Photocatalytic Investigation of Aerosol-Assisted Atmospheric Pressure Plasma Deposited Hybrid TiO2 Containing Nanocomposite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Deposition and Treatment of Coatings
2.3. Characterization of the Coatings
2.4. Photocatalytic Activity Evaluation
3. Results and Discussion
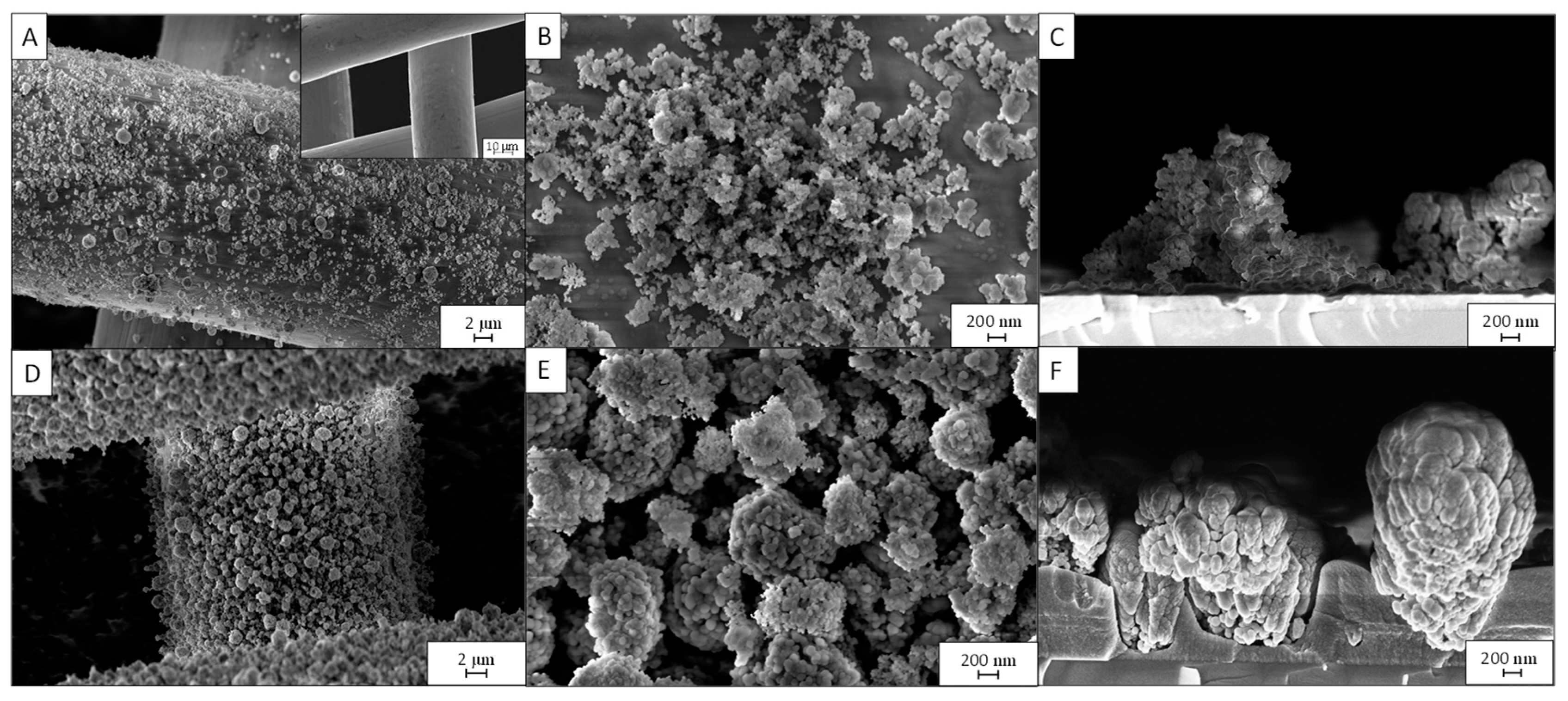
