Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems
Abstract
1. Introduction
2. Materials and Methods
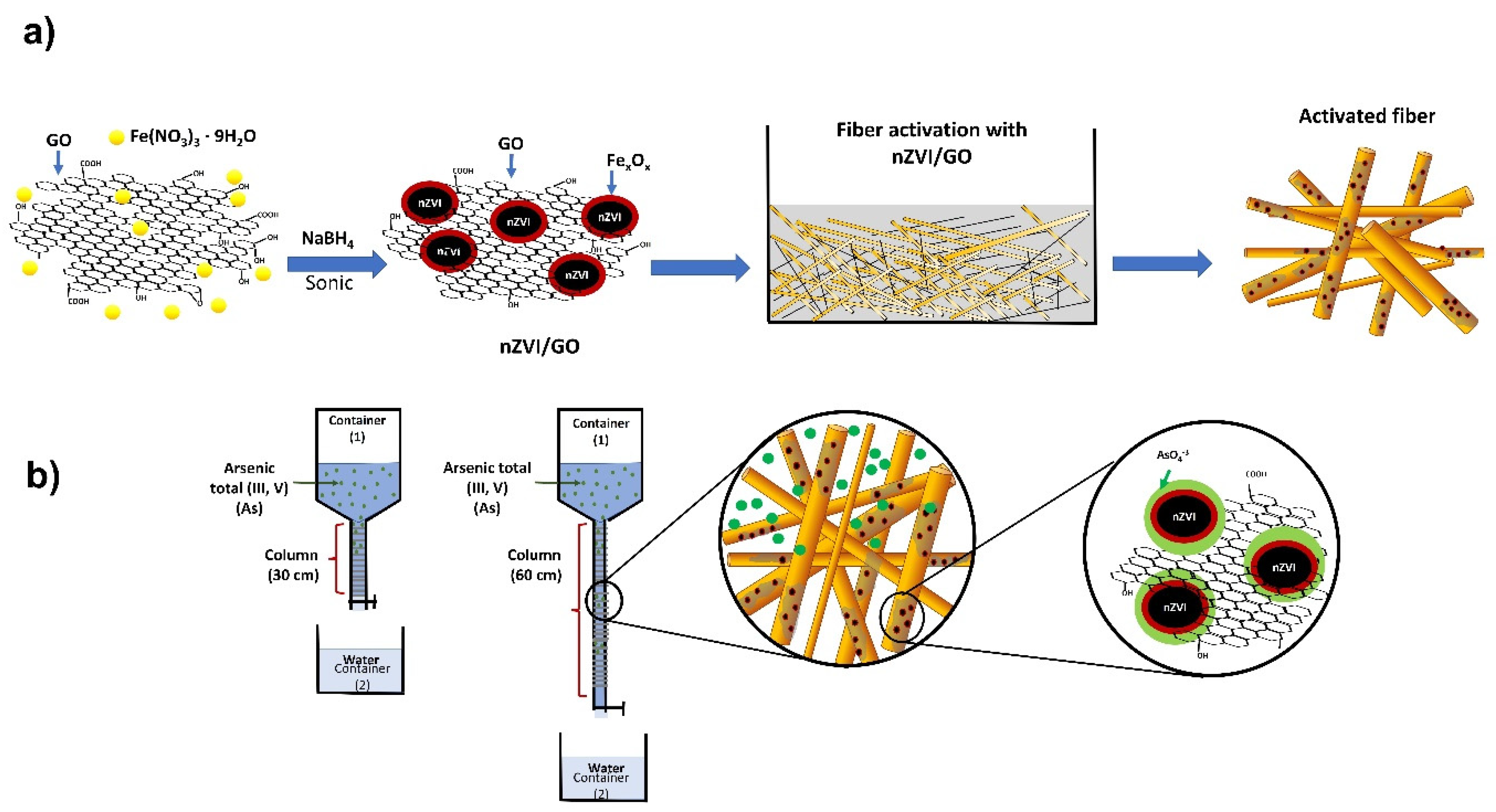
2.1. Synthesis: GO, nZVI, nZVI/GO and J-nZVI/GO
2.2. Fiber Evaluation: Preparation, nZVI/GO Retention, and Arsenic Adsorption
2.3. Removal Test in a Dynamic Flow
3. Results and Discussion
3.1. Fiber Evaluation: Preparation, nZVI/GO Retention, and Arsenic Adsorption
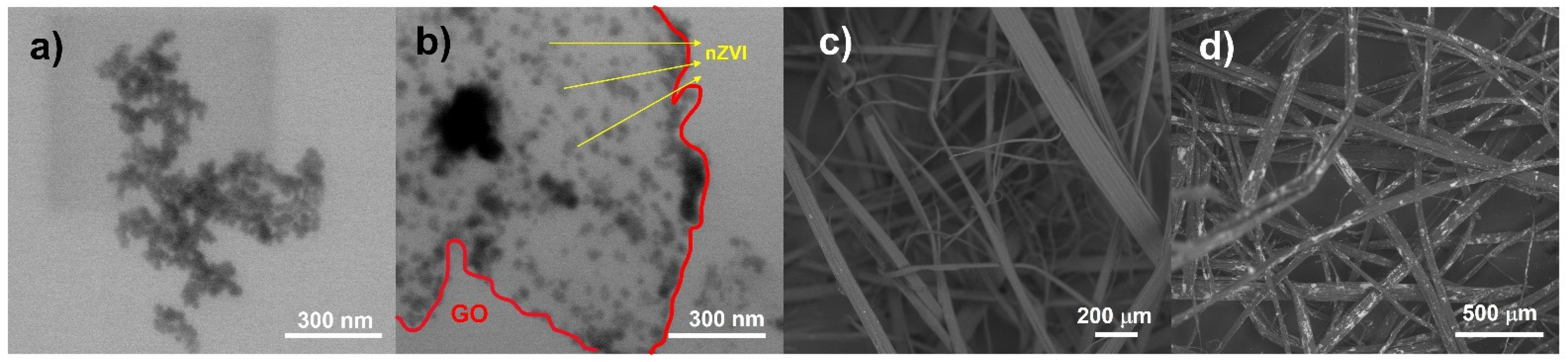
3.1.1. Morphological Analysis
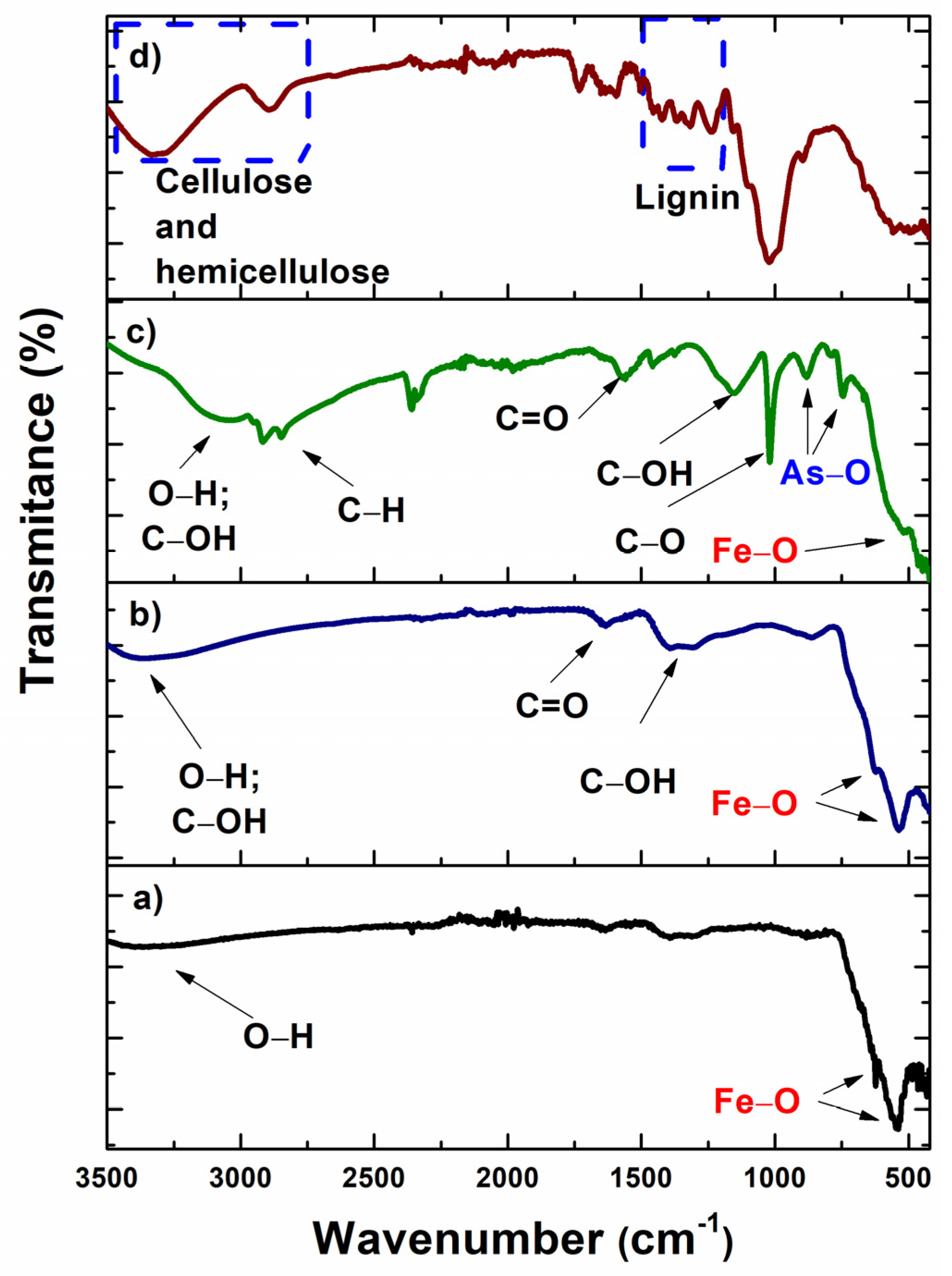
3.1.2. FTIR Spectroscopy
3.2. Removal Test in a Dynamic Flow: Testing Batch
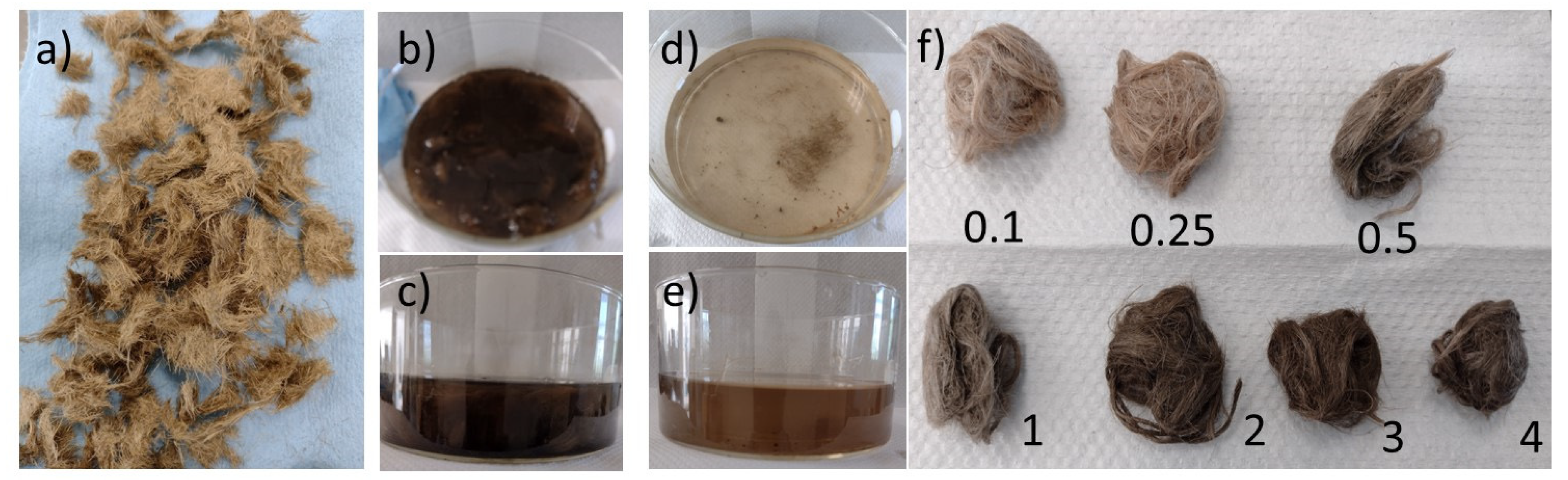
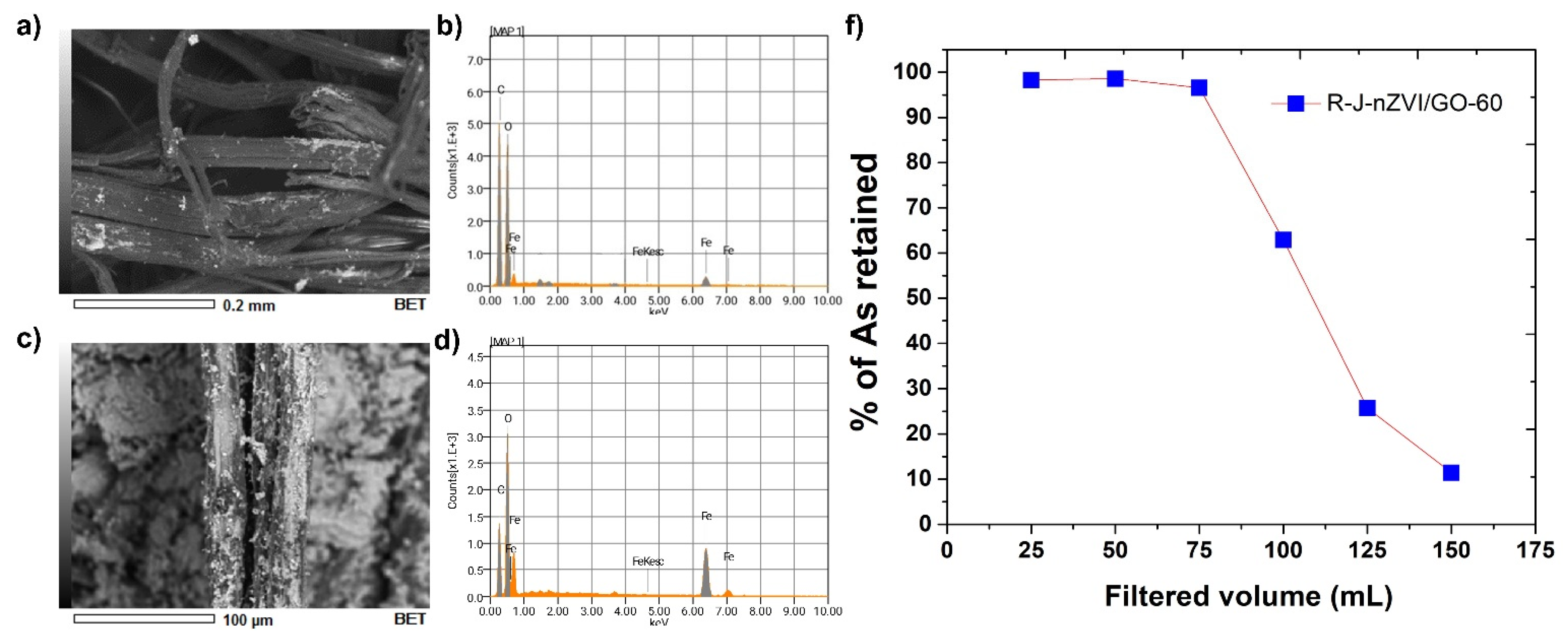
3.3. Fiber Reactivation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.M.; Goh, P.S.; Ismail, A.F. Highly adsorptive polysulfone/hydrous iron-nickel-manganese (PSF/HINM) nanocomposite hollow fiber membrane for synergistic arsenic removal. Sep. Purif. Technol. 2019, 213, 162–175. [Google Scholar] [CrossRef]
- Sabbatini, P.; Yrazu, F.; Rossi, F.; Thern, G.; Marajofsky, A.; Fidalgo de Cortalezzi, M.M. Fabrication and characterization of iron oxide ceramic membranes for arsenic removal. Water Res. 2010, 44, 5702–5712. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Kumari, N.; Tyagi, M.; Jagadevan, S. Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics. Chem. Eng. J. 2018, 347, 91–100. [Google Scholar] [CrossRef]
- Sikder, M.T.; Tanaka, S.; Saito, T.; Kurasaki, M. Application of zerovalent iron impregnated chitosan-caboxymethyl- b-cyclodextrin composite beads as arsenic sorbent. Biochem. Pharmacol. 2014, 2, 370–376. [Google Scholar]
