Optical Properties and Concentration Quenching Mechanism of Er3+ Heavy Doped Gd2(MoO4)3 Phosphor for Green Light-Emitting Diode
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure and Morphology
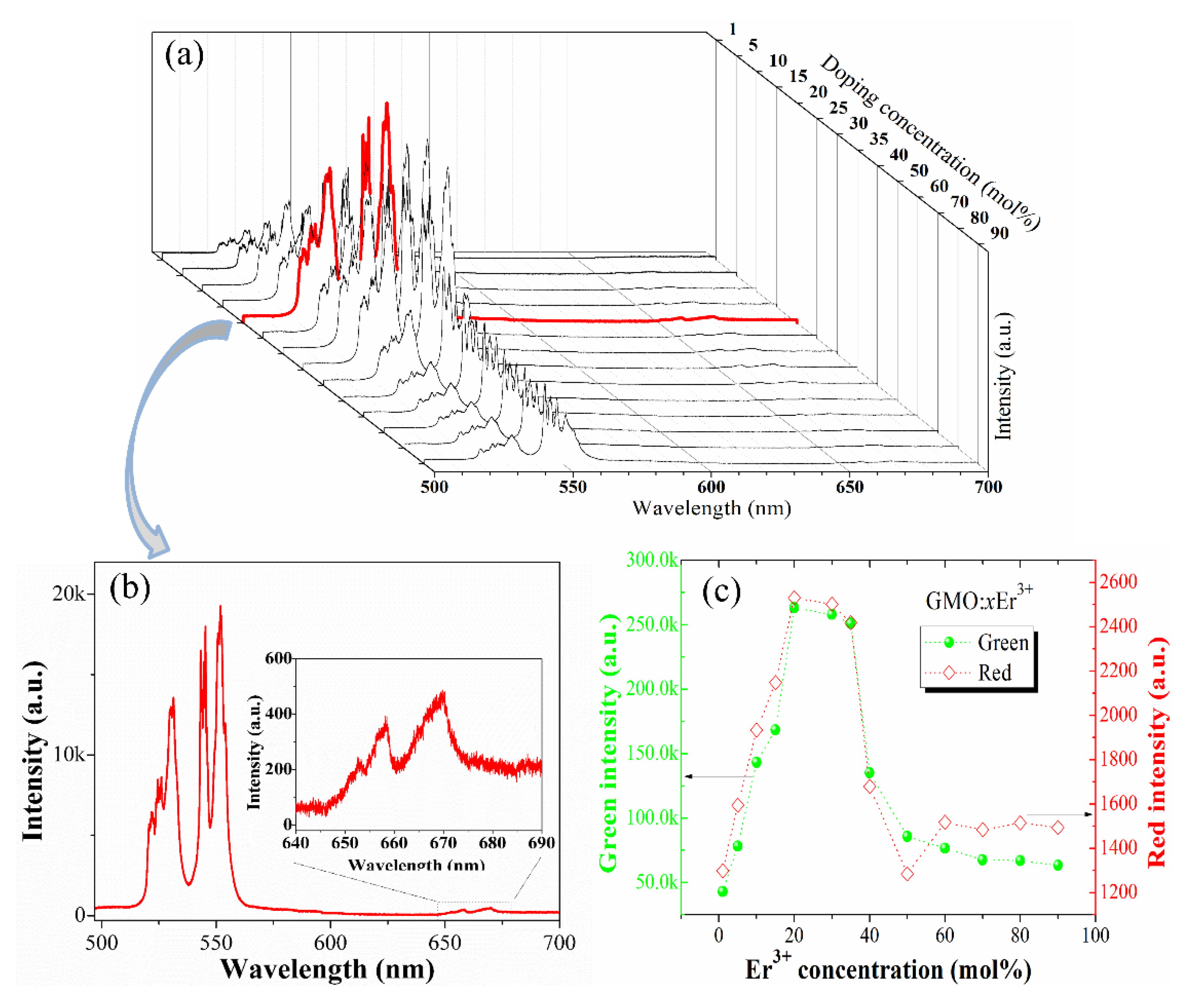
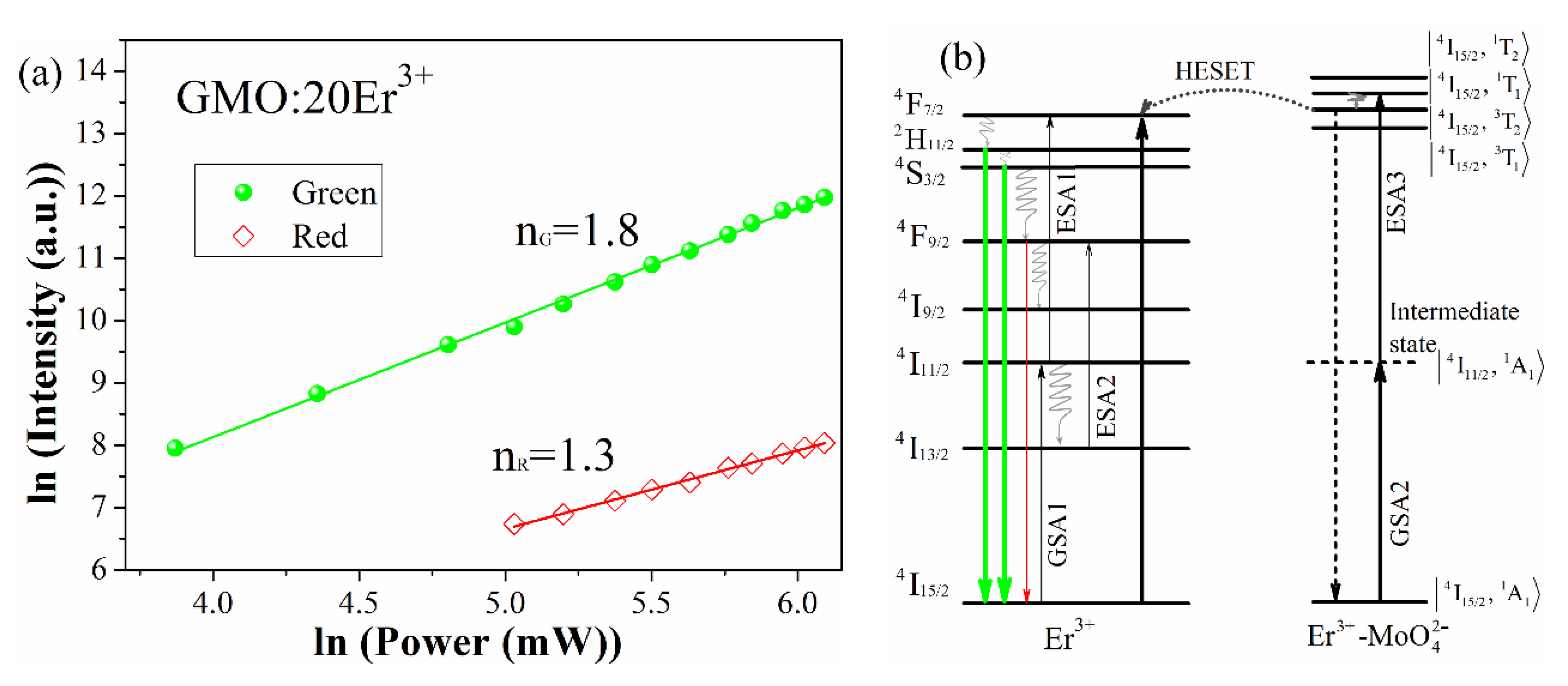
3.2. Upconversion Mechanism Analysis
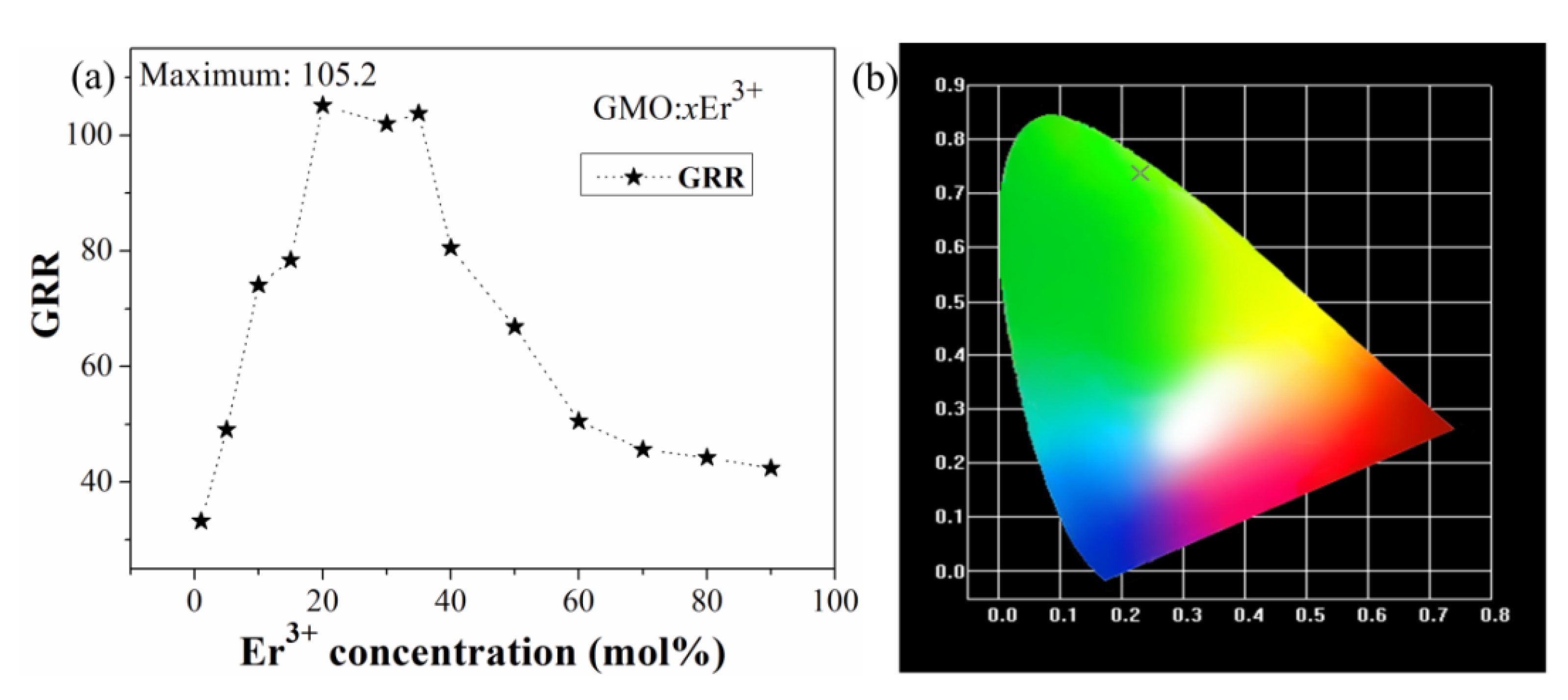
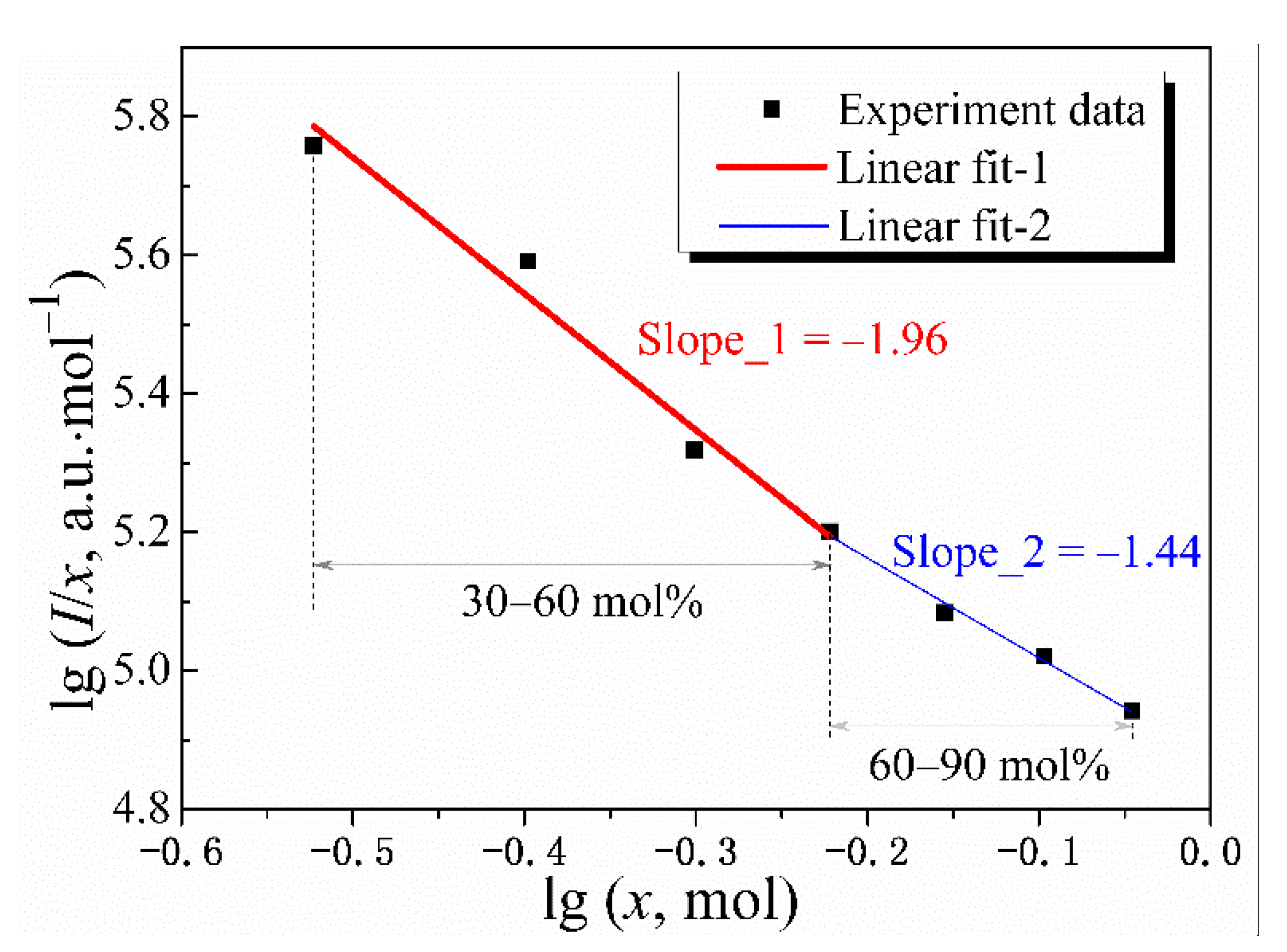
3.3. Concentration Quenching Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, C.; Li, Z.; Ma, X.; Chen, D.; Wan, X.; Deng, Z.; Deng, R.; Peng, X. Near-Infrared-Light Emitting Diode Driven White Light Emission: Upconversion Nanoparticles Decorated Metal-Organic Frame-works Thin Film. Chem. Eng. J. 2021, 409, 128220. [Google Scholar] [CrossRef]
- Scheps, R. Upconversion Laser Processes. Prog. Quant. Electron. 1996, 20, 271–358. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.; Shao, L.; Wu, S.; Huang, Z.; Jin, X.; Zhang, Q.; Li, Q. Tailoring Solar Energy Spectrum for Efficient Organic/Inorganic Hybrid Solar Cells by Up-conversion Luminescence Nanophosphors. Electrochim. Acta 2015, 182, 416–423. [Google Scholar] [CrossRef]
