The Effects of the Addition of Polyurethane–MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PU-MgO Nanohybrids
2.3. Production of Cement Pastes
2.4. Mechanical Tests
2.5. Characterizations
3. Results and Discussion
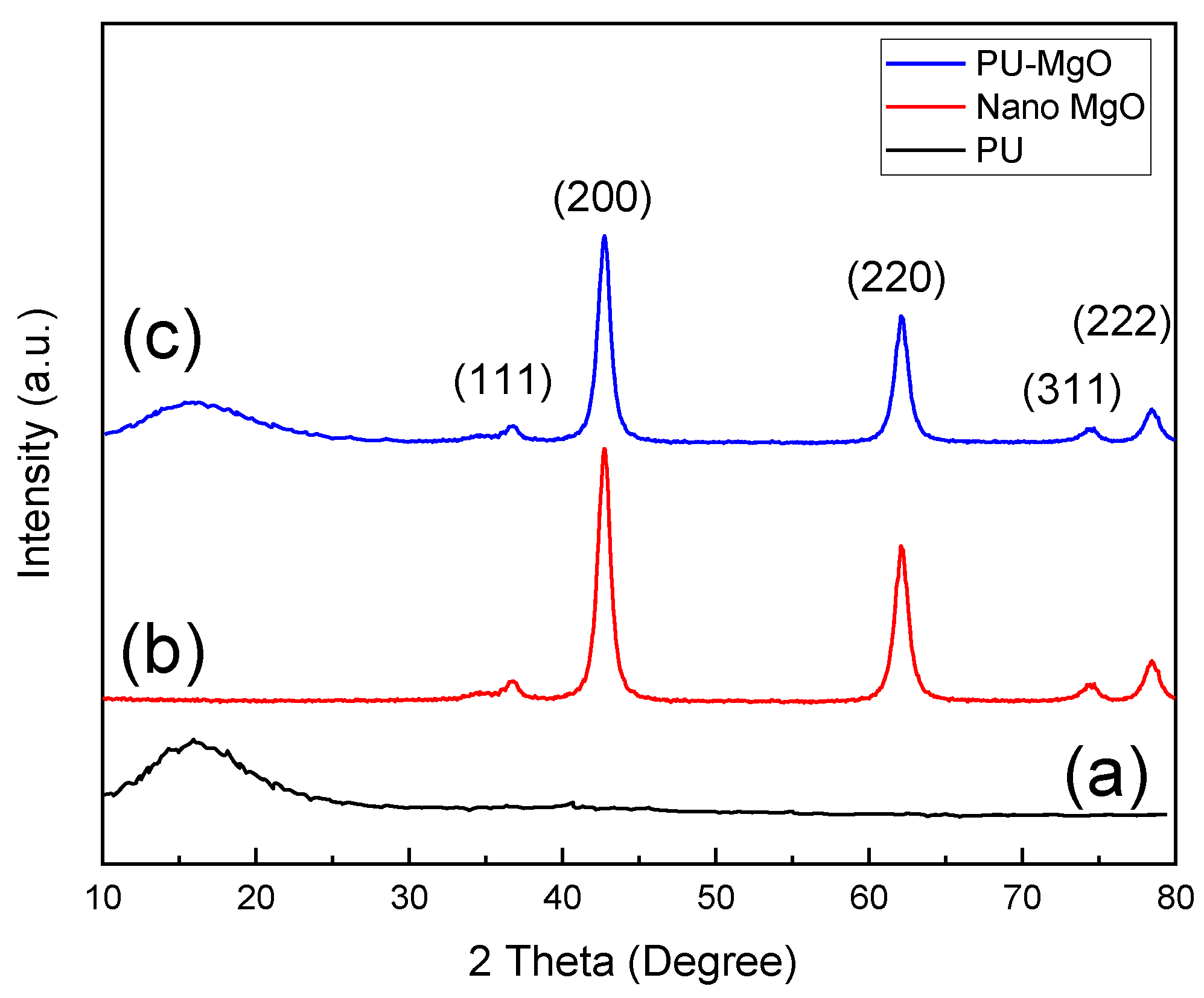
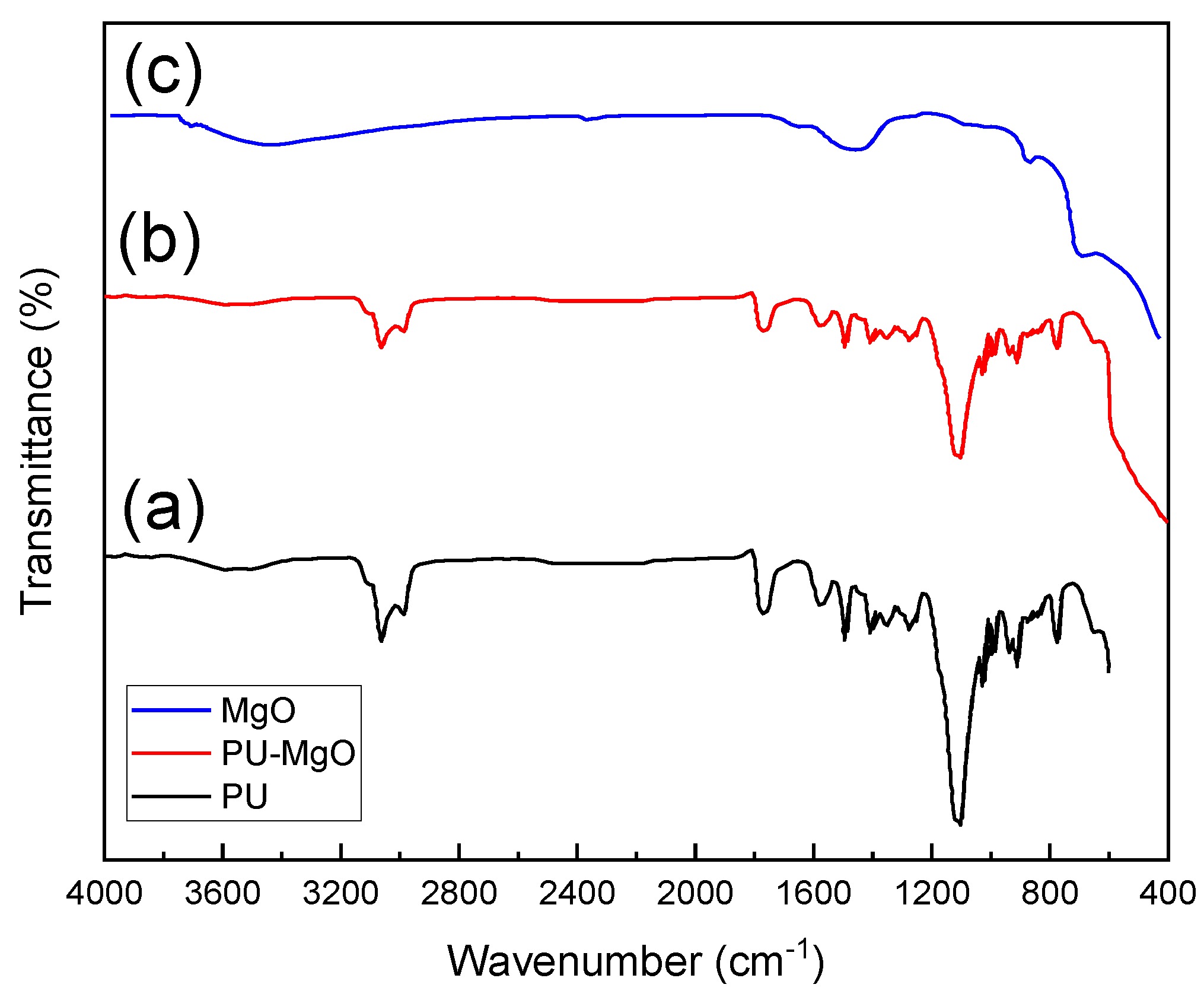
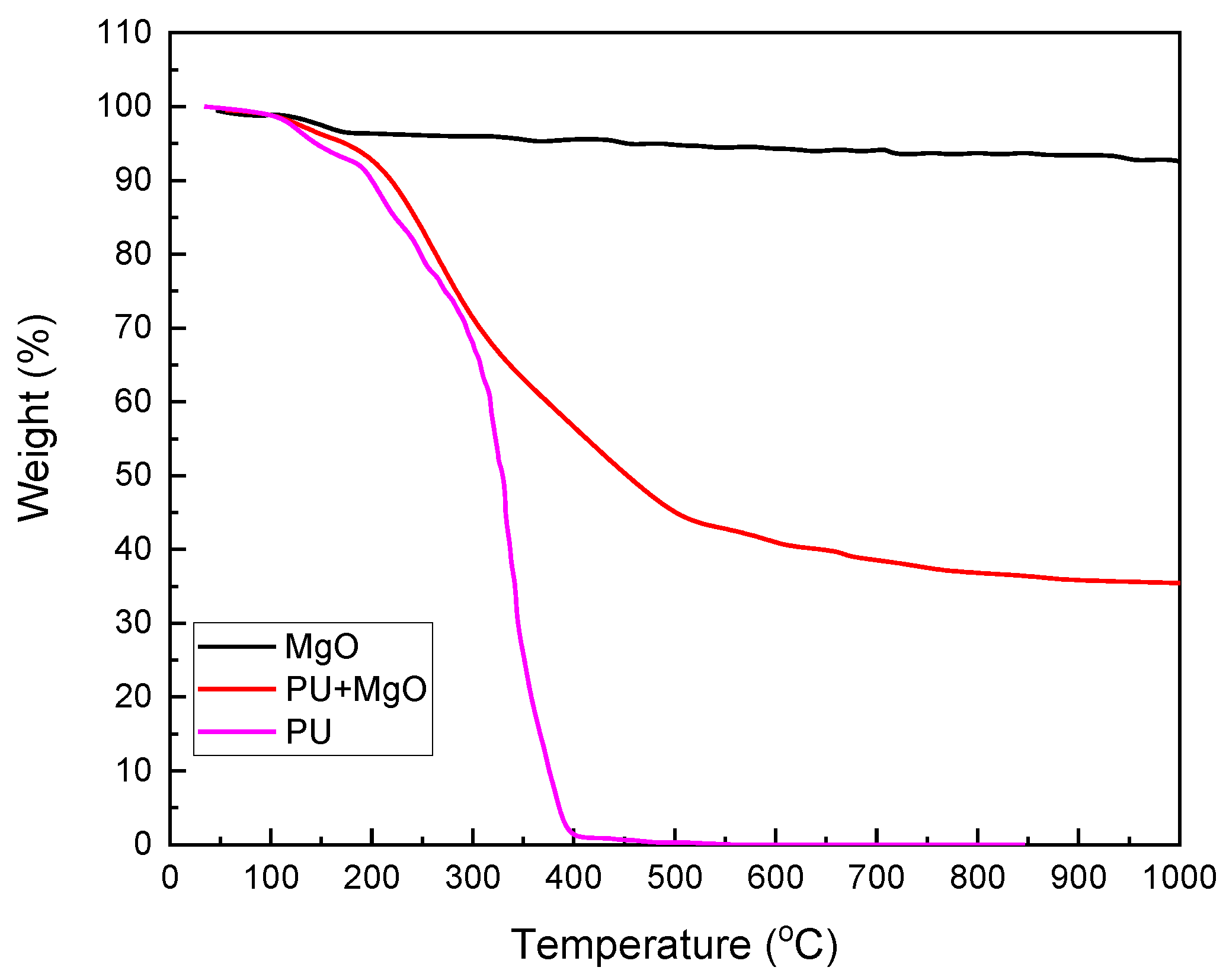
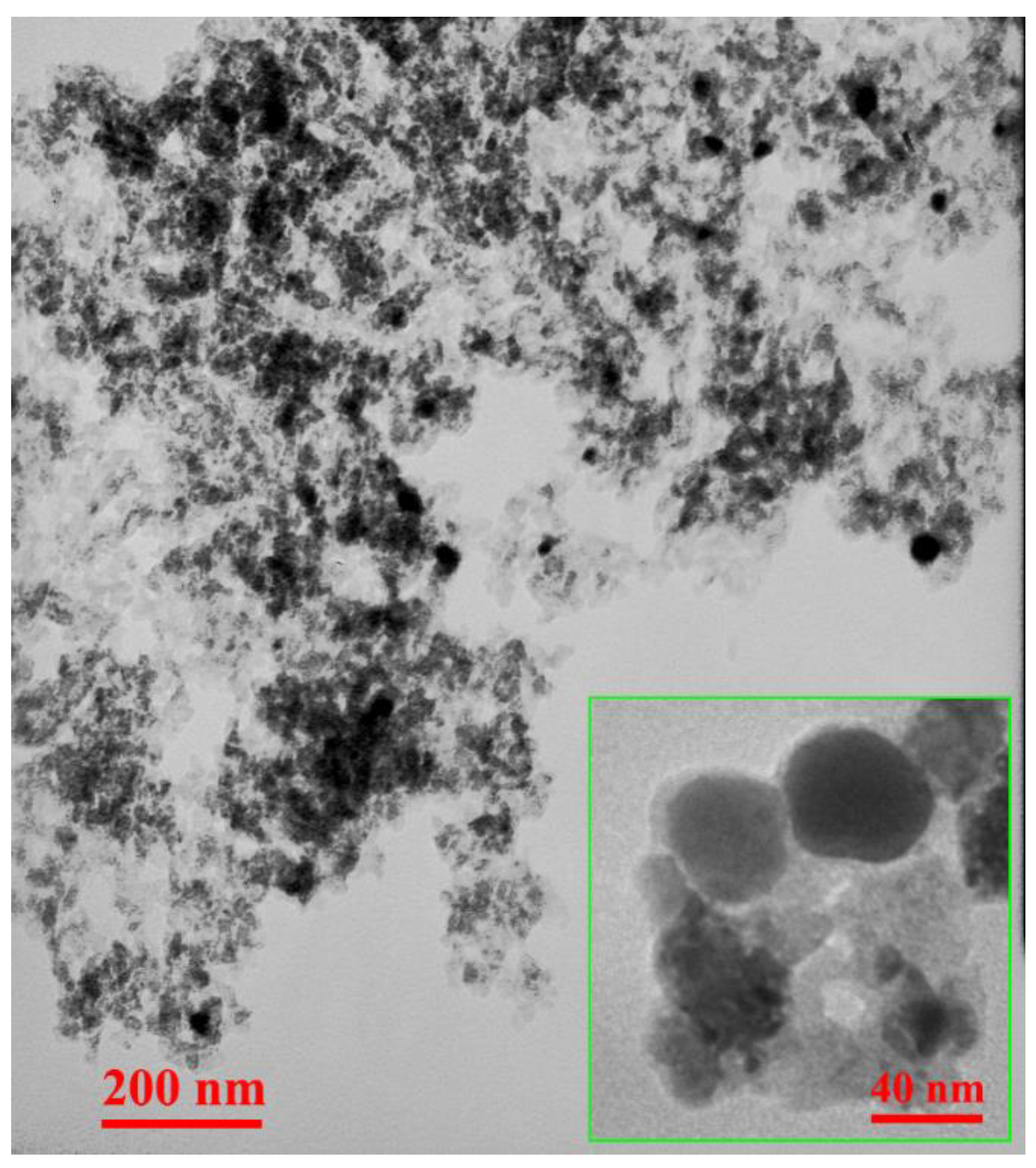
3.1. Characterization of PU-MgO
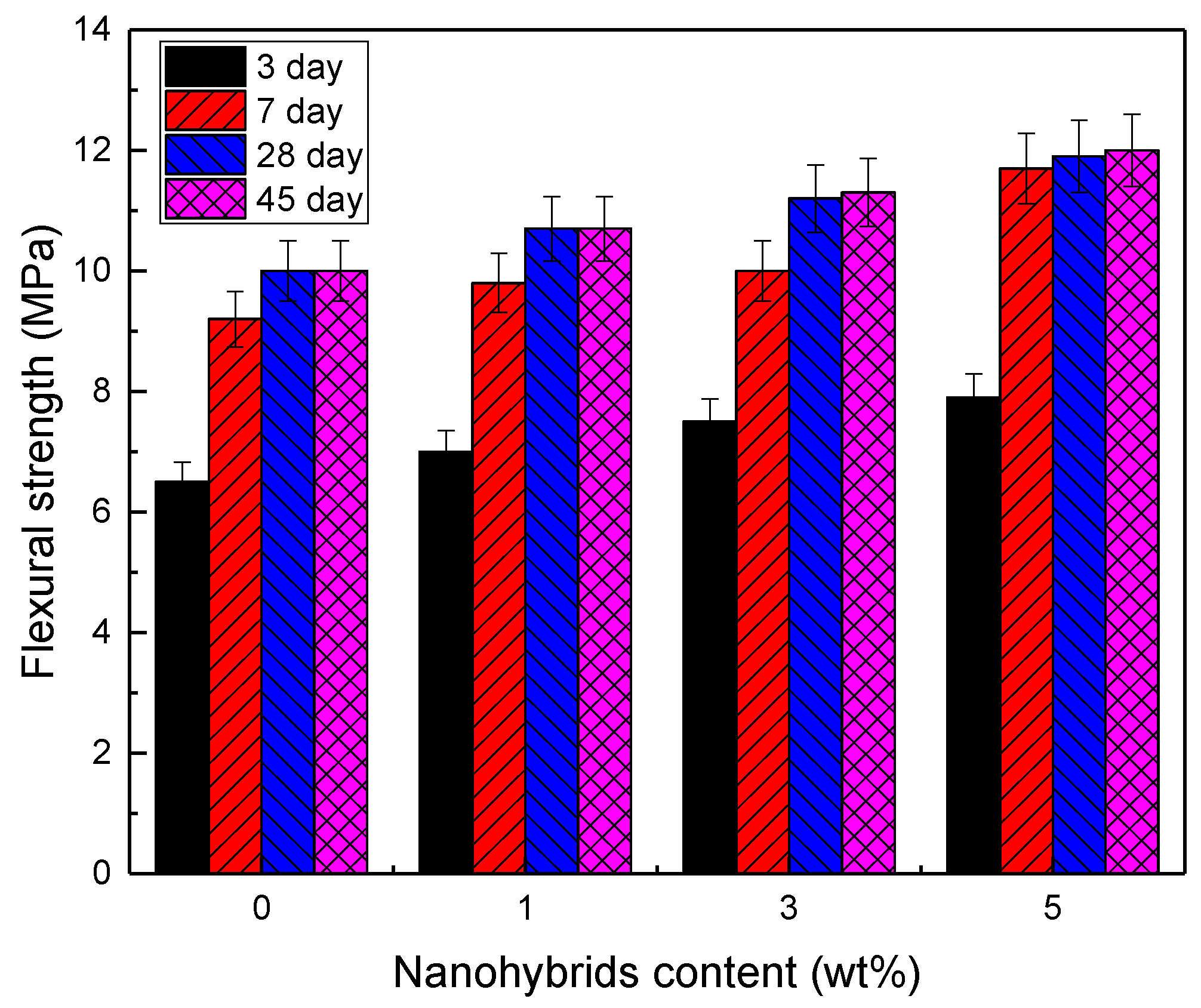
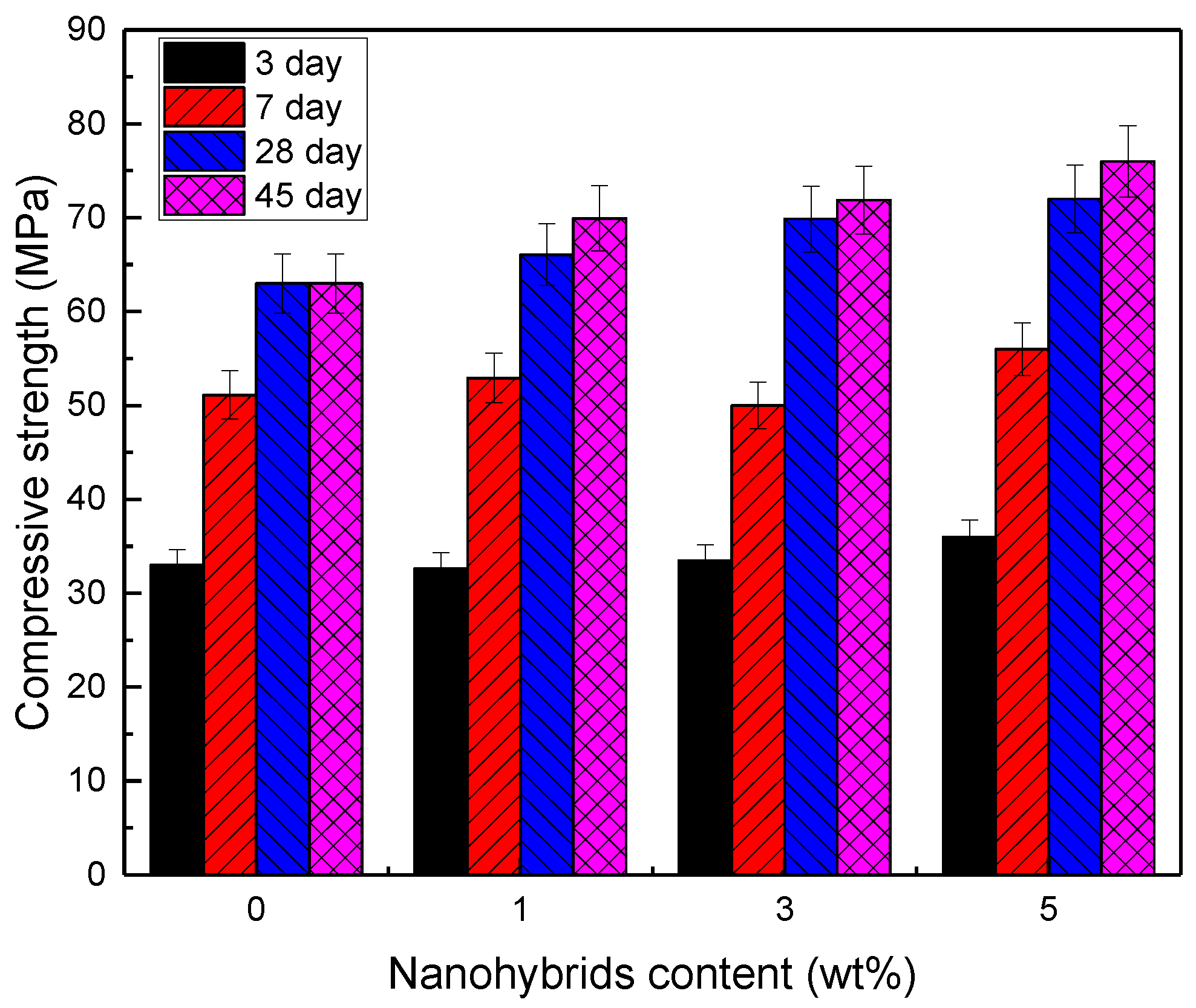
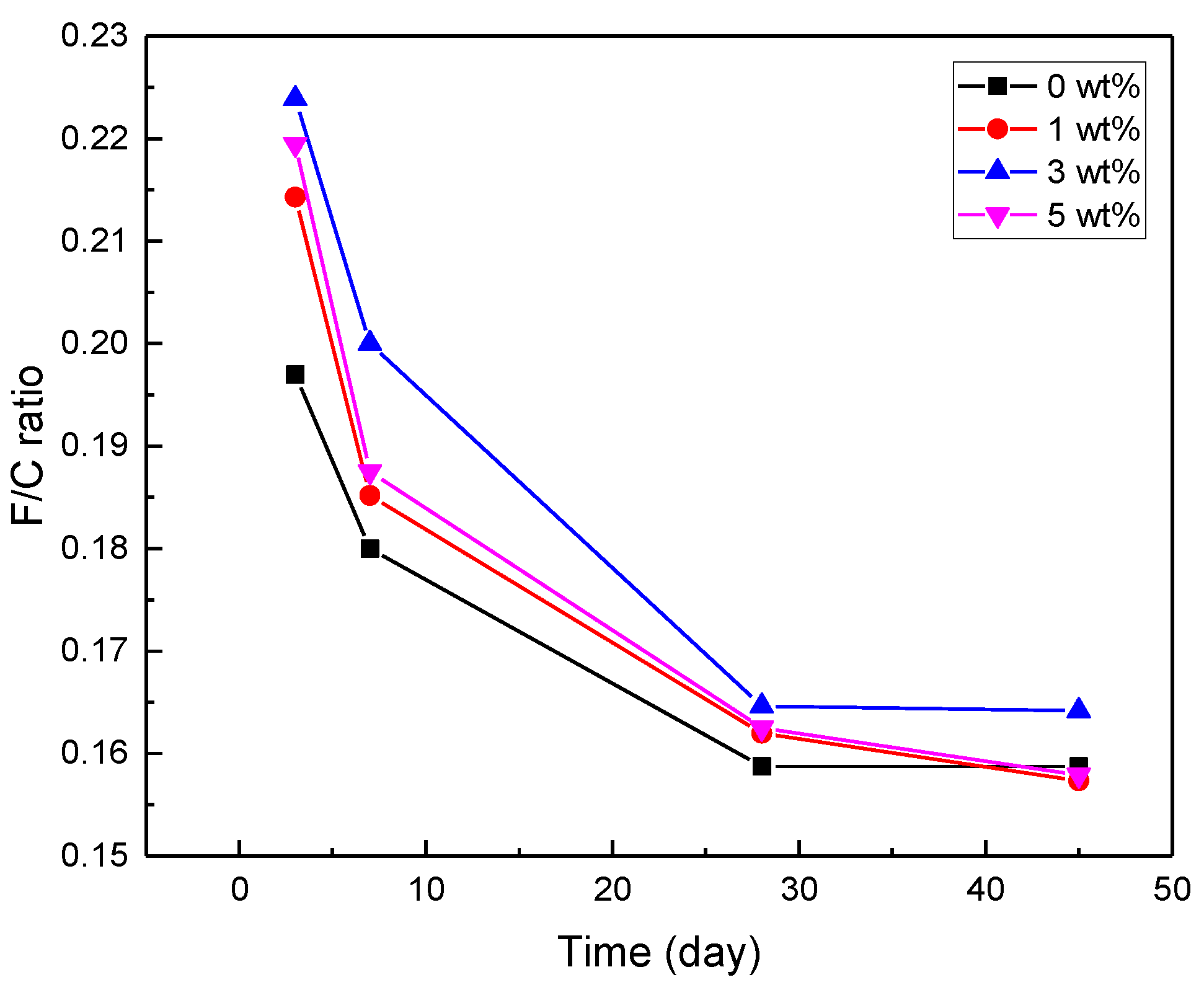
3.2. Mechanical Properties
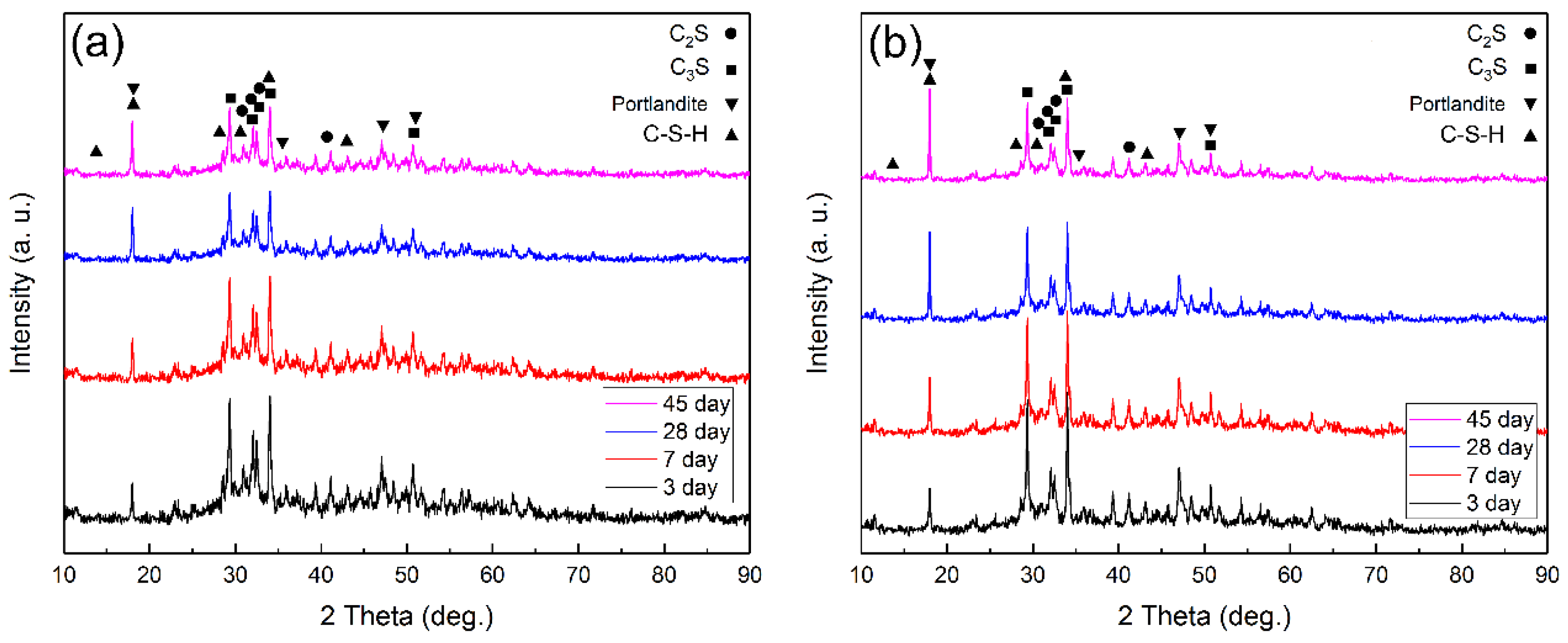
3.3. Phase Characterization
3.4. Cement Hydration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Shu, X.; Zheng, S.; Qi, S.; Ran, Q. Effects of polyurethane–silica nanohybrids as additives on the mechanical performance enhancement of ordinary Portland cement paste. Constr. Build. Mater. 2022, 338, 127666. [Google Scholar] [CrossRef]
- Zhang, P.; Sha, D.; Li, Q.; Zhao, S.; Ling, Y. Effect of Nano Silica Particles on Impact Resistance and Durability of Concrete Containing Coal Fly Ash. Nanomaterials 2021, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, D.; Zhang, S.; Cui, H.; Li, D. Effect of Nano-SiO2 on the Hydration and Microstructure of Portland Cement. Nanomaterials 2016, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-Silica-Modified Concrete: A Bibliographic Analysis and Comprehensive Review of Material Properties. Nanomaterials 2022, 12, 1989. [Google Scholar] [CrossRef]
- Berktas, I.; Chaudhari, O.; Ghafar, A.N.; Menceloglu, Y.; Okan, B.S. Silanization of SiO2 Decorated Carbon Nanosheets from Rice Husk Ash and Its Effect on Workability and Hydration of Cement Grouts. Nanomaterials 2021, 11, 655. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Velay-Lizancos, M. Impact of nano-TiO2 addition on the reduction of net CO2 emissions of cement pastes after CO2 curing. Cem. Concr. Compos. 2021, 123, 104160. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Lin, S.; Yan, J.; Du, S. Effects of nano-SiO2 coated multi-walled carbon nanotubes on mechanical properties of cement-based composites. Constr. Build. Mater. 2021, 281, 122577. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Z.; Liu, H.; Zhuge, Y.; Zhang, D. A new in-situ growth strategy to achieve high performance graphene-based cement material. Constr. Build. Mater. 2022, 335, 127451. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, H.; Du, M.; Chen, W. Dispersion of Multi-Walled Carbon Nanotubes Stabilized by Humic Acid in Sustainable Cement Composites. Nanomaterials 2018, 8, 858. [Google Scholar] [CrossRef]