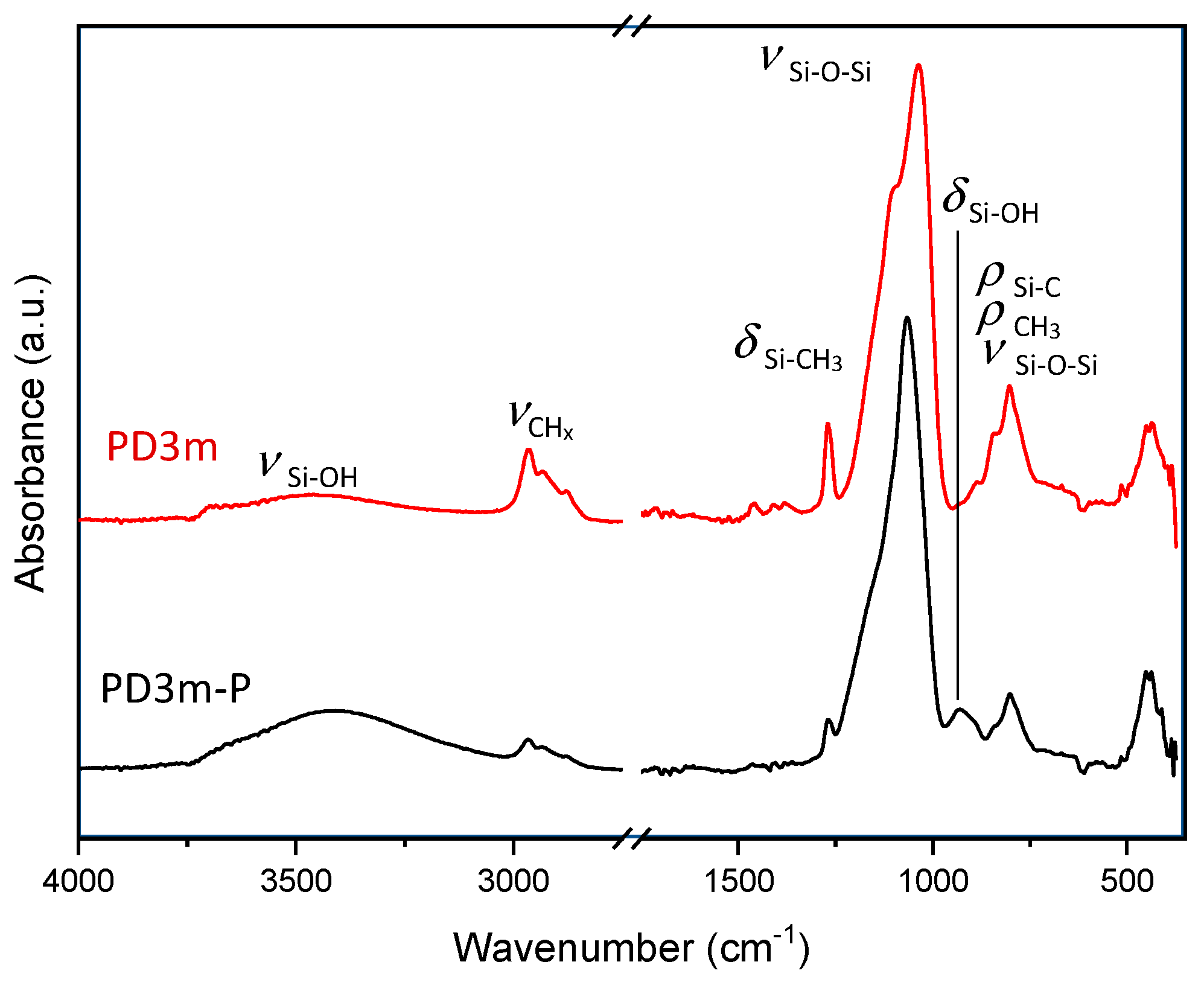
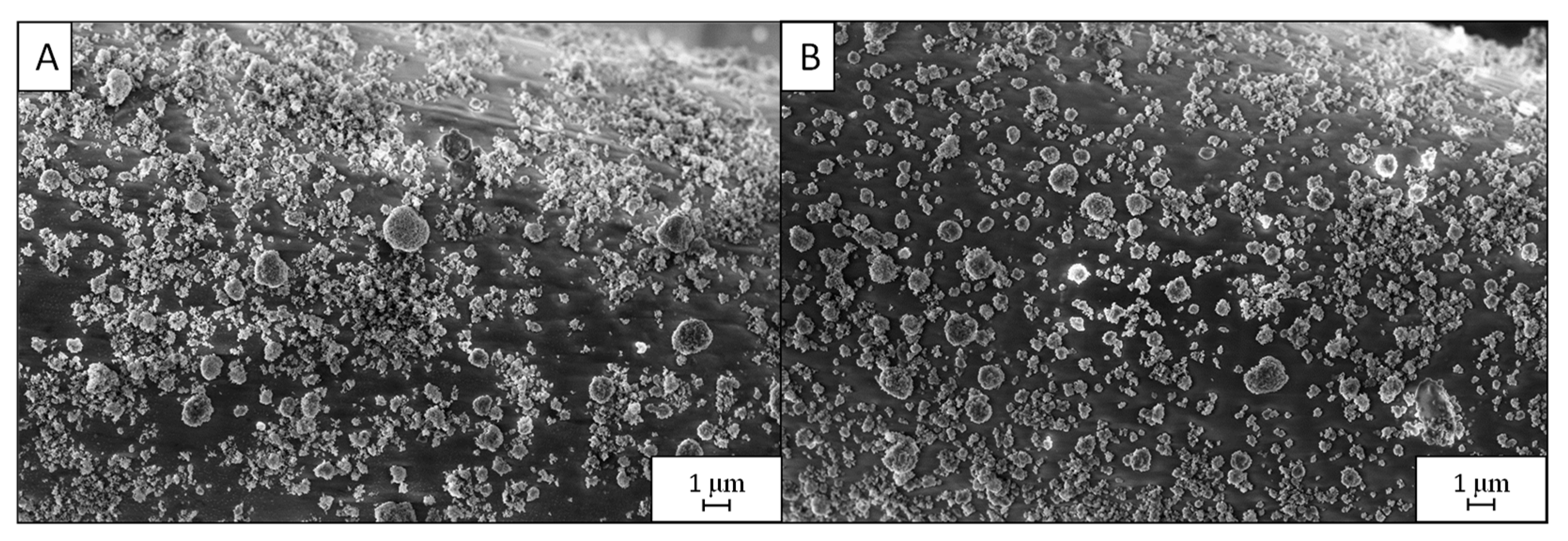
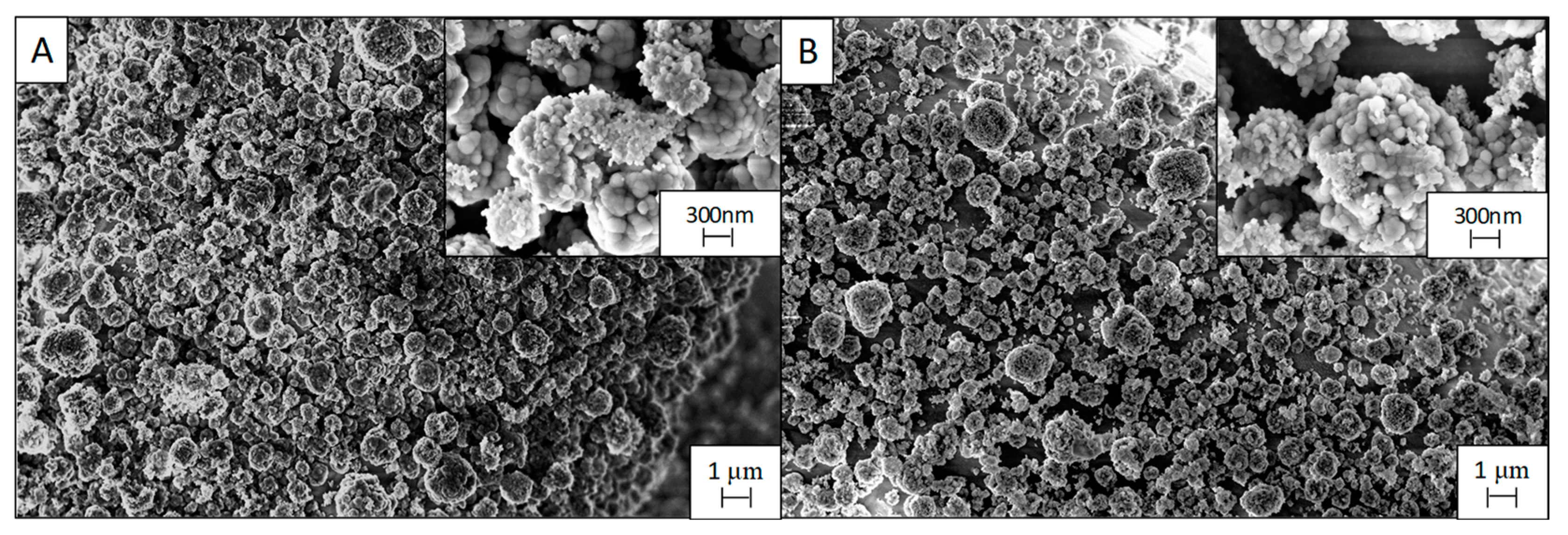
3.1. Effect of the Deposition Time
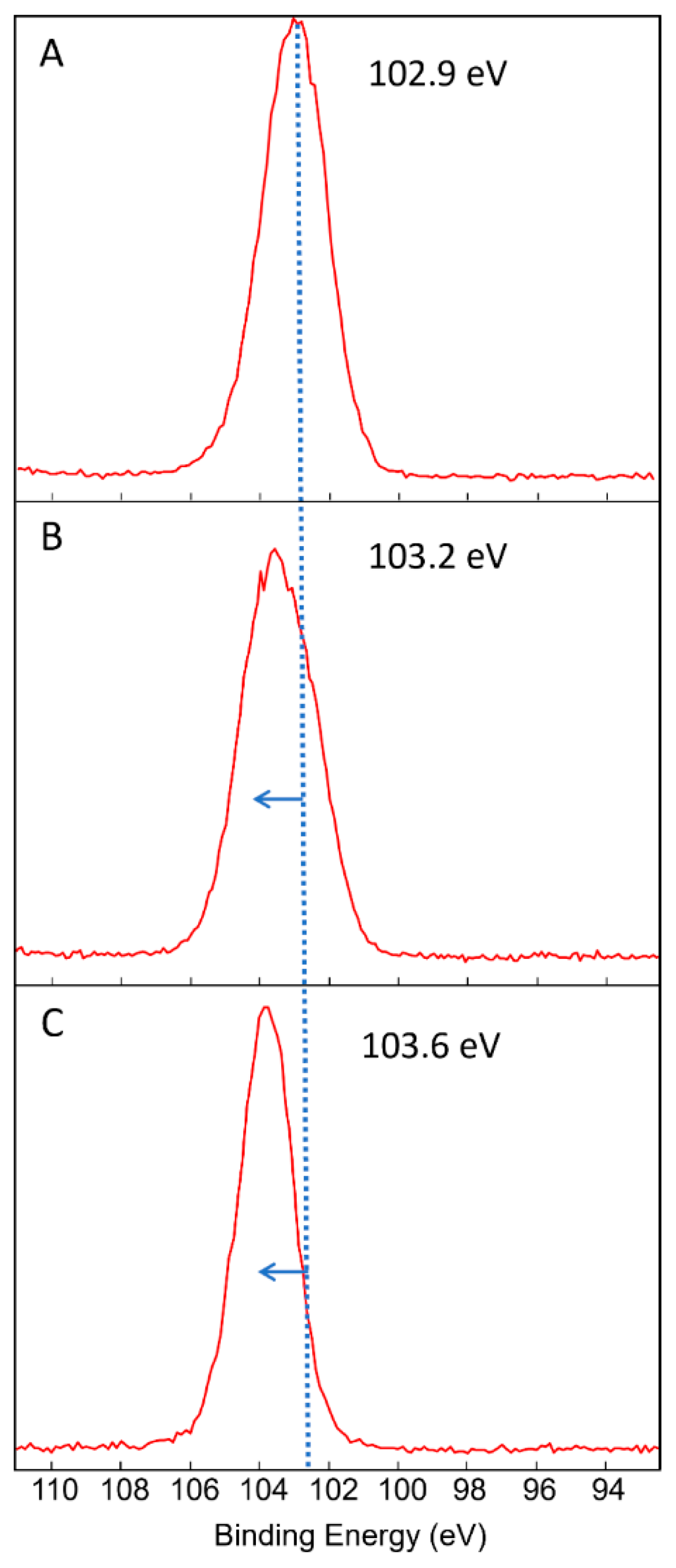
3.2. Effect of the Postdeposition Plasma Treatment
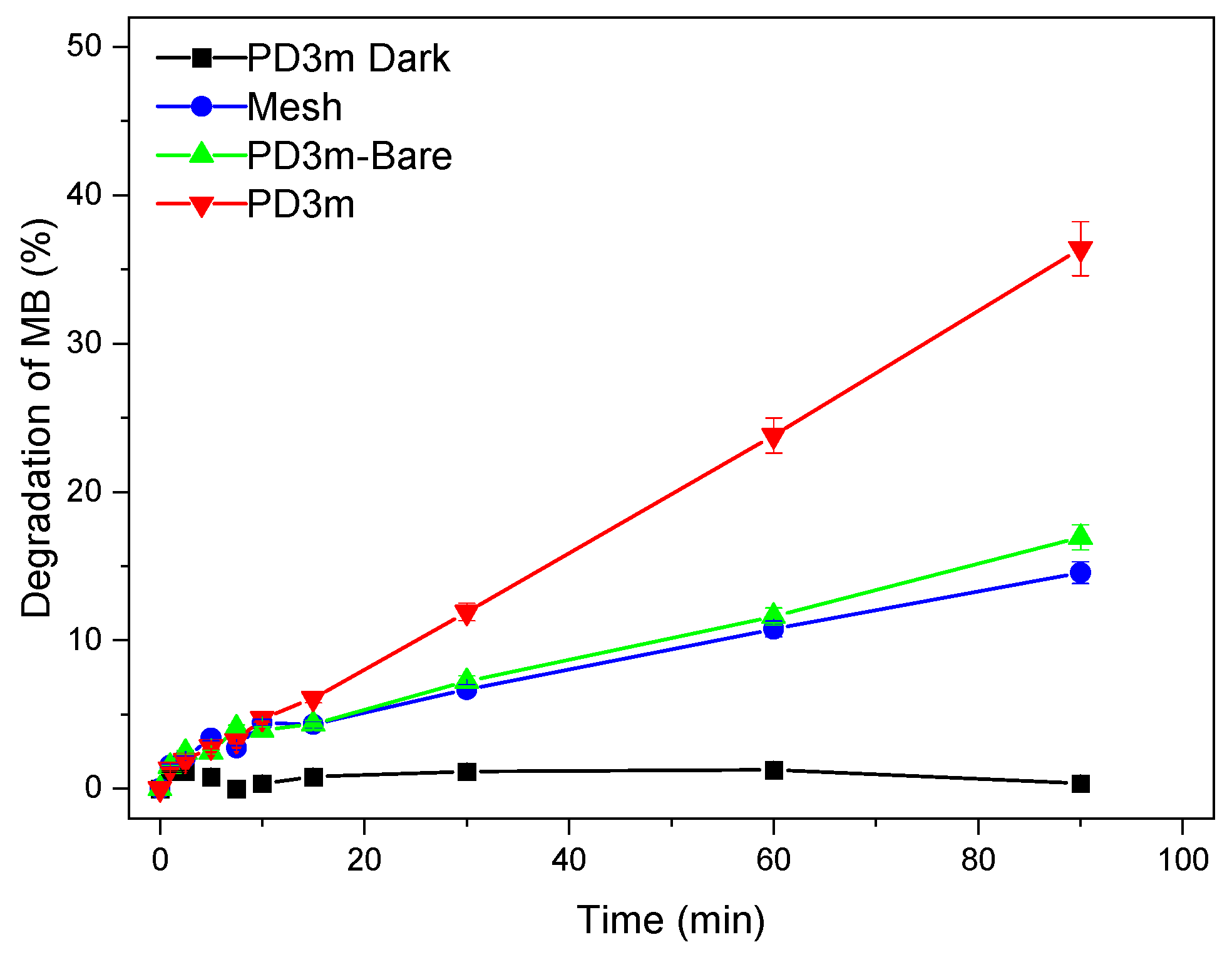
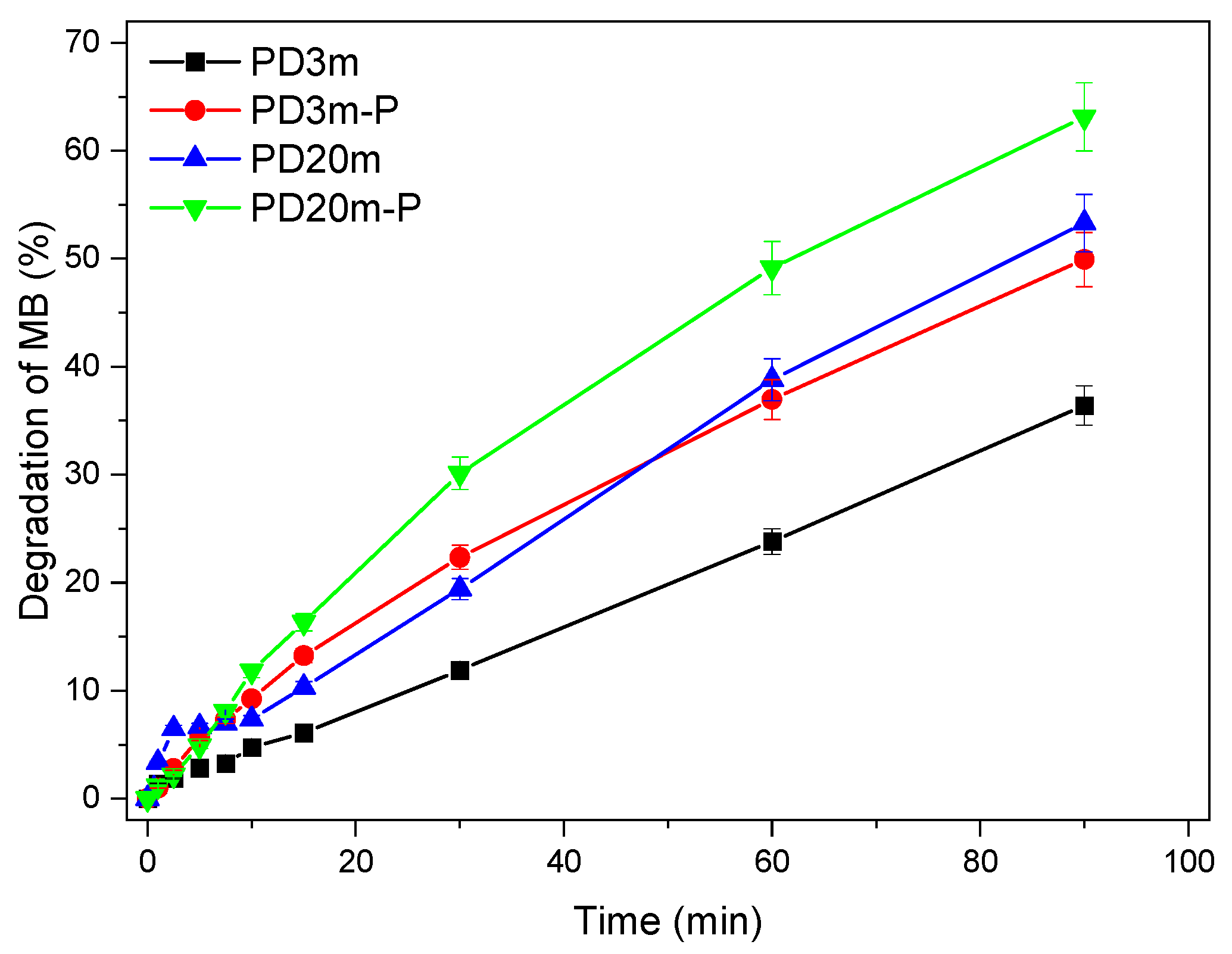
