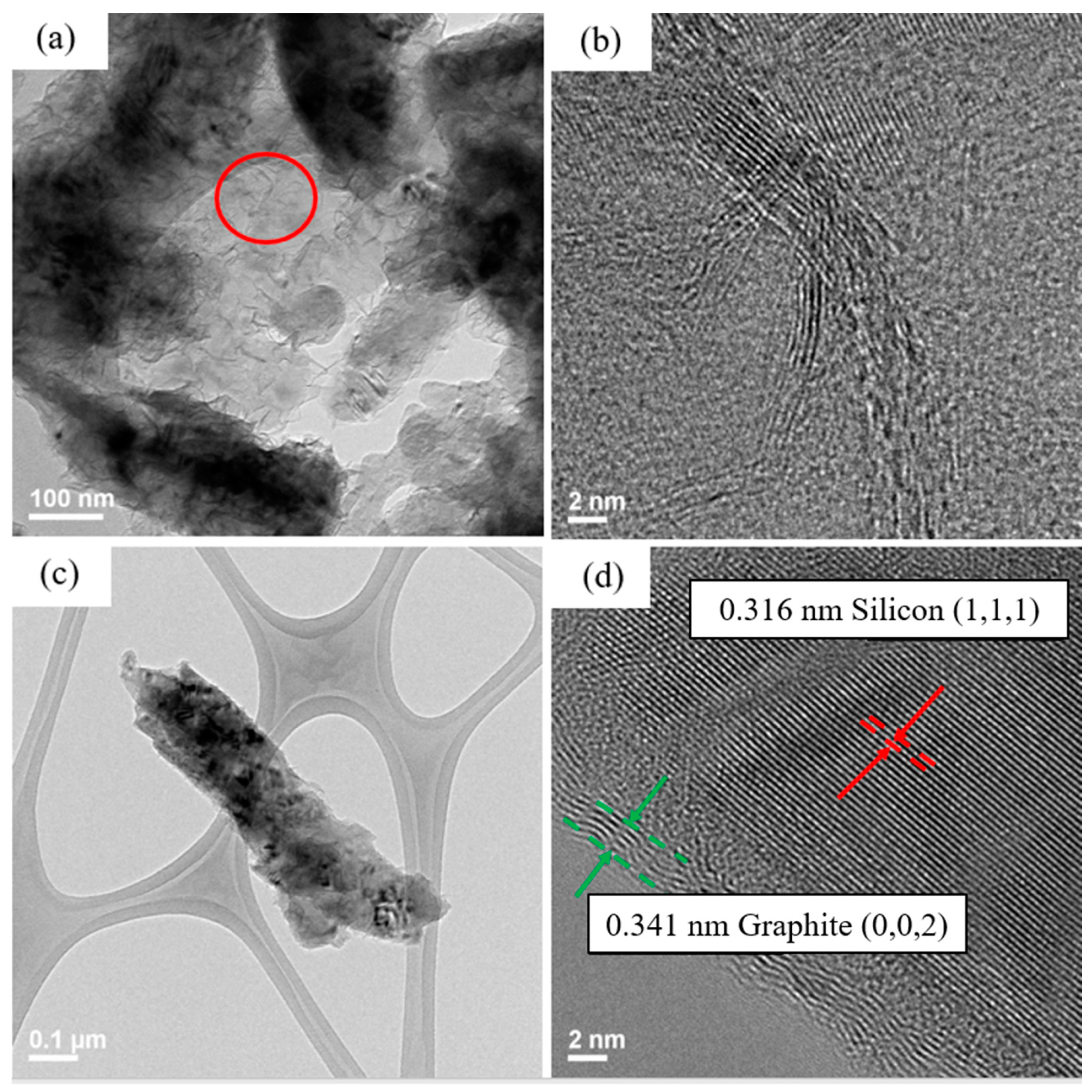
Figure 4a,b shows silicon flakes.
Figure 4b shows that a 3–8 nm oxide layer are present on the surface of silicon flake.
Figure 4c,e show the morphology of CMC and SBR binders without pyrolysis, respectively. Both CMC and SBR exhibit amorphous nature.
Figure 4d,f show that, after pyrolysis, CMC and SBR exhibited partial crystalline structural arrangements. The carbon matrix is considered to contribute to the overall conductivity of the electrode. The pyrolytic SBR shown in
Figure 4f exhibits a higher degree of structural arrangement than that of CMC shown in
Figure 4d. Long chain-like SBR molecules were converted by the pyrolysis process to a more crystalline carbon structure than that of CMC.
Pyrolytic electrode was dissolved in alcohol and subjected to ultrasonic vibration. The solution was then dropped on a copper net and dried in a vacuum oven. The microscopic structure of the silicon powder and the pyrolytic binders were analyzed by TEM images shown in
Figure 5.
The darker portion of the TEM image shown in
Figure 5a is silicon flake. The lighter part is amorphous and ordered carbon produced by pyrolysis. Shown in
Figure 5b is a TEM image of the carbon structure after pyrolysis showing ordered carbon structure embedded in the disordered carbon. Orderly arranged carbon material is believed to contribute to the electrical conductivity of the anode. A silicon flake after pyrolysis is shown in
Figure 5c with the surface layers shown in
Figure 5d. Binder that adhered to the surface of silicon flake was converted to graphitic carbon coatings on silicon by the pyrolysis process. Graphitic carbon coating on silicon is desirable for reducing irreversible reactions between silicon and the electrolyte in a battery [
19,
20].
Raman spectroscopy was applied to analyze the binder before and after pyrolysis. Binder made by a ratio of CMC/SBR = 1:1 in weight was prepared to coat on a copper foil without silicon powder. After the binders were dried, Raman spectroscopy was applied to differentiate the carbon contents of the binders before and after pyrolysis.
