NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors
Abstract
1. Introduction
2. Experimental Study
2.1. Materials
2.2. Preparation of Ni and NiO Aerogels
2.3. Characterizations
2.4. Electrochemical Measurements for NiO/Ni/NF Electrode
2.5. Fabrication of the NiO/Ni/NF//AC Asymmetric Supercapacitor
2.6. Calculation of Specific Capacitance: ED and PD
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crane, M.J.; Lim, M.B.; Zhou, X.; Pauzauskie, P.J. Rapid synthesis of transition metal dichalcogenide–carbon aerogel composites for supercapacitor electrodes. Microsyst. Nanoeng. 2017, 3, 17032. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Chun, J.; Jo, C.; Hwang, J. Recent advances in the synthesis of mesoporous materials and their application to lithium-ion batteries and hybrid supercapacitors. Korean J. Chem. Eng. 2021, 38, 227–247. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, M.V. Conducting polymers-based electrochemical supercapacitors—Progress and prospects. Electrochim. Acta 2013, 101, 109–129. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Wang, R.; Xuan, H.; Zhang, G.; Li, H.; Guan, Y.; Liang, X.; Zhang, S.; Wu, Z.; Han, P.; Wu, Y. Applied Surface Science Design and fabrication of free-standing Ni3S2/NiV-LDH nanosheets arrays on reduced graphene oxide / Ni foam as a novel electrode for asymmetric supercapacitor. Appl. Surf. Sci. 2020, 526, 146641. [Google Scholar] [CrossRef]
- Hu, W.; Wang, B.; Yu, Y.; Wang, N.; Wu, X. Biomass derived carbon containing in-situ constructed nickel-based hydroxide nanostructures based on MnO2 template for high performance asymmetric supercapacitors. J. Alloys Compd. 2021, 884, 161149. [Google Scholar] [CrossRef]
- Li, L.; Xiao, R.; Tao, X.; Wu, Y.; Jiang, L.; Zhang, Z.; Qing, Y. Free-standing electrodes via coupling nanostructured Ni–NiO with hierarchical wood carbon for high-performance supercapacitors and Ni–Zn batteries. J. Power Sources 2021, 491, 229618. [Google Scholar] [CrossRef]
- Muthu, N.S.; Gopalan, M. Applied Surface Science Mesoporous nickel sulphide nanostructures for enhanced supercapacitor performance. Appl. Surf. Sci. 2019, 480, 186–198. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, D.; Meng, W.; Wu, Y.; Yang, Y.; Pu, H.; Gao, R. Facile synthesis of yolk-shelled NiO/Ni composites as cathode material for high-performance hybrid supercapacitors. J. Power Sources 2019, 438, 226977. [Google Scholar] [CrossRef]
- Hao, P.; Tian, J.; Sang, Y.; Tuan, C.; Cui, G.; Shi, X.; Wong, C.P.; Tang, B.; Liu, H. 1D Ni–Co oxide and sulfide nanoarray/carbon aerogel hybrid nanostructures for asymmetric supercapacitors with high energy density and excellent cycling stability. Nanoscale 2016, 8, 16292–16301. [Google Scholar] [CrossRef]
- Yan, S.X.; Luo, S.H.; Wang, Q.; Zhang, Y.H.; Liu, X. Rational design of hierarchically sulfide and MXene-reinforced porous carbon nanofibers as advanced electrode for high energy density flexible supercapacitors. Compos. Part B Eng. 2021, 224, 109246. [Google Scholar] [CrossRef]
- Yan, S.X.; Luo, S.H.; Feng, J.; Li, P.W.; Guo, R.; Wang, Q.; Zhang, Y.H.; Liu, Y.G.; Bao, S. Rational design of flower-like FeCo2S4/reduced graphene oxide films: Novel binder-free electrodes with ultra-high conductivity flexible substrate for high-performance all-solid-state pseudocapacitor. Chem. Eng. J. 2020, 381, 122695. [Google Scholar] [CrossRef]
