Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of CdS/CdCO3 Nanocomposites
2.3. Preparation of CdS/CdCO3@SDS Nanocomposites
2.4. Characterizations
2.5. Photocatalytic Reduction Studies of Cr(VI)
3. Results and Discussion
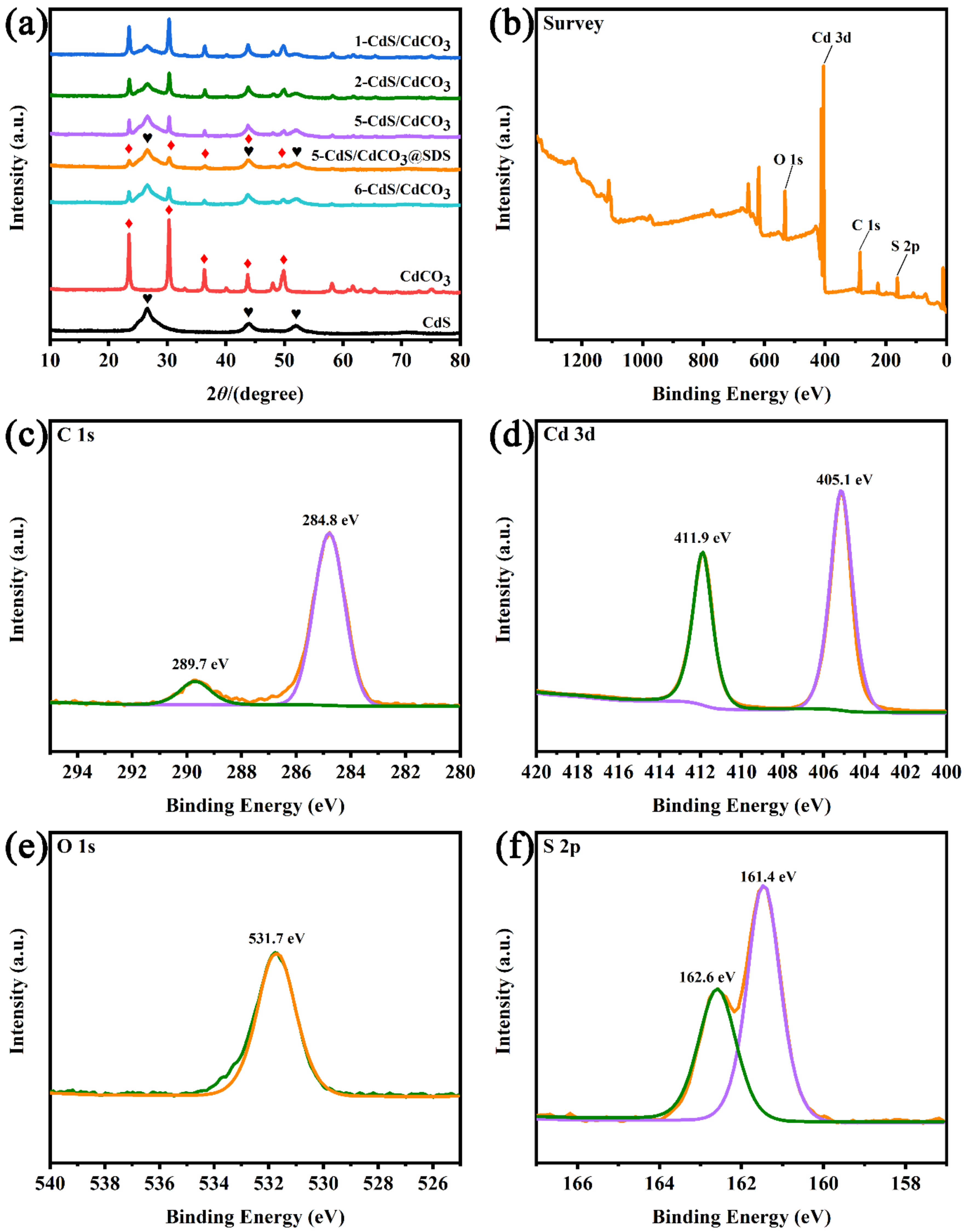
3.1. XRD and XPS Analysis
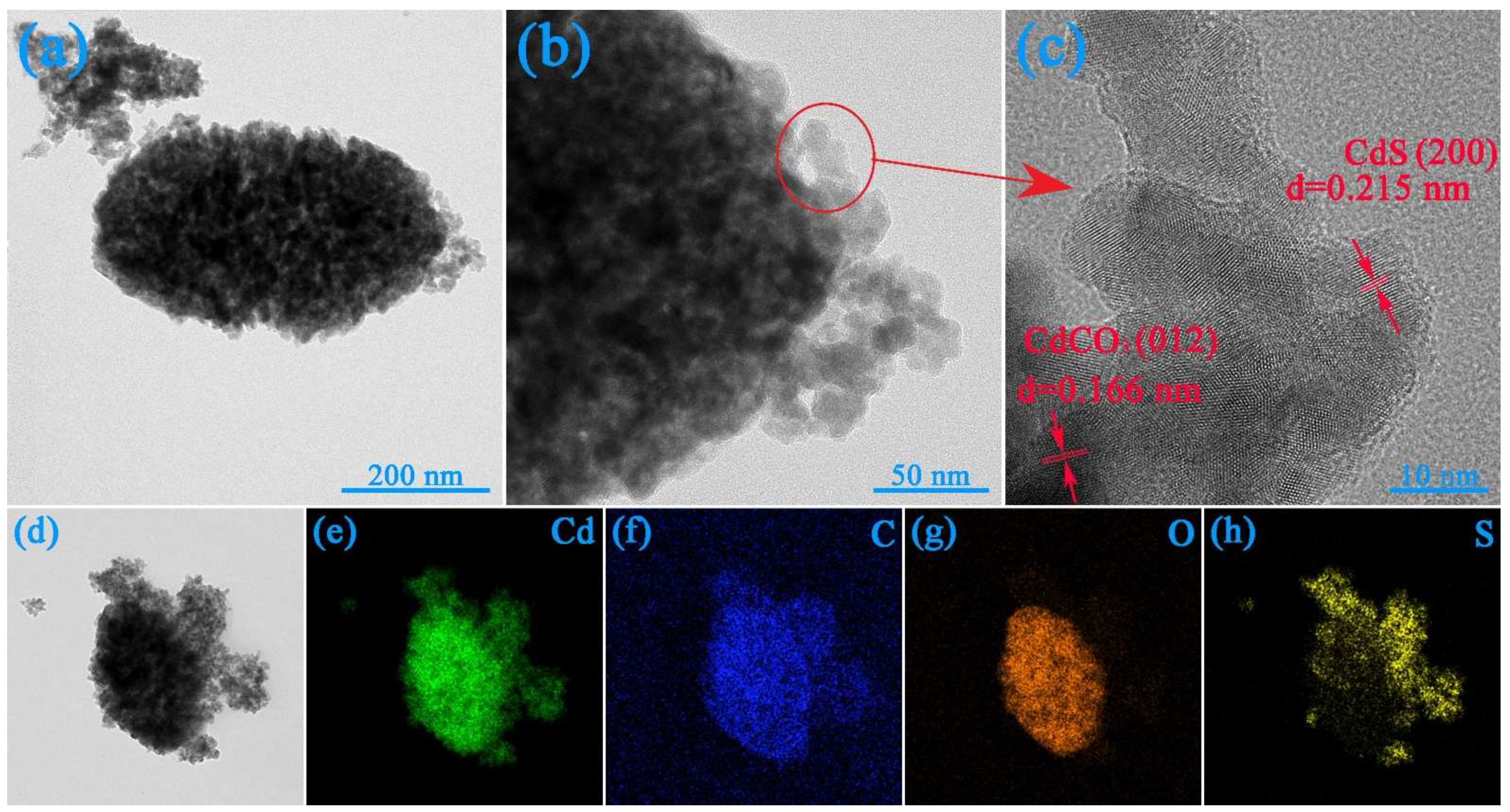
3.2. SEM, TEM and BET Analyses
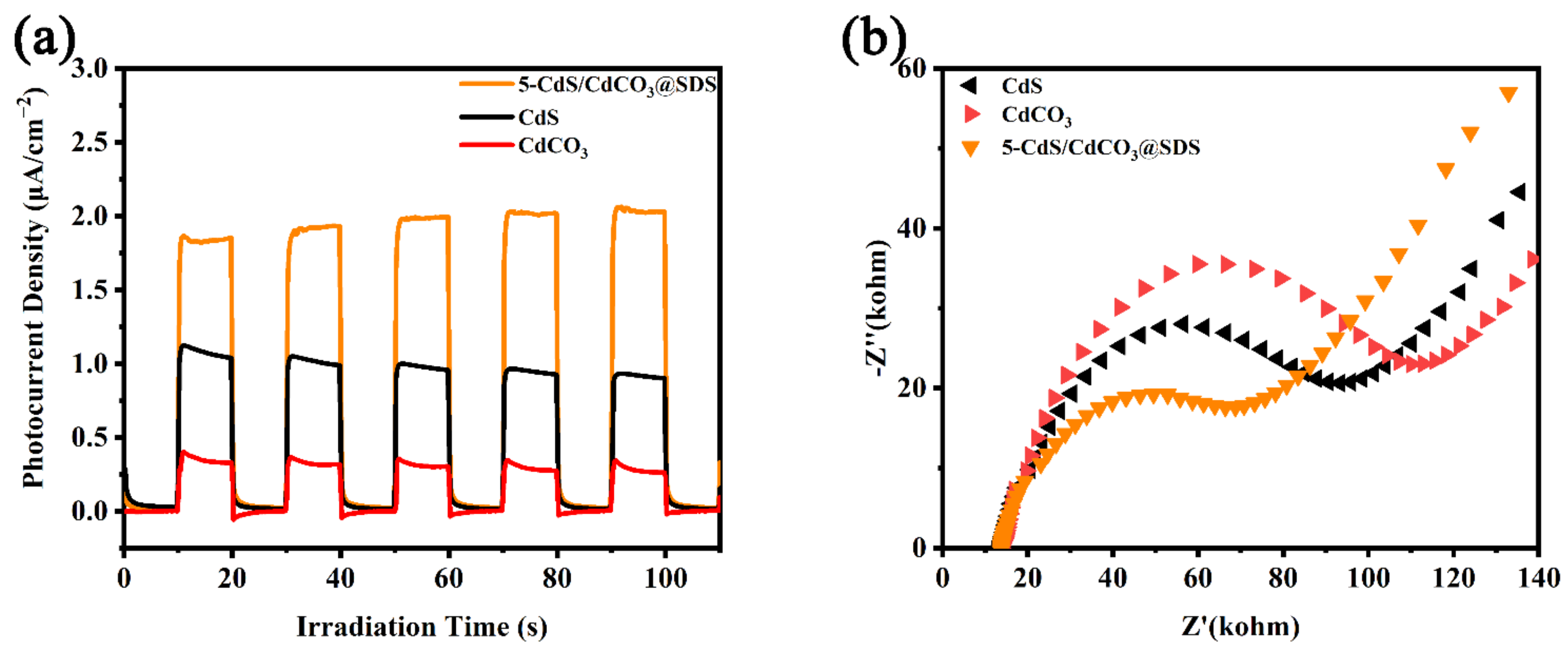
3.3. Photocatalytic Reduction and Influence Factor
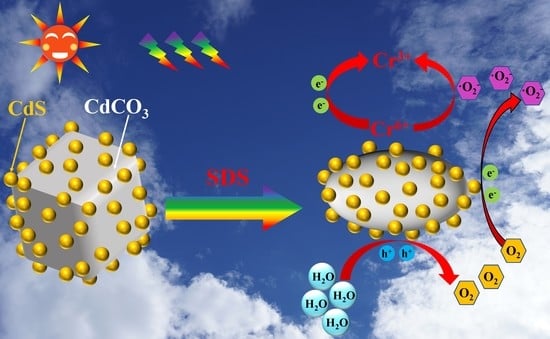
3.4. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Shan, G.; Wang, T.; Yin, D.; Chen, Y. Photothermal Enhanced Photocatalytic Activity Based on Ag-Doped CuS Nanocomposites. J. Alloys Compd. 2021, 864, 158591. [Google Scholar] [CrossRef]
- Liu, G.; Xia, H.; Yan, M.; Song, L.; Li, H.; Niu, Y. Performance and Mechanism of Self-Cleaning Synergistic Photocatalytic Coating Inhibiting NO2 for Green Degradation of NO. Appl. Surf. Sci. 2022, 586, 152787. [Google Scholar] [CrossRef]
- Wen, X.J.; Shen, C.H.; Fei, Z.H.; Fang, D.; Liu, Z.T.; Dai, J.T.; Niu, C.G. Recent Developments on AgI Based Heterojunction Photocatalytic Systems in Photocatalytic Application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. Recent Advances in Crystalline Carbon Nitride for Photocatalysis. J. Mater. Sci. Technol. 2021, 91, 224–240. [Google Scholar] [CrossRef]
- Zhou, Y.; Elchalakani, M.; Du, P.; Sun, C. Cleaning up Oil Pollution in the Ocean with Photocatalytic Concrete Marine Structures. J. Clean. Prod. 2021, 329, 129636. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, Z.; Guo, M.; Qiu, Y.; Fang, B.; Li, Z.; Yang, S.; Zhou, W. Journal of Colloid and Interface Science Hollow Core-Shell Potassium Phosphomolybdate@Cadmium Sulfide@Bismuth Sulfide Z-Scheme Tandem Heterojunctions toward Optimized Photothermal-Photocatalytic Performance. J. Colloid Interface Sci. 2022, 607, 942–953. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Nguyen, T.N.; Van Tran, V.; Hur, J.; Kim, I.T.; Park, D.; Lee, Y.C. Photocatalytic Materials for Indoor Air Purification Systems: An Updated Mini-Review. Environ. Technol. Innov. 2021, 22, 1–29. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yuan, J.; Xiang, W.; Xin, X.; Liu, H.; Luo, H.; Li, L.; Chen, M.; Zhong, D.; et al. Photocatalytic Optical Fibers for Degradation of Organic Pollutants in Wastewater: A Review. Environ. Chem. Lett. 2021, 19, 1335–1346. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, Y.; Luo, J.; Darling, S.B. Drawing on Membrane Photocatalysis for Fouling Mitigation. ACS Appl. Mater. Interfaces 2021, 13, 14844–14865. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Wen, X.; Chen, S.; Cheng, M.; Deng, R.; Li, B.; Yang, Y.; Liu, X. Silver-Based Semiconductor Z-Scheme Photocatalytic Systems for Environmental Purification. J. Hazard. Mater. 2020, 390, 122128. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Li, Y.; Ling, Y.; Zhang, Y.; Zhang, X.; Huang, S. New Insights into the Efficient Charge Transfer by Construction of Adjustable Dominant Facet of BiOI/CdS Heterojunction for Antibiotics Degradation and Chromium Cr(VI) Reduction under Visible-Light Irradiation. Chemosphere 2022, 302, 134862. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Zuo, Y.; He, G.; Chen, Q.; Meng, Q.; Chen, H. Reduced Graphene Oxide Supported ZnO/CdS Heterojunction Enhances Photocatalytic Removal Efficiency of Hexavalent Chromium from Aqueous Solution. Chemosphere 2022, 286, 131738. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A Noble Metal-Free Reduced Graphene Oxide-Cds Nanorod Composite for the Enhanced Visible-Light Photocatalytic Reduction of CO2 to Solar Fuel. J. Mater. Chem. A 2014, 2, 3407–3416. [Google Scholar] [CrossRef]
- Zhukovskyi, M.; Tongying, P.; Yashan, H.; Wang, Y.; Kuno, M. Efficient Photocatalytic Hydrogen Generation from Ni Nanoparticle Decorated CdS Nanosheets. ACS Catal. 2015, 5, 6615–6623. [Google Scholar] [CrossRef]
- Liu, S.; Weng, B.; Tang, Z.R.; Xu, Y.J. Constructing One-Dimensional Silver Nanowire-Doped Reduced Graphene Oxide Integrated with CdS Nanowire Network Hybrid Structures toward Artificial Photosynthesis. Nanoscale 2015, 7, 861–866. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Seeta Rama Raju, G.; Pavitra, E.; Kwak, C.H.; Han, Y.K.; Huh, Y.S. Controlled Synthesis of Hierarchical α-Nickel Molybdate with Enhanced Solar-Light-Responsive Photocatalytic Activity: A Comprehensive Study on the Kinetics and Effect of Operational Factors. Ceram. Int. 2019, 45, 12041–12052. [Google Scholar] [CrossRef]
- Dharamalingam, K.; Arjun Kumar, B.; Ramalingam, G.; Sasi Florence, S.; Raju, K.; Senthil Kumar, P.; Govindaraju, S.; Thangavel, E. The Role of Sodium Dodecyl Sulfate Mediated Hydrothermal Synthesis of MoS2 Nanosheets for Photocatalytic Dye Degradation and Dye-Sensitized Solar Cell Application. Chemosphere 2022, 294, 133725. [Google Scholar] [CrossRef]