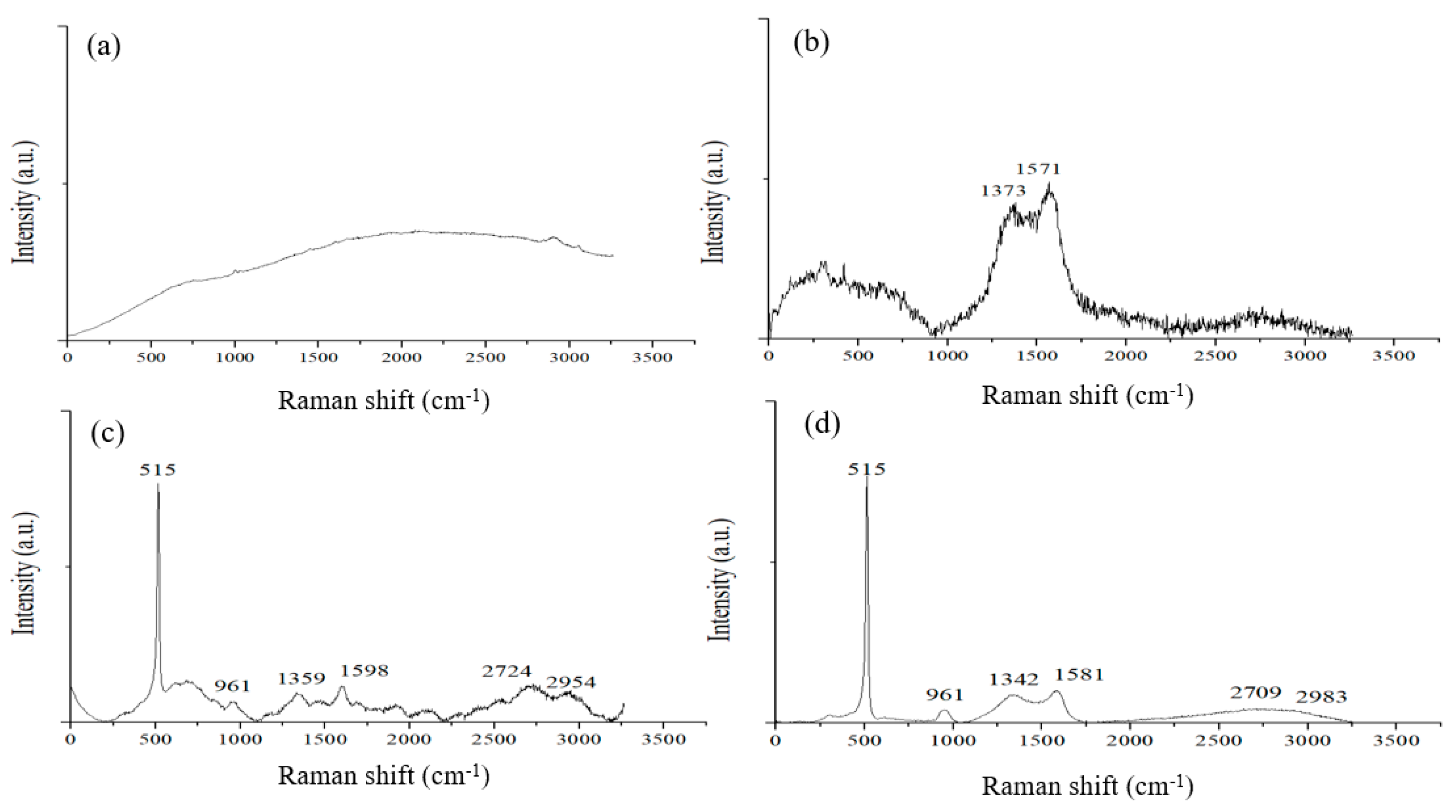
Figure 6a shows a Raman spectrum of a CMC/SBR film before pyrolysis. The as-coated binder mixture is of amorphous nature and does not exhibit distinct characteristic peaks. After pyrolysis, the polymer film changed color to black. Broad D and G bands appeared in the Raman spectrum shown in
Figure 6b. This is consistent with the structured carbon displayed by TEM images [
21].
Figure 6c,d shows Raman spectra of an electrode made of silicon flake and the binder mixture, of which the Raman spectra are shown in
Figure 6a,b before and after pyrolysis, respectively. The characteristic peaks of silicon crystals are clearly shown at the wavenumbers of 515 and 961 cm
−1. Before pyrolysis, the electrode exhibits several characteristic peaks of carbon phases in the range of 550–900 and 1200–3000 cm
−1 as shown in
Figure 6c. After pyrolysis, most of the peaks disappeared, leaving broad D and G bands as shown in
Figure 6d. The characteristic D and G bands prove that disordered graphitic structures were produced by the pyrolysis.
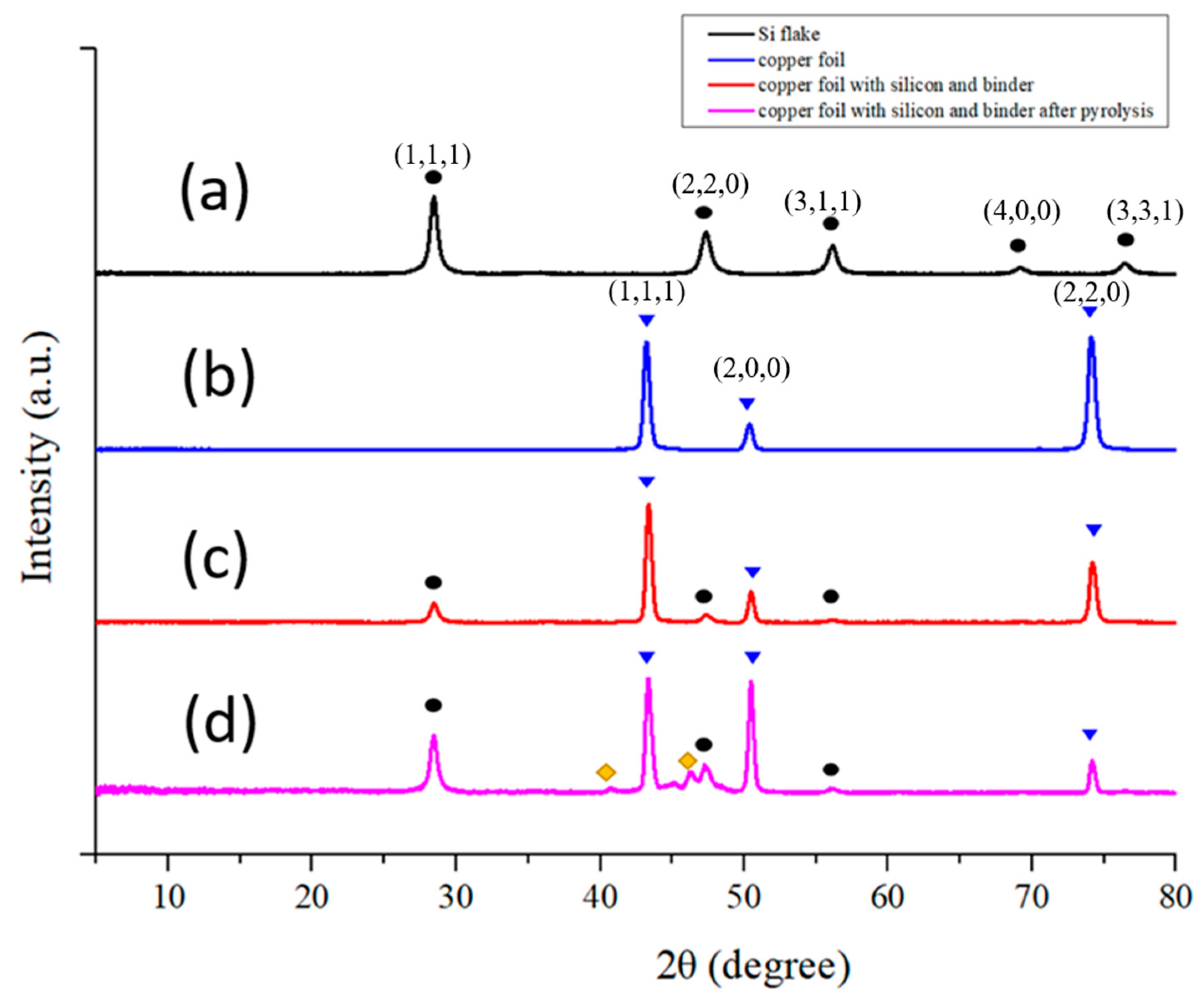
Figure 7 shows XRD diffraction patterns of (a) a Si flake, (b) a copper foil, (c) a Si-based anode without conductivity enhancement additive, and (d) a Si-based anode without conductivity enhancement additive after pyrolysis, respectively.
Figure 7a shows 2θ = 23°, 47°, 56, 69°, and 76° corresponding to interlayer space and planes of crystalline silicon.