- Yan, S.X.; Luo, S.H.; Feng, J.; Yang, L.; Li, P.W.; Wang, Q.; Zhang, Y.H.; Liu, X.; Chang, L.J. Asymmetric, Flexible Supercapacitor Based on Fe-Co Alloy@Sulfide with High Energy and Power Density. ACS Appl. Mater. Interfaces 2021, 13, 49952–49963. [Google Scholar] [CrossRef]
- Lv, Z.; Luo, Y.; Tang, Y.; Wei, J.; Zhu, Z.; Zhou, X.; Li, W.; Zeng, Y.; Zhang, W.; Zhang, Y.; et al. Editable Supercapacitors with Customizable Stretchability Based on Mechanically Strengthened Ultralong MnO2 Nanowire Composite. Adv. Mater. 2018, 30, 1704531. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, C.; Wan, C.; Wang, R.; Dai, X.; Wei, J.; Xia, H.; Li, W.; Zhang, W.; Cao, S.; et al. Strain-Driven Auto-Detachable Patterning of Flexible Electrodes. Adv. Mater. 2022, 34, 2202877. [Google Scholar] [CrossRef]
- Wei, J.; Zhong, L.; Xia, H.; Lv, Z.; Diao, C.; Zhang, W.; Li, X.; Du, Y.; Xi, S.; Salanne, M.; et al. Metal-Ion Oligomerization Inside Electrified Carbon Micropores and its Effect on Capacitive Charge Storage. Adv. Mater. 2022, 34, 2107439. [Google Scholar] [CrossRef]
- Georgi, M.; Klemmed, B.; Benad, A. MATERIALS CHEMISTRY A versatile ethanolic approach to metal aerogels. Mater. Chem. Front. 2019, 1586–1592. [Google Scholar] [CrossRef]
- Mayer, S.T.; Pekala, R.W.; Kaschmitter, J.L. The Aerocapacitor: An Electrochemical Double-Layer Energy-Storage Device. J. Electrochem. Soc. 1993, 140, 446–451. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, D.W.; Jung, H.Y. Which is the most effective pristine graphene electrode for energy storage devices: Aerogel or xerogel? Nanoscale 2019, 11, 17563–17570. [Google Scholar] [CrossRef] [PubMed]
- Talande, S.V.; Bakandritsos, A.; Zdražil, L.; Jakubec, P.; Mohammadi, E.; Tomanec, O.; Otyepka, M.; Presser, V.; Zbořil, R.; Tuček, J. Pinning ultrasmall greigite nanoparticles on graphene for effective transition-metal-sulfide supercapacitors in an ionic liquid electrolyte. J. Mater. Chem. A 2020, 8, 25716–25726. [Google Scholar] [CrossRef]
- Yan, X.; Wang, X.; Dai, Y.; He, Y.; Cai, Z.; Wang, Y.; Wang, X. In situ self-assembly of SiO2 coating Co3O4/graphene aerogel and its enhanced electrochemical performance for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 17218–17226. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Comparison of hydrophobicity and durability of functionalized aluminium oxide nanoparticle coatings with magnetite nanoparticles–links between morphology and wettability. J. Colloid Interface Sci. 2019, 555, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Anandan, K.; Rajendran, V. Morphological and size effects of NiO nanoparticles via solvothermal process and their optical properties. Mater. Sci. Semicond. Process. 2011, 14, 43–47. [Google Scholar] [CrossRef]
- Ebin, B. Simple Preparation of Ni and NiO Nanoparticles Using Raffinate Solution Originated from Spent NiMH Battery Recycling. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2554–2563. [Google Scholar] [CrossRef]