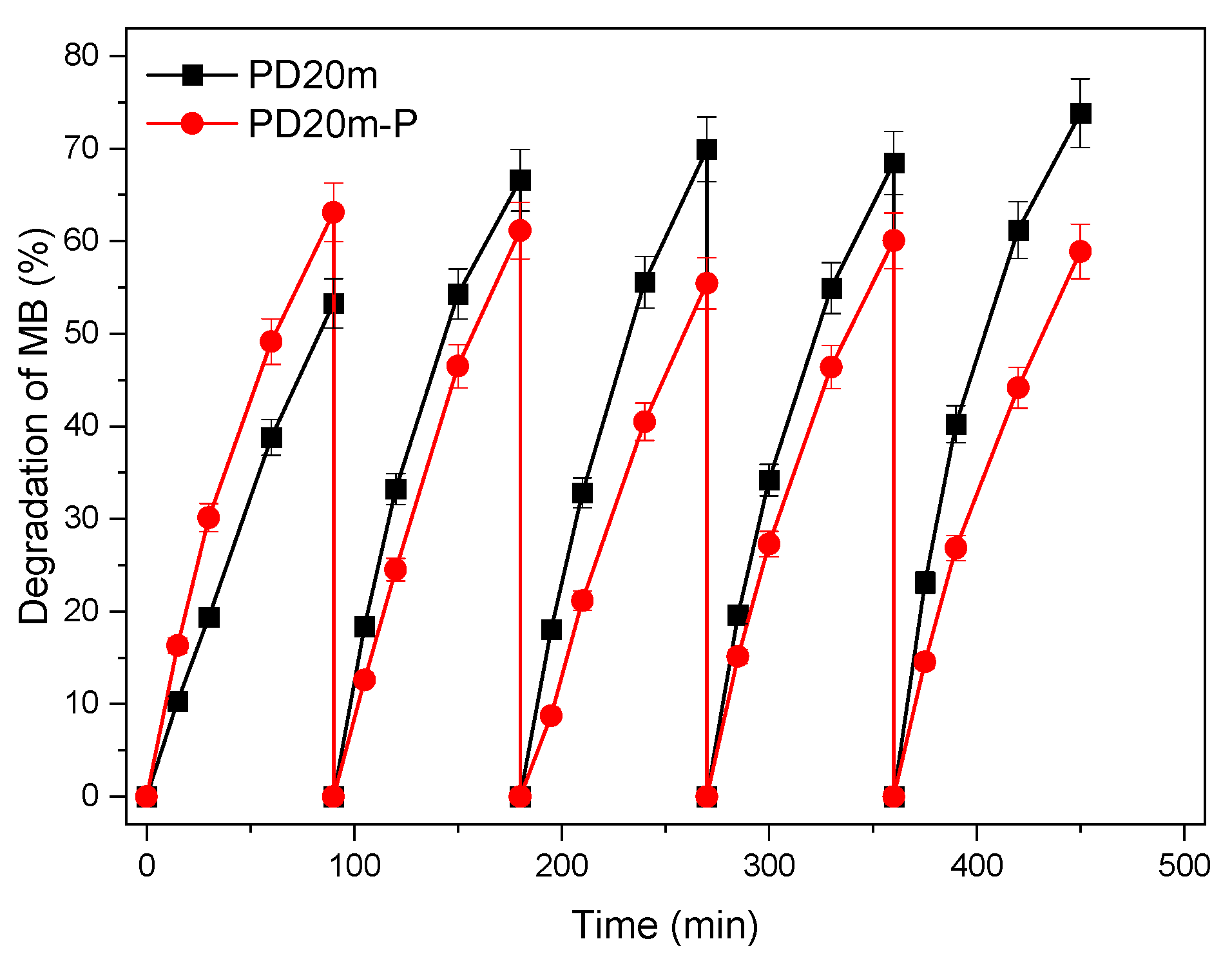
3.3. Photocatalytic Activity of the Nanocomposite Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present Applications of Titanium Dioxide for the Photocatalytic Removal of Pollutants from Water: A Review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic Remediation of Persistent Organic Pollutants (POPs): A Review. Arab. J. Chem. 2020, 13, 8309–8337. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar Photocatalytic Degradation of Pesticides over TiO2-RGO Nanocomposites at Pilot Plant Scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Removal of Organic Pollutants from Water by Fe2O3/TiO2 Based Photocatalytic Degradation: A Review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2-Based Photocatalysts: A Review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced Oxidation Processes for Water Disinfection: Features, Mechanisms and Prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical Development and Prospects of Photocatalysts for Pollutant Removal in Water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.-H. A Review of Photochemical Approaches for the Treatment of a Wide Range of Pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2022/63 of 14 January 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2022.011.01.0001.01.ENG (accessed on 25 October 2022).