Figure 7b shows 2θ = 43°, 51°, and 74° corresponding to the d-space and planes of crystalline copper. Both CMC and SBR exhibit a broad peak near 2θ = 21°, indicating amorphous nature of both binders [
22].
After pyrolysis, most of the binder was lost, leaving only 14 wt% of its original weight. In other words, the weight ratio of silicon powder and carbon material on the electrode became 94.3%:5.7%. Before pyrolysis, the conductivity enhancement additive is in an amorphous nature and there is no specific signal on XRD diffraction pattern. After pyrolysis, most of the Raman scattering signal from conductivity enhancement additive disappeared. The silicon contents and signal intensity of silicon powder are stronger as shown in
Figure 7d.
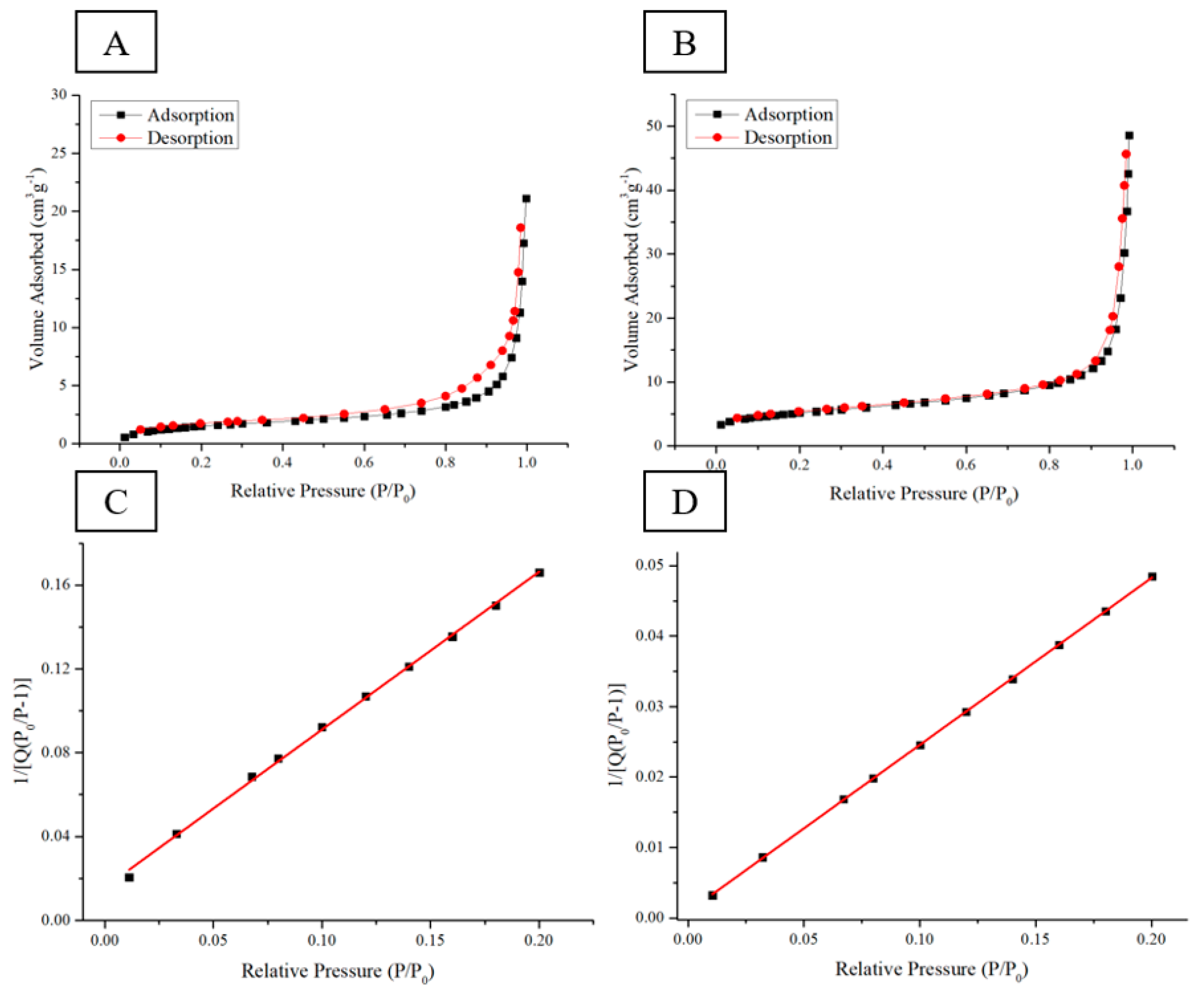
Two circular ingot samples with a weight of 0.5 g and the weight ratio of Si–CMC–SBR being 70%:15%:15% were prepared for measuring the effective surface area before and after pyrolysis.
Figure 8A,C shows nitrogen adsorption–desorption isotherms of electrode with Si–CMC/SBR = 70%:30% in wt before pyrolysis.
Figure 8B,D shows nitrogen adsorption–desorption isotherms of electrode with Si–CMC/SBR = 70%:30% in wt after pyrolysis. The sample after having been subjected to pyrolysis lost weight with the remaining weight being 0.36 g. BET isotherm adsorption method was applied to examine the specific surface area. The pyrolysis process increased the specific surface area from 5.66 m
2/g to 18.3 m
2/g. It showed that the thermogravimetric loss of the binder does result in a porous electrode. The pores provide buffer space for silicon volume expansion during the formation of silicon–lithium compounds.
EIS measurement provides a variety of effective, time-varying information, including lithium-ion migration, carrier exchange, phase transfer, solid material transfer phenomenon, and the performance of capacitance effect. It is the most effective tool for observing the changes in the interface between the electrolyte and the electrode.