- Barani, A.; Aghazadeh, M.; Ganjali, M.R.; Sabour, B.; Barmi, A.A.M.; Dalvand, S. Nanostructured nickel oxide ultrafine nanoparticles: Synthesis, characterization, and supercapacitive behavior. Mater. Sci. Semicond. Process. 2014, 23, 85–92. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, X.; Ding, S.; Li, B.Q. Preparation and electrochemical characteristics of porous hollow spheres of NiO nanosheets as electrodes of supercapacitors. J. Power Sources 2014, 256, 440–448. [Google Scholar] [CrossRef]
- Wu, M.S.; Huang, Y.A.; Jow, J.J.; Yang, W.D.; Hsieh, C.Y.; Tsai, H.M. Anodically potentiostatic deposition of flaky nickel oxide nanostructures and their electrochemical performances. Int. J. Hydrogen Energy 2008, 33, 2921–2926. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, J.; Wang, Y.; Ma, J.; Song, B.; Ma, H. Interfacial Deposition of Three-Dimensional Nickel Hydroxide Nanosheet-Graphene Aerogel on Ni Wire for Flexible Fiber Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 821–827. [Google Scholar] [CrossRef]
- Hassan, K.T.; Wang, J.; Han, X.; Sharp, J.J.; Bhaduri, G.A.; Martis, V.; Šiller, L. Catalytic Performance of Nickel Nanowires Immobilized in Silica Aerogels for the CO2 Hydration Reaction. ACS Omega 2019, 4, 1824–1830. [Google Scholar] [CrossRef]
- Lv, Z.; Tang, Y.; Zhu, Z.; Wei, J.; Li, W.; Xia, H.; Jiang, Y.; Liu, Z.; Luo, Y.; Ge, X.; et al. Honeycomb-Lantern-Inspired 3D Stretchable Supercapacitors with Enhanced Specific Areal Capacitance. Adv. Mater. 2018, 30, 1805468. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Marzolo, F.; Rubino, A.; Zanoni, R.; Pagnanelli, F. Optimizing the structure of Ni-Ni(OH)2/NiO core-shell nanowire electrodes for application in pseudocapacitors: The influence of metallic core, Ni(OH)2/NiO ratio and nanowire length. J. Alloys Compd. 2021, 856, 157718. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Srihasam, S.; Thyagarajan, K.; Korivi, M.; Lebaka, V.R.; Mallem, S.P.R. Phytogenic generation of NiO nanoparticles using stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar] [CrossRef]
- Li, H.; Xie, F.; Li, W.; Fahlman, B.D.; Chen, M.; Li, W. Preparation and adsorption capacity of porous MoS2 nanosheets. RSC Adv. 2016, 6, 105222–105230. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Wang, X.; Liang, K.; Tay, B.K. In situ fabrication of three-dimensional, ultrathin graphite/carbon nanotube/NiO composite as binder-free electrode for high-performance energy storage. J. Mater. Chem. A 2015, 3, 624–633. [Google Scholar] [CrossRef]
- Du, H.; Zhou, C.; Xie, X.; Li, H.; Qi, W.; Wu, Y.; Liu, T. Pseudocapacitance of nanoporous Ni@NiO nanoparticles on Ni foam substrate: Influence of the annealing temperature. Int. J. Hydrogen Energy 2017, 42, 15236–15245. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Vishnu Kamath, P. Suppression of the α → β-nickel hydroxide transformation in concentrated alkali: Role of dissolved cations. J. Appl. Electrochem. 2001, 31, 1315–1320. [Google Scholar] [CrossRef]
- Song, Y.; Hwang, J.; Lee, S.; Thirumalraj, B.; Kim, J.H.; Jenei, P.; Gubicza, J.; Choe, H. Synthesis of a High-Capacity NiO/Ni Foam Anode for Advanced Lithium-Ion Batteries. Adv. Eng. Mater. 2020, 22, 2000351. [Google Scholar] [CrossRef]
- Brisse, A.; Stevens, P. Ni(OH)2 and NiO Based Composites: Battery Type Electrode Materials for Hybrid Supercapacitor Devices. Materials 2018, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries; Springer: Singapore; Volume 12, ISBN 0123456789.