- Fang, P.; Wang, Z.; Wang, W. Enhanced Photocatalytic Performance of ZnTi-LDHs with Morphology Control. CrystEngComm 2019, 21, 7025–7031. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Wang, T.; Wang, B.; Meng, D.; Wang, Y. Synthesis, Growth Mechanism and Photocatalytic Property of CdS with Different Kinds of Surfactants. New J. Chem. 2019, 43, 10126–10133. [Google Scholar] [CrossRef]
- Estrada-Flores, S.; Martínez-Luévanos, A.; Perez-Berumen, C.M.; García-Cerda, L.A.; Flores-Guia, T.E. Relationship between Morphology, Porosity, and the Photocatalytic Activity of TiO2 Obtained by Sol–Gel Method Assisted with Ionic and Nonionic Surfactants. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 209–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Zhang, S.; Cai, Y.; Lv, Z.; Fang, M.; Tan, X.; Wang, X. Highly Efficient Removal of U(VI) by the Photoreduction of SnO2/CdCO3/CdS Nanocomposite under Visible Light Irradiation. Appl. Catal. B Environ. 2020, 279, 119390. [Google Scholar] [CrossRef]
- Xuan, Y.; Quan, H.; Shen, Z.; Zhang, C.; Yang, X.; Lou, L.; Liu, S.; Yu, K. Band-Gap and Charge Transfer Engineering in Red Phosphorus-Based Composites for Enhanced Visible-Light-Driven H2 Evolution. Chem.–A Eur. J. 2020, 26, 2285–2292. [Google Scholar] [CrossRef]
- Vidyasagar, D.; Ghugal, S.G.; Kulkarni, A.; Shende, A.G.; Umare, S.S.; Sasikala, R. Microwave Assisted: In Situ Decoration of a g-C3N4 Surface with CdCO3 Nanoparticles for Visible Light Driven Photocatalysis. New J. Chem. 2018, 42, 6322–6331. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Chen, G.; Li, D.; Meng, S.; Chen, S. Synthesis of BiPO4 by Crystallization and Hydroxylation with Boosted Photocatalytic Removal of Organic Pollutants in Air and Water. J. Hazard. Mater. 2020, 399, 122999. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, T.; Qu, G.; Deng, F.; Liang, D.; Yang, W.; Liu, M. In Situ Synthesis of g-C3N4/TiO2 Heterojunction Nanocomposites as a Highly Active Photocatalyst for the Degradation of Orange II under Visible Light Irradiation. Environ. Sci. Pollut. Res. 2018, 25, 19122–19133. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, G.; Du, H.; Zhu, J.; Li, J. Preparation and Application of BiOBr-Bi2S3 Heterojunctions for Efficient Photocatalytic Removal of Cr(VI). J. Hazard. Mater. 2021, 407, 124394. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Huang, H.; Wang, Y.; Tong, N.; Lin, J.; Liu, D.; Wang, X. Oxygen Vacancy Modulation of Two-Dimensional γ-Ga2O3 Nanosheets as Efficient Catalysts for Photocatalytic Hydrogen Evolution. Nanoscale 2018, 10, 21509–21517. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, N.; Fang, F.; Liu, G.; Chen, L.; Meng, J.; Chen, C.; Wang, L.; Wan, H.; Guan, G. Facile Synthesis of Three-Dimensional Ordered Macroporous Sr1-XKxTiO3 Perovskites with Enhanced Catalytic Activity for Soot Combustion. Catal. Sci. Technol. 2018, 8, 5462–5472. [Google Scholar] [CrossRef]
- Abbasi Moud, A.; Hatzikiriakos, S.G. Kaolinite Colloidal Suspensions under the Influence of Sodium Dodecyl Sulfate. Phys. Fluids 2022, 34, 013107. [Google Scholar] [CrossRef]