- Keshavarz, L.; Ghaani, M.R.; MacElroy, J.M.D.; English, N.J. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation. Chem. Eng. J. 2021, 412, 128604. [Google Scholar] [CrossRef]
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Tuutijärvi, T.; Bhatnagar, A.; Vahala, R. Adsorptive removal of arsenic(V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 2016, 214, 149–156. [Google Scholar] [CrossRef]
- Mautner, A.; Kwaw, Y.; Weiland, K.; Mvubu, M.; Botha, A. Natural fibre-nanocellulose composite filters for the removal of heavy metal ions from water. Ind. Crops Prod. 2019, 133, 325–332. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic(V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, A.; Zhao, D.; Su, K.; Li, Z.; Wang, Y.; Shen, X.-C.; Liu, K.; Ruan, C. Facile synthesis of widened MoS2 nanosheets vertically anchored on natural cellulose fibers for efficient removal of mercury ions from aquatic systems. J. Environ. Chem. Eng. 2022, 10, 108229. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Nam, A.; Park, S.J.; Do, T.; Choi, U.S.; Lee, S.H. Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J. Hazard. Mater. 2017, 325, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Islam, T.; Hasan, M.M.; Chowdhury, A.N.; Ahammad, A.J.S.; Reaz, A.H.; Roy, C.K.; Shah, S.S.; Al-Imran; Aziz, M.A. Evaluating the electrochemical detection of nitrite using a platinum nanoparticle coated jute carbon modified glassy carbon electrode and voltametric analysis. J. Phys. Chem. Solids 2022, 165, 110659. [Google Scholar] [CrossRef]