- Sciancalepore, C.; Rosa, R.; Barrera, G.; Tiberto, P.; Allia, P.; Bondioli, F. Microwave-assisted nonaqueous sol-gel synthesis of highly crystalline magnetite nanocrystals. Mater. Chem. Phys. 2014, 148, 117–124. [Google Scholar] [CrossRef]
- Barazandeh, M.; Kazemi, S.H. High-performance freestanding supercapacitor electrode based on polypyrrole coated nickel cobalt sulfide nanostructures. Sci. Rep. 2022, 12, 4628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, Q.; Gao, H.; Shi, Z.; Wu, J.; Zhi, M.; Hong, Z. Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv. 2016, 6, 112620–112624. [Google Scholar] [CrossRef]
- Guo, S.; Li, H.; Zhang, X.; Nawaz, H.; Chen, S.; Zhang, X.; Xu, F. Lignin carbon aerogel/nickel binary network for cubic supercapacitor electrodes with ultra-high areal capacitance. Carbon 2021, 174, 500–508. [Google Scholar] [CrossRef]
- Ye, S.; Feng, J.; Wu, P. Deposition of three-dimensional graphene aerogel on nickel foam as a binder-free supercapacitor electrode. ACS Appl. Mater. Interfaces 2013, 5, 7122–7129. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, J.C.; Park, S.; Seo, J.G.; Baeck, S.H.; Yoon, J.R.; Yi, J.; Song, I.K. Nano-sized Ni-doped carbon aerogel for supercapacitor. J. Nanosci. Nanotechnol. 