Fabrication of Cu Micromembrane as a Flexible Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
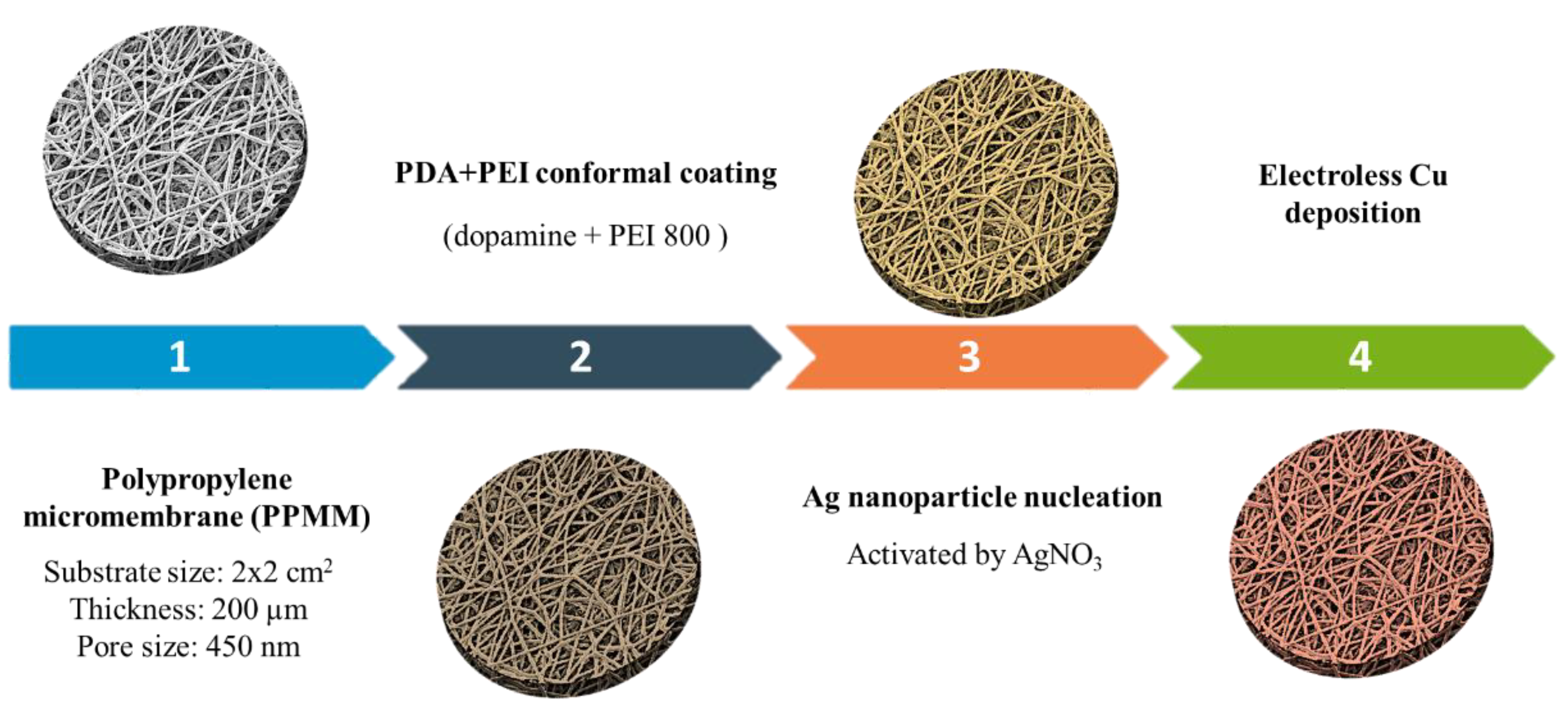
2.2. Fabrication of Cu Micromembrane
2.3. Materials Characterization
3. Results
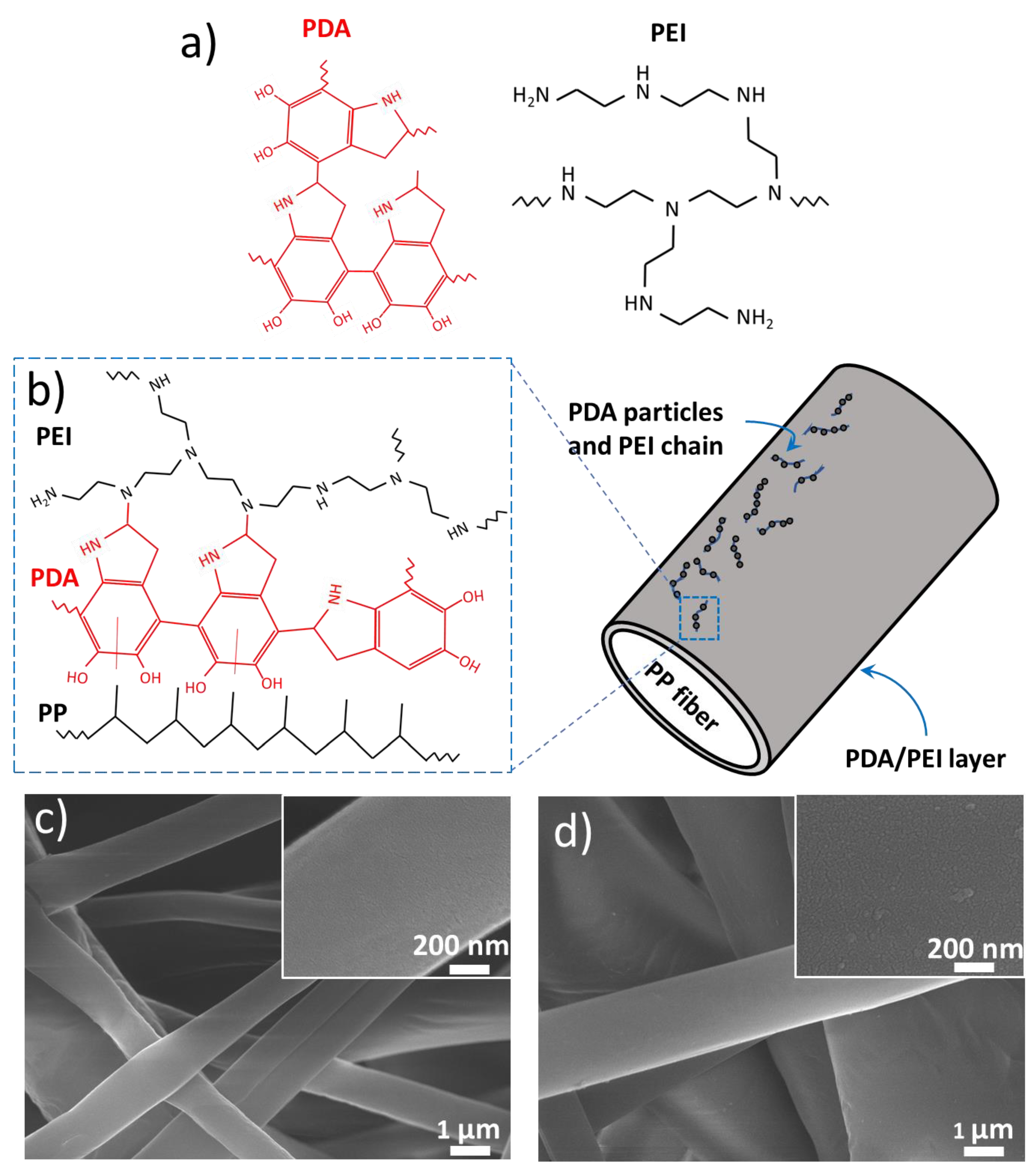
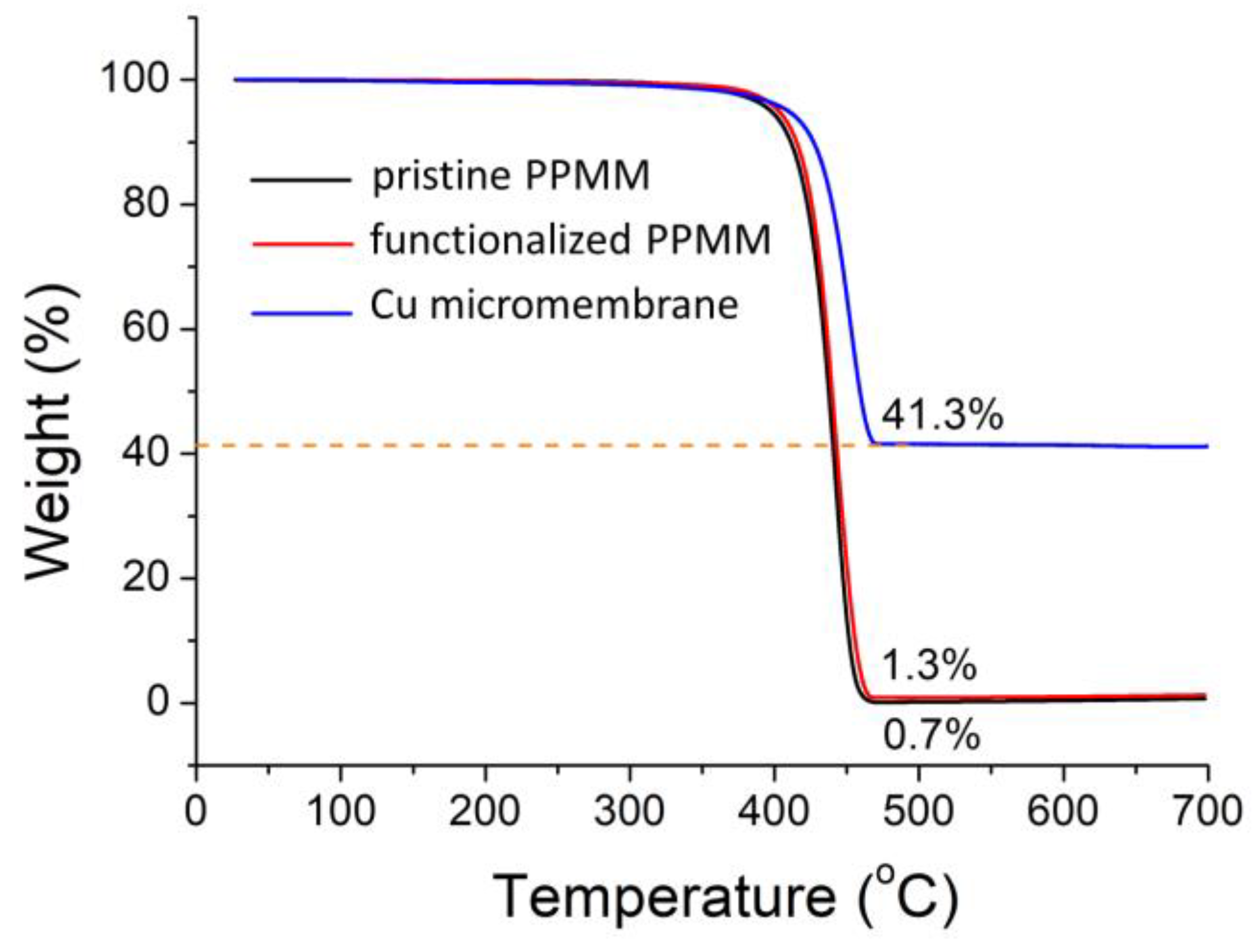
3.1. Functionalization of PPMM
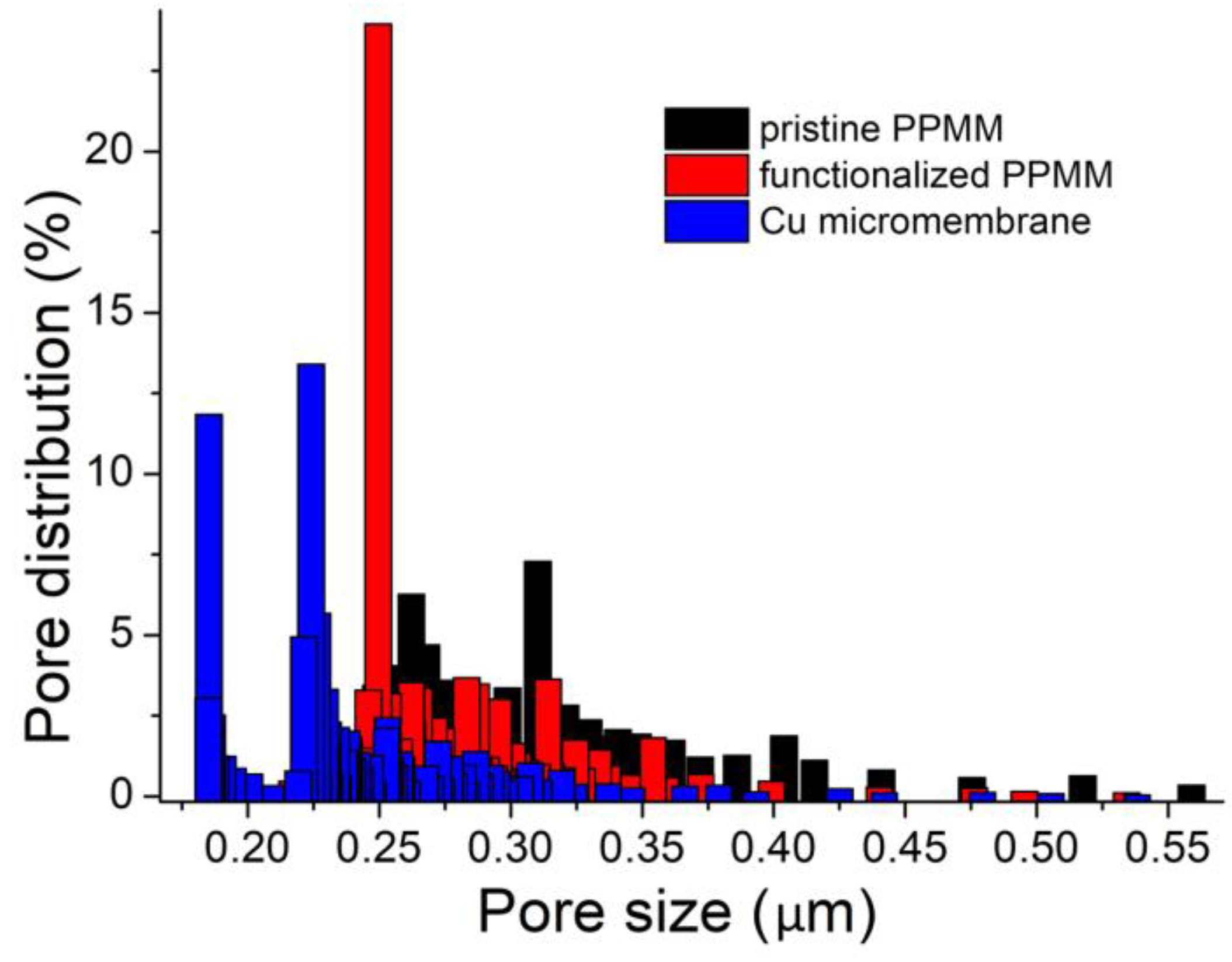
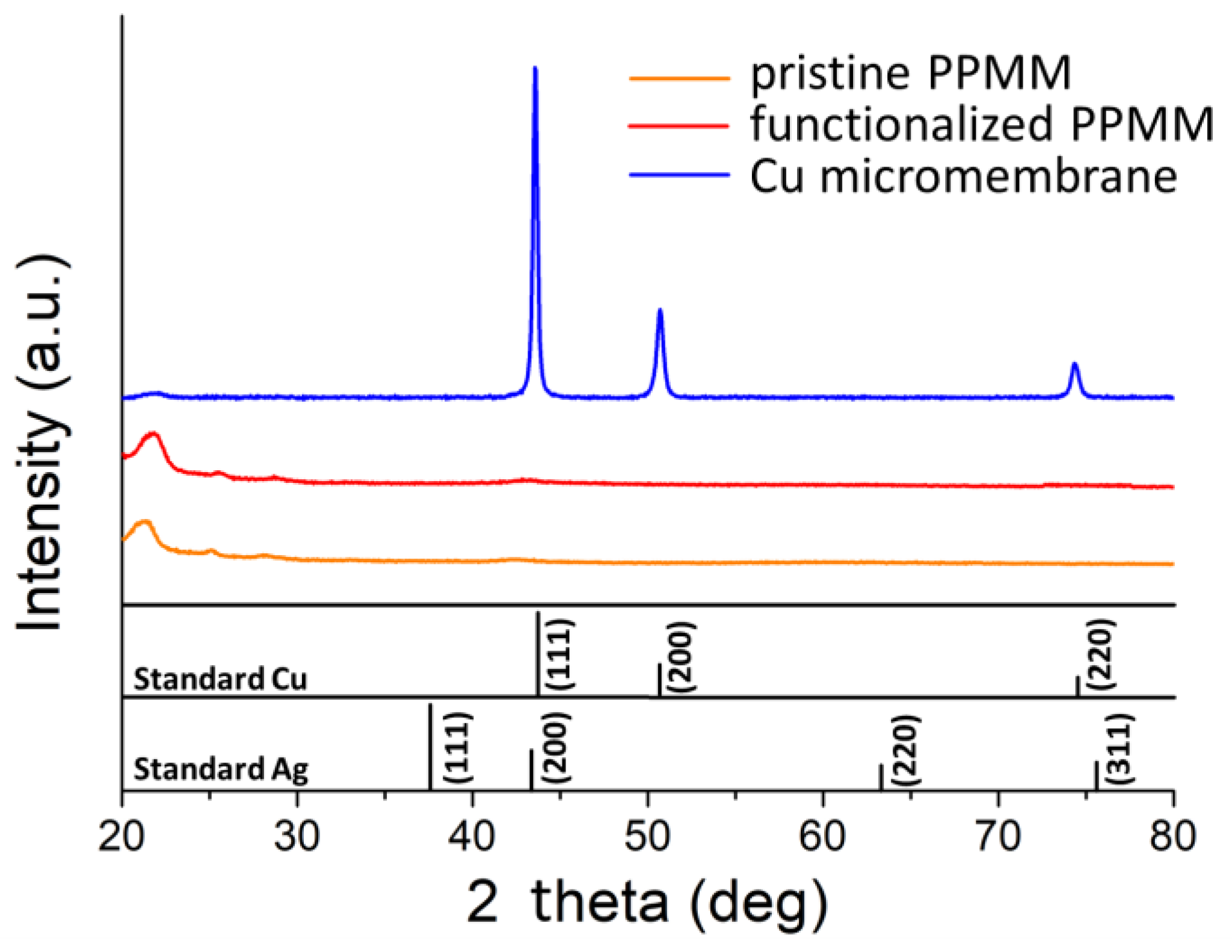
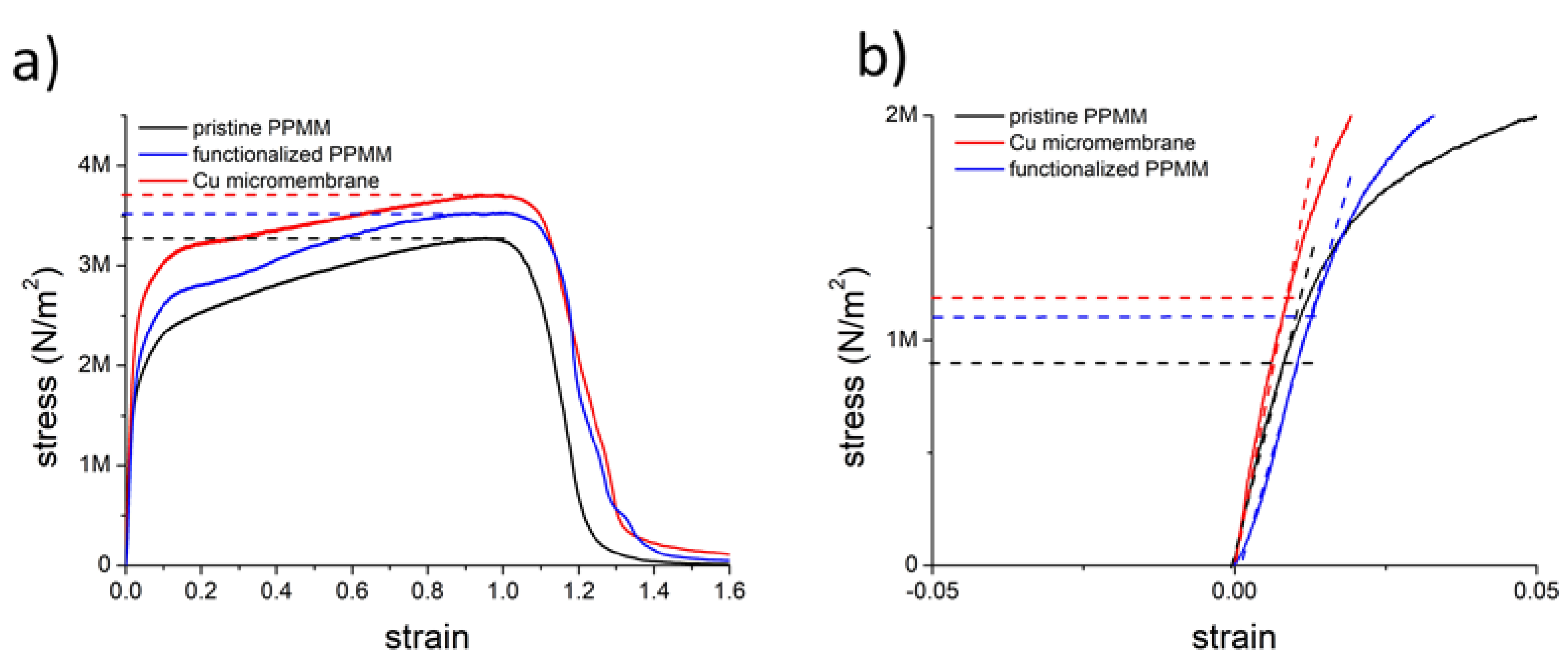
3.2. Material Characterization of Cu Micromembrane
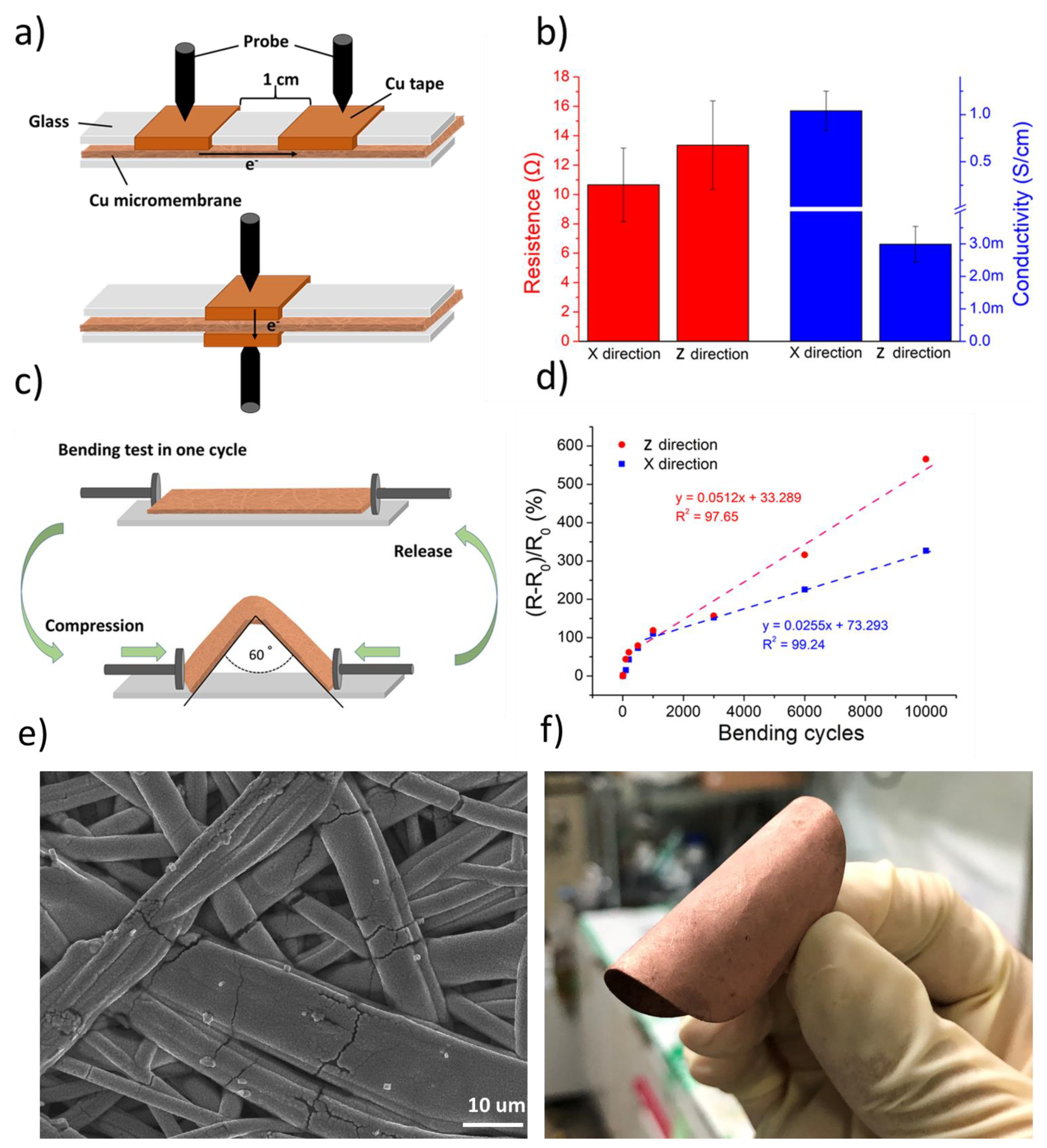
3.3. Electrical and Electrochemical Characterization of Cu Micromembrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Hu, M.; Wang, H.; Chen, Z.; Feng, Y.; Wang, J.; Ling, W.; Huang, Y. The Evolution of Flexible Electronics: From Nature, Beyond Nature, and to Nature. Adv. Sci. 2020, 7, 2001116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Zhao, Y.; Du, S.; Zhi, C. Recent Advances in Electrode Fabrication for Flexible Energy-Storage Devices. Adv. Mater. Technol. 