- Ivanovska, A.; Maletić, S.; Djokić, V.; Tadić, N.; Kostić, M. Effect of chemical modifications and coating with Cu-based nanoparticles on the electro-physical properties of jute fabrics in a condition of high humidity. Ind. Crops Prod. 2022, 180, 114792. [Google Scholar] [CrossRef]
- Karmaker, A.C.; Youngquist, J.A. Injection molding of polypropylene reinforced with short jute fibers. J. Appl. Polym. Sci. 1996, 62, 1147–1151. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M. Studies on Jute Composites—A Literature Review. Polym. Plast. Technol. Eng. 1995, 34, 729–792. [Google Scholar] [CrossRef]
- Islam, M.S.; Alauddin, M. World Production of Jute: A Comparative Analysis of Bangladesh. Int. J. Manag. Bus. Stud. 2012, 2, 14–22. [Google Scholar]
- Wang, L.; Guan, H.; Hu, J.; Huang, Q.; Dong, C.; Qian, W.; Wang, Y. Jute-based porous biomass carbon composited by Fe3O4 nanoparticles as an excellent microwave absorber. J. Alloys Compd. 2019, 803, 1119–1126. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Zhang, Z.; Wu, Y.; Zhang, J.; Chen, S. Removal of As(III) and As(V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J. Hazard. Mater. 2014, 268, 124–131. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.; Zhang, H.B.; Cao, H.Z.; Hou, G.Y.; Tang, Y.P.; Zheng, G.Q. Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem. Eng. J. 2018, 334, 1808–1819. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Dalanta, F.; Aryanti, N.; Othman, N.H. Intensifying separation and antifouling performance of PSf membrane incorporated by GO and ZnO nanoparticles for petroleum refinery wastewater treatment. J. Water Process Eng. 2021, 41, 102030. [Google Scholar] [CrossRef]