- Abdelbasir, S.M.; Shalan, A.E. An Overview of Nanomaterials for Industrial Wastewater Treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-Scale Synthesis of UV–Vis Light Active Plasmonic Photocatalytic Nanocomposite Based on TiO2/Au Nanorods for Degradation of Pollutants in Water. Appl. Catal. B Environ. 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic Membrane Reactor Performance towards Oxytetracycline Removal from Synthetic and Real Matrices: Suspended vs. Immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Nicosia, A.; Vento, F.; Di Mari, G.M.; D’Urso, L.; Mineo, P.G. TiO2-Based Nanocomposites Thin Film Having Boosted Photocatalytic Activity for Xenobiotics Water Pollution Remediation. Nanomaterials 2021, 11, 400. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Wang, Y.; Wu, Z. 2D Graphene-TiO2 Composite and Its Photocatalytic Application in Water Pollutants. Front. Energy Res. 2021, 8, 612512. [Google Scholar] [CrossRef]
- Xiao, L.; Youji, L.; Feitai, C.; Peng, X.; Ming, L. Facile Synthesis of Mesoporous Titanium Dioxide Doped by Ag-Coated Graphene with Enhanced Visible-Light Photocatalytic Performance for Methylene Blue Degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Lo Porto, C.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-Based Coatings for Environmental Applications. Catal. Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Wang, D.; Mueses, M.A.; Márquez, J.A.C.; Machuca-Martínez, F.; Grčić, I.; Moreira, R.P.M.; Li Puma, G. Engineering and Modeling Perspectives on Photocatalytic Reactors for Water Treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic Reactors with Suspended and Immobilized TiO2: Comparative Efficiency Evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of Heterogeneous Photocatalytic Degradation of Reactive Dyes in an Immobilized TiO2 Photocatalytic Reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite Materials for Photocatalytic Degradation of Pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Palumbo, F.; Lo Porto, C.; Fracassi, F.; Favia, P. Recent Advancements in the Use of Aerosol-Assisted Atmospheric Pressure Plasma Deposition. Coatings 2020, 10, 440. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Aerosol-Assisted Atmospheric Pressure Cold Plasma Deposition of Organic–Inorganic Nanocomposite Coatings. Plasma Chem. Plasma Process. 2014, 34, 473–487. [Google Scholar] [CrossRef]
- Brunet, P.; Rincón, R.; Margot, J.; Massines, F.; Chaker, M. Deposition of Homogeneous Carbon-TiO2 Composites by Atmospheric Pressure DBD. Plasma Process. Polym. 2017, 14, 1600075. [Google Scholar] [CrossRef]
- Brunet, P.; Rincón, R.; Martinez, J.-M.; Matouk, Z.; Fanelli, F.; Chaker, M.; Massines, F. Control of Composite Thin Film Made in an Ar/Isopropanol/TiO2 Nanoparticles Dielectric Barrier Discharge by the Excitation Frequency. Plasma Process. Polym. 2017, 14, 1700049. [Google Scholar] [CrossRef]
- Dembele, A.; Rahman, M.; Reid, I.; Twomey, B.; MacElroy, J.M.D.; Dowling, D.P. Deposition of Hybrid Organic-Inorganic Composite Coatings Using an Atmospheric Plasma Jet System. J Nanosci. Nanotechnol. 2021, 11, 8730–8737. [Google Scholar] [CrossRef]
- Mitronika, M.; Profili, J.; Goullet, A.; Gautier, N.; Stephant, N.; Stafford, L.; Granier, A.; Richard-Plouet, M. TiO2–SiO2 Nanocomposite Thin Films Deposited by Direct Liquid Injection of Colloidal Solution in an O2/HMDSO Low-Pressure Plasma. J. Phys. Appl. Phys. 2020, 54, 085206. [Google Scholar] [CrossRef]
- Uricchio, A.; Nadal, E.; Plujat, B.; Plantard, G.; Massines, F.; Fanelli, F. Low-Temperature Atmospheric Pressure Plasma Deposition of TiO2-Based Nanocomposite Coatings on Open-Cell Polymer Foams for Photocatalytic Water Treatment. Appl. Surf. Sci. 2021, 561, 150014. [Google Scholar] [CrossRef]