- Hong, X.; Lee, J.C.; Qian, B. Mechanical Properties and Microstructure of High-Strength Lightweight Concrete Incorporating Graphene Oxide. Nanomaterials 2022, 12, 833. [Google Scholar] [CrossRef]
- Dehkordi, B.A.; Nilforoushan, M.R.; Talebian, N.; Tayebi, M. A comparative study on the self-cleaning behavior and antibacterial activity of Portland cement by addition of TiO2 and ZnO nanoparticles. Mater. Res. Express 2021, 8, 35403. [Google Scholar] [CrossRef]
- Parvan, M.-G.; Voicu, G.; Badanoiu, A.-I.; Nicoara, A.-I.; Vasile, E. CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions. Nanomaterials 2021, 11, 875. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.; Rajaee, A. Characterization of zeolite/bioglass nanocomposites for surface coating of stainless steel material for bone implantation. J. Sol-Gel Sci. Technol. 2022, 104, 365–379. [Google Scholar] [CrossRef]
- Yao, K.; Wang, W.; Li, N.; Zhang, C.; Wang, L. Investigation on strength and microstructure characteristics of nano-MgO admixed with cemented soft soil. Constr. Build. Mater. 2019, 206, 160–168. [Google Scholar] [CrossRef]
- Díaz, J.; Gálvez, J.C.; Alberti, M.G.; Enfedaque, A. Achieving Ultra-High Performance Concrete by Using Packing Models in Combination with Nanoadditives. Nanomaterials 2021, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.H.; Chung, W.; Nam, B.H. Molecular Dynamics Simulation of Calcium-Silicate-Hydrate for Nano-Engineered Cement Composites—A Review. Nanomaterials 2020, 10, 2158. [Google Scholar] [CrossRef]
- Lu, L.; Ouyang, D. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials 2017, 7, 187. [Google Scholar] [CrossRef]
- Sikora, P.; Abd Elrahman, M.; Stephan, D. The Influence of Nanomaterials on the Thermal Resistance of Cement-Based Composites—A Review. Nanomaterials 2018, 8, 465. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Q.; Wang, Z.; Tayebi, M.; Hamawandi, B. The Effect of Eco-Friendly Inhibitors on the Corrosion Properties of Concrete Reinforcement in Harsh Environments. Materials 2022, 15, 4746. [Google Scholar] [CrossRef]