2019, 4, 1900083. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; McBride, S.A.; Warsinger, D.M.; Dizge, N.; Hasan, S.W.; Arafat, H.A. Thin Film Deposition Techniques for Polymeric Membranes—A Review. J. Membr. Sci. 2020, 610, 118258. [Google Scholar] [CrossRef]
- Melentiev, R.; Yudhanto, A.; Tao, R.; Vuchkov, T.; Lubineau, G. Metallization of Polymers and Composites: State-of-the-Art Approaches. Mater. Des. 2022, 221, 110958. [Google Scholar] [CrossRef]
- Xu, L.J.; Shi, X.Y.; Chai, M.Y.; Ji, J.; Xu, Z.K.; Wan, L.S. Surface Metallization of Porous Polymer Materials for Multifunctional Applications. Langmuir 2020, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Mehdi, M.; Yousif, M.; Ali, A.; Ullah, S.; Hussain Siyal, S.; Hussain, T.; Kim, I.S. Synthesis of Highly Conductive Electrospun Recycled Polyethylene Terephthalate Nanofibers Using the Electroless Deposition Method. Nanomaterials 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Gou, X.; Jin, X.; Wang, S.; He, W.; Wang, S.; Deng, L.; Chen, S.; Liu, G. Low Fractal Dimension Modified Drilling-Hole Wall for Ptfe High-Frequency Board Copper Plating with Plasma Treatment. J. Appl. Polym. Sci. 2019, 136, 48052. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, J.F.; Chuang, K.J.; Cheng, H.Y.; Chang, K.C.; Ma, C.M. Preparing Copper Nanoparticles and Flexible Copper Conductive Sheets. Nanomaterials 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, L.; Huang, Z.; Liu, J.; Xu, Y.; Li, R.; Zhang, M.; Hong, H.; Lin, H. A Novel in-Situ Micro-Aeration Functional Membrane with Excellent Decoloration Efficiency and Antifouling Performance. J. Membr. Sci. 2022, 641, 119925. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, J.; Pan, Y.; Liu, E.; Liu, D.; Meng, J. Hierarchical Porous Polyimide Nanocomposite Membrane for Flow-through CO2 Cycloaddition at Mild Conditions. Chem. Eng. J. 2020, 383, 123166. [Google Scholar] [CrossRef]
