Progress in Studies of Surface Nanotextures and Coatings with Nanomaterials on Glass for Anti-Dust Functionality
Abstract
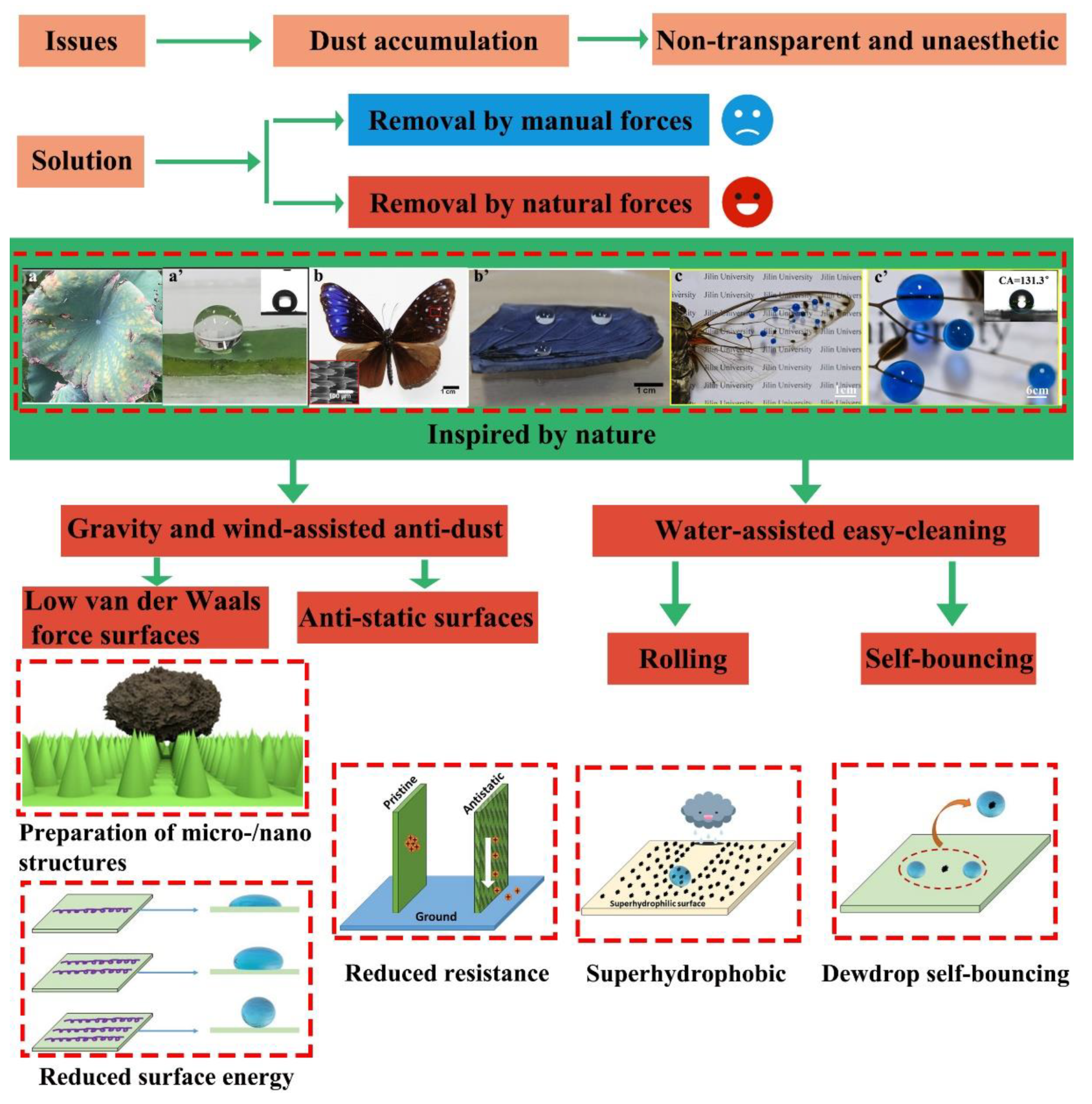
1. Introduction
- The adhesion mechanism of dust particles on glass surface
- An overview of the various properties of dust particles, including composition, particle size, electrostatic charges, and the natural settling and adhesion processes of dust particles.
- Processes to create easy-cleaning surfaces under natural forces, including low van der Waals force surfaces, anti-static surfaces, and superhydrophobic surfaces.
- An analysis of the pros and cons of various dust prevention strategies, which include creating surface micro-/nanotextures, dust-repelling nanocoatings, and nanomaterials with anti-static properties.
- Future prospects in anti-dust research.
2. Adhesion Mechanism
2.1. Van der Waals Forces
2.2. Electrostatic Attraction
2.3. Capillary Forces
3. Properties of Dust Particles
4. Methods for Creating Anti-Dust and Easy-Cleaning Surfaces
4.1. Gravity- and Wind-Assisted Anti-Dust Surface
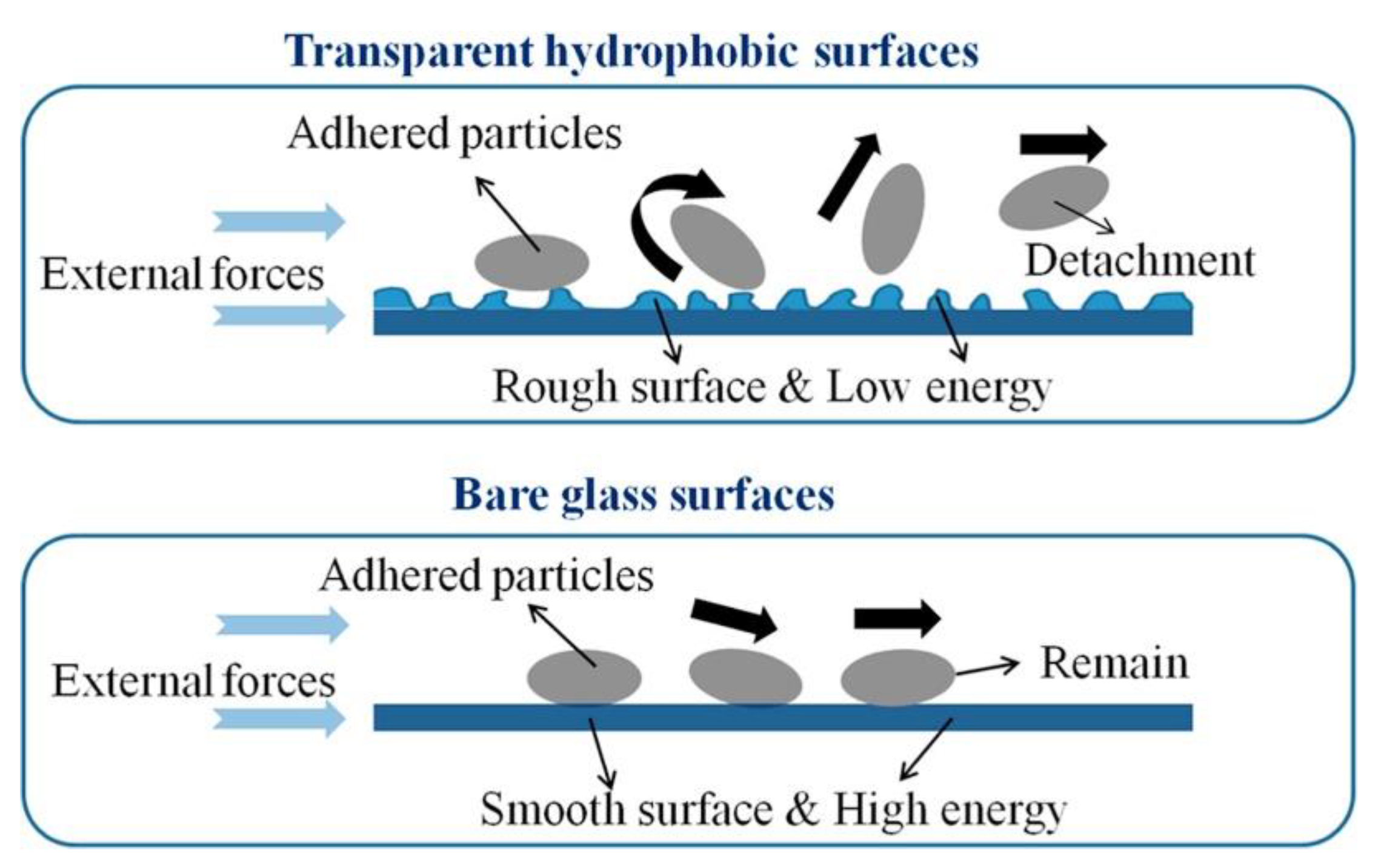
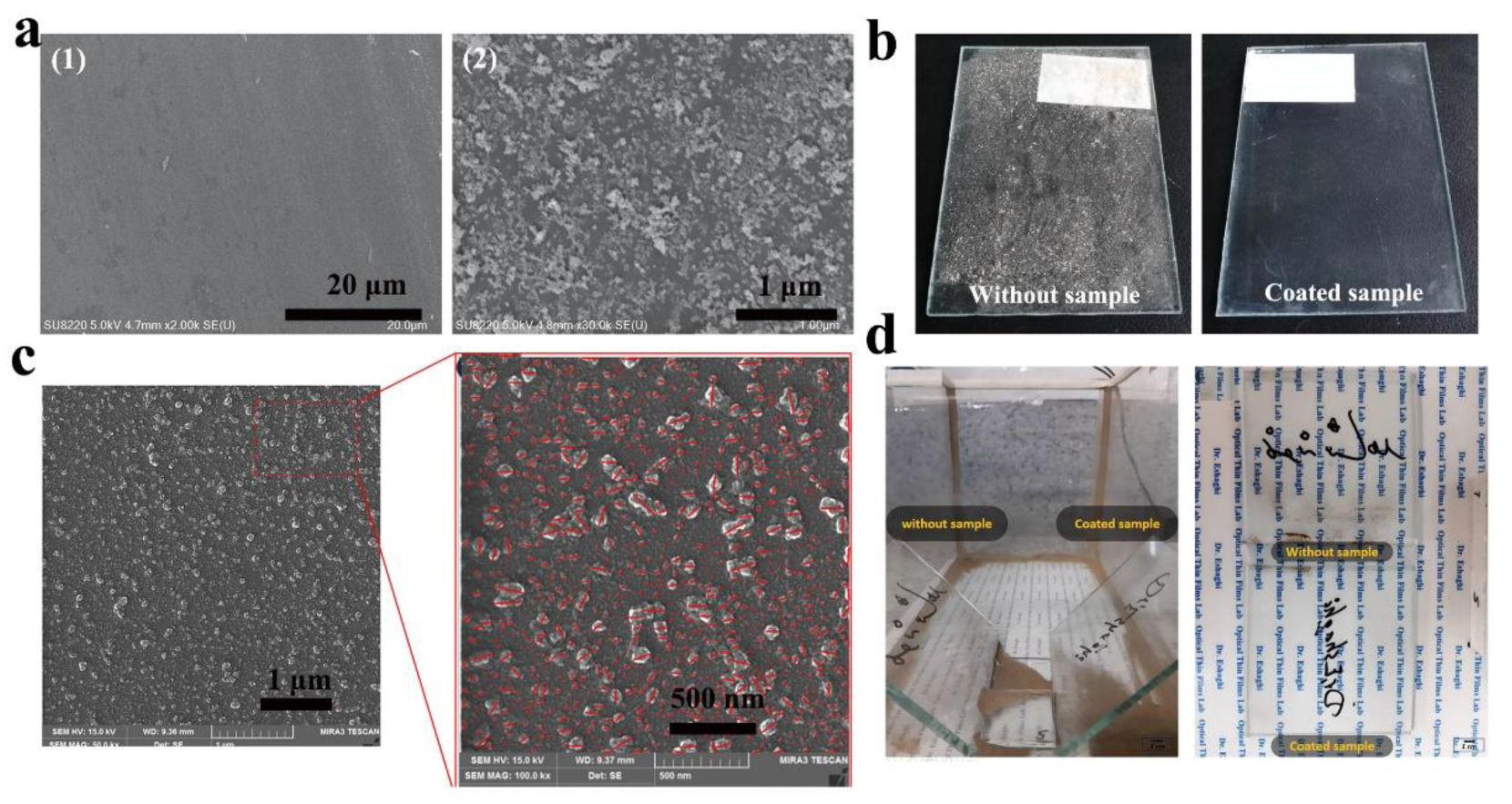
4.1.1. Low van der Waals Force Surfaces
Surfaces with Micro- and Nanostructures
Low Surface Energy Surface
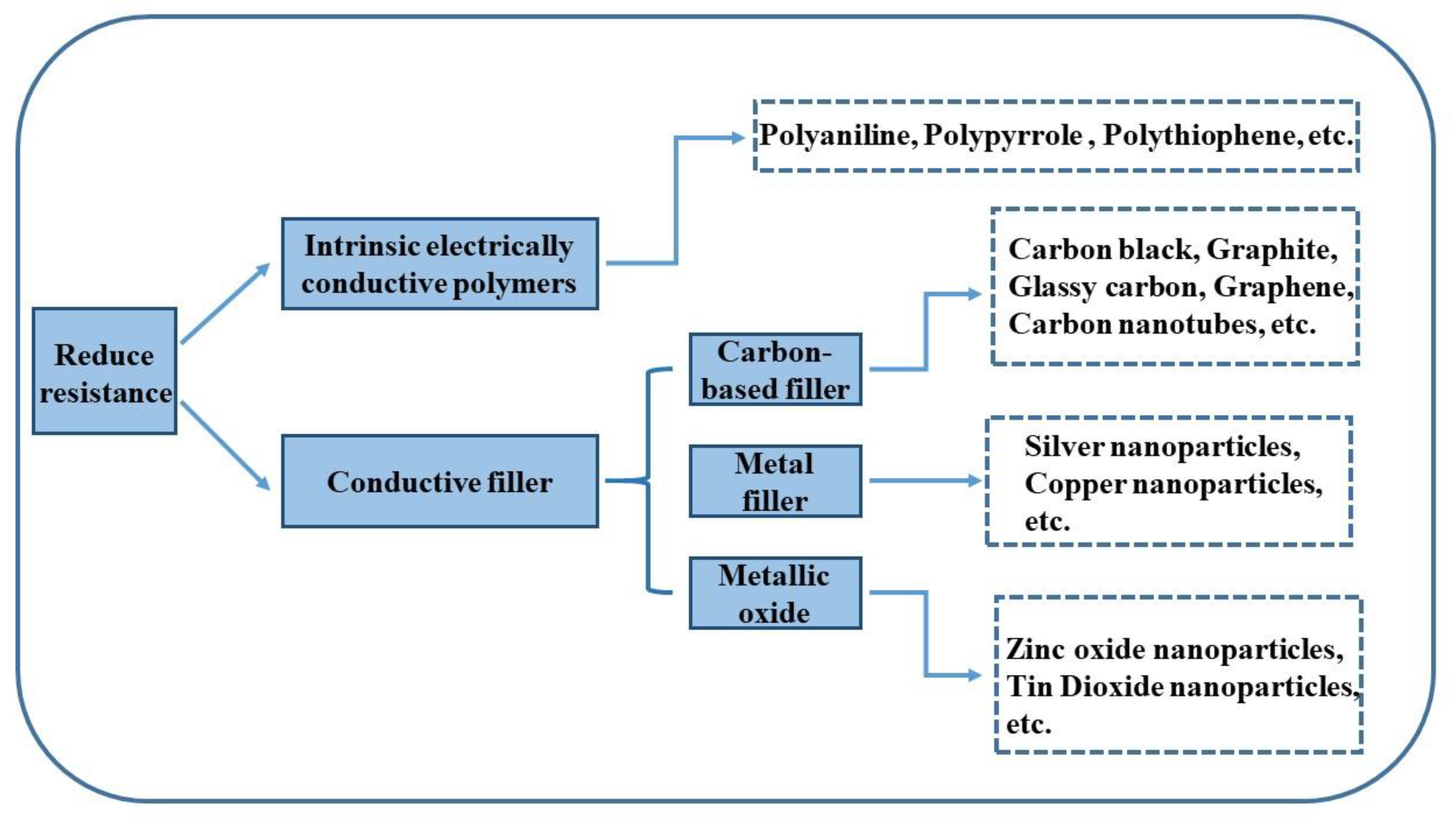
4.1.2. Low Electrical Resistance Surface
4.2. Water-Assisted Easy-Cleaning Surface
4.2.1. Dust Removal Using the Motion of Water Droplets
4.2.2. Dust Removal Using Dewdrop’s Self-Bounce
5. Summary and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sutha, S.; Suresh, S.; Raj, B.; Ravi, K. Transparent alumina based superhydrophobic self–cleaning coatings for solar cell cover glass applications. Sol. Energy Mater. Sol. Cells 2017, 165, 128–137. [Google Scholar] [CrossRef]
- Bergin, M.H.; Ghoroi, C.; Dixit, D.; Schauer, J.J.; Shindell, D. Large Reductions in Solar Energy Production Due to Dust and Particulate Air Pollution. Environ. Sci. Technol. Lett. 2017, 4, 339–344. [Google Scholar] [CrossRef]
- Taheri, F.; Forouzani, M.; Yazdanpanah, M.; Ajili, A. How farmers perceive the impact of dust phenomenon on agricultural production activities: A Q-methodology study. J. Arid Environ. 2020, 173, 104028. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Wu, M.; Hong, S.; Wang, P. Photovoltaic panel cooling by atmospheric water sorption–evaporation cycle. Nat. Sustain. 2020, 3, 636–643. [Google Scholar] [CrossRef]
- Lu, H.; Cai, R.; Zhang, L.Z.; Lu, L.; Zhang, L. Experimental investigation on deposition reduction of different types of dust on solar PV cells by self-cleaning coatings. Sol. Energy 2020, 206, 365–373. [Google Scholar] [CrossRef]
- Li, M. Study on Mechanism of Adhesion and Removal of Solid Microparticles and Surface Cleaning Technology. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Shehri, A.A.; Parrott, B.; Carrasco, P.; Saiari, H.A.; Taie, I. Impact of dust deposition and brush-based dry cleaning on glass transmittance for PV modules applications. Sol. Energy 2016, 135, 317–324. [Google Scholar] [CrossRef]
- Kim, M.; Choi, P.; Jo, J.H.; Kim, K. Glass Substrate Dust Removal Using 233 fs Laser-Generated Shockwave. Micromachines 2021, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, V.S.; Darvekar, S.K. Solar Photovoltaic Panels Cleaning Methods A Review. Int. J. Pure Appl. Math. 2018, 118, 1–17. [Google Scholar]
- Marmur, A. The Lotus Effect: Superhydrophobicity and Metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef]
- Wu, W.; Liang, R.; Lu, L.; Wang, W.; Ran, X.; Yue, D. Preparation of superhydrophobic laser-induced graphene using taro leaf structure as templates. Surf. Coat. Technol. 2020, 393, 125744. [Google Scholar] [CrossRef]
- Du, T.; Ma, S.; Pei, X.; Wang, X.; Zhou, F. Bio-Inspired Design and Fabrication of Micro/Nano-Brush Dual Structural Surfaces for Switchable Oil Adhesion and Antifouling. Small 2017, 13, 1602020. [Google Scholar] [CrossRef] [PubMed]
- Hansen, W.R.; Autumn, K. Evidence for self-cleaning in gecko setae. Proc. Natl. Acad. Sci. USA 2005, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Z.; Liu, W. Biomimetic Multi-functional Superamphiphobic FOTS-TiO2 Particles beyond Lotus Leaf. ACS Appl. Mater. Interfaces 2016, 8, 27188–27198. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ge, L.; Ci, L.; Ajayan, P.M.; Dhinojwala, A. Gecko-inspired carbon nanotube-based self-cleaning adhesives. Nano Lett. 2008, 8, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Ekhlas, H.; Anna, Z.; Ludmila, A.; Ofer, M. Signatures of Van Der Waals and Electrostatic Forces in the Deposition of Nanoparticle Assemblies. J. Phys. Chem. Lett. 2018, 9, 5226–5232. [Google Scholar]
- Klauser, W.; Bartenwerfer, M.; Fatikow, S. Measurement of sub-nanonewton forces inside a scanning electron microscope. Rev. Sci. Instrum. 2020, 91, 43701. [Google Scholar] [CrossRef]