- Zhang, X.; Ali, R.F.; Boyer, J.C.; Branda, N.R.; Gates, B.D. Direct Photolithographic Deposition of Color-Coded Anti-Counterfeit Patterns with Titania Encapsulated Upconverting Nanoparticles. Adv. Opt. Mater. 2020, 8, 2000664. [Google Scholar] [CrossRef]
- Du, P.; Ran, W.; Wang, C.; Luo, L.; Li, W. Facile Realization of Boosted Near-Infrared-Visible Light Driven Photocatalytic Activities of BiOF Nanoparticles through Simultaneously Exploiting Doping and Upconversion Strategy. Adv. Mater. Inter. 2021, 8, 2100749. [Google Scholar] [CrossRef]
- Hong, A.R.; Kyhm, J.H.; Kang, G.; Jang, H.S. Orthogonal R/G/B Upconversion Luminescence-Based Full-Color Tunable Upconversion Nanophosphors for Transparent Displays. Nano Lett. 2021, 21, 4838–4844. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Huang, Z.; Jin, X.; Xu, B.; Zhang, Z.; Zhang, T.; Wang, D.; Liu, X.; Suo, H.; et al. Simultaneous Enhancement of Emission and Thermal Sensitivity via Phase Transition in Gd2(MoO4)3:Yb3+/Er3+ Crystals. J Am Ceram Soc. 2022, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Yang, X.; Zheng, X.; Wen, S.; Wang, F.; Vidal, X.; Zhao, J.; Liu, D.; Zhou, Z. Amplified Stimulated Emission in Upconversion Nanoparticles for Super-Resolution Nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion Nanoparticles in Biological Labeling, Imaging, and Therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in Highly Doped Upconversion Nanoparticles. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Emerging Frontiers of Upconversion Nanoparticles. Tren. Chem. 2020, 2, 427–439. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Combating Concentration Quenching in Upconversion Nanoparticles. Acc. Chem. Res. 2019, 53, 358–367. [Google Scholar] [CrossRef]
- Liu, L.; Lu, K.; Xu, L.; Tang, D.; Liu, C.; Shahzad, M.K.; Yan, D.; Khan, F.; Zhao, E.; Li, H. Highly Efficient Upconversion Luminescence of Er Heavily Doped Nanocrystals through 1530 nm Excitation. Opt. Lett. 2019, 44, 711–714. [Google Scholar] [CrossRef]
- Wen, S.; Li, D.; Liu, Y.; Chen, C.; Wang, F.; Zhou, J.; Bao, G.; Zhang, L.; Jin, D. Power-Dependent Optimal Concentrations of Tm3+ and Yb3+ in Upconversion Nanoparticles. J. Phys. Chem. Lett. 2022, 13, 5316–5323. [Google Scholar] [CrossRef]
- Johnson, N.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.; Almutairi, A. Direct Evidence for Coupled Surface and Concentration Quenching Dynamics in Lanthanide-Doped Nanocrystals. J. Am. Chem. Soc. 2017, 139, 3275–3282. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, G.; Gao, H.; Zhang, H.; Ao, T.; Gao, W.; Mao, Y. Single Band Red Emission of Er3+ Ions Heavily Doped Upconversion Nanoparticles Realized by Active-Core/Active-Shell Structure. Ceram. Int. 2021, 47, 18824–18830. [Google Scholar] [CrossRef]