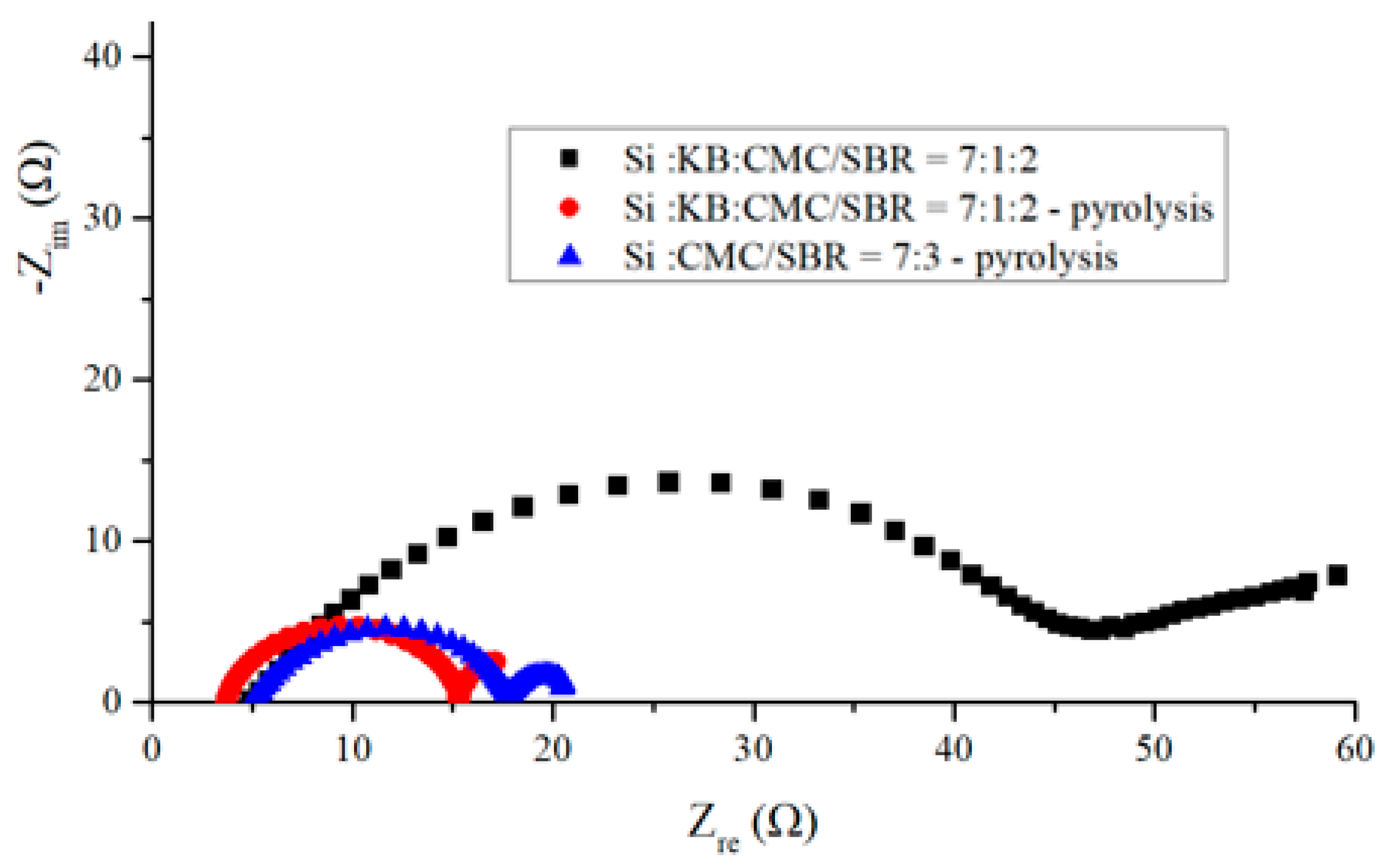
Figure 9 shows the electrochemical impedance spectra of three anodes before cycling. The black squared curve was measured from an anode made according to the weight ratio of Si–KB–CMC/SBR = 70%:10%:20%. The spectrum representing the same anode after pyrolysis is presented by the red dotted curve. The blue triangle curve represents the spectrum for an anode made according to the weight ratio of Si–CMC/SBR = 70%:30% without KB additive and after pyrolysis.
The left side of each curve was measured at higher frequency than the right side. The bottom left point on the real number axis represents the contact resistance. The radius of the EIS semicircle stands for the carrier transfer resistance. The above impedance reflects the rate of insertion of lithium ions through the electric double-layer interface. The slope of the oblique line in the low-frequency region on the right side of the graph is related to the diffusion rate of lithium ions. The higher the slope of the oblique line is, the faster the diffusion rate of lithium ions in the sample [
23].
For contact resistance, pyrolytic anode with conductivity enhancement additive exhibited the lowest value, and the pyrolytic anode without conductivity enhancement additive exhibits the largest value. This is consistent with the contents of conductive carbon in the anode. The contact impedances of three kinds of anodes are all below 5 ohms. On the other hand, pyrolytic binder provides a conductive network, which decreased the charge transfer resistance effectively.
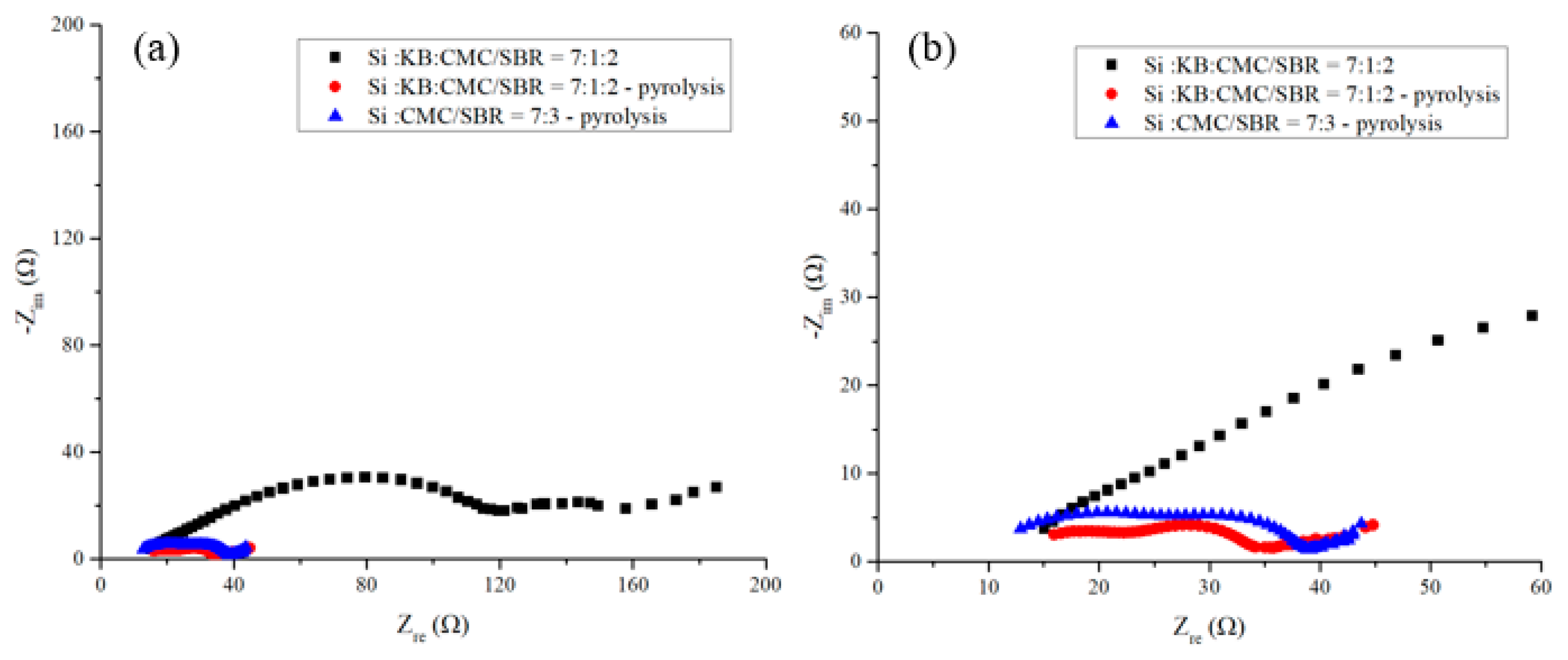
Figure 10 shows the EIS spectra of anodes after 200 cycles of charge–discharge. The carrier transfer resistance of the silicon-based anode without pyrolysis increases to about 3 times of that before cycling. The sum of the radius of the first and second semicircles of the anode doubled. The slopes of the oblique straight lines in the low frequency area of the three curves declined. It indicates that the lithium-ion diffusion capability of the three anode decreased after 200 cycles of charging and discharging. Among them, the pyrolytic sample with 10 wt% KB exhibits the highest slope of the diagonal line and survives the longest cycling life. Pyrolytic anodes with conductivity enhancement additive exhibit improved rate of carrier transfer and reduced interface resistance effectively. The carrier transfer impedance in intermediate frequency of pyrolytic electrode with KB is slightly lower than that of the electrode without conductive enhancement agent. This is attributed to the promotion of the conductivity enhancement additives on the carrier transport. The cycling life of the silicon-based anode improves, too.