- Hassan, G.; Yilbas, B.S.; Al-Qahtani, H. Droplet fluid infusion into a dust layer in relation to self-cleaning. RSC Adv. 2020, 10, 32034–32042. [Google Scholar] [CrossRef] [PubMed]
- Yilbas, B.S.; Yousaf, M.R.; Al-Sharafi, A.; Ali, H.; Al-Sulaiman, F.; Abu-Dheir, N.; Khaled, M.; Al-Aqeeli, N. Silicone oil impregnated nano silica modified glass surface and influence of environmental dust particles on optical transmittance. RSC Adv. 2017, 7, 29762–29771. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S. Study on preparation and performance of dust-proof coatings for solar cells of mars rover. J. Fudan Univ. 2019, 58, 740–746. [Google Scholar]
- Chang, H.; Li, X.; Zhang, J.; Lv, N. Preparation and characteristics of glass dust-proof coating. Sci. Technol. Inf. 2008, 13, 14. [Google Scholar]
- Lee, Y.; You, E.A.; Ha, Y. Transparent, self-cleaning and waterproof surfaces with tunable micro/nano dual-scale structures. Nanotechnology 2016, 27, 355701. [Google Scholar] [CrossRef]
- Fenero, M.; Palenzuela, J.; Azpitarte, I.; Knez, M.; Rodriguez, J.; Tena-Zaera, R. Laponite-Based Surfaces with Holistic Self-Cleaning Functionality by Combining Antistatics and Omniphobicity. ACS Appl. Mater. Interfaces 2017, 9, 39078–39085. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sano, T.; Ito, H. Antistatic properties of transparent plastics using a donor-accepter molecular compound antistatic agent. J. Polym. Eng. 2018, 38, 555–561. [Google Scholar] [CrossRef]
- Chen, S.; Wu, D.; Xu, C.; Ma, M.; Shi, Y.; Yuan, K.; Xu, R.; Wang, X. The preparation and mechanism of permanently flame retardancy, antistatic, good toughness and high transparent poly(methyl methacrylate). Polym. Adv. Technol. 2020, 32, 1230–1238. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, K.; Jiao, Z.; Wu, M.; He, M.; Su, Y.; Jiang, Z. Constructing dual-defense mechanisms on membrane surfaces by synergy of PFSA and SiO2 nanoparticles for persistent antifouling performance. Appl. Surf. Sci. 2018, 440, 113–124. [Google Scholar] [CrossRef]
- Sidharam, P.P.; Luc, S.; Antonius, T.; Han, Z. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar]
- Król, B.E.; Król, P.; Byczyński, U.; Szalański, P. Methods of increasing hydrophobicity of polyurethane materials: Important applications of coatings with low surface free energy. Colloid. Polym. Sci. 2017, 295, 2309–2321. [Google Scholar] [CrossRef]
- Matin, A.; Merah, N.; Ibrahim, A. Superhydrophobic and self-cleaning surfaces prepared from a commercial silane using a single-step drop-coating method. Prog. Org. Coat. 2016, 99, 322–329. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Development of hybrid hydrophobic molybdenum disulfide (MoS2) nanoparticles for super water repellent self-cleaning. Prog. Org. Coat. 2021, 153, 106161. [Google Scholar] [CrossRef]
- Syafiq, A.; Pandey, A.K.; Adzman, N.N.; Rahim, A. Advances in approaches and methods for self-cleaning of solar photovoltaic panels. Sol. Energy 2018, 162, 597–619. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H. Self-Cleaning Performance of Super-Hydrophilic Coatings for Dust Deposition Reduction on Solar Photovoltaic Cells. Coatings 2021, 11, 1059. [Google Scholar] [CrossRef]
- Afshari, F.; Bafghi, Z.G.; Manavizadeh, N. Unsophisticated one-step synthesis super hydrophilic self-cleaning coating based on ZnO nanosheets. Appl. Phys. A 2022, 128, 75. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent Progress in Super Hydrophobic/Hydrophilic Self-Cleaning Surfaces for Various Industrial Applications: A Review. Polym. Plast. Technol. Mater. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Piliougine, M.; Canete, C.; Moreno, R.; Carretero, J.; Hirose, J.; Ogawa, S.; Sidrach-De-Cardona, M. Comparative analysis of energy produced by photovoltaic modules with anti-soiling coated surface in arid climates. Appl. Energy 2013, 112, 626–634. [Google Scholar] [CrossRef]
- Aïssa, A.H.; Puzenat, E.; Plassais Herrmann, J.M.; Haehnel, C.; Guillard, C. Characterization and photocatalytic performance in air of cementitious materials containing TiO2 Case study of formaldehyde removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Tang, X.; Rosseler, O.; Chen, S.; L’Aulnoit, S.; Destaillats, H. Self-cleaning and de-pollution efficacies of photocatalytic architectural membranes. Langmuir 2019, 35, 119260. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Z. Superhydrophobic Plant Leaves: The Variation in Surface Morphologies and Wettability during the Vegetation Period. Appl. Phys. A 2019, 125, 1047–1053. [Google Scholar]
- Li, P.; Zhang, B.; Zhao, H.; Zhang, L.; Wang, Z.; Xu, X.; Fu, T.; Wang, X.; Hou, Y.; Fan, Y. Unidirectional Droplet Transport on the Biofabricated Butterfly Wing. Langmuir 2018, 34, 12482–12487. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Z.; Li, B.; Feng, X.; Jiao, Z.; Zhang, J.; Zhao, J.; Niu, S.; Ren, L. Flexible Self-Cleaning Broadband Antireflective Film Inspired by the Transparent Cicada Wings. ACS Appl. Mater. Interfaces 2019, 11, 17019–17027. [Google Scholar] [CrossRef]