- Chou, S.C.; Chung, W.A.; Fan, T.L.; Dordi, Y.; Koike, J.; Wu, P.-W. Polydopamine and Its Composite Film as an Adhesion Layer for Cu Electroless Deposition on SiO2. J. Electrochem. Soc. 2020, 167, 042507. [Google Scholar] [CrossRef]
- Chou, S.-C.; Lin, C.; Sun, B.-Y.; Tso, K.-C.; Chan, T.-S.; Wu, P.-W. Formation of RuO2 Thin Film Using Dopamine as a Reducing, Chelating, and Adhesive Agent Simultaneously. J. Taiwan Inst. Chem. Eng. 2021, 119, 196–203. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation. Nanomaterials 2021, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Qian, B.; Uto, K.; Chen, J.; Liu, X.; Minari, T. Functional Biomaterials Towards Flexible Electronics and Sensors. Biosens. Bioelectron. 2018, 119, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dyke, J.C.; Bowman, B.A.; Ko, C.C.; You, W. Investigation of Dopamine Analogues: Synthesis, Mechanistic Understanding, and Structure-Property Relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent Progress in Synthesis and Application of Mussel-Inspired Adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, S.; Hu, Q.; Yu, F.; Cheng, Y.; Huang, K.; Yuan, H.; Jiang, J.; Li, W.; Li, J.; et