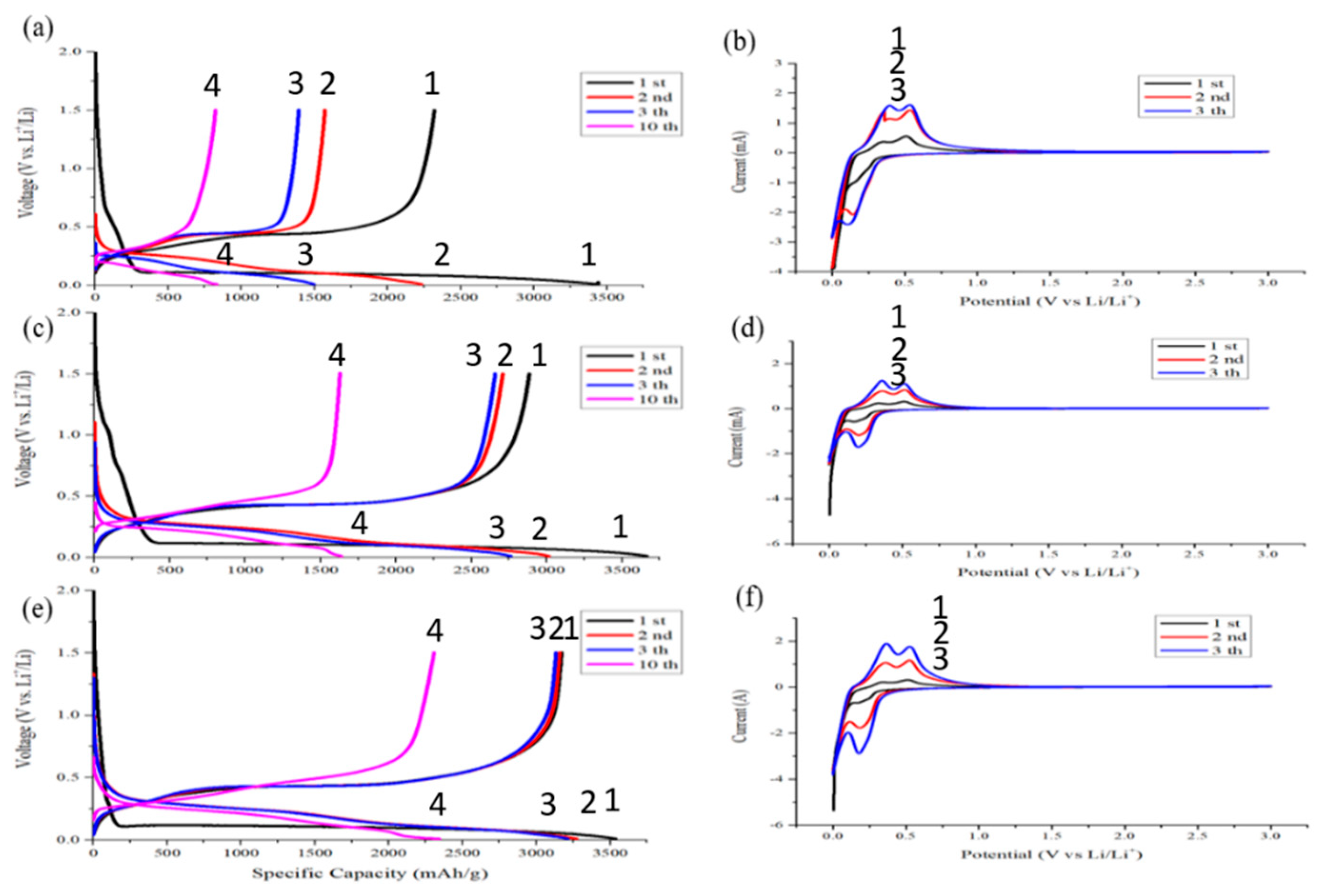
The initial coulombic efficiency of the pristine silicon anode is only 67.4%, and the capacity decay in the second and third cycles is fast. The 3rd charging capacity is only 60.8% of the initial charging. After the 10th charging and discharging, the capacity of this sample has only 780 mAh/g left. After pyrolysis, the same sample exhibits an initial coulombic efficiency that increased by 10.6%. The rate of capacity decline also slows down. The 3rd charging capacity is 96.4% of that of the first charging. After 10 times of cycling, this sample still retained a capacity of 1750 mAh/g. Moreover, the initial coulombic efficiency of pyrolytic anode even without conductive agent is as high as 89.6%. The 3rd charging capacity of the sample is almost the same as the first one as shown in
Figure 11e. The pyrolysis process not only increases the first cycle coulombic efficiency but also extends the cycling life of the silicon-based anode.
Figure 11a,c,e shows that the area of the 1st corresponding capacitance before the potential drops to 0.01 V is proportional to the nanocarbon contents, which are present in the anode. Less nano-carbon is desirable to avoid the side effects between nano-carbon and electrolyte in the first stage of the cycling. The less conductive carbon in the anode, the higher the initial coulombic efficiency will be. To optimize the ICE, either the nano-carbon contents need to decrease, or a better choice of nano-carbon phase is desirable.
Figure 11b,d,f shows discharge–charge CV curves. The discharge begins with the lithiation of the crystalline silicon near zero volt. The lithiation forms lithium–silicon alloys. When alloys dissociate to release lithium, silicon does not recrystallize but instead becomes amorphous silicon. The delithiation curves exhibit two peaks near 0.4 V and 0.6 V. These peaks are attributed to a two-step delithiation process, i.e., the anode first delithiates to become an intermediate lithium silicon alloy [
24].
The cyclic voltammetry patterns of the pyrolytic samples are similar. The scanning patterns of the two and three circles are basically unchanged. This indicates that the reversibility of the pyrolytic anode is good. The pyrolysis process does improve the electrical performance of an anode.