- Wen, T.; Luo, J.; Jiao, K.; Lu, L. Comparison of Pool Boiling Heat Transfer Performance between Aqueous Cationic and Anionic Surfactant Solutions with Similar Ionic Group. Appl. Therm. Eng. 2022, 216, 119136. [Google Scholar] [CrossRef]
- Xu, L.; Lu, C.; Zhang, Z.; Yang, X.; Hou, W. Various Self-Assembled Three-Dimensional Hierarchical Architectures of La2(MoO4)3: Controlled Synthesis, Growth Mechanisms, Luminescence Properties and Adsorption Activities. Nanoscale 2010, 2, 995–1005. [Google Scholar] [CrossRef]
- Jia, M.; Yang, Z.; Xu, H.; Song, P.; Xiong, W.; Cao, J.; Zhang, Y.; Xiang, Y.; Hu, J.; Zhou, C.; et al. Integrating N and F Co-Doped TiO2 Nanotubes with ZIF-8 as Photoelectrode for Enhanced Photo-Electrocatalytic Degradation of Sulfamethazine. Chem. Eng. J. 2020, 388, 124388. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.; Liu, W.; Liu, F.; Ye, J.; Liang, J.; Li, Y. Carbon Quantum Dots Modified Tubular g-C3N4 with Enhanced Photocatalytic Activity for Carbamazepine Elimination: Mechanisms, Degradation Pathway and DFT Calculation. J. Hazard. Mater. 2020, 381, 120957. [Google Scholar] [CrossRef]
- He, D.; Zhang, C.; Zeng, G.; Yang, Y.; Huang, D.; Wang, L.; Wang, H. A Multifunctional Platform by Controlling of Carbon Nitride in the Core-Shell Structure: From Design to Construction, and Catalysis Applications. Appl. Catal. B Environ. 2019, 258, 117957. [Google Scholar] [CrossRef]
- Qiao, X.Q.; Zhang, Z.W.; Li, Q.H.; Hou, D.; Zhang, Q.; Zhang, J.; Li, D.S.; Feng, P.; Bu, X. In Situ Synthesis of N-n Bi2MoO6 & Bi2S3 Heterojunctions for Highly Efficient Photocatalytic Removal of Cr(VI). J. Mater. Chem. A 2018, 6, 22580–22589. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Sun, Y.; Wang, H.; Jiang, G.; Lee, S.C.; Dong, F. Enhancing ROS Generation and Suppressing Toxic Intermediate Production in Photocatalytic NO Oxidation on O/Ba Co-Functionalized Amorphous Carbon Nitride. Appl. Catal. B Environ. 2018, 237, 938–946. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; He, W.; Zhou, Y.; Lee, S.C.; Dong, F. Visible Light Induced Electron Transfer from a Semiconductor to an Insulator Enables Efficient Photocatalytic Activity on Insulator-Based Heterojunctions. Nanoscale 2018, 10, 15513–15520. [Google Scholar] [CrossRef]
- Hu, X.; Lu, P.; He, Y.; Wang, C.; Chen, J.; Fu, M. Anionic/Cationic Synergistic Action of Insulator BaCO3 Enhanced the Photocatalytic Activities of Graphitic Carbon Nitride. Appl. Surf. Sci. 2020, 528, 146924. [Google Scholar] [CrossRef]
- Krukowska, A.; Trykowski, G.; Lisowski, W.; Klimczuk, T.; Winiarski, M.J.; Zaleska-Medynska, A. Monometallic Nanoparticles Decorated and Rare Earth Ions Doped KTaO3/K2Ta2O6 Photocatalysts with Enhanced Pollutant Decomposition and Improved H2 Generation. J. Catal. 2018, 364, 371–381. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Ranjith, K.S.; Park, B.; Hwang, S.K.; Hosseini, R.; Behjatmanesh-Ardakani, R.; Pourmortazavi, S.M.; Lee, H.U.; Son, B.; Mirsadeghi, S.; et al. Full-Spectrum-Responsive Bi2S3@CdS S-Scheme Heterostructure with Intimated Ultrathin RGO toward Photocatalytic Cr(VI) Reduction and H2O2 Production: Experimental and DFT Studies. Chem. Eng. J. 2021, 419, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, C.-C.; Fu, H.; Liu, Y.; Wang, P.; Zhao, C. S-TiO2/UiO-66-NH2 Composite for Boosted Photocatalytic Cr(VI) Reduction and Bisphenol A Degradation under LED Visible Light. J. Hazard. Mater. 