- Rifai, A.; Dheir, N.A.; Yilbas, B.S.; Khaled, M. Mechanics of dust removal from rotating disk in relation to self-cleaning applications of PV protective cover. Sol. Energy 2016, 130, 193–206. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Peukert, W. London-van der Waals adhesiveness of rough particles. Powder Technol. 2006, 161, 248–255. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Khaled, M.M.; Al-Aqeeli, N.; Abu-Dheir, N.; Varanasi, K.K. Influence of dust and mud on the optical, chemical, and mechanical properties of a pv protective glass. Sci. Rep. 2015, 5, 15833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lv, Y.; Zhou, Q.; Yan, W. Investigation on particle deposition criterion and dust accumulation impact on solar PV module performance. Energy 2021, 233, 121240. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Adler, J.J.; Ata, A.; Singh, R.K.; Moudgil, B.M. Adhesion between Nanoscale Rough Surfaces. J. Colloid Interface Sci. 2000, 232, 10–16. [Google Scholar] [CrossRef]
- Dove, A.; Devaud, G.; Xu, W.; Crowder, M.; Lawitzke, A.; Haley, C. Mitigation of lunar dust adhesion by surface modification. Planet. Space Sci. 2011, 59, 1784–1790. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, L. Experimental investigation of the anti-dust effect of transparent hydrophobic coatings applied for solar cell covering glass. Sol. Energy Mater. Sol. Cells 2017, 160, 382–389. [Google Scholar] [CrossRef]
- Kim, O.V.; Dunn, P.F. A microsphere-surface impact model for implementation in computational fluid dynamics. J. Aerosol Sci. 2007, 38, 532–549. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Dong, B. Research progress on adhesion mechanism and self-cleaning of dust on glass surface. Shandong Ceram. 2020, 43, 3–8. [Google Scholar]
- Naga, A.; Vollmer, D.; Butt, H.J. Capillary Torque on a Particle Rotating at an Interface. Langmuir 2021, 37, 7457–7463. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.A.S.; Tyrrell, J.W.G. The Influence of Relative Humidity on Particle Adhesion- a Review of Previous Work and the Anomalous Behaviour of Soda-lime Glass. KONA Powder Part J. 2004, 22, 9–22. [Google Scholar] [CrossRef]
- Abdelmagid, G.; Yilbas, B.S.; Al-Sharafi, A.; Al-Qahtani, H.; Al-Aqeeli, N. Water droplet on inclined dusty hydrophobic surface: Influence of droplet volume on environmental dust particles removal. RSC Adv. 2019, 9, 3582–3596. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, R.; Al-Othman, A.; Aidan, A.A.; Tawalbeh, M. A characterization study for the properties of dust particles collected on photovoltaic (PV) panels in Sharjah, United Arab Emirates. Renew. Energy 2021, 171, 133–140. [Google Scholar] [CrossRef]
- Wu, Z.; Yan, S.; Wang, Z.; Ming, T.; Zhao, X.; Ma, R.; Wu, Y. The effect of dust accumulation on the cleanliness factor of a parabolic trough solar concentrator. Renew. Energy 2020, 152, 529–539. [Google Scholar] [CrossRef]
- Al-Nassar, A.; Al-Nassar, W.; Al-Hemoud, A.; Alsaleh, A.; Ramadan, A.; Al-Dousari, N.; Ahmed, M. Solar and wind energy: Challenges and solutions in desert regions. Energy 2019, 176, 184–194. [Google Scholar]
- Gholami, A.; Khazaee, I.; Eslami, S.; Zandi, M.; Akrami, E. Experimental investigation of dust deposition effects on photo-voltaic output performance. Sol. Energy 2018, 159, 346–352. [Google Scholar] [CrossRef]
- Hachicha, A.A.; Ai-Sawafta, I.; Said, Z. Impact of dust on the performance of solar photovoltaic (PV) systems under United Arab Emirates weather conditions. Renew. Energy 2019, 141, 287–297. [Google Scholar] [CrossRef]
- Yoshifumi, S. A simple method of dust charge estimation using an externally applied oscillating electric field. Phys. Plasmas 2018, 25, 73701. [Google Scholar]
- Wiese, R.; Sushkov, V.; Kersten, H.; Ikkurthi, V.R.; Schneider, R.; Hippler, R. Behavior of a porous particle in a radiofrequency plasma under pulsed argon ion beam bombardment. New J. Phys. 2010, 12, 1062–1065. [Google Scholar] [CrossRef]
- Merrison, J.; Jensen, J.; Kinch, K.; Mugford, R.; Planetary, P. The electrical properties of Mars analogue dust. Planet. Space Sci. 2004, 52, 279–290. [Google Scholar] [CrossRef]
- Izvekova, Y.N.; Popel, S.I. Charged Dust Motion in Dust Devils on Earth and Mars. Contrib. Plasm. Phys. 2016, 56, 263–269. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, G.; Shen, M.; Liu, Y.; Lam, P. Characteristics of Indoor Dust in an Industrial City: Comparison with Outdoor Dust and Atmospheric Particulates. Chemosphere 2021, 272, 129952. [Google Scholar] [CrossRef]
- Lu, H.; Liang, S. Reduction of Dust Deposition on Solar Photovoltaic Cells by Self-Cleaning Coating: Experimental Study of Influencing Factors. Energy Technol. 2021, 9, 2000795. [Google Scholar] [CrossRef]