- Shi, T.; Lan, Y.; Hu, Z.; Wang, H.; Xu, J.; Zheng, B. Tensile and Fracture Properties of Silicon Carbide Whisker-Modified Cement-Based Materials. Int. J. Concr. Struct. Mater. 2022, 16, 2. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, T.; Cen, M.; Wang, J.; Zhao, X.; Zeng, C.; Zhou, Y.; Fan, Y.; Liu, Y.; Zhao, Z. Research progress on lunar and Martian concrete. Constr. Build. Mater. 2022, 343, 128117. [Google Scholar] [CrossRef]
- Hasanova, I.; Hasanova, U.; Gasimov, E.; Rzayev, F.; Hajiyev, E.; Eyvazova, G.; Shaliyev, M.; Mehdiyeva, A.; Aliyeva, N.; Yusifov, Y.; et al. PEG-assisted controlled precipitation of calcium hydroxide and calcium carbonate nanostructures for cement reinforcement. Mater. Chem. Phys. 2021, 271, 124865. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, Q.; Cai, Y.; Zhang, Y.; Wang, Z.; Dong, B.; Xing, F. Influences of styrene-acrylate latexes on cement hydration in oil well cement system at different temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 46–57. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, R.; Zhang, L.; Guan, X. Molecular insights into the adsorption of chloride ions in calcium silicate hydrate gels: The synergistic effect of calcium to silicon ratio and sulfate ion. Microporous Mesoporous Mater. 2022, 345, 112248. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y. Three-dimensional numerical investigation of mixed-mode debonding of FRP-concrete interface using a cohesive zone model. Constr. Build. Mater. 2022, 350, 128818. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Yu, C.; Wang, R. Influence of cationic polyurethane on mechanical properties of cement based materials and its hydration mechanism. Constr. Build. Mater. 2017, 137, 494–504. [Google Scholar] [CrossRef]
- Wang, J.; Seidi, F.; Huang, Y.; Xiao, H. Smart lignin-based polyurethane conjugated with corrosion inhibitor as bio-based anticorrosive sublayer coating. Ind. Crops Prod. 2022, 188, 115719. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Green and facile procedure for the preparation of liquid and gel-like polyurethanes based on castor oil and lignin: Effect of processing conditions on the rheological properties. J. Clean. Prod. 2020, 277, 123367. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Zheng, B.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, T.; Wang, Q. Synthesis of lignin-based polyols via thiol-ene chemistry for high-performance polyurethane anticorrosive coating. Compos. Part B Eng. 2020, 200, 108295. [Google Scholar] [CrossRef]
