Room-Temperature Synthesis of Highly-Efficient Eu3+-Activated KGd2F7 Red-Emitting Nanoparticles for White Light-Emitting Diode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Eu3+-Activated KGd2F7 Nanoparticles at Room Temperature
2.2. Characterization
3. Results
3.1. Phase Structure and Morphology
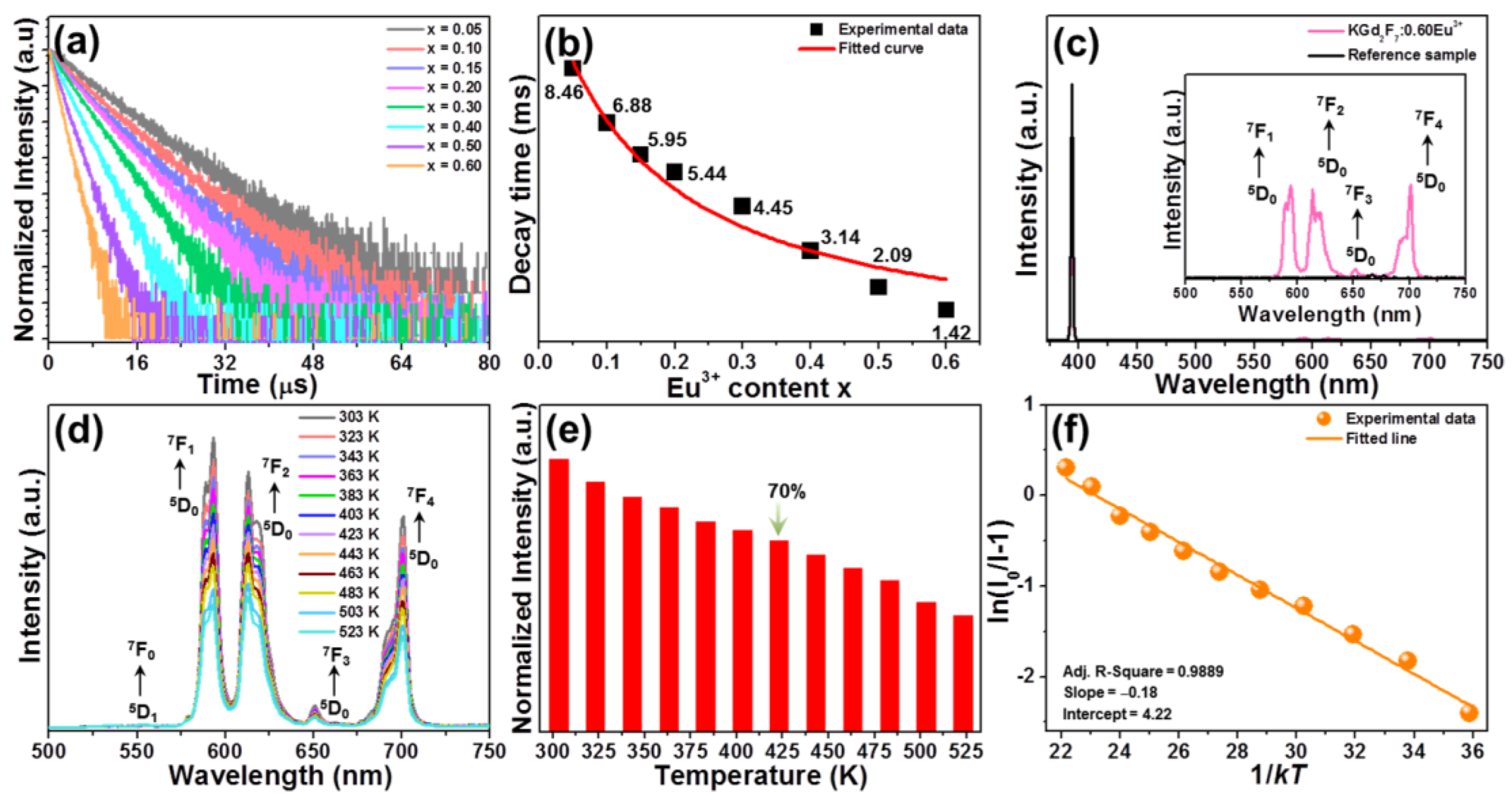
3.2. Luminescence Behaviors and Thermal Stability
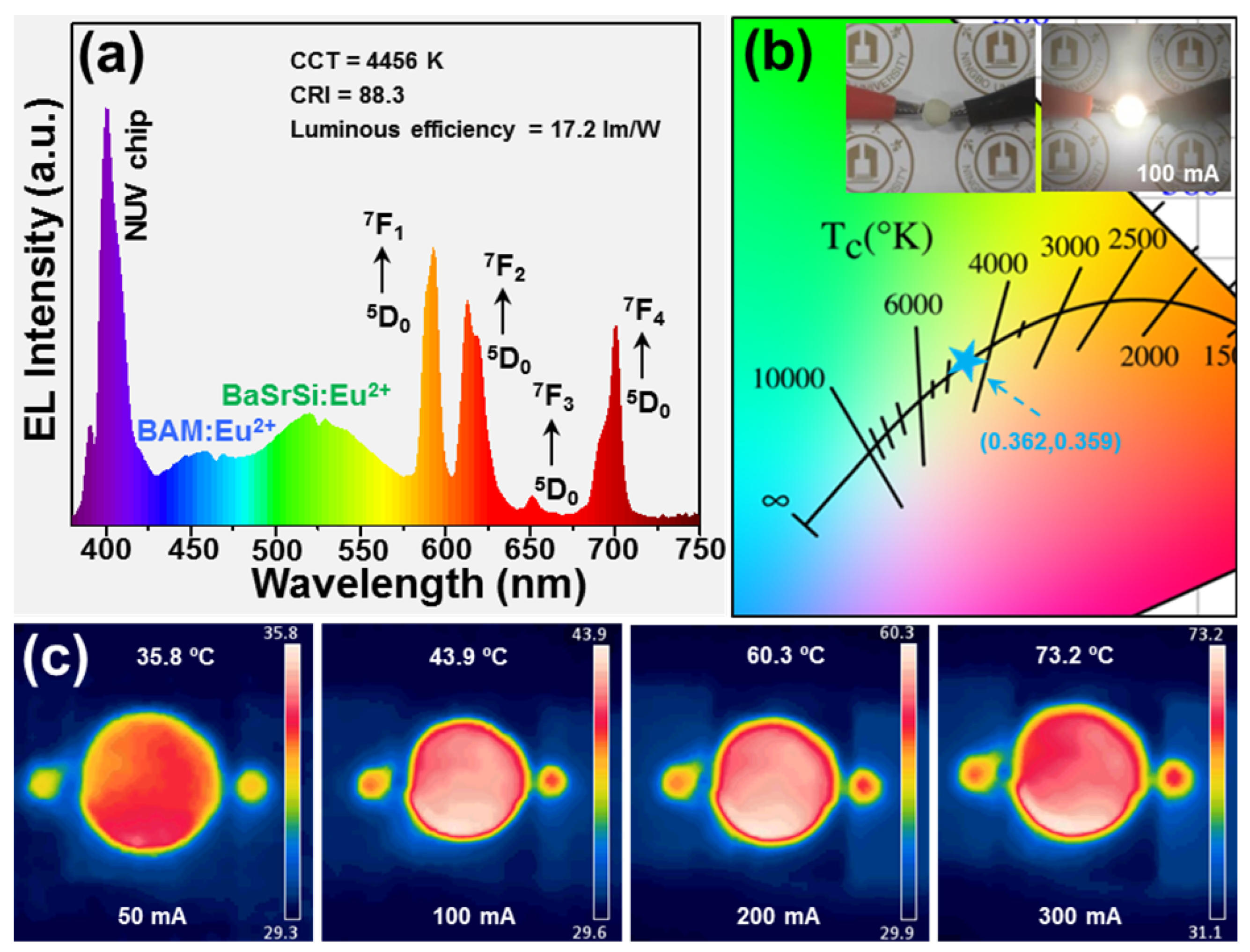
3.3. EL Performance of Fabricated White-LED
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, T.; Luo, L.; Du, P.; Lis, S.; Rodríguez-Mendoza, U.R.; Lavín, V.; Martín, I.R.; Runowski, M. Pressure-triggered enormous redshift and enhanced emission in Ca2Gd8Si6O26:Ce3+ phosphors: Ultrasensitive, thermally-stable and ultrafast response pressure monitoring. Chem. Eng. J. 2022, 443, 136414. [Google Scholar] [CrossRef]
- Lv, H.; Du, P.; Li, W.; Luo, L. Tailoring of Upconversion Emission in Tm3+/Yb3+-Codoped Y2Mo3O12 Submicron Particles Via Thermal Stimulation Engineering for Noninvasive Thermometry. ACS Sustain. Chem. Eng. 2022, 10, 2450–2460. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, X.; Gao, W.; Liu, H.; Nie, L.; Guo, H.; Zhao, F.; Yu, S.; Wang, T. Optical enhancement of nonstoichiometry-induced heterojunction in lanthanide doped double perovskite phosphors for WLEDs and scintillation applications. Chem. Eng. J. 2022, 442, 136235. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Qiao, J.; Molokeev, M.S.; Xia, Z. Rapid Synthesis of Red-Emitting Sr2Sc0.5Ga1.5O5:Eu2+ Phosphors and the Tunable Photoluminescence Via Sr/Ba Substitution. Adv. Opt. Mater. 2021, 9, 2100131. [Google Scholar] [CrossRef]
- Wang, T.; Xu, L.; Cui, J.; Wu, J.; Li, Z.; Wu, Y.; Tian, B.; Tian, Y. Enhanced Charge Separation for Efficient Photocatalytic H2 Production by Long-Lived Trap-State-Induced Interfacial Charge Transfer. Nano Lett. 2022, 22, 6664–6670. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, H.; Ji, R.; Wang, H.; Seto, T.; Wang, Y. Ca2YHf2Al3O12:Ce3+, Mn2+: Energy transfer and PL/CL properties of an efficient emission-tunable phosphor for LEDs and FEDs. Inorg. Chem. Front. 2021, 8, 5113–5123. [Google Scholar] [CrossRef]