- Liang, X.; Huang, X.; Zhang, Q. Gd2(MoO4)3:Er3+ Nanophosphors for an Enhancement of Silicon Solar-Cell Near-Infrared Response. J. Fluoresc. 2009, 19, 285–289. [Google Scholar] [CrossRef]
- Li, D.; Huang, Z.; Nie, Z.; Zhang, L.; Bai, Y.; Zhang, X.; Song, Y.; Wang, Y. Anomalous Upconversion Luminescence of SrMoO4:Yb3+/Er3+ Nanocrystals by High Excited State Energy Transfer. J. Alloys Comp. 2015, 650, 799–804. [Google Scholar] [CrossRef]
- Jin, X.; Li, H.; Li, D.; Zhang, Q.; Li, F.; Sun, W.; Chen, Z.; Li, Q. Role of Ytterbium-Erbium Co-Doped Gadolinium Molybdate (Gd2(MoO4)3:Yb/Er) Nanophosphors in Solar Cells. Opt. Express 2016, 24, A1276–A1287. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. The Modulated Structure and Frequency Upconversion Properties of CaLa2(MoO4)4:Ho3+/Yb3+ Phosphors Prepared by Microwave Synthesis. Phys. Chem. Chem. Phys. 2015, 17, 19278–19287. [Google Scholar] [CrossRef]
- Chang Sung, L.; Victor, V.A.; Aleksandr, S.A.; Yuriy, G.D.; Maxim, S.M.; Aleksandr, S.O. Fabrication of Microcrystalline NaPbLa(WO4)3:Yb3+/Ho3+ Phosphors and Their Upconversion Photoluminescent Characteristics. J. Korean Mater. Res. 2019, 29, 741–746. [Google Scholar]