- Rivera, L.M.; Betancur, A.F.; Zarate, D.G.; Torres, D.; Hoyos, L.; García, A. Simultaneous N doping and reduction of GO: Compositional, structural characterization and its effects in negative electrostatic charges repulsion. Diam. Relat. Mater. 2019, 97, 107447. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhang, R.; Wang, X. Nanoscale Zero-Valent Iron Particles Supported on Reduced Graphene Oxides by Using a Plasma Technique and Their Application for Removal of Heavy-Metal Ions. Chem. Asian J. 2015, 10, 1410–1417. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Roncevic, S.; Ivan, N.; Tea, Z.F.; Dubravka, M.-C. Characterization of nZVI nanoparticles functionalized by EDTA and dipicolinic acid: A comparative study of metal ion removal from aqueous solutions. R. Soc. Chem. 2019, 9, 31043–31051. [Google Scholar]
- Chen, W.; Pan, L.; Chen, L.; Wang, Q.; Yan, C. Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: Iron loading, kinetics and pathway †. RSC Adv. 2014, 4, 46689–46696. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K. Characterization of Alkali-Treated Jute Fibers for Physical. J. Appl. Polym. Sci. 2001, 80, 1013–1020. [Google Scholar] [CrossRef]
- Wang, W.; Cai, Z.; Yu, J.; Xia, Z. Changes in Composition, Structure, and Properties of Jute Fibers after Chemical Treatments. Fibers Polym. 2009, 10, 776–780. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Arancibia-Miranda, N.; Altbir, D. Surface rearrangement of nanoscale zerovalent iron: The role of pH and its implications in the kinetics of arsenate sorption. Environ. Technol. 2014, 35, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, W.A.; Alam, M.A.; Alam, M.O.; Chakraborty, S.; Owens, G.; Bhattacharya, T.; Mondal, N.K. Enhanced aqueous phase arsenic removal by a biochar based iron nanocomposite. Environ. Technol. Innov. 2020, 19, 100936. [Google Scholar] [CrossRef]
- Liu, W.; Huang, C.; Han, J.; Qin, W. Removal and reuse of arsenic from arsenic-bearing purified residue by alkaline pressure oxidative leaching and reduction of As (V). Hydrometallurgy 2021, 199, 105541. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Yan, J.; Ye, S. Selective extraction of arsenic and antimony from gold-bearing sludge using two-stage alkaline leaching. Resour. Conserv. Recycl. 2021, 167, 105388. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Han, J.; Li, W.; Wang, X.; Wang, N.; Qin, W. Selective separation of arsenic from lead smelter flue dust by alkaline pressure oxidative leaching. Minerals 2019, 9, 308. [Google Scholar] [CrossRef]



| Test | Fiber Weight (g) | Flow | Colum Length (cm) |
|---|---|---|---|
| J-nZVI/GO-1.6 | 1.6 | free | 60 |
| J-nZVI/GO-4 | 4 | free | 60 |
| J-30 | 6 | Controlled | 30 |
| J-60 | 6 | Controlled | 60 |
| J-nZVI/GO-30 | 6 | Controlled | 30 |
| J-nZVI/GO-60 | 6 | Controlled | 60 |
| J-nZVI/GO-60-TR | 6 | controlled + Retention time | 60 |
| Reactivated fiber | |||