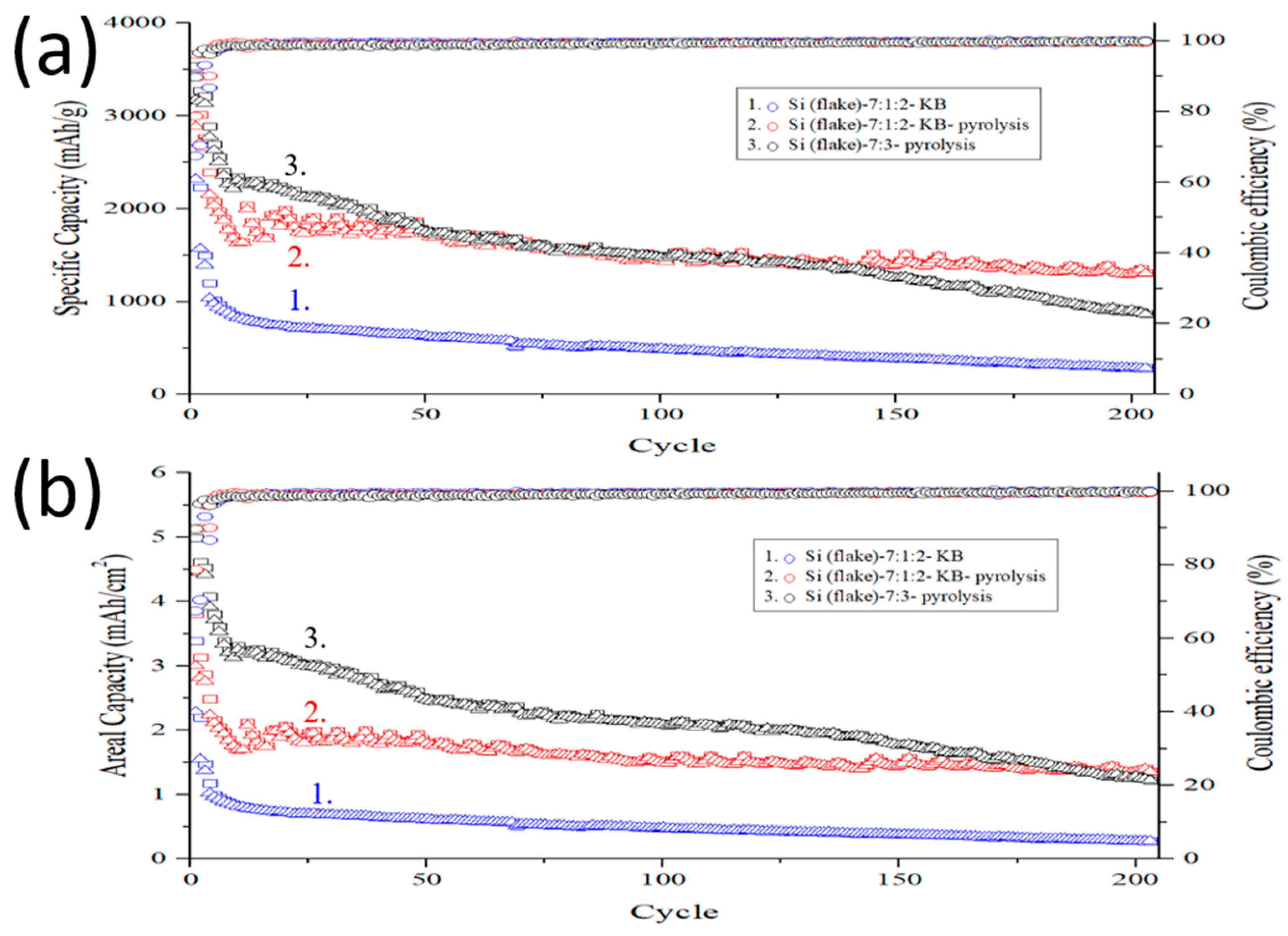
Figure 12 shows cycling performance of an anode made of Si flake–KB–CMC/SBR = 70%:10%:20% in weight ratio, a pyrolytic anode made of Si flake–KB–CMC/SBR = 70%:10%:20% in weight ratio, and a pyrolytic anode made of Si flake–CMC/SBR = 70%:30% in weight ratio. The mass loading of these anodes is in the range of 1.2–1.5 mg/cm
2. The charge–discharge cycling current is 200 mA/g for the first three cycles and 1000 mA/g for the subsequent cycles. Graphitic carbon coating on silicon flake reduces direct contact of silicon with the electrolyte and their undesirable irreversible reactions. It results in much improved initial coulombic efficiency.
When conductivity enhancement additive (KB) was not added, there was only 5.7 wt% of carbon that was formed by conversion from the binder by the pyrolysis process. The low nanocarbon contents further reduce undesirable reactions with electrolyte catalyzed by nano carbon. The achieved initial coulombic efficiency was as high as approximately 90%.
The anode made of silicon flake, binder, and conductivity enhancement additives but without pyrolysis process lacks graphitic carbon coatings on silicon flakes and a porous structure of pyrolyzed binder. The conductivity of this anode is the lowest among three anodes. Silicon volume expansion leads to high internal stresses, which tend to cause cracks in the anode and losses of electrical connectivity between cracked anode pieces. As a result, such an anode exhibits poor electrochemical performance with the capacity rapidly declining to 500 mAh/g after only about 10 cycles of charging and discharging. After 200 charging and discharging cycles, the capacity of silicon anode decreases to 250 mAh/g.
In contrast, pyrolytic anodes exhibit slow decay in the capacity by cycling. This is attributed to the graphitic carbon coatings on silicon flake and the porous conductive carbon structure formed by the binder pyrolysis process. The electrochemical performance of two pyrolytic anodes studied are different. In the stage of the 1st–10th cycles, the pyrolytic anode with Si flake–KB–CMC/SBR = 70%:10%:20% in weight ratio decayed faster than the pyrolytic anode with Si flake–CMC/SBR = 70%:30% in weight ratio. The KB conductivity enhancement additive acts as an undesirable catalyst for reactivity of the electrolyte and results in reduced ICE and faster decay in the capacity cycling. In this respect, an anode without KB is desirable. A different conductivity enhancement additive than KB or a less amount of KB should have been applied.
However, in the later stage of the capacity cycling, the capacity of the pyrolytic anode with Si flake–KB–CMC/SBR = 70%:10%:20% in weight ratio remained basically constant with only a smaller decrease than the anode without KB additive. After 200 cycles, the anode still maintained a high capacity of nearly 1200 mAh/g, and a high areal capacity of 1.4 mAh/cm2. As a comparison, the capacity of the pyrolytic anode made of Si flake–CMC/SBR = 70%:30% in weight ratio without KB additive declines rapidly in the initial 50 cycles. Without conductivity enhancement additive, KB, although the anode performs well, the conductivity of the anode is too low to handle the high cycling current of 1 A/g. In the 50th–150th cycles, the capacity of this sample remained stable with only slightly decreased to 1.75 mAh/cm2. After 150 cycles, the capacity of the sample gradually decreased. After 200 cycles, the sample retains a capacity of 900 mAh/g (1.3 mAh/cm2). Without the conductivity enhancement additive, KB, the internal resistance gradually increases with repetitive charging and discharging causing the capacity to decline.