- Lo Porto, C.; Palumbo, F.; Buxadera-Palomero, J.; Canal, C.; Jelinek, P.; Zajickova, L.; Favia, P. On the Plasma Deposition of Vancomycin-Containing Nano-Capsules for Drug-Delivery Applications. Plasma Process. Polym. 2018, 15, 1700232. [Google Scholar] [CrossRef]
- Lo Porto, C.; Palumbo, F.; Palazzo, G.; Favia, P. Direct Plasma Synthesis of Nano-Capsules Loaded with Antibiotics. Polym. Chem. 2017, 8, 1746–1749. [Google Scholar] [CrossRef]
- Lo Porto, C.; Palumbo, F.; Somma, S.; Masiello, M.; Moretti, A.; Fracassi, F.; Favia, P. Plasma-Assisted Deposition of Fungicide Containing Coatings for Encapsulation and Protection of Maize Seeds. Plasma Process. Polym. 2019, 16, 1900022. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.-H.; Monteiro, P.J.M.; Yu, L.E. Characterization of Photocatalytic TiO2 Powder under Varied Environments Using near Ambient Pressure X-Ray Photoelectron Spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Gao, J.; Wang, Y. Synthesis of Highly Active H2O2-Sensitized Sulfated Titania Nanoparticles with a Response to Visible Light. J. Photochem. Photobiol. Chem. 2009, 202, 128–135. [Google Scholar] [CrossRef]
- Creatore, M.; Palumbo, F.; d’Agostino, R. Deposition of SiOx Films from Hexamethyldisiloxane/Oxygen Radiofrequency Glow Discharges: Process Optimization by Plasma Diagnostics. Plasmas Polym. 2002, 7, 291–310. [Google Scholar] [CrossRef]


| Sample | Deposition Time (min) | Aerosol Composition | Post Deposition O2 Plasma Treatment |
|---|---|---|---|
| PD3M | 3 | TiO2 (10 mg/mL) HMDSO/IPA (10/90 v/v) | No |
| PD20M | 20 | TiO2 (10 mg/mL) HMDSO/IPA (10/90 v/v) | No |
| PD3M-P | 3 | TiO2 (10 mg/mL) HMDSO/IPA (10/90 v/v) | Yes |
| PD20M-P | 20 | TiO2 (10 mg/mL) HMDSO/IPA (10/90 v/v) | Yes |
| PD3M-BARE | 3 | HMDSO/IPA (10/90 v/v) | No |
| Elements | PD3m (%) | PD20m (%) | PD3m-P (%) |
|---|---|---|---|
| C | 53 ± 3 | 8.8 ± 0.4 | 8.0 ± 0.4 |
| O | 29 ± 1 | 63 ± 3 | 67 ± 3 |
| Si | 17.3 ± 0.9 | 27 ± 1 | 24 ± 1 |
| Ti | 0.4 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 |
| Sample | Ti/Fe | Ti/Si |
|---|---|---|
| PD3m | 0.10 ± 0.03 | 0.21 ± 0.02 |
| PD3m-P | 0.09 ± 0.03 | 0.49 ± 0.03 |
| Sample | Ti/Fe | Ti/Si |
|---|---|---|
| PD3m before | 0.10 ± 0.03 | 0.21 ± 0.02 |
| PD3m after | 0.07 ± 0.03 | 0.18 ± 0.03 |
| Sample | k (min−1) | R2 | Degradation after 90 min |
|---|---|---|---|
| PD3m | 0.0039 | 0.98 | 36±2% |
| PD20m | 0.0087 | 0.98 | 53±3% |
| PD3m-P | 0.0094 | 0.99 | 50±2% |
| PD20m-P | 0.0122 | 0.99 | 63±3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Porto, C.; Dell’Edera, M.; De Pasquale, I.; Milella, A.; Fracassi, F.; Curri, M.L.; Comparelli, R.; Palumbo, F. Photocatalytic Investigation of Aerosol-Assisted Atmospheric Pressure Plasma Deposited Hybrid TiO2 Containing Nanocomposite Coatings. Nanomaterials 2022, 12, 3758. https://doi.org/10.3390/nano12213758
Lo Porto C, Dell’Edera M, De Pasquale I, Milella A, Fracassi F, Curri ML, Comparelli R, Palumbo F. Photocatalytic Investigation of Aerosol-Assisted Atmospheric Pressure Plasma Deposited Hybrid TiO2 Containing Nanocomposite Coatings. Nanomaterials. 2022; 12(21):3758. https://doi.org/10.3390/nano12213758
Chicago/Turabian StyleLo Porto, Chiara, Massimo Dell’Edera, Ilaria De Pasquale, Antonella Milella, Francesco Fracassi, Maria Lucia Curri, Roberto Comparelli, and Fabio Palumbo. 2022. "Photocatalytic Investigation of Aerosol-Assisted Atmospheric Pressure Plasma Deposited Hybrid TiO2 Containing Nanocomposite Coatings" Nanomaterials 12, no. 21: 3758. https://doi.org/10.3390/nano12213758
APA StyleLo Porto, C., Dell’Edera, M., De Pasquale, I., Milella, A., Fracassi, F., Curri, M. L., Comparelli, R., & Palumbo, F. (2022). Photocatalytic Investigation of Aerosol-Assisted Atmospheric Pressure Plasma Deposited Hybrid TiO2 Containing Nanocomposite Coatings. Nanomaterials, 12(21), 3758. https://doi.org/10.3390/nano12213758