al. Vertical Graphene-Coated Cu Wire for Enhanced Tolerance to High Current Density in Power Transmission. Nano Res. 2021, 15, 9727–9733. [Google Scholar] [CrossRef]
- An, B.S.; Kwon, Y.; Oh, J.S.; Lee, C.; Choi, S.; Kim, H.; Lee, M.; Pae, S.; Yang, C.W. Characteristics of an Amorphous Carbon Layer as a Diffusion Barrier for an Advanced Copper Interconnect. ACS Appl. Mater. Interfaces 2020, 12, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.W.; Tran, D.P.; Chen, C. Effect of Cu Ion Concentration on Microstructures and Mechanical Properties of Nanotwinned Cu Foils Fabricated by Rotary Electroplating. Nanomaterials 2021, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, Y.; Tian, H.; Li, Z.; Huang, P.; Jiang, J.; Wang, C. In-Situ Growth of Flexible 3D Hollow Tubular Cu2S Nanorods on Cu Foam for High Electrochemical Performance Supercapacitor. J. Mater. 2020, 6, 192–199. [Google Scholar] [CrossRef]
- Gu, Z.; Shen, H.; Chen, Z.; Yang, Y.; Yang, C.; Ji, Y.; Wang, Y.; Zhu, C.; Liu, J.; Li, J.; et al. Efficient Electrocatalytic CO2 Reduction to C2+ Alcohols at Defect-Site-Rich Cu Surface. Joule 2021, 5, 429–440. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, N.; Wang, C.; Zhang, X.; Liu, Z. Oleylamine-Mediated Hydrothermal Growth of Millimeter-Long Cu Nanowires and Their Electrocatalytic Activity for Reduction of Nitrate. Nanomaterials 2018, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.T.; Chou, S.C.; Sun, B.Y.; Wu, P.W. A Conductive Silver Membrane for Electrochemical Detection of Free Chlorine in Aqueous Solution. Sens. Actuators B Chem. 2021, 348, 130724. [Google Scholar] [CrossRef]
- Chou, S.C.; Hsieh, Y.C.; Cheang, W.H.; Sun, B.Y.; Chu, C.Y.; Chen, S.Y.; Chiao, J.C.; Wu, P.W. A Flexible Iro2 Membrane for pH Sensing. Sci. Rep. 2022, 12, 11712. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, J.; Li, M.; Tang, Y.; Gao, C. Studies on a Novel Nanofiltration Membrane Prepared by Cross-Linking of Polyethyleneimine on Polyacrylonitrile Substrate. J. Membr. Sci. 2014, 451, 103–110. [Google Scholar] [CrossRef]