- Lim, C.S.; Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S. Preparation of NaSrLa(WO4)3:Ho3+/Yb3+ Ternary Tungstates and Their Upconversion Photoluminescence Properties. Mater. Lett. 2016, 181, 38–41. [Google Scholar] [CrossRef]
- Bae, H.; Lee, E.; Lee, K.T. Power-Dependent Photophysical Pathways of Upconversion in BaTiO3:Er3+. Phys. Chem. Chem. Phys. 2021, 23, 14587–14591. [Google Scholar] [CrossRef] [PubMed]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power Dependence of Upconversion Luminescence in Lanthanide and Transition-Metal-Ion Systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Van Uitert, L. Characterization of energy transfer interactions between rare earth ions. J. Electrochem. Soc. 1967, 114, 1048. [Google Scholar] [CrossRef]
- Ozawa, L.; Jaffe, P.M. The Mechanism of the Emission Color Shift with Activator Concentration in Eu+3 Activated Phosphors. J. Electrochem. Soc. 1971, 118, 1678. [Google Scholar] [CrossRef]
- Li, H.; Zhao, R.; Jia, Y.; Sun, W.; Fu, J.; Jiang, L.; Zhang, S.; Pang, R.; Li, C. Sr1.7Zn0.3CeO4: Eu3+ Novel Red-Emitting Phosphors: Synthesis and Photoluminescence Properties. ACS Appl. Mater. Interf. 2014, 6, 3163–3169. [Google Scholar] [CrossRef]