- Dang, P.; Li, G.; Yun, X.; Zhang, Q.; Liu, D.; Lian, H.; Shang, M.; Lin, J. Thermally stable and highly efficient red-emitting Eu3+-doped Cs3GdGe3O9 phosphors for WLEDs: Non-concentration quenching and negative thermal expansion. Light Sci. Appl. 2021, 10, 29. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Wang, Y. Cyan Broad-Band Emission Phosphor with Scandium Silicon Multiple-Ring Structure for White Light-Emitting Diodes and Field Emission Displays. Inorg. Chem. 2021, 60, 8870–8879. [Google Scholar] [CrossRef]
- Panigrahi, K.; Nag, A. Challenges and Strategies to Design Phosphors for Future White Light Emitting Diodes. J. Phys. Chem. C 2022, 126, 8553–8564. [Google Scholar] [CrossRef]
- Khan, W.U.; Zhou, L.; Li, X.; Zhou, W.; Khan, D.; Niaz, S.; Wu, M. Single phase white LED phosphor Ca3YAl3B4O15:Ce3+,Tb3+,Sm3+ with superior performance: Color-tunable and energy transfer study. Chem. Eng. J. 2021, 410, 128455. [Google Scholar] [CrossRef]
- Cao, M.; Tian, J.; Zhuang, W.; Liu, R.; Liu, Y.; Chen, G.; Zhou, G.; Wang, L.; Wang, J. Multisite Cation Regulation of Broadband Cyan-Emitting (Ba1-xSrx)9Lu2Si6O24/Eu2+ Phosphors for Full-Spectrum wLEDs. Inorg. Chem. 2022, 61, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiong, P.; Liu, X.; Wang, X.; Wu, S.; Liu, Q.; Peng, M.; Chen, Y. A promising blue-emitting phosphor CaYGaO4:Bi3+ for near-ultraviolet (NUV) pumped white LED application and the emission improvement by Li+ ions. J. Mater. Chem. C 2021, 9, 303–312. [Google Scholar] [CrossRef]
- Dang, P.; Zhang, Q.; Liu, D.; Li, G.; Lian, H.; Shang, M.; Lin, J. Hetero-valent substitution strategy toward orange-red luminescence in Bi3+ doped layered perovskite oxide phosphors for high color rendering index white light-emitting diodes. Chem. Eng. J. 2021, 420, 127640. [Google Scholar] [CrossRef]