2011, 11, 6528–6532. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Na, H.; Pan, C.; Xu, X.; Huang, G.; Liu, Y.; Liu, Y.; Gao, J. Self-Assembly Method to Fabricate Reduced Graphene Oxide Aerogels Loaded with Nickel Hydroxyl Nanoparticles and Their Excellent Properties in Absorbing and Supercapacitors. Ind. Eng. Chem. Res. 2016, 55, 6553–6562. [Google Scholar] [CrossRef]
- Chen, W.; Gui, D.; Liu, J. Nickel oxide/graphene aerogel nanocomposite as a supercapacitor electrode material with extremely wide working potential window. Electrochim. Acta 2016, 222, 1424–1429. [Google Scholar] [CrossRef]
- Pilban Jahromi, S.; Pandikumar, A.; Goh, B.T.; Lim, Y.S.; Basirun, W.J.; Lim, H.N.; Huang, N.M. Influence of particle size on performance of a nickel oxide nanoparticle-based supercapacitor. RSC Adv. 2015, 5, 14010–14019. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Wan, L.; He, C.; Chen, D.; Liu, J.; Zhang, Y.; Du, C.; Xie, M.; Chen, J. In situ grown NiFeP@NiCo2S4 nanosheet arrays on carbon cloth for asymmetric supercapacitors. Chem. Eng. J. 2020, 399, 125778. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Ramadoss, A. Nickel–zinc sulfide nanocomposite thin film as an efficient cathode material for high-performance hybrid supercapacitors. Mater. Sci. Semicond. Process. 2020, 105, 104709. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, X.; She, P.; Shang, Y.; Sun, H.; Liu, Z. Macroscopic porous MnO2 aerogels for supercapacitor electrodes. Inorg. Chem. Front. 2016, 3, 1043–1047. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Liang, M.; He, Z.; Li, K.; Song, W.; Tian, S.; Duan, W.; Zhao, Y.Z.; Miao, Z. Novel Dealloying-Fabricated NiS/NiO Nanoparticles with Superior Cycling Stability for Supercapacitors. ACS Omega 2021, 6, 17999–18007. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Sarcheshmeh, H.M.; Naderi, H.; Farbod, F.; Sabaghian, F. Fabrication of a high-performance hybrid supercapacitor using a modified graphene aerogel/cerium oxide nanoparticle composite. J. Energy Storage 2019, 26, 100998. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, I.A.; Karaman, O.; Karaman, C. The production of rGO/RuO2 aerogel supercapacitor and analysis of its electrochemical performances. Ceram. Int. 2021, 47, 34514–34520. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, Z.; Xue, K.; Ye, Z.; Hong, Z.; Yu, X.; Zhi, M. Cobalt sulfide aerogel prepared by anion exchange method with enhanced pseudocapacitive and water oxidation performances. Nanotechnology 2018, 29, 215601. [Google Scholar] [CrossRef]

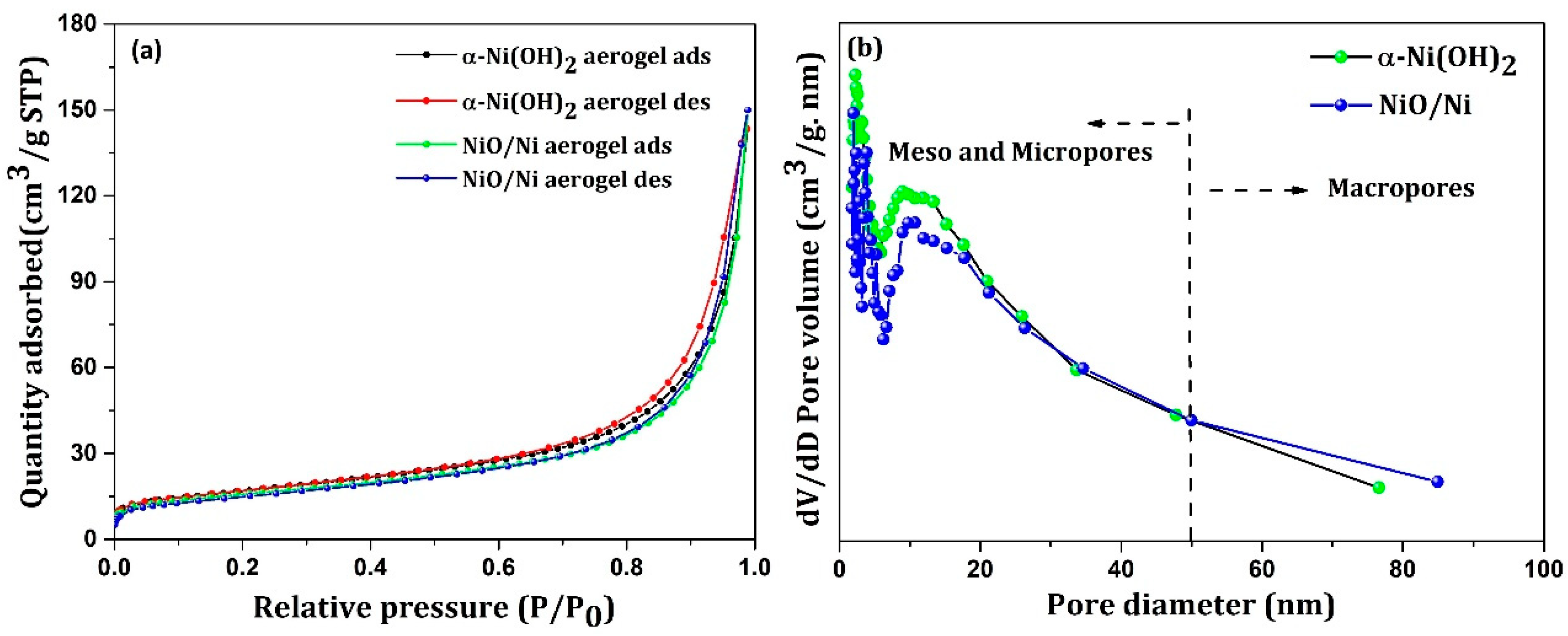
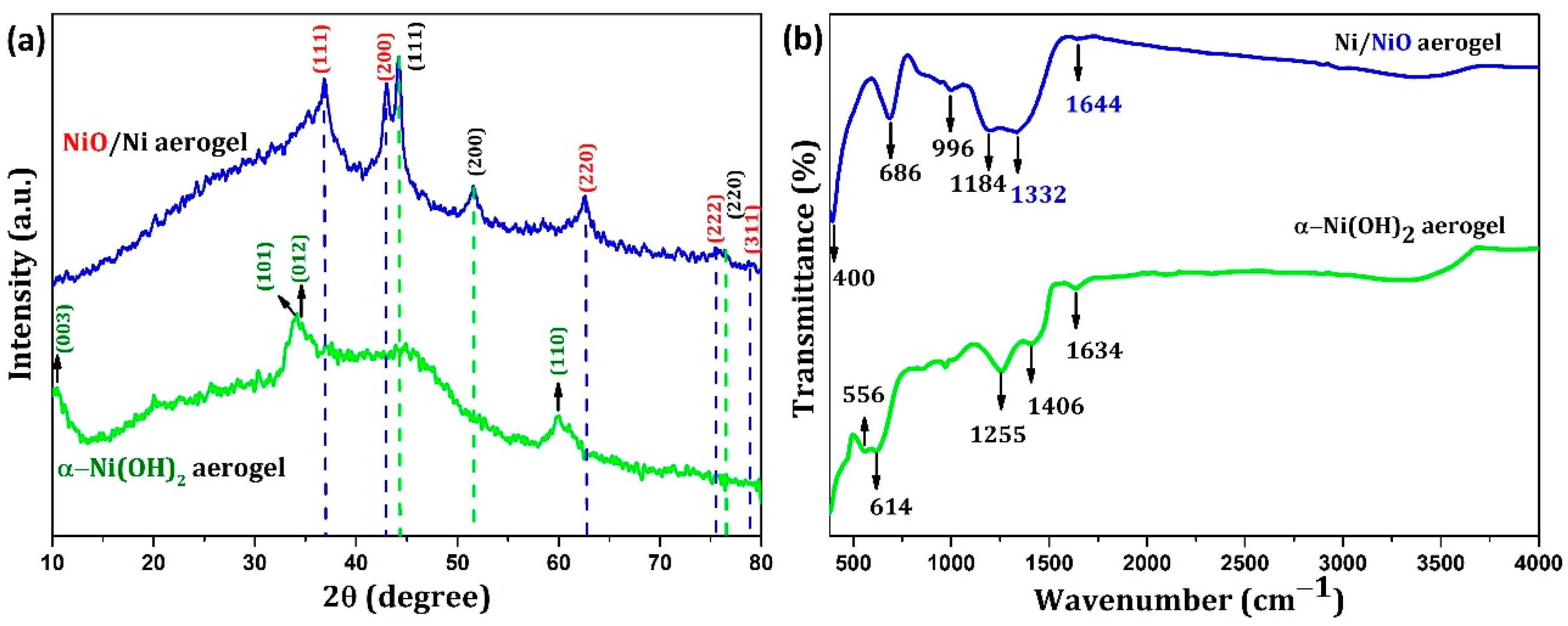


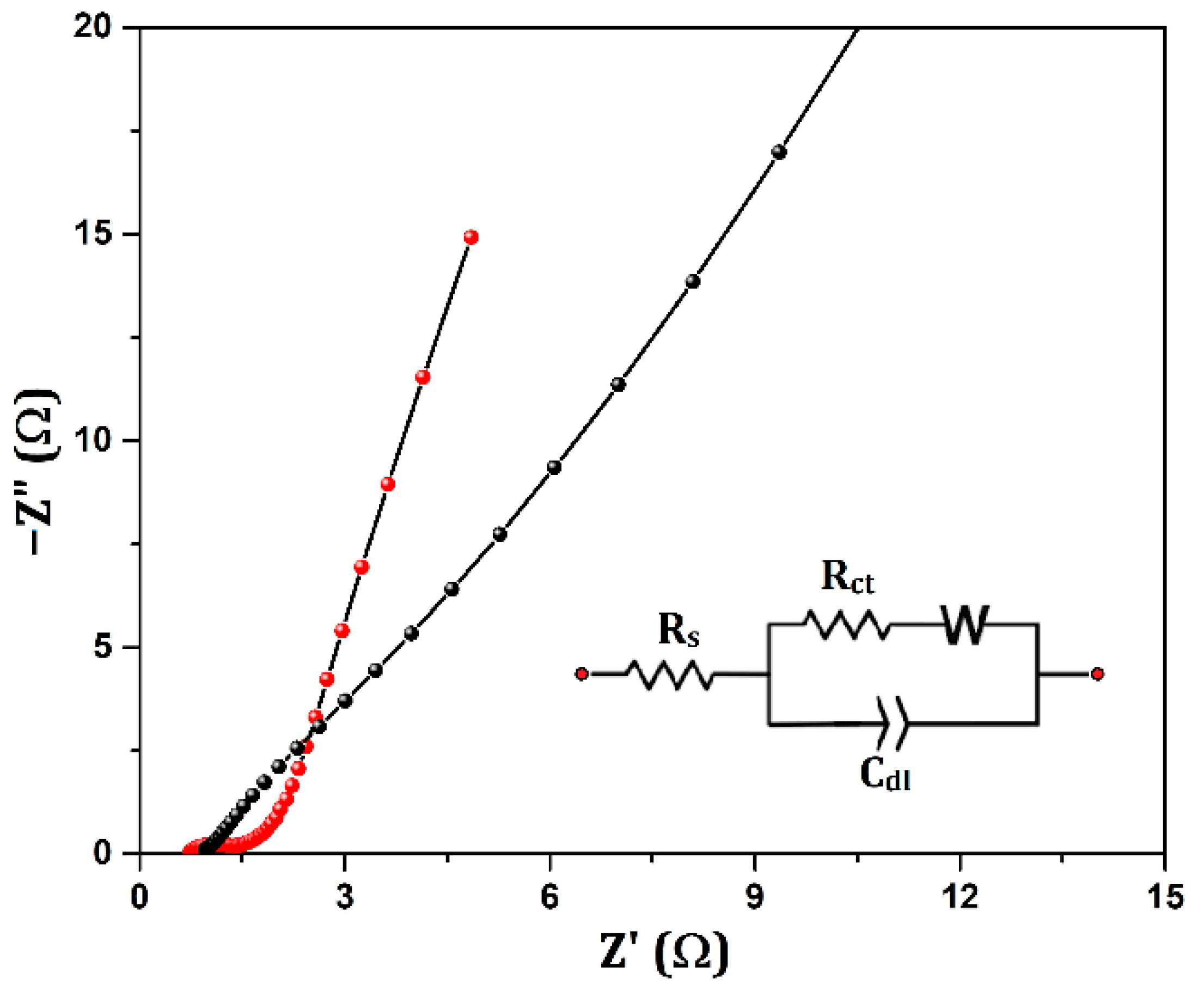

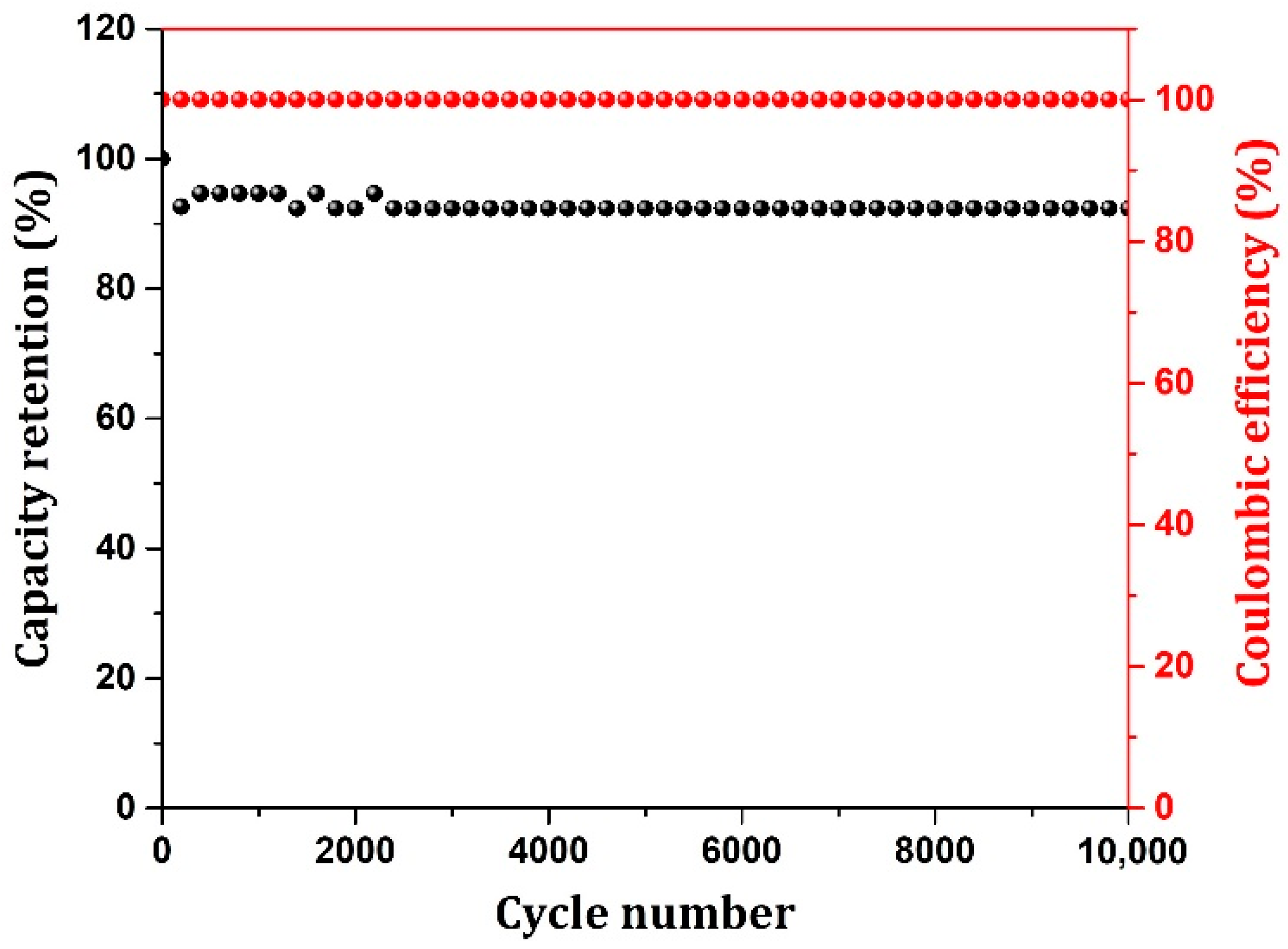
| System | Capacitance Calculation Method | Capacitance (F/g)/ Capacity(C/g) | Electrolyte | Reference |
|---|---|---|---|---|
| NiO aerogel | CV | 797 (10 mV/s) | 6M KOH | [45] |
| Carbon aerogel/Ni | GCD | 181 (1 mA/cm2) | 6M KOH | [46] |
| Ni foam/Graphene aerogel | GCD | 366 (2 A/g) | 6M KOH | [47] |
| Nano Ni-doped carbon aerogel | GCD | 110 (1 A/g) | 6M KOH | [48] |
| rGO and Ni(OH)2 | GCD | 561 (2 A/g) | 1M KOH | [49] |
| NiO/Graphene aerogel | GCD | 587 (1 A/g) | 6M KOH | [50] |
| NiO NPs | CV | 549 (1 mV/s) | 1M KOH | [51] |
| NiO NPs/CC | CV | 132 (5 mV/s) | 1M KOH | [52] |
| NiFeP@NiCo2S4 | GCD | 1602/720 (10 A/g) | 2M KOH | [53] |
| Ni-ZnS | CV | 191/131 (10 mV/s) | 2M KOH | [54] |
| NiO/Ni/NF | GCD | 1060/422 (1 A/g) | 3M KOH | This Study |