- Zhao, W.; Lv, Y.; Zhou, Q.; Yan, W. Collision-adhesion mechanism of particles and dust deposition simulation on solar PV modules. Renew. Energy 2021, 176, 169–182. [Google Scholar] [CrossRef]
- Liu, X.; Yue, S.; Lu, L.; Li, J. Investigation of the Dust Scaling Behaviour on Solar Photovoltaic Panels. J. Clean. Prod. 2021, 295, 126391. [Google Scholar] [CrossRef]
- Lu, H.; Lu, L.; Wang, Y. Numerical investigation of dust pollution on a solar photovoltaic (PV) system mounted on an isolated building. Appl. Energy 2016, 180, 27–36. [Google Scholar] [CrossRef]
- Liu, X.; Yue, S.; Li, J.; Lu, L. Study of a dust deposition mechanism dominated by electrostatic force on a solar photovoltaic module. Sci. Total Environ. 2021, 754, 142241. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L. Numerical study of dry deposition of monodisperse and polydisperse dust on building-mounted solar photovoltaic panels with different roof inclinations. Sol. Energy 2018, 176, 535–544. [Google Scholar] [CrossRef]
- Liu, X.; Yue, S.; Lu, L.; Li, J. Study on Dust Deposition Mechanics on Solar Mirrors in a Solar Power Plant. Energies 2019, 12, 4550. [Google Scholar] [CrossRef]
- Gong, X.; He, S. Highly Durable Superhydrophobic Polydimethylsiloxane/Silica Nanocomposite Surfaces with Good Self-Cleaning Ability. ACS Omega 2020, 5, 4100–4108. [Google Scholar] [CrossRef]
- Maharjan, S.; Liao, K.-S.; Wang, A.J.; Barton, K.; Haldar, A.; Alley, N.J.; Byrne, H.J.; Curran, S.A. Self-cleaning hydrophobic nanocoating on glass: A scalable manufacturing process. Mater. Chem. Phys. 2020, 239, 122000. [Google Scholar] [CrossRef]
- Rimai, D.S.; Ezenyilimba, M.C.; Quesnel, D.J. Effects of Electrostatic and van der Waals Interactions on the Adhesion of Spherical 7m Particles. J. Adhes. 2005, 81, 245–269. [Google Scholar] [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Ali, M.S.; Dhoble, S.J.; Rahim, N.A.; Omar, A.; Bakar, A.H.A. Application of transparent self-cleaning coating for photovoltaic panel: A review. Curr. Opin. Chem. Eng. 2022, 36, 100801. [Google Scholar] [CrossRef]
- Salehi, H.; Eshaghi, A.; Rezazadeh, M.; Zabolian, H. Antireflective and anti-dust modified silica based thin film on solar cell cover glass. J. Alloys Compd. 2022, 892, 162228. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Zhou, S.; Yang, H.; Chen, C. Transparent dust removal coatings for solar cell on mars and its Anti-dust mechanism. Prog. Org. Coat. 2019, 134, 312–322. [Google Scholar] [CrossRef]
- Pan, A.; Lu, H.; Zhang, L.Z. Experimental Investigation of Dust Deposition Reduction on Solar Cell Covering Glass by Different Self-cleaning Coatings. Energy 2019, 181, 645–653. [Google Scholar] [CrossRef]
- Lin, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and robust transparent superhydrophobic glass surfaces fabricated by a femtosecond laser with exceptional water repellency and thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Karunakaran, R.G.; Lu, C.H.; Zhang, Z.; Yang, S. Highly Transparent Superhydrophobic Surfaces from the Coassembly of Nanoparticles (≤100 nm). Langmuir 2011, 27, 4594–4602. [Google Scholar] [CrossRef]
- Peng, P.P.; Ke, Q.; Zhou, G.; Tang, T. Fabrication of microcavity-array superhydrophobic surfaces using an improved template method. J. Colloid Interface Sci. 2013, 395, 326–328. [Google Scholar] [CrossRef]
- An, H.; Wang, S.; Li, D.; Peng, Z.; Chen, S. Self-Cleaning Performance of the Micropillar-Arrayed Surface and Its Micro-Scale Mechanical Mechanism. Langmuir 2021, 37, 10079–10088. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Wen, F.; Huang, Y.; Wang, B. Abrasion resistant semitransparent self-cleaning coatings based on porous silica microspheres and polydimethylsiloxane. Ceram. Int. 2018, 45, 401–408. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Yin, K.; Zhu, Z.; Duan, J.A. Robust Hierarchical Porous PTFE Film Fabricated via Femtosecond Laser for Self-Cleaning Passive Cooling. Nano Lett. 2021, 21, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.L.; Ruan, M.; Li, W.; Li, H.; Hu, L.Y.; Ma, F.M.; Yu, Z.L.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Chu, D.; Yao, P.; Huang, C. Anti-reflection silicon with self-cleaning processed by femtosecond laser. Opt. Laser Technol. 2021, 136, 106790. [Google Scholar] [CrossRef]
- Kang, H.; Zhao, B.; Li, L.; Zhang, J. Durable superhydrophobic glass wool@polydopamine@PDMS for highly efficient oil/water separation. J. Colloid Interface Sci. 2019, 544, 257–265. [Google Scholar] [CrossRef]
- Mosayebi, E.; Azizian, S.; Noei, N. Preparation of Robust Superhydrophobic Sand by Chemical Vapor Deposition of Polydimethylsiloxane for Oil/Water Separation. Macromol. Mater. Eng. 2020, 305, 2000425. [Google Scholar] [CrossRef]