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials 2022, 15, 2308. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, C.; Chen, D.; Wu, Z.; Li, S.; Sun, T.; Liu, X. Synthesis of a coupling agent containing polyurethane chain and its influence on improving the dispersion of SiO2 nanoparticles in epoxy/amine thermoset. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106573. [Google Scholar] [CrossRef]
- Hu, F.; Qi, F.; Xiang, Z.; Zhang, B.; Qi, F.; Zhao, N.; Ouyang, X. Synergistic enhancement effect of nano-SiO2 and ionic liquids on mechanical properties and impact resistance of polyurethane elastomer. Compos. Commun. 2021, 27, 100876. [Google Scholar] [CrossRef]
- Bahramnia, H.; Semnani, H.M.; Habibolahzadeh, A.; Abdoos, H. Epoxy/polyurethane hybrid nanocomposite coatings reinforced with MWCNTs and SiO2 nanoparticles: Processing, mechanical properties and wear behavior. Surf. Coat. Technol. 2021, 415, 127121. [Google Scholar] [CrossRef]
- Babar, M.; Sharma, A.; Kakkar, P.; Arora, A.; Arora, T.; Verma, G. Correlating thermal properties of polyurethane/clay nanocomposite coatings with processing. Prog. Org. Coat. 2022, 165, 106743. [Google Scholar] [CrossRef]
- Ramesh, S.; Punithamoorthy, K. Synthesis, characterization and gas permeability properties of a novel nanocomposite based on poly(ethylene-co-vinyl acetate)/polyurethane acrylate/clay. J. Mater. Res. Technol. 2019, 8, 4173–4181. [Google Scholar] [CrossRef]
- Verma, G. Weathering, salt spray corrosion and mar resistance mechanism of clay (nano-platelet) reinforced polyurethane nanocomposite coatings. Prog. Org. Coat. 2019, 129, 260–270. [Google Scholar] [CrossRef]
- SUN, M.; LIU, F.; SHI, H.; HAN, E. A study on water absorption in freestanding polyurethane films filled with nano-TiO2 pigments by capacitance measurements. Acta Metall. Sin. (Engl. Lett.) 2009, 22, 27–34. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Wang, C.; Ding, Z.; Babar, A.A.; Wei, H.; Wang, X.; Yu, J.; Ding, B. Tailoring high anti-UV performance polypropylene based geotextiles with homogeneous waterborne polyurethane-TiO2 composite emulsions. Compos. Commun. 2020, 22, 100529. [Google Scholar] [CrossRef]
- Król, P.; Szlachta, M.; Pielichowska, K. Hydrophilic and hydrophobic films based on polyurethane cationomers containing TiO2 nanofiller. Prog. Org. Coat. 2022, 162, 106524. [Google Scholar] [CrossRef]
- Tu, H.; Zhou, M.; Gu, Y.; Gu, Y. Conductive, self-healing, and repeatable graphene/carbon nanotube/polyurethane flexible sensor based on Diels-Alder chemothermal drive. Compos. Sci. Technol. 2022, 225, 109476. [Google Scholar] [CrossRef]