- Chien, H.W.; Tsai, M.Y.; Kuo, C.J.; Lin, C.L. Well-Dispersed Silver Nanoparticles on Cellulose Filter Paper for Bacterial Removal. Nanomaterials 2021, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.E.; Schmitt, R.; Dittler, A.; Kasper, G. On the Accuracy of Capillary Flow Porometry for Fibrous Filter Media. Sep. Purif. Technol. 2018, 199, 198–205. [Google Scholar] [CrossRef]
- Wei, Q.; Xiao, X.; Hou, D.; Ye, H.; Huang, F. Characterization of Nonwoven Material Functionalized by Sputter Coating of Copper. Surf. Coat. Technol. 2008, 202, 2535–2539. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Estimating Surface Area of Copper Powder: A Comparison between Electrochemical, Microscopy and Laser Diffraction Methods. Adv. Powder Technol. 2018, 29, 3520–3526. [Google Scholar] [CrossRef]


| PPMM | Cu Micromembrane | ||
|---|---|---|---|
| Pristine | Functionalized | ||
| Bubble point pore diameter (µm) | 3.633 | 3.585 | 3.489 |
| Bubble point pressure (psi) | 1.816 | 1.84 | 1.891 |
| Mean flow pore diameter (µm) | 0.482 | 0.436 | 0.266 |
| Mean flow pore pressure (psi) | 13.691 | 15.136 | 24.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.-Y.; Cheang, W.-H.; Chou, S.-C.; Chiao, J.-C.; Wu, P.-W. Fabrication of Cu Micromembrane as a Flexible Electrode. Nanomaterials 2022, 12, 3829. https://doi.org/10.3390/nano12213829
Sun B-Y, Cheang W-H, Chou S-C, Chiao J-C, Wu P-W. Fabrication of Cu Micromembrane as a Flexible Electrode. Nanomaterials. 2022; 12(21):3829. https://doi.org/10.3390/nano12213829
Chicago/Turabian StyleSun, Bo-Yao, Wai-Hong Cheang, Shih-Cheng Chou, Jung-Chih Chiao, and Pu-Wei Wu. 2022. "Fabrication of Cu Micromembrane as a Flexible Electrode" Nanomaterials 12, no. 21: 3829. https://doi.org/10.3390/nano12213829
APA StyleSun, B.-Y., Cheang, W.-H., Chou, S.-C., Chiao, J.-C., & Wu, P.-W. (2022). Fabrication of Cu Micromembrane as a Flexible Electrode. Nanomaterials, 12(21), 3829. https://doi.org/10.3390/nano12213829