- Shen, Y.; Wei, Y.; Li, J.; Li, Q.; Ma, J.; Wang, P.; Li, B.; He, W.; Du, X. Preparation of microwave absorbing Co-C nanofibers with robust superhydrophobic properties by electrospinning. J. Mater. Sci. Mater. Electron. 2019, 30, 3365–3377. [Google Scholar] [CrossRef]
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning superhydrophobic nanofibrous poly(vinylidene fluoride)/stearic acid coatings with excellent corrosion resistance. Thin Solid Film. 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, Z.; Wang, Z.; Tian, D. Application of Nanocrystalline Composite Coating in Dust Prevention of Martian Glass Cover. Surf. Technol. 2019, 48, 167–171. [Google Scholar]
- Khodaei, M.; Shadmani, S. Superhydrophobicity on aluminum through reactive-etching and TEOS/GPTMS/nano-Al2O3 silane-based nanocomposite coating. Surf. Coat. Technol. 2019, 374, 1078–1090. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; He, J. CuO/Cu based superhydrophobic and self-cleaning surfaces. Scr. Mater. 2016, 118, 60–64. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Pan, A.J.; Cai, R.R.; Lu, H. Indoor experiments of dust deposition reduction on solar cell covering glass by transparent super-hydrophobic coating with different tilt angles. Sol. Energy 2019, 188, 1146–1155. [Google Scholar] [CrossRef]
- Datta, A.; Singh, V.K.; Das, C.; Halder, A.; Ghoshal, D.; Ganguly, R. Fabrication and characterization of transparent, self-cleaning glass covers for solar photovoltaic cells. Mater. Lett. 2020, 277, 128350. [Google Scholar] [CrossRef]
- Wang, P.; Xie, J.; Ni, L.; Wan, L.; Ou, K.; Zheng, L.; Sun, K. Reducing the effect of dust deposition on the generating efficiency of solar PV modules by super-hydrophobic films. Sol. Energy 2018, 169, 277–283. [Google Scholar] [CrossRef]
- Özmen, E.; Durán, A.; Castro, Y. Hydrophobic and oleophobic sol-gel coatings on glass substrates for usage at high temperatures. Int. J. Appl. Glass Sci. 2018, 9, 413–420. [Google Scholar] [CrossRef]
- Polizos, G.; Sharma, J.K.; Smith, D.B.; Tuncer, E.; Park, J.; Voylov, D.; Sokolov, A.P.; Meyer, H.M.; Aman, M. Anti-soiling and highly transparent coatings with multi-scale features. Sol. Energy Mater. Sol. Cells 2018, 188, 255–262. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Fang, X.; Zhang, Z.; Deng, M. Transparent hydrophobic thermal insulation CsxWO3-ZnO-SiO2 coatings: Energy saving, anti-dust and anti-fogging performance. Mater. Res. Express 2021, 8, 25004. [Google Scholar] [CrossRef]
- Cwa, B.; Jg, A.; Hy, A.; Han, L.A.; Zhen, W.A.; Min, Z.A.; Jl, A.; Xia, L.C. Preparation and self-cleaning property of a superhydrophobic coating based on micro–nano integrated TiO2 microspheres. Ceram. Int. 2021, 47, 32456–32459. [Google Scholar]
- Sun, Y.; Cheng, M.; Sun, S.; Hu, S. Fabrication and characterization of a TiO2/polysiloxane resin composite coating with full-thickness super-hydrophobicity. Chem. Eng. J. 2018, 333, 361–369. [Google Scholar]
- Guo, J.; Wang, C.; Yu, H.; Li, X. Preparation of a wear-resistant, superhydrophobic SiO2/silicone-modified polyurethane composite coating through a two-step spraying method. Prog. Org. Coat. 2020, 146, 105710. [Google Scholar] [CrossRef]
- Syafiq, A.; Pandey, A.K.; Balakrishnan, V.; Rahim, N.A. Study on self-cleaning performance and hydrophobicity of TiO2/silane coatings. Pigment. Resin Technol. 2019. [Google Scholar] [CrossRef]
- Lee, Y.; You, E.A.; Ha, Y.G. Facile one-step construction of covalently networked, self-healable, and transparent superhydrophobic composite films. Appl. Surf. Sci. 2018, 445, 368–375. [Google Scholar] [CrossRef]
- Al-Shatty, W.; Lord, A.M.; Alexander, S.; Barron, A.R. Tunable Surface Properties of Aluminum Oxide Nanoparticles from Highly Hydrophobic to Highly Hydrophilic. ACS Omega 2017, 2, 2507–2514. [Google Scholar] [CrossRef]
- Mayengbam, R.; Mazumder, J.T.; Singh, N.K. Catalyst-free Synthesis of Hydrophobic ZnO Nanowires for Self-cleaning Applications. Braz. J. Phys. 2022, 52, 102. [Google Scholar] [CrossRef]
- Taheri, S.; Motlagh, F.H.; Dehestanizad, S.; Yahyaei, H.; Motallebzadeh, A.; Zarrabi, A.; Tehrani, A.G.; Khodabakhsh, M.; Makki, H. The effect of surface chemistry on anti-soiling properties of transparent perfluoroalkyl and alkyl modified silica coatings. Surf. Interfaces 2022, 30, 101824. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Johnson, D.; Ackermann, L.; Figgis, B.; Ayoub, M. Evaluation of the adhesion forces between dust particles and photovoltaic module surfaces. Sol. Energy Mater. Sol. Cells 2019, 191, 413–421. [Google Scholar] [CrossRef]
- Dong, B.; Gao, Y.; Liu, J. Preparation of SnO2-SiO2 film with high transmittance and strong dust-removing by sol-gel. Optik 2021, 245, 167727. [Google Scholar] [CrossRef]
- Cai, W.; Ma, X.; Guo, J.; Peng, X.; Zhang, S.; Qiu, Z.; Ying, J.; Wang, J. Preparation and performance of a transparent poly(3,4-ethylene dioxythiophene)-poly(p-styrene sulfonate-co-acrylic acid sodium) film with a high stability and water resistance. J. Appl. Polym. Sci. 2017, 134, 45163. [Google Scholar] [CrossRef]