- Dehghani, F.; Khorasani, M.T.; Movahedi, M. Fabrication of polyurethane—Heparinized carbon nanotubes composite for heart valves application. Mater. Chem. Phys. 2022, 280, 125819. [Google Scholar] [CrossRef]
- Li, Y.; Shang, Y.; He, J.; Li, M.; Yang, M. Low-loading oxidized multi-walled carbon nanotube grafted waterborne polyurethane composites with ultrahigh mechanical properties improvement. Diam. Relat. Mater. 2022, 130, 109427. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Ding, M.; Tan, H.; Ling, Q.; Zhong, Y.; Fu, Q. Synthesis and degradation of nontoxic biodegradable waterborne polyurethanes elastomer with poly(ε-caprolactone) and poly(ethylene glycol) as soft segment. Eur. Polym. J. 2007, 43, 1838–1846. [Google Scholar] [CrossRef]
- Sun, X.; Gao, H.; Wu, G.; Wang, Y.; Fan, Y.; Ma, J. Biodegradable and temperature-responsive polyurethanes for adriamycin delivery. Int. J. Pharm. 2011, 412, 52–58. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K.; Khudzari, A.Z.M.; Prabhakaran, P. Development of advanced nanostructured polyurethane composites comprising hybrid fillers with enhanced properties for regenerative medicine. Polym. Test. 2019, 73, 12–20. [Google Scholar] [CrossRef]
- Pournajaf, R.; Hassanzadeh-Tabrizi, S.A.; Ebrahimi-Kahrizsangi, R.; Alhaji, A.; Nourbakhsh, A.A. Polycrystalline infrared-transparent MgO fabricated by spark plasma sintering. Ceram. Int. 2019, 45, 18943–18950. [Google Scholar] [CrossRef]
- Li, G.; Li, P.; Qiu, H.; Li, D.; Su, M.; Xu, K. Synthesis, characterizations and biocompatibility of alternating block polyurethanes based on P3/4HB and PPG-PEG-PPG. J. Biomed. Mater. Res. Part A 2011, 98, 88–99. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasneen, A.; Chung, I.; Kim, H.; Lee, W.-K.; Chun, J.H. Synthesis and properties of polyurethane coatings: The effect of different types of soft segments and their ratios. Compos. Interfaces 2013, 20, 15–26. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Macossay, J.; Cantu, T.; Zhang, X.; Shamshi Hassan, M.; Esther Salinas, M.; Farhangi, C.S.; Ahmad, H.; Kim, H.; Bowlin, G.L. Imaging, spectroscopy, mechanical, alignment and biocompatibility studies of electrospun medical grade polyurethane (CarbothaneTM 3575A) nanofibers and composite nanofibers containing multiwalled carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2015, 41, 189–198. [Google Scholar] [CrossRef]