| System | Capacitance (F/g) | Capacity Retention (Number of Cycles) | Reference |
|---|---|---|---|
| MnO2 aerogel | 139 @ 1 A/g | 93% (5000) | [55] |
| NiS/NiO | 91 @ 1 A/g | 93% (30,000) | [56] |
| Graphene aerogel/CeO2 | 156 @ 1 A/g | 91% (4000) | [57] |
| rGO/RuO2 aerogels | 310 @ 1 A/g | 83% (5000) | [58] |
| Cobalt sulfide aerogel | 72 @ 1 A/g | - | [59] |
| NiO/Ni/NF | 169 @ 1 A/g | 92% (10,000) | This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramkumar, R.; Dhakal, G.; Shim, J.-J.; Kim, W.K. NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors. Nanomaterials 2022, 12, 3813. https://doi.org/10.3390/nano12213813
Ramkumar R, Dhakal G, Shim J-J, Kim WK. NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors. Nanomaterials. 2022; 12(21):3813. https://doi.org/10.3390/nano12213813
Chicago/Turabian StyleRamkumar, Ramya, Ganesh Dhakal, Jae-Jin Shim, and Woo Kyoung Kim. 2022. "NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors" Nanomaterials 12, no. 21: 3813. https://doi.org/10.3390/nano12213813
APA StyleRamkumar, R., Dhakal, G., Shim, J.-J., & Kim, W. K. (2022). NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors. Nanomaterials, 12(21), 3813. https://doi.org/10.3390/nano12213813