- Farahani, R.D.; Gagne, M.; Klemberg-Sapieha, J.E.; Therriault, D. Electrically Conductive Silver Nanoparticles-Filled Nanocomposite Materials as Surface Coatings of Composite Structures. Adv. Eng. Mater. 2016, 18, 1189–1199. [Google Scholar] [CrossRef]
- Li, X.; Qian, J.; Xu, J.; Xing, J.; Tao, E. Synthesis, characterization and electrical properties of TiO2 modified with SiO2 and antimony-doped tin oxide. J. Mater. Sci. Mater. Electron. 2018, 29, 12100–12108. [Google Scholar] [CrossRef]
- Huang, J.R.; Yang, X.X.; Her, S.C.; Liang, Y.M. Carbon Nanotube/Graphene Nanoplatelet Hybrid Film as a Flexible Multifunctional Sensor. Sensors 2019, 19, 317. [Google Scholar] [CrossRef]
- Ma, L.; Dong, S.; Chen, S.; Ma, W.; Sun, D.; Gao, Y.; Ma, T.; Cheng, H.; Ren, W. UV-Epoxy-Enabled Simultaneous Intact Transfer and Highly Efficient Doping for Roll-to-Roll Production of High-Performance Graphene Films. ACS Appl. Mater. Interfaces 2018, 10, 40756–40763. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, L.; Bao, J.; He, L. Durable superhydrophobic/high-oleophobic coatings from multi-dome SiO2 nanoparticles and fluoroacrylate block copolymers on flat substrates. J. Mater. Chem. A 2015, 3, 20134–20144. [Google Scholar]
- Chen, K.; Wu, Y.; Zhou, S.; Wu, L. Recent Development of Durable and Self-Healing Surfaces with Special Wettability. Macromol. Rapid Commun. 2016, 37, 463. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xiao, J.; Zeng, J.; Wang, C.; Tang, J. Facile method to prepare a novel honeycomb-like superhydrophobic Polydimethylsiloxan surface. Surf. Coat. Technol. 2010, 205, 1947–1952. [Google Scholar] [CrossRef]
- Golovin, K.; Boban, M.; Joseph, M.; Mabry, J.; Tuteja, A. Designing Self-Healing Superhydrophobic Surfaces with Exceptional Mechanical Durability. ACS Appl. Mater. Interfaces 2017, 9, 11212–11223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Xia, Z.; Ning, T.; Zhang, J. Totally Waterborne, Nonfluorinated, Mechanically Robust and Self-Healing Superhydrophobic Coatings for Actual Anti-Icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Xiong, D.S.; Zhou, H. TMES-modified SiO2 matrix non-fluorinated superhydrophobic coating for long-term corrosion resistance of aluminium alloy. Ceram. Int. 2020, 46, 1211. [Google Scholar] [CrossRef]
- Ellis-Terrell, C.; Wei, R.; Mcknight, R.; Huang, X.; Lin, K. Thermal Stability of Superhydrophobic and Oleophobic Silica Nanoparticle Spray Coating. Mater. Today Commun. 2020, 25, 101370. [Google Scholar] [CrossRef]
- Mcdonald, B.; Cholewinski, A.; Zhao, B. Bio-inspired polydimethylsiloxane-functionalized silica particles—Epoxy bilayer as a robust superhydrophobic surface coating. Surf. Coat. Technol. 2014, 254, 230–237. [Google Scholar]
- Heckenthaler, T.; Sadhujan, S.; Morgenstern, Y.; Natarajan, P.; Kaufman, Y. The Self-Cleaning Mechanism: Why Nanotexture and Hydrophobicity Matter. Langmuir 2019, 35, 15526–15534. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
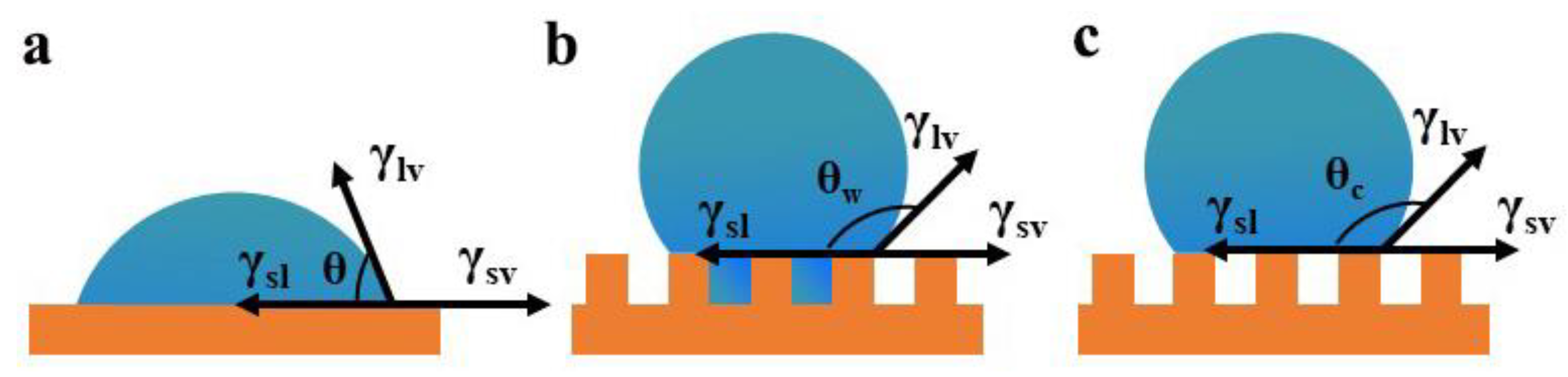
- Hazlett, R.D. Fractal applications: Wettability and contact angle. J. Colloid Interface Sci. 1990, 137, 527–533. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
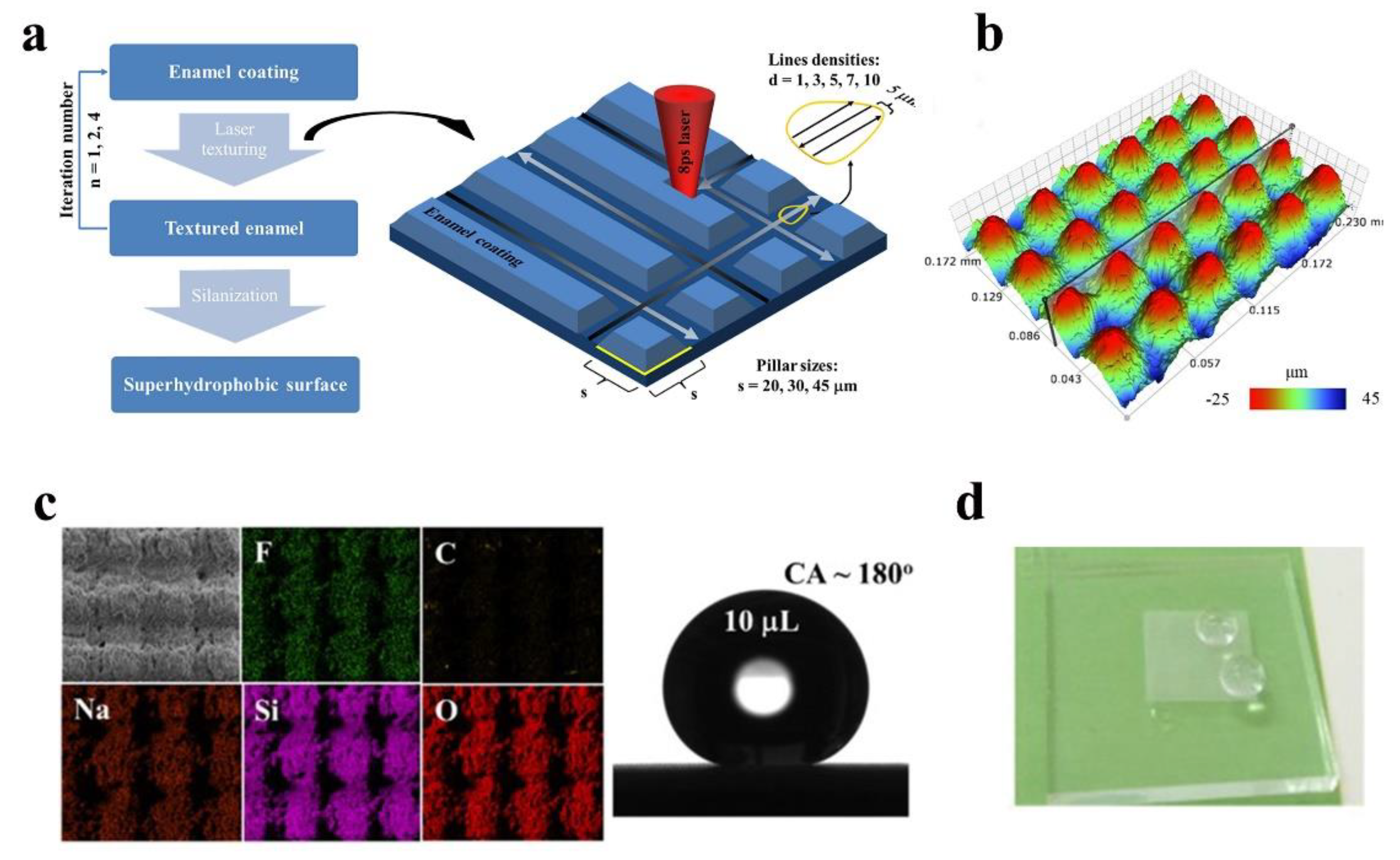
- Nguyen, H.H.; Tieu, A.K.; Wan, S.; Zhu, H.; Johnston, B. Surface characteristics and wettability of superhydrophobic silanized inorganic glass coating surfaces textured with a picosecond laser. Appl. Surf. Sci. 2021, 537, 147808. [Google Scholar] [CrossRef]
- Kontziampasis, D.; Boulousis, G.; Smyrnakis, A.; Ellinas, K.; Tserepi, A.; Gogolides, E. Biomimetic, antireflective, superhydrophobic and oleophobic PMMA and PMMA-coated glass surfaces fabricated by plasma processing. Microelectron. Eng. 2014, 121, 33–38. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Cao, B.X.; Do, T.C.; Trinh, H.B.; Nguyen, T.B. Interfacial parameters in correlation with anti-icing performance. J. Adhes. 2021, 97, 860–872. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Feng, X.; Jiao, Z.; Zhang, J.; Niu, S.; Han, Z.; Ren, L. Rapid Fabrication of Bio-inspired Antireflection Film Replicating from Cicada Wings. J. Bionic Eng. 2020, 17, 34–44. [Google Scholar] [CrossRef]
- Borjak, S.K.; Rafee, R.; Valipour, M. Experimental Investigation of Water Droplet Impact on the Electrospun Superhydrophobic Cylindrical Glass: Contact Time, Maximum Spreading Factor, and Splash Threshold. Langmuir 2020, 36, 13498–13508. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.A.; Dinachali, S.S.; Raut, H.K.; Walsh, T.M.; Nair, A.S.; Ramakrishna, S. Electrospun SiO2 nanofibers as a template to fabricate a robust and transparent superamphiphobic coating. RSC Adv. 2013, 3, 3819–3824. [Google Scholar] [CrossRef]