- Bekhti, H.; Boucheffa, Y.; Blal, A.H.A.; Travert, A. In situ FTIR investigation of CO2 adsorption over MgO–Impregnated NaY zeolites. Vib. Spectrosc. 2021, 117, 103313. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Pichiah, P.B.T.; Gnanasekaran, G.; Seenivasan, K.; Barakat, N.A.M.; Cha, Y.-S.; Jung, C.-H.; Shanmugam, A.; Kim, H.Y. Emu oil-based electrospun nanofibrous scaffolds for wound skin tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 454–460. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Subash, M.; Perumal, V.; Panimalar, S.; Aravindan, S.; Uthrakumar, R.; Inmozhi, C.; Isaev, A.B.; Muniyasamy, S.; Raja, A.; et al. Specific charge separation of Sn doped MgO nanoparticles for photocatalytic activity under UV light irradiation. Sep. Purif. Technol. 2022, 294, 121189. [Google Scholar] [CrossRef]
- Dabhane, H.; Ghotekar, S.; Zate, M.; Kute, S.; Jadhav, G.; Medhane, V. Green synthesis of MgO nanoparticles using aqueous leaf extract of Ajwain (Trachyspermum ammi) and evaluation of their catalytic and biological activities. Inorg. Chem. Commun. 2022, 138, 109270. [Google Scholar] [CrossRef]
- Siyanbola, T.O.; Sasidhar, K.; Rao, B.V.S.K.; Narayan, R.; Olaofe, O.; Akintayo, E.T.; Raju, K.V.S.N. Development of Functional Polyurethane–ZnO Hybrid Nanocomposite Coatings from Thevetia peruviana Seed Oil. J. Am. Oil Chem. Soc. 2015, 92, 267–275. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, R.S.; Narayan, R.; Raju, K.V.S.N. Effect of nano ZnO on the phase mixing of polyurethane hybrid dispersions. Prog. Org. Coat. 2010, 67, 405–413. [Google Scholar] [CrossRef]
- Faraj, R.H.; Mohammed, A.A.; Omer, K.M. Self-compacting concrete composites modified with nanoparticles: A comprehensive review, analysis and modeling. J. Build. Eng. 2022, 50, 104170. [Google Scholar] [CrossRef]
- Beigi, M.H.; Berenjian, J.; Lotfi Omran, O.; Sadeghi Nik, A.; Nikbin, I.M. An experimental survey on combined effects of fibers and nanosilica on the mechanical, rheological, and durability properties of self-compacting concrete. Mater. Des. 2013, 50, 1019–1029. [Google Scholar] [CrossRef]
- Shahbazpanahi, S.; Faraj, R.H. Feasibility study on the use of shell sunflower ash and shell pumpkin ash as supplementary cementitious materials in concrete. J. Build. Eng. 2020, 30, 101271. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Wang, P.; Xu, L. Effect of HEMC on the early hydration of Portland cement highlighted by isothermal calorimetry. J. Therm. Anal. Calorim. 2015, 119, 1833–1843. [Google Scholar] [CrossRef]