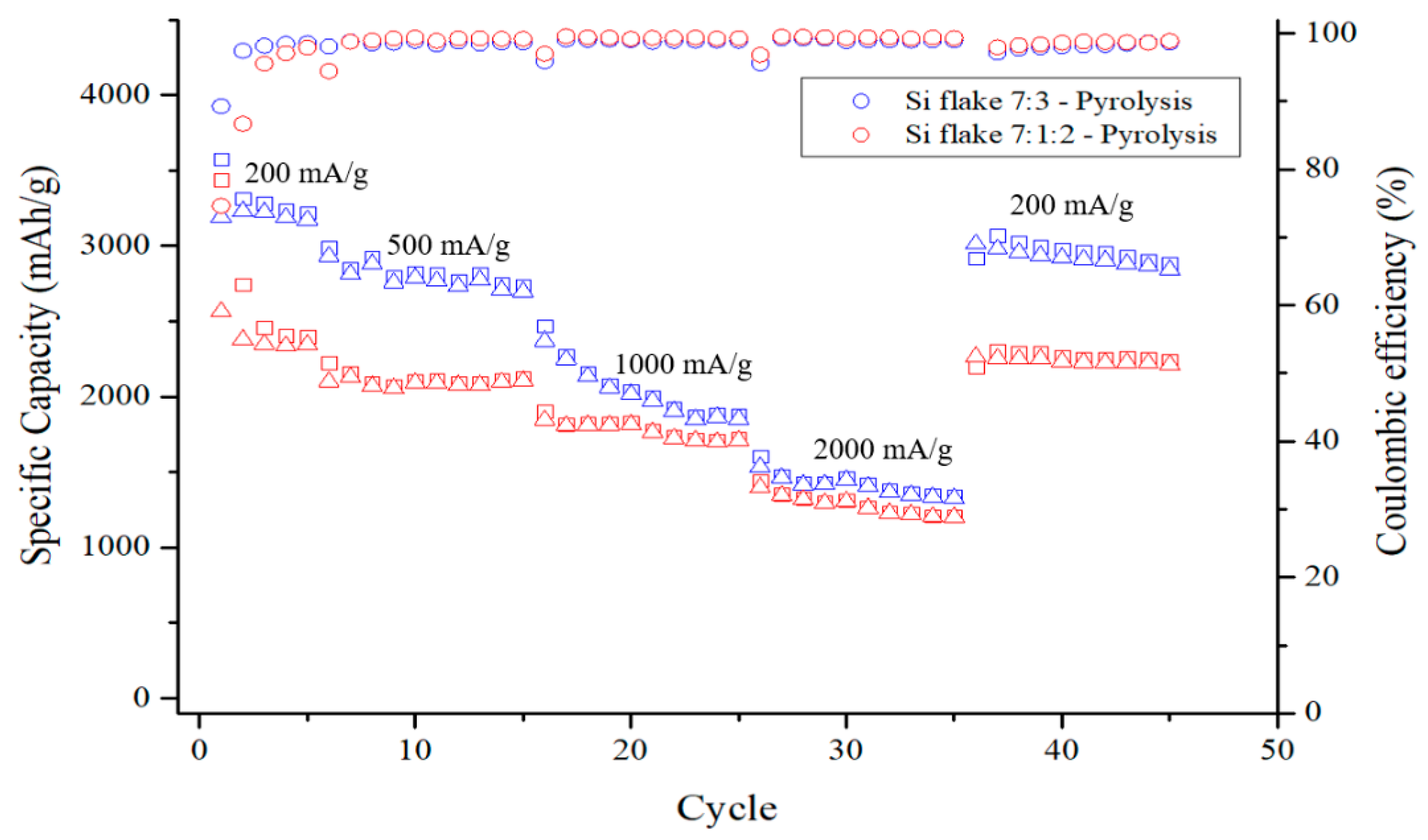
Figure 13 shows the capacity cycling and the coulombic efficiencies of two kinds of anodes after having been subjected to pyrolysis at different charging–discharging rates. The test current of the measurement is 200 mA/g for the first 5 cycles, followed by 500 mA/g, 1000 mA/g, and 2000 mA/g for 10 cycles each. The specific capacity of the pyrolytic anodes is higher than 2200 mAh/g with the anode without conductivity enhancement additive, KB, being as high as 3000 mAh/g. The difference is due to the different ICE of these two anodes. The ICE of anode containing KB is only 75%, while the anode without KB exhibits an ICE is as high as 89%. The conductivity enhancement additive promotes the conductivity of the anode but also consumes significant lithium during the first discharge–charge cycles. An optimized small amount of conductivity enhancement additive is desirable. When the discharge–charge current is increased to 1000 mA/g, the capacity of the anode with conductivity enhancement additive, KB, does not decline significantly, while the anode without KB exhibits a faster decline in capacity due to low internal conductivity of the anode. When the current is restored to 200 mAh/g, the capacity increased to 2300 mAh/g and 3000 mAh/g, for anode without and with KB, respectively. The capacity decreased by less than 10% after the cycling at different current rate up to 2 A/g. These results show that pyrolytic silicon-based anodes exhibit excellent electrochemical performance and capacity retention.
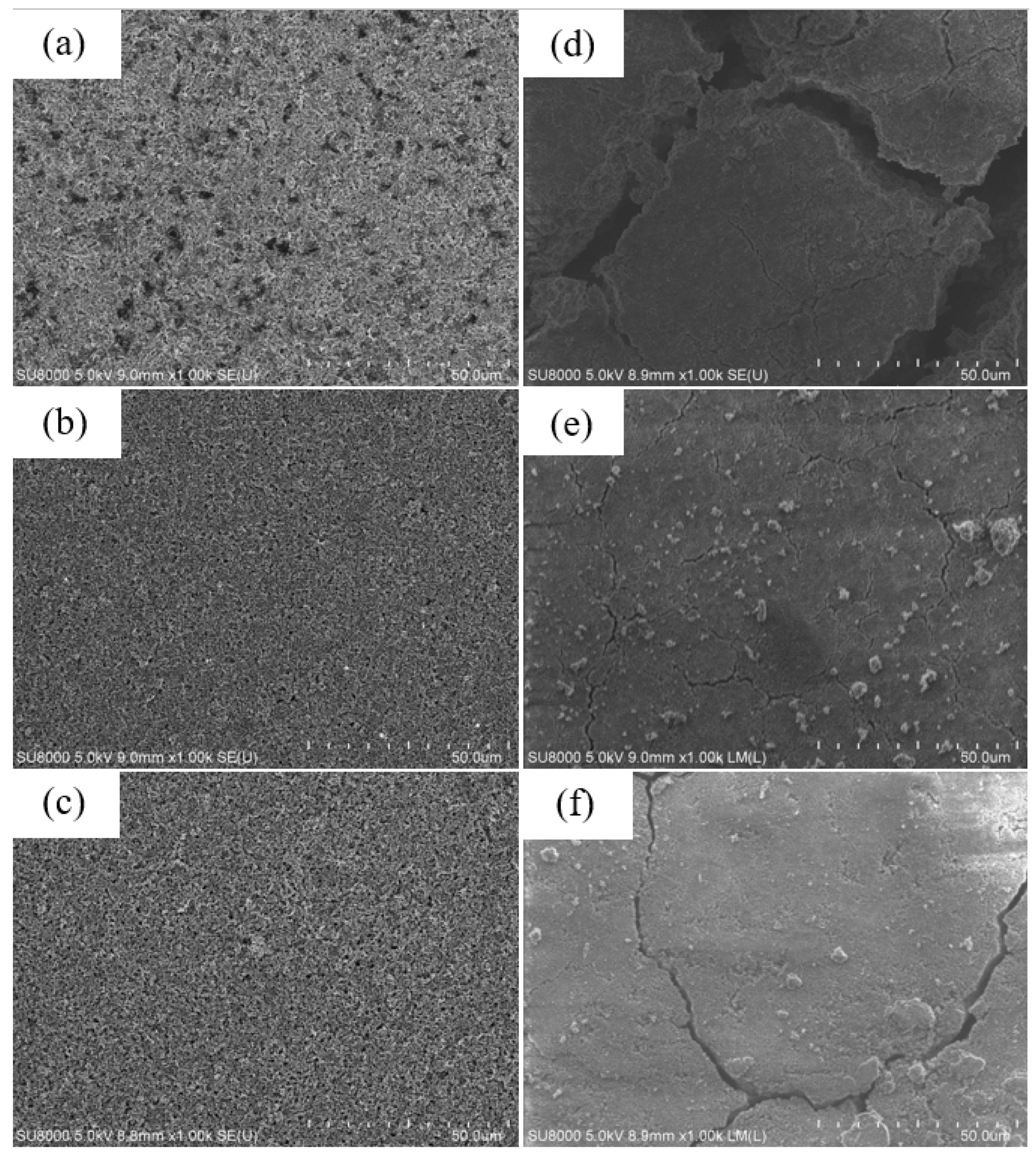
Figure 14 shows SEM images of the surface of (a) as-fabricated anode made of Si–KB–CMC/SBR = 70%:10%:20% in weight ratio, (b) pyrolytic anode made of Si–KB–CMC/SBR = 70%:10%:20% in weight ratio, and (c) pyrolytic anode made of Si–CMC/SBR = 70%:30% in weight ratio. The anodes were cleaned by DEC solvent, in order to achieve the consistency of the image. The surfaces of the as-fabricated anodes were homogeneous without cracks.