- Blasse, G. Energy Transfer in Oxidic Phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, B.; Huang, Z.; Jin, X.; Zhang, Z.; Zhang, T.; Wang, D.; Liu, X.; Li, Q. Optical Properties and Concentration Quenching Mechanism of Er3+ Heavy Doped Gd2(MoO4)3 Phosphor for Green Light-Emitting Diode. Nanomaterials 2022, 12, 3641. https://doi.org/10.3390/nano12203641
Li D, Xu B, Huang Z, Jin X, Zhang Z, Zhang T, Wang D, Liu X, Li Q. Optical Properties and Concentration Quenching Mechanism of Er3+ Heavy Doped Gd2(MoO4)3 Phosphor for Green Light-Emitting Diode. Nanomaterials. 2022; 12(20):3641. https://doi.org/10.3390/nano12203641
Chicago/Turabian StyleLi, Dongyu, Bing Xu, Zhen Huang, Xiao Jin, Zhenghe Zhang, Tingting Zhang, Deng Wang, Xuping Liu, and Qinghua Li. 2022. "Optical Properties and Concentration Quenching Mechanism of Er3+ Heavy Doped Gd2(MoO4)3 Phosphor for Green Light-Emitting Diode" Nanomaterials 12, no. 20: 3641. https://doi.org/10.3390/nano12203641
APA StyleLi, D., Xu, B., Huang, Z., Jin, X., Zhang, Z., Zhang, T., Wang, D., Liu, X., & Li, Q. (2022). Optical Properties and Concentration Quenching Mechanism of Er3+ Heavy Doped Gd2(MoO4)3 Phosphor for Green Light-Emitting Diode. Nanomaterials, 12(20), 3641. https://doi.org/10.3390/nano12203641