- El-Diadamony, H.; Amer, A.A.; Sokkary, T.M.; El-Hoseny, S. Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC J. 2018, 14, 150–158. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Liu, L.; Xiao, J.; Zhang, G.; Guo, F.; Li, L. Influence of MgO on the Hydration and Shrinkage Behavior of Low Heat Portland Cement-Based Materials via Pore Structural and Fractal Analysis. Fractal Fract. 2022, 6, 40. [Google Scholar] [CrossRef]
- Zheng, L.; Xuehua, C.; Mingshu, T. Hydration and setting time of MgO-type expansive cement. Cem. Concr. Res. 1992, 22, 1–5. [Google Scholar] [CrossRef]
- Kabir, H.; Hooton, R.D. Evaluating soundness of concrete containing shrinkage-compensating MgO admixtures. Constr. Build. Mater. 2020, 253, 119141. [Google Scholar] [CrossRef]
- Mo, L.; Liu, M.; Al-Tabbaa, A.; Deng, M.; Lau, W.Y. Deformation and mechanical properties of quaternary blended cements containing ground granulated blast furnace slag, fly ash and magnesia. Cem. Concr. Res. 2015, 71, 7–13. [Google Scholar] [CrossRef]
- Behazin, E.; Misra, M.; Mohanty, A.K. Sustainable biocarbon from pyrolyzed perennial grasses and their effects on impact modified polypropylene biocomposites. Compos. Part B Eng. 2017, 118, 116–124. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Kishar, E.A.; Abo-El-Enein, S.A. FTIR study and cation exchange capacity of Fe3+- and Mg2+-substituted calcium silicate hydrates. J. Alloys Compd. 2009, 473, 538–542. [Google Scholar] [CrossRef]
- Moradpour, R.; Taheri-Nassaj, E.; Parhizkar, T.; Ghodsian, M. The effects of nanoscale expansive agents on the mechanical properties of non-shrink cement-based composites: The influence of nano-MgO addition. Compos. Part B Eng. 2013, 55, 193–202. [Google Scholar] [CrossRef]
- Yao, K.; An, D.; Wang, W.; Li, N.; Zhang, C.; Zhou, A. Effect of nano-MgO on mechanical performance of cement stabilized silty clay. Mar. Georesour. Geotechnol. 2020, 38, 250–255. [Google Scholar] [CrossRef]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef]
- Kalaivani, S.S.; Muthukrishnaraj, A.; Sivanesan, S.; Ravikumar, L. Novel hyperbranched polyurethane resins for the removal of heavy metal ions from aqueous solution. Process Saf. Environ. Prot. 2016, 104, 11–23. [Google Scholar] [CrossRef]