| RJ-nZVI/GO-60 | 6 | controlled | 60 |
| Bond | Vibration | Wavelength Number (cm−1) | Ref. |
|---|---|---|---|
| Fe-O | Stretching | 540 and 620 1650–1624 | [5,24,25,26,27] |
| O-H | Stretching | 3000–3700 | |
| O-H | Bending | 1636 | |
| C=C | Stretching | 1600–1613 | |
| C-H | Flexion Bending | 2867 1352 | |
| CH2 | Bending | 1389 | |
| -COOH | Stretching | 1712 | |
| C-O-C | Stretching | 1036–1004 | |
| As-O | Symmetrical and antisymmetrical stretching | 710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Bárcenas, A.; Arizpe-Zapata, J.A.; Rivera Haro, J.A.; Sepúlveda, P.; Garcia-Garcia, A. Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems. Nanomaterials 2022, 12, 3974. https://doi.org/10.3390/nano12223974
Moreno-Bárcenas A, Arizpe-Zapata JA, Rivera Haro JA, Sepúlveda P, Garcia-Garcia A. Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems. Nanomaterials. 2022; 12(22):3974. https://doi.org/10.3390/nano12223974
Chicago/Turabian StyleMoreno-Bárcenas, Alejandra, Jesús Alejandro Arizpe-Zapata, Julio Alejandro Rivera Haro, Pamela Sepúlveda, and Alejandra Garcia-Garcia. 2022. "Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems" Nanomaterials 12, no. 22: 3974. https://doi.org/10.3390/nano12223974
APA StyleMoreno-Bárcenas, A., Arizpe-Zapata, J. A., Rivera Haro, J. A., Sepúlveda, P., & Garcia-Garcia, A. (2022). Jute Fibers Synergy with nZVI/GO: Superficial Properties Enhancement for Arsenic Removal in Water with Possible Application in Dynamic Flow Filtration Systems. Nanomaterials, 12(22), 3974. https://doi.org/10.3390/nano12223974