For an anode without pyrolysis, after repetitive discharge–charge cycling, volume changes of silicon flake might cause cracks appearing on the surface of the anode, as shown in
Figure 14d. In the contrast, the pyrolyzed anodes contain carbonized binder, which was not only beneficial to the anode conductivity, but also coated on the surface of the silicon flake with graphitic carbon as a barrier against undesirable reactions between silicon surface and the electrolyte, reduced side reactions, and provided room in the porous carbon structure as a buffer for the volume changes of silicon flakes. As a result, the surface cracks of pyrolytic anodes shown in
Figure 14e,f after 200 cycles are relatively small compared to those of the anode without pyrolysis as shown in
Figure 14d. Small cracks are more likely shallow without completely losing electrical connectivity. Large cracks cause two sides of anode to lose electrical contacts.
Figure 14f shows the surface morphology of a pyrolytic electrode made of Si flake–CMC/SBR = 70%:30% in weight ratio without conductivity enhancement additive, KB, but instead, only containing 5.7% of conductive carbon by weight that was formed by the pyrolysis of the binder. Although a smaller amount of conductive carbon reduces the reactions catalyzed by nano-carbon and effectively improve the ICE, the lithium-ion diffusion rate is gradually reduced during the capacity cycling. This is not desirable for long-term cycling. By adding a small but appropriate amount of conductivity enhancement additive, desirable diffusion rate of lithium ions during the cycling is preserved to increase the cycling life of the anode while keeping the ICE near 90%.
Figure 14e shows the surface morphology of an example of such an optimized anode.
Figure 15 compares this work with selected high-performance silicon-based anodes which have been published in the literature in recent years [
25,
26,
27,
28,
29,
30,
31,
32,
33,
34]. The initial coulomb efficiency and the 100th areal capacity are compared. Research groups #1 [
25], #2 [
26], and #4 [
28] reported higher areal capacity using silicon powder of smaller than 100 nm in size to reduce the stress issues induced by silicon volume expansion. Group #3 [
27] used magnesium powder to reduce the coating layer of silicon dioxide, followed by reducing silicon dioxide to form silicon as the active material. Group #5 [
29] used commercial SiOx powder as the active material for the anode. High-areal capacity silicon-based anode was achieved by some groups partially by means of choosing appropriate silicon materials and sizes to overcome the problems of silicon volume expansion and pulverization during charging and discharging cycling. However, the costs of the synthesis and purification processes for producing nano-sized silicon powder and SiOx and the magnesium reduction process are high.
Silicon flake (800 nm × 800 nm × 100 nm) recycled from silicon wafer manufacturing waste was used as the active material in this work. By pyrolyzing the binder, excellent initial coulombic efficiency of silicon-based anode of about 90% was achieved. This is higher than or as good as data reported in recent publications. We attribute this excellent performance to firstly, micron-sized flat silicon flake exhibits smaller effective surface area than silicon nanoparticles of the same total volume. Secondly, the graphitic coating on silicon by pyrolyzing the binder protects silicon surface from undesirable reactions with the electrolyte to form irreversible silicon-lithium compounds. The silicon anode prepared in this work retains a high areal capacity of 2.1 mAh/cm2 after 100 discharge–charge cycles. It maintained an areal capacity of more than 1.4 mAh/cm2 after 200 cycles.
Mass loading of silicon-based anodes are typically in the range of 1–2 mg/cm
2 [
25,
26,
27,
28,
29,
30,
31,
32,
33,
34]. An anode with a higher loading suffers from long lithium ions diffusion time. Group #3 [
27] made the anode with a mass loading of more than 4 mg/cm
2, but the electrochemical performance of the two samples with 4.0 mg/cm
2 and 4.9 mg/cm
2 is not as good as the sample with only 3 mg/cm
2. In addition, group #9 [
33] also made the electrode with a loading of more than 10 mg/cm
2. As a result, the capacity of this anode dropped significantly near the beginning stage of the discharge–charge cycling. In this work, mass loading in the range of 1.2–1.5 mg/cm
2 was chosen. KB was used as the conductivity enhancement additive. There is no pre-fabrication coating of carbon or other protective layer on silicon. For our anodes made without pyrolysis, the ICE was only 67.4%, and the areal capacity decreased to only 0.35 mAh/cm
2 after 100 cycles.
After pyrolyzing the anode, The ICE increased to 78.0%, and after the 100th cycle, the areal capacity was 1.4 mAh/cm2. There was little decline in capacity afterwards. The excellent electrochemical performance may be attributed to the graphitic carbon coatings on the surface of the silicon flake. The coatings serve as protective layers to avoid side reactions of silicon, especially at the initial stage of the cycling, with the electrolyte. At the same time, the binder transformed into a porous carbon structure, providing additional buffer room for volume expansion of the silicon flake, improving the cycling lifetime of the anode.
Higher ICE than 78% is desirable. Reactions of silicon with electrolyte is not the only major cause to the consumption of lithium in the first cycle. To investigate the roles played by the conductivity enhancement additive, KB, on the ICE, pyrolytic anode without KB was fabricated. Without KB, the ICE increased from 78% to 89.6%, and after the 100th cycle the areal capacity was 2.1 mAh/cm2. We attribute the significant increase in ICE to the absence of KB as a catalyst for undesirable reactions with the electrolyte during the initial discharging and charging cycle. Therefore, some active carbon contents in the anode have adverse effects on ICE. The pyrolytic anode made of Si flake–KB–CMC/SBR = 70%:10%:20% in weight ratio contains 15.5 wt% nano carbon and exhibits ICE of 78.0%. As a comparison, pyrolytic anode made of Si flake–CMC/SBR = 70%:30% in weight ratio contains only 5.7 wt% nanocarbon and exhibits a higher ICE of 89.6%.
However, from
Figure 9 and
Figure 10 it shows that the lithium-ion diffusion rate of the anode without KB additive is poorer than that with KB and the capacity retention after long cycling time is lower than that of the pyrolytic anode with KB. Although the porous carbon structure provides sufficient conductivity and physical integrity for the anode to perform better than the anode with KB but without pyrolysis, the conductivity was not adequate for the high demanding support of the charge–discharge cycling. When the anode internal impedance increases with cycling time, cracks appeared and the capacity decay rate increased in the cycling. KB was chosen as the conductivity enhancement additive for this study because of its high electrical conductivity. An optimized amount of KB additive or by choosing a different inert conductivity enhancement additive for the pyrolytic anode is believed to be able to achieve a long-cycling-life anode with a high ICE on the order of 90%. Indeed, when the KB is replaced by Super P conductivity enhancement additive, the pyrolytic anode made of Si flake–Super P–CMC/SBR = 70%:10%:20% in weight ratio exhibited an excellent ICE of 89.92%. Research is being undertaken to study the effects of different kinds and amounts of conductivity enhancement additives on ICE. The detailed results will be published in the near future.