2020, 399, 123085. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Duoerkun, G.; Cao, W.; Liu, T.; Zhang, L.; Liu, J.; Li, M.; Chen, Z. Fabrication of MoS2/BiOBr Heterojunctions on Carbon Fibers as a Weaveable Photocatalyst for Tetracycline Hydrochloride Degradation and Cr(VI) Reduction under Visible Light. Environ. Sci. Nano 2020, 7, 2708–2722. [Google Scholar] [CrossRef]



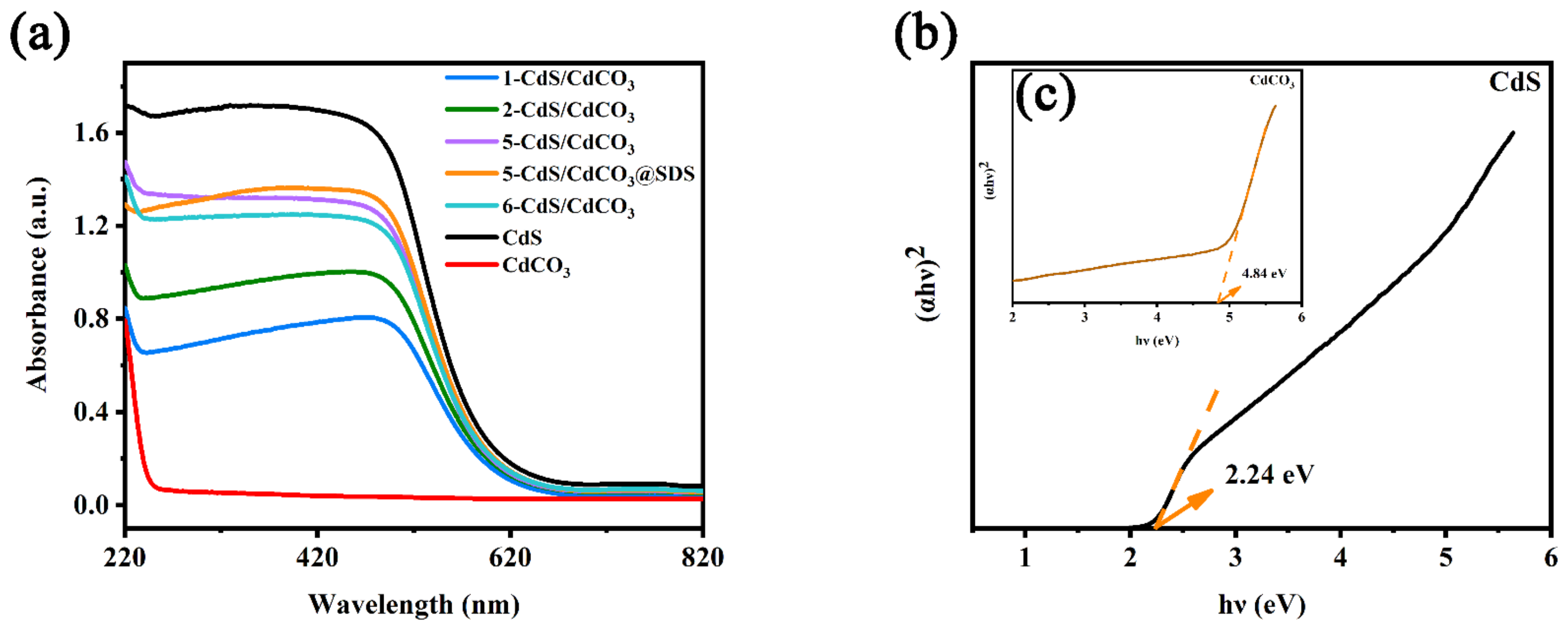
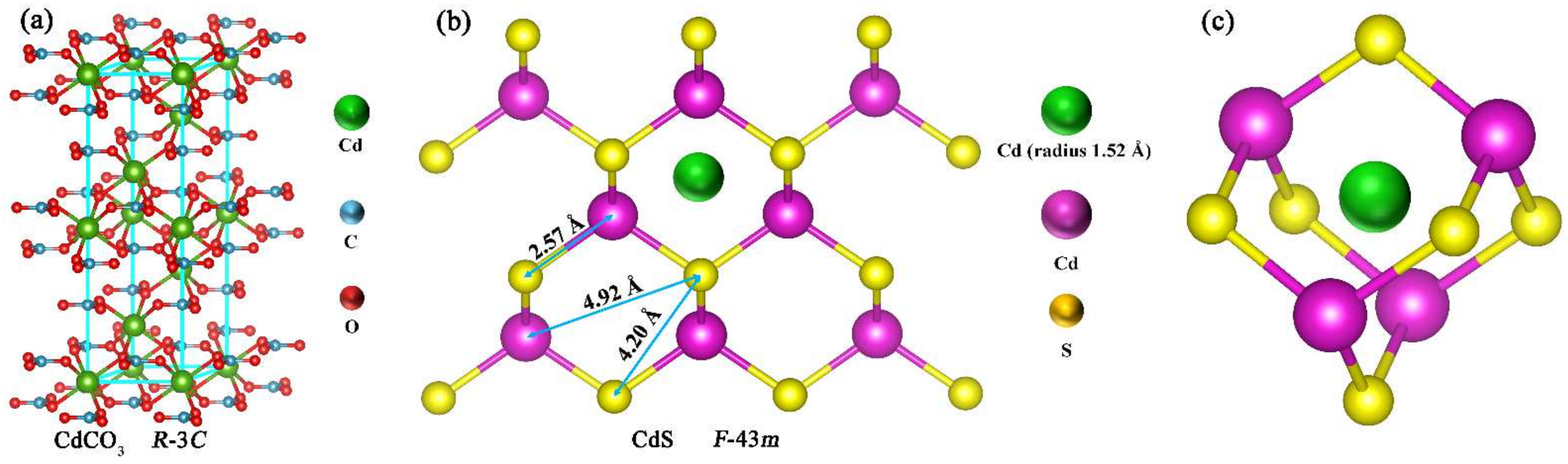
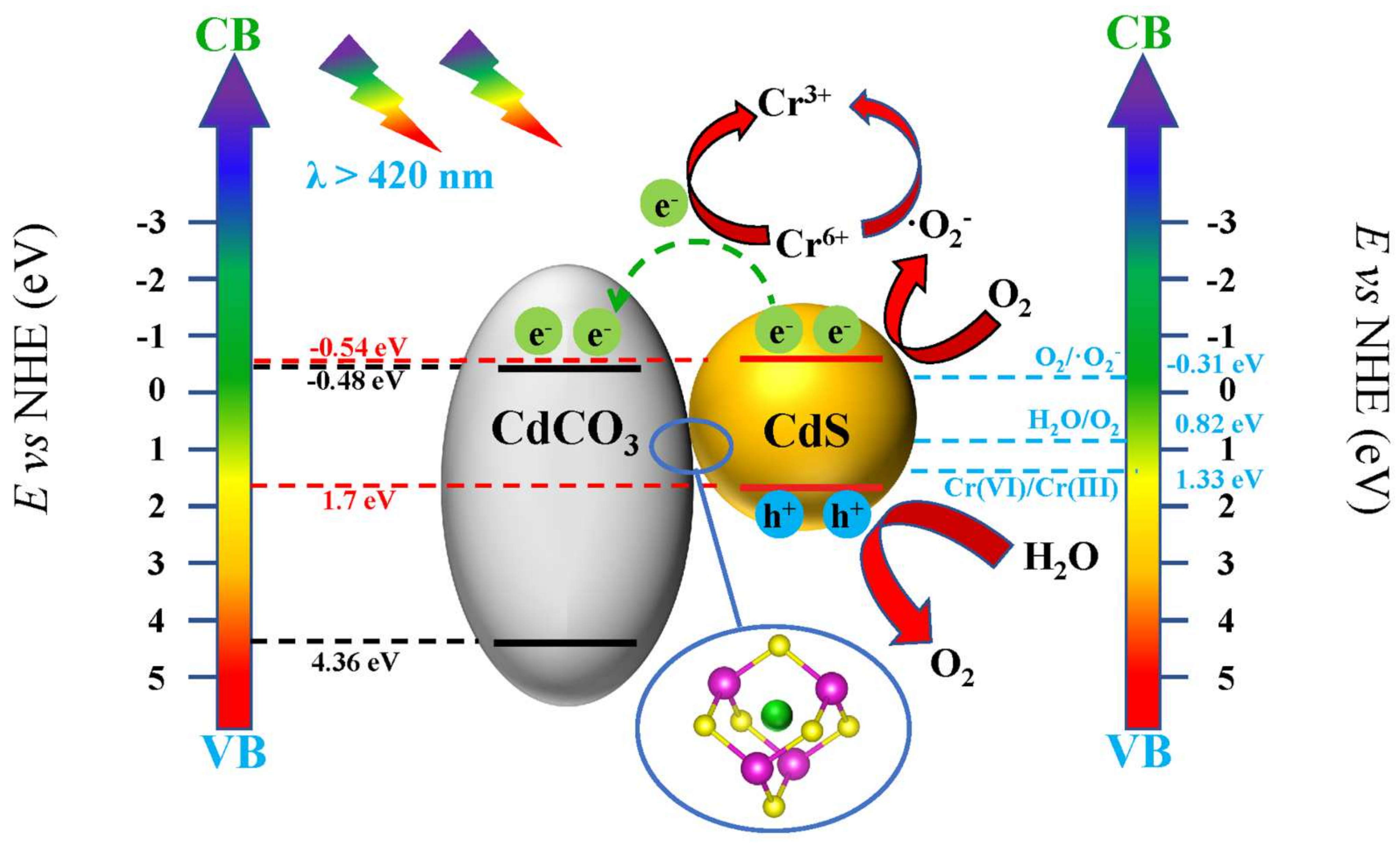
| Sample | SBET/(m2g−1) |
|---|---|
| CdS | 75 |
| CdCO3 | 7 |
| 5-CdS/CdCO3 | 50 |
| 5-CdS/CdCO3@SDS | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Y.; Sang, T.; Zhong, Y.; Hu, C.-H.; Wang, D.-H.; Ye, J.-C.; Wei, N.-N.; Liu, H. Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance. Nanomaterials 2022, 12, 3923. https://doi.org/10.3390/nano12213923
Wang W-Y, Sang T, Zhong Y, Hu C-H, Wang D-H, Ye J-C, Wei N-N, Liu H. Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance. Nanomaterials. 2022; 12(21):3923. https://doi.org/10.3390/nano12213923
Chicago/Turabian StyleWang, Wen-Yi, Tian Sang, Yan Zhong, Chao-Hao Hu, Dian-Hui Wang, Jun-Chen Ye, Ni-Ni Wei, and Hao Liu. 2022. "Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance" Nanomaterials 12, no. 21: 3923. https://doi.org/10.3390/nano12213923
APA StyleWang, W.-Y., Sang, T., Zhong, Y., Hu, C.-H., Wang, D.-H., Ye, J.-C., Wei, N.-N., & Liu, H. (2022). Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance. Nanomaterials, 12(21), 3923. https://doi.org/10.3390/nano12213923