- Land, G.; Stephan, D. Controlling cement hydration with nanoparticles. Cem. Concr. Compos. 2015, 57, 64–67. [Google Scholar] [CrossRef]
- Jo, B.-W.; Kim, C.-H.; Tae, G.; Park, J.-B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]

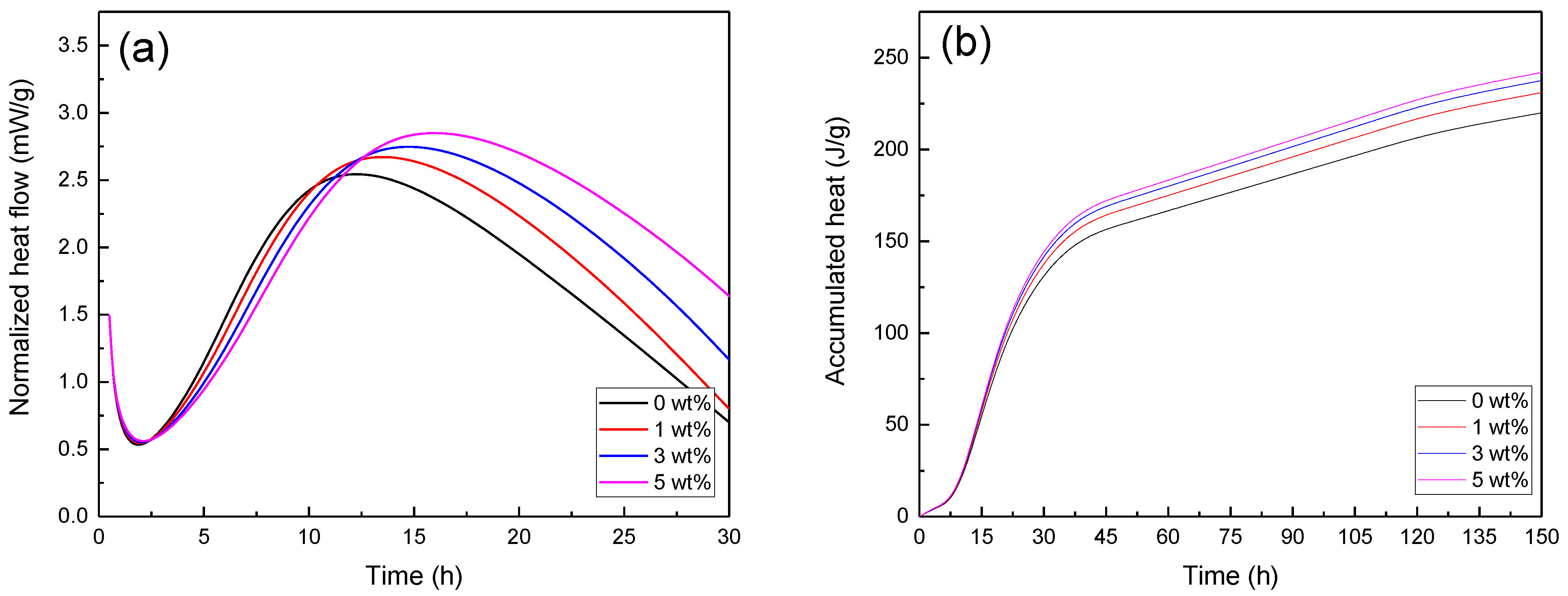
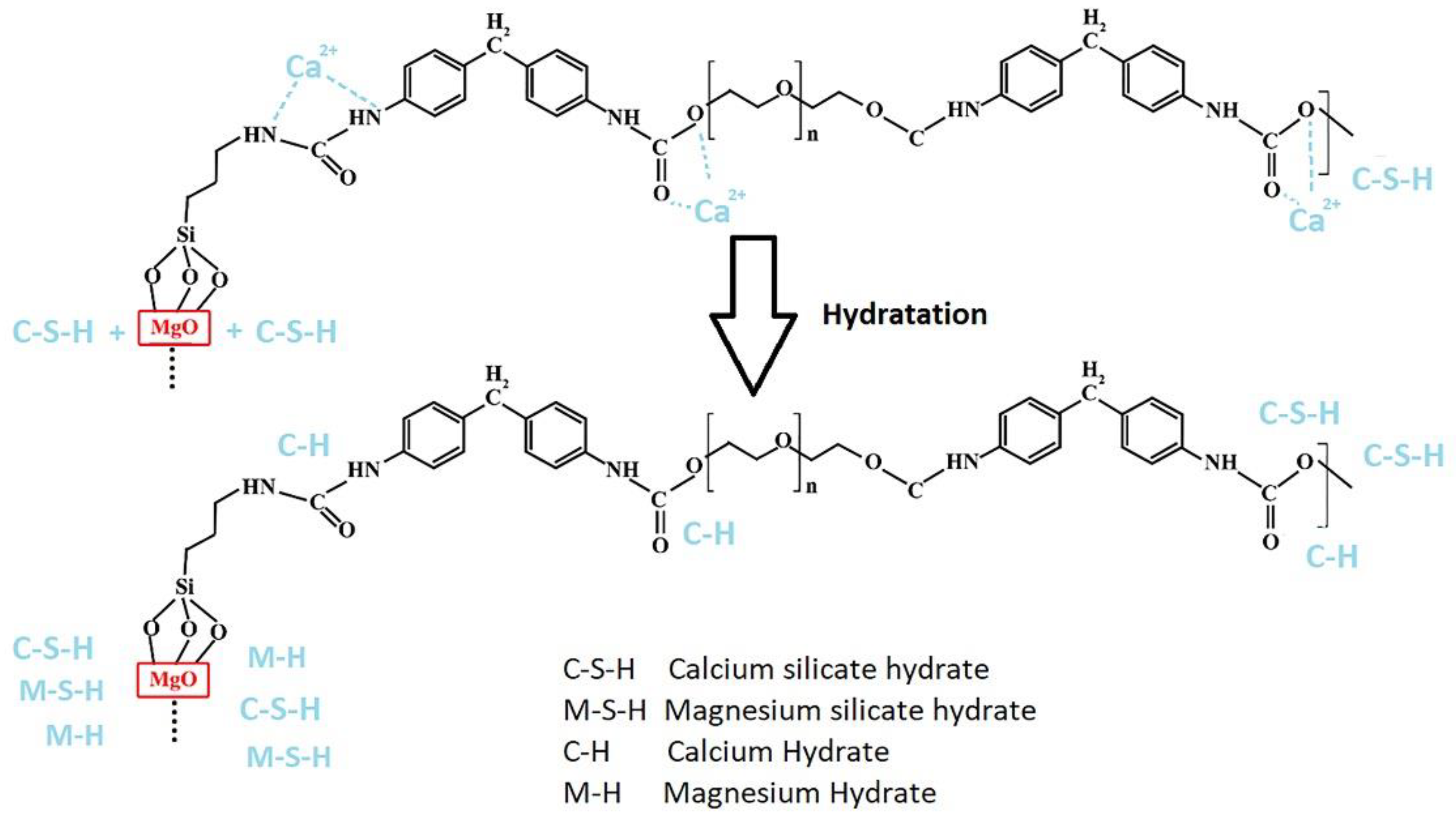
| Component | CaO | SiO2 | Al2O3 | MgO | SO3 | Na2O | K2O | Fe2O3 | L.O.I * |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 65 | 23.2 | 4.9 | 1.4 | 1.6 | 0.3 | 0.35 | 0.35 | 2.6 |
| Name | Abbreviation | Chemical Formula |
|---|---|---|
| Alite | C3S | Ca3SiO5 |
| Belite | C2S | Ca2SiO4 |
| Aluminate | C3A | Ca3Al2O6 |
| Portlandite | P | Ca(OH)2 |
| Ettringite | E | [Ca6[Al(OH)6]2·24H2O] [(SO4)3·1.5H2O] |
| Calcite | CaO | CaO |
| Calcium–Silicates–Hydrates | C-S-H | (CaO) × (SiO)-(2H2O)y |
| Curing Time | Strength (MPa) | Nanohybrids (wt%) | |||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | ||
| 3 Days | Flexural | 6.5 | 7 | 7.5 | 7.9 |
| Compressive | 33 | 32.6 | 33.5 | 36 | |
| F/C Ratio | 0.197 | 0.215 | 0.224 | 0.219 | |
| 7 Days | Flexural | 9.2 | 9.8 | 10 | 10.5 |
| Compressive | 51 | 53 | 50 | 56 | |
| F/C Ratio | 0.180 | 0.185 | 0.200 | 0.187 | |
| 28 Days | Flexural | 10 | 10.7 | 11.5 | 11.7 |
| Compressive | 63 | 66 | 69.8 | 72 | |
| F/C Ratio | 0.159 | 0.162 | 0.165 | 0.162 | |
| 45 Days | Flexural | 10 | 11 | 11.8 | 12 |
| Compressive | 63 | 70 | 71.8 | 76 | |
| F/C Ratio | 0.159 | 0.157 | 0.164 | 0.158 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Ning, W.; Li, Y.; Li, F.; Pournajaf, R.; Hamawandi, B. The Effects of the Addition of Polyurethane–MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste. Nanomaterials 2022, 12, 3978. https://doi.org/10.3390/nano12223978
Fang Y, Ning W, Li Y, Li F, Pournajaf R, Hamawandi B. The Effects of the Addition of Polyurethane–MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste. Nanomaterials. 2022; 12(22):3978. https://doi.org/10.3390/nano12223978
Chicago/Turabian StyleFang, Yu, Weiqing Ning, Yuan Li, Fang Li, Reza Pournajaf, and Bejan Hamawandi. 2022. "The Effects of the Addition of Polyurethane–MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste" Nanomaterials 12, no. 22: 3978. https://doi.org/10.3390/nano12223978
APA StyleFang, Y., Ning, W., Li, Y., Li, F., Pournajaf, R., & Hamawandi, B. (2022). The Effects of the Addition of Polyurethane–MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste. Nanomaterials, 12(22), 3978. https://doi.org/10.3390/nano12223978