- Li, W.; Liang, Z.; Dong, B.; Tang, H. Transmittance and self-cleaning polymethylsiloxane coating with superhydrophobic surfaces. Surf. Eng. 2019, 36, 1–9. [Google Scholar] [CrossRef]
- Czyzyk, S.; Dotan, A.; Dodiuk, H.; Kenig, S. Processing effects on the kinetics morphology and properties of hybrid sol-gel superhydrophobic coatings. Prog. Org. Coat. 2020, 140, 105501. [Google Scholar] [CrossRef]
- Jumrus, N.; Chaisen, T.; Sriboonruang, A.; Panthawan, A.; Thongsuwan, W. A facile methodology to make the glass surface superhydrophobic. Mater. Lett. 2020, 264, 127347. [Google Scholar] [CrossRef]
- Fu, H.; Liu, S.; Yi, L.; Jiang, H.; Chen, Y. A Durable and Self-Cleaning Superhydrophobic Surface Prepared by Precipitating FlowerLike Crystals on a Glass-Ceramic Surface. Materials 2020, 13, 1642. [Google Scholar] [CrossRef]
- Zuo, Z.; Gao, J.; Liao, R.; Zhao, X.; Yuan, Y. A novel and facile way to fabricate transparent superhydrophobic film on glass with self-cleaning and stability. Mater. Lett. 2019, 239, 48–51. [Google Scholar] [CrossRef]
- Yuan, Y.; Duan, Y.; Zuo, Z.; Yang, L.; Liao, R. Novel, stable and durable superhydrophobic film on glass prepared by RF magnetron sputtering. Mater. Lett. 2017, 199, 97–100. [Google Scholar] [CrossRef]
- Zhuang, A.; Liao, R.; Dixon, S.C.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Transparent superhydrophobic PTFE films via one-step aerosol assisted chemical vapor deposition. RSC Adv. 2017, 7, 29275–29283. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.; Parkin, I.P. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar] [CrossRef]
- Tang, M.; Huang, X.; Guo, Z.; Yu, J.; Li, X.; Zhang, Q. Fabrication of robust and stable superhydrophobic surface by a convenient, low-cost and efficient laser marking approach. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 449–456. [Google Scholar] [CrossRef]
- Fan, P.; Pan, R.; Zhong, M. Ultrafast Laser Enabling Hierarchical Structures for Versatile Superhydrophobicity with Enhanced Cassie-Baxter Stability and Durability. Langmuir 2019, 35, 16693–16711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, H.; Liu, J.; Zhang, J.; Jin, Z. A green, maskless, and universal preparation method for patterned surfaces on various metal substrates. Appl. Surf. Sci. 2020, 514, 145838. [Google Scholar] [CrossRef]
- Baumann, R.; Milles, S.; Leupolt, B.; Kleber, S.; Lasagni, A. Tailored wetting of copper using precise nanosecond direct laser interference patterning. Opt. Laser Eng. 2020, 137, 106364. [Google Scholar] [CrossRef]
- Stroj, S.; Kasemann, S.; Domke, M.; Piredda, G.; Zehetner, J.; Matylitskaya, V. Transparent superhydrophobic surfaces with high adhesion generated by the combination of femtosecond laser structuring and wet oxidation. Appl. Surf. Sci. 2017, 420, 550–557. [Google Scholar] [CrossRef]
- Wang, B.; Hua, Y.; Ye, Y.; Chen, R.; Li, Z. Transparent superhydrophobic solar glass prepared by fabricating groove-shaped arrays on the surface. Appl. Surf. Sci. 2017, 426, 957–964. [Google Scholar] [CrossRef]
- Xi, R.; Wang, Y.; Li, X.; Zhang, X.; Du, X. A facile strategy to form three-dimensional network structure for mechanically robust superhydrophobic nanocoatings with enhanced transmittance. J. Colloid Interface Sci. 2019, 563, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Bake, A.; Merah, N.; Matin, A.; Gondal, M.; Qahtan, T.; Abu-Dheir, N. Preparation of transparent and robust superhydrophobic surfaces for self-cleaning applications. Prog. Org. Coat. 2018, 122, 170–179. [Google Scholar] [CrossRef]
- Cong, S.; Yongjun, Z.; Yanqing, Z.; Weina, S.; Xiudi, X.; Gang, X.; Runqiang, C. Preparation of smart glass with superhydrophobic and thermochromic properties. Chem. Phys. Lett. 2019, 723, 65–68. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Mahadik, S.S. Surface morphological and topographical analysis of multifunctional superhydrophobic sol-gel coatings. Ceram. Int. 2021, 47, 29475–29482. [Google Scholar] [CrossRef]
- Hashjin, R.N.R.; Ranjbar, Z.; Yari, H.; Momen, G. Tuning up sol-gel process to achieve highly durable superhydrophobic coating. Surf. Interfaces 2022, 33, 102282. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Bioinspired rice leaf and butterfly wing surface structures combining shark skin and lotus effects. Soft Matter 2012, 8, 11271–11284. [Google Scholar] [CrossRef]
- Drotlef, D.M.; Stepien, L.; Kappl, M.; Barnes, W.; Butt, H.J.; Campo, A.D. Insights into the Adhesive Mechanisms of Tree Frogs using Artificial Mimics. Adv. Funct. Mater. 2012, 23, 1137–1146. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Yang, X.; Xu, X.; Zhang, L.; Zhao, N.; Xu, J. Antifogging and antireflective silica film and its application on solar modules. Surf. Coat. Technol. 2011, 206, 1490–1494. [Google Scholar] [CrossRef]
- Tao, C.; Zou, X.; Du, K.; Zhou, G.; Yan, H.; Yuan, X.; Zhang, L. Fabrication of robust, self-cleaning, broadband TiO2-SiO2 double-layer antireflective coatings with closed-pore structure through a surface sol-gel process. J. Alloys Compd. 2018, 747, 43–49. [Google Scholar] [CrossRef]
- Lei, F.; Chen, S.; Sun, H.; Han, H.; Yang, J.; Huang, J.; Li, D.; Sun, D. Fabrication of highly transparent and superhydrophilic coatings on glass by modified α-zirconium phosphate nanoplatelets. Mater. Chem. Phys. 2021, 263, 124377. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, C. Comparison of Dust Deposition Reduction Performance by Super-Hydrophobic and Super-Hydrophilic Coatings for Solar PV Cells. Coatings 2022, 12, 502. [Google Scholar] [CrossRef]
- Bakri, H.A.; Elhaija, W.A.; Zyoud, A.A. Solar photovoltaic panels performance improvement using active self-cleaning nanotechnology of SurfaShield G. Energy 2021, 223, 119908. [Google Scholar] [CrossRef]
- Park, K.C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, K.M.; Watson, J.A.; Qu, X.; Liu, F.; Chen, C.H. Self-cleaning of superhydrophobic surfaces by self-propelled jumping condensate. Proc. Natl. Acad. Sci. USA 2013, 110, 7992–7997. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, W.; Xiao, Z.; Yu, X.; Zhang, Y. Water-free dedusting on antireflective glass with durable superhydrophobicity. Surf. Coat. Technol. 2018, 356, 123–131. [Google Scholar] [CrossRef]
- Cho, H.J.; Preston, D.J.; Zhu, Y.; Wang, E.N. Nanoengineered materials for liquid–vapour phase-change heat transfer. Nat. Rev. Mater. 2016, 2, 16092. [Google Scholar] [CrossRef]
- Gong, X.; Gao, X.; Jiang, L. Recent Progress in Bionic Condensate Microdrop Self-Propelling Surfaces. Adv. Mater. 2017, 29, 1703002. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, M.; Shang, Y.; Zhou, P.; Song, R.; Xu, X.; Chen, X.; Wang, Z.; Yao, S. Suppressing Ice Nucleation of Supercooled Condensate with Biphilic Topography. Phys. Rev. Lett. 2018, 120, 75902. [Google Scholar] [CrossRef] [PubMed]
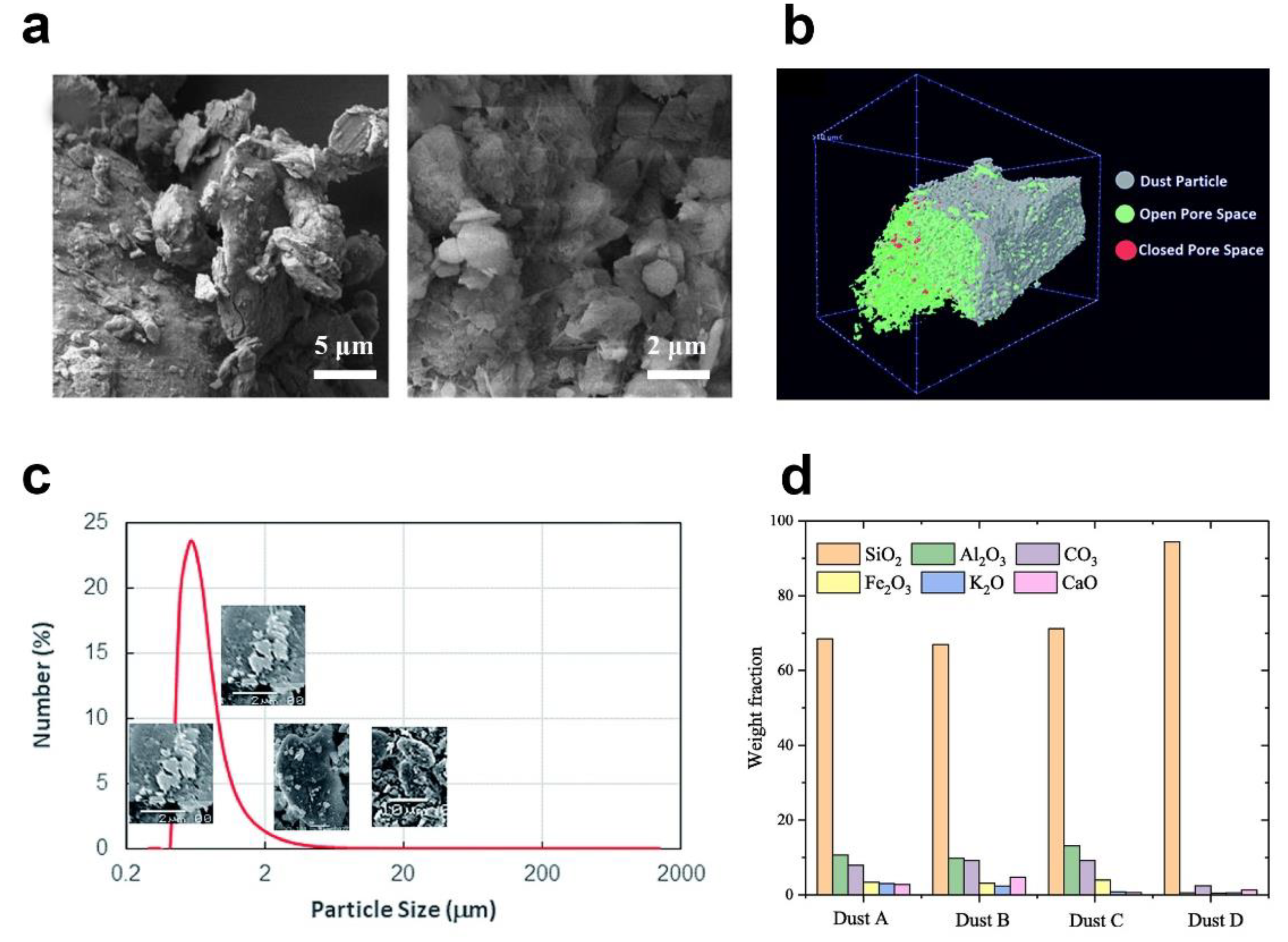


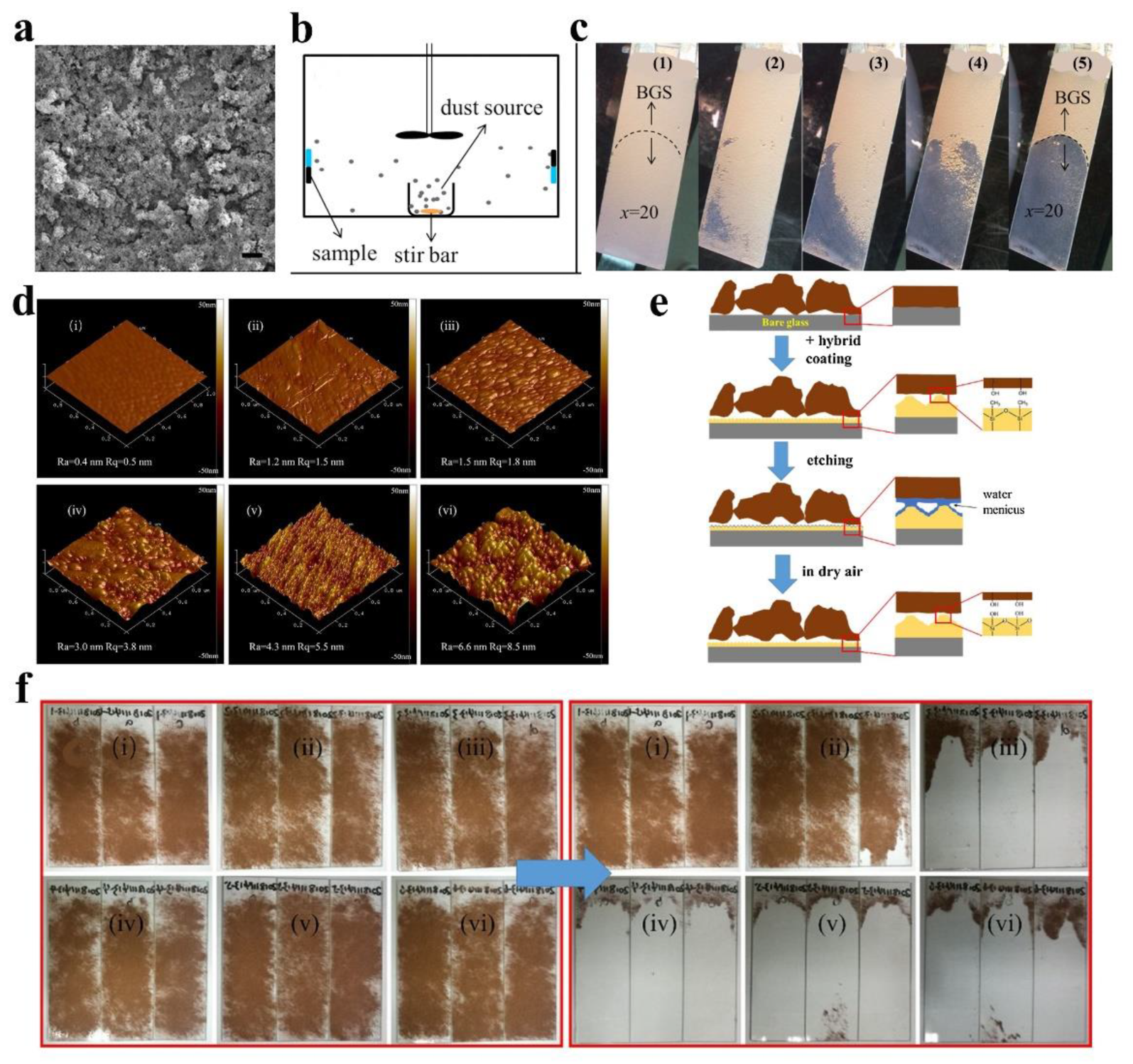

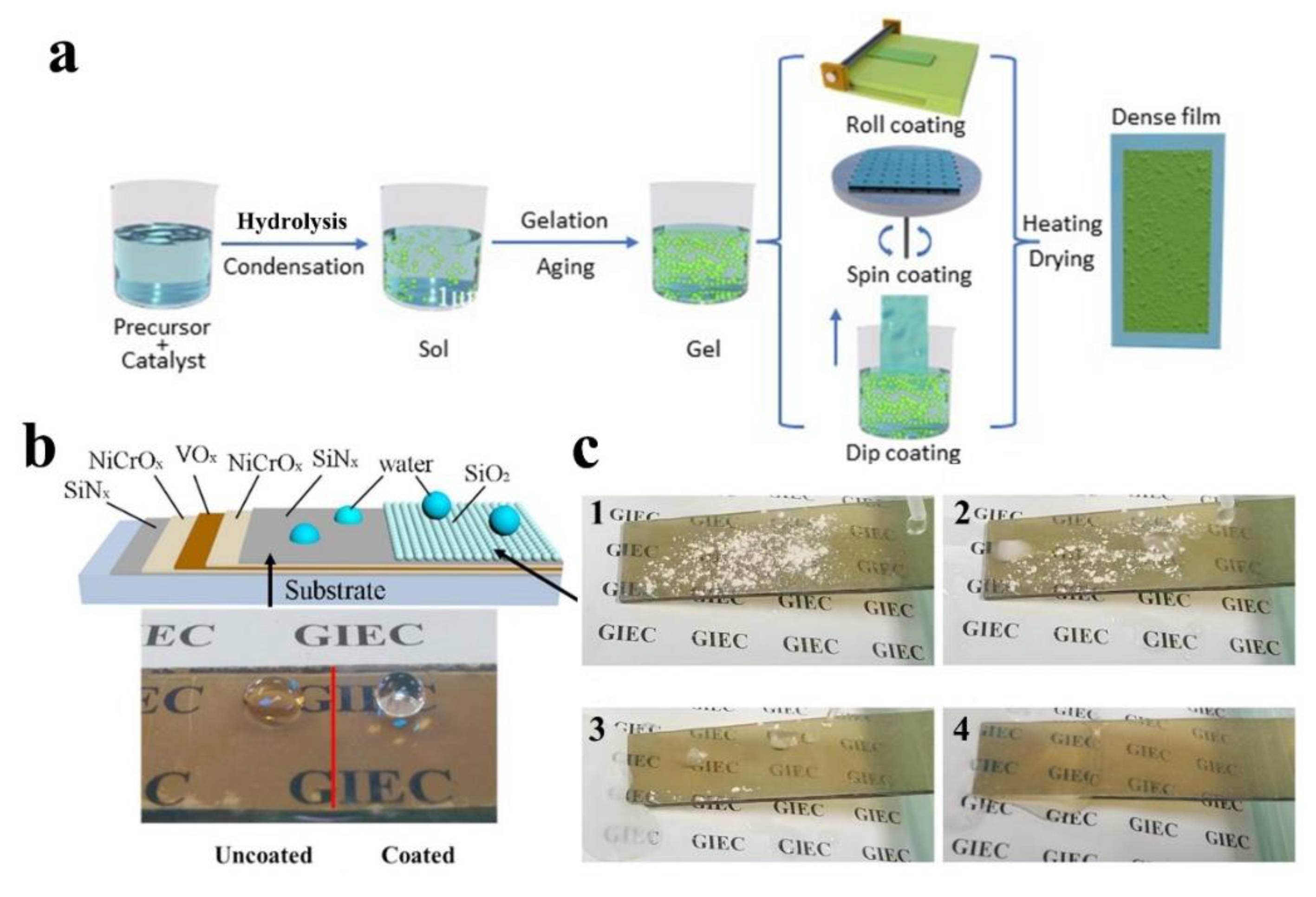
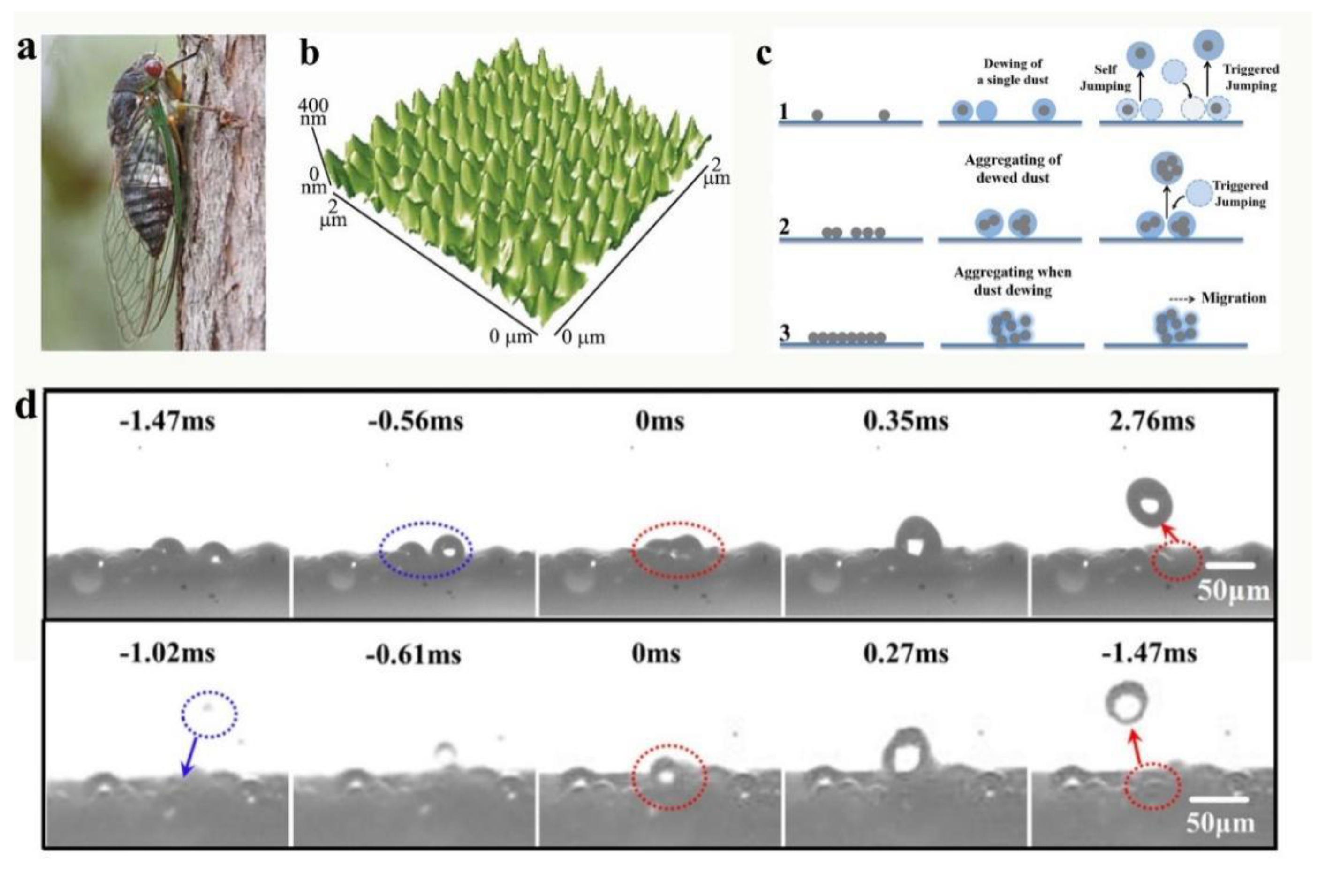
| Author | Location | Chemical Composition of Dust Particles | Ref. |
|---|---|---|---|
| Dhaouadi | United Arab Emirates | CaSO4, SiO2, Mg2(SiO4), Ca(CO3), Fe2O3, Ca2Mg(Si2O7), | [53] |
| Lu | China | SiO2, Al2O3, Fe2O3, CaO, K2O | [5] |
| Wu | China | SiO2, CaCO3, NaAlSi3O8 | [54] |
| Al-Dousari | Kuwait | Quartz, Carbonates, Feldspars, Clay | [55] |
| Gholami | Iran | SiO2, CaO, Al2O3, Fe2O3, MgO, K2O, TiO2, SO3, MnO2, Cr2O3, SrO and NiO. | [56] |
| Hachicha | UAE | SiO2, CaO, Fe2O3, MgO, Al2O3 | [57] |
| Methods | Low Surface Energy Materials | Contact Angle (°) | Rolling Angle (°) | Light Transmittance (%) | Ref |
|---|---|---|---|---|---|
| Laser machining | 1H, 1H, 2H, 2H-perfluorooctyltrichlorosilane | ~180 | - | - | [129] |
| 1H, 1H, 2H, 2H-perfluorodecyltriethoxysilane | 161 | 2 | 92 | [77] | |
| Plasma etching | 1H, 1H, 2H, 2H-perfluorooctyltrichlorosilane | ~150 | - | - | [130] |
| perfluorooctyl triethoxysilane | 166 | - | - | [131] | |
| Template transfer technology | Polymeric Methyl Methacrylate | 152 | 3 | - | [40] |
| polydimethylsiloxane | 152 | 4 | 93 | [132] | |
| Electrospinning | Trichlorosilane | 158 | - | - | [133] |
| (Tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane | 161 | 1 | 85 | [134] | |
| Sol-gel method | Triethoxymethylsilane | 164 | 5 | 91.13 | [135] |
| Triethoxy (3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl) silane | 160 | 0 | 90 | [136] | |
| Acid etching | methyltrichlorosilane | 154 | 3 | - | [137] |
| 1H, 1H, 2H, 2H-perfluorodecyltriethoxysilane | 170 | 2 | - | [138] | |
| RF magnetron sputtering | hexadecyltrimethoxysilane | 155 | - | - | [139] |
| hexadecyltrimethoxysilane | 169 | 1 | - | [140] | |
| AACVD | Polytetrafluoroethylene | 168 | 1 | 90 | [141] |
| Polydimethylsiloxane | 160 | 1 | 80 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, M.; Wu, Y.; Zheng, H. Progress in Studies of Surface Nanotextures and Coatings with Nanomaterials on Glass for Anti-Dust Functionality. Nanomaterials 2022, 12, 3677. https://doi.org/10.3390/nano12203677
Wang L, Liu M, Wu Y, Zheng H. Progress in Studies of Surface Nanotextures and Coatings with Nanomaterials on Glass for Anti-Dust Functionality. Nanomaterials. 2022; 12(20):3677. https://doi.org/10.3390/nano12203677
Chicago/Turabian StyleWang, Liyong, Mingming Liu, Yongling Wu, and Hongyu Zheng. 2022. "Progress in Studies of Surface Nanotextures and Coatings with Nanomaterials on Glass for Anti-Dust Functionality" Nanomaterials 12, no. 20: 3677. https://doi.org/10.3390/nano12203677
APA StyleWang, L., Liu, M., Wu, Y., & Zheng, H. (2022). Progress in Studies of Surface Nanotextures and Coatings with Nanomaterials on Glass for Anti-Dust Functionality. Nanomaterials, 12(20), 3677. https://doi.org/10.3390/nano12203677