- Gupta, S.K.; Abdou, H.; Segre, C.U.; Mao, Y. Excitation-Dependent Photoluminescence of BaZrO3:Eu3+ Crystals. Nanomaterials 2022, 12, 3028. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Tang, J.; Li, W.; Luo, L. Exploiting the diverse photoluminescence behaviors of NaLuF4:xEu3+ nanoparticles and g-C3N4 to realize versatile applications in white light-emitting diode and optical thermometer. Chem. Eng. J. 2021, 406, 127165. [Google Scholar] [CrossRef]
- Luo, M.; Sha, X.; Chen, B.; Zhang, X.; Yu, H.; Li, X.; Zhang, J.; Xu, S.; Cao, Y.; Wang, Y.; et al. Optical transition properties, internal quantum efficiencies, and temperature sensing of Er3+ doped BaGd2O4 phosphor with low maximum phonon energy. J. Am. Ceram. Soc. 2022, 105, 3353–3363. [Google Scholar] [CrossRef]
- Du, P.; Tang, J.; Li, W.; Luo, L.; Runowski, M. Manipulating concentration quenching and thermal stability of Eu3+-activated NaYbF4 nanoparticles via phase transition strategy toward diversified applications. Mater. Today Chem. 2022, 26, 101013. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Liu, Y.; Jiang, F.; Hong, M. Lanthanide-Doped KGd2F7 Nanocrystals: Controlled Synthesis, Optical Properties, and Spectroscopic Identification of the Optimum Core/Shell Architecture for Highly Enhanced Upconverting Luminescence. Cryst. Growth Des. 2019, 19, 2340–2349. [Google Scholar] [CrossRef]
- Cao, J.; Chen, W.; Xu, D.; Hu, F.; Chen, L.; Guo, H. Wide-range thermometry based on green up-conversion of Yb3+/Er3+ co-doped KLu2F7 transparent bulk oxyfluoride glass ceramics. J. Lumin. 2018, 194, 219–224. [Google Scholar] [CrossRef]
- Ding, Y.; Teng, X.; Zhu, H.; Wang, L.; Pei, W.; Zhu, J.; Huang, L.; Huang, W. Orthorhombic KSc2F7:Yb/Er nanorods: Controlled synthesis and strong red upconversion emission. Nanoscale 2013, 5, 11928–11932. [Google Scholar] [CrossRef]
- Chen, S.; Song, W.; Cao, J.; Hu, F.; Guo, H. Highly sensitive optical thermometer based on FIR technique of transparent NaY2F7:Tm3+/Yb3+ glass ceramic. J. Alloys Compd. 2020, 825, 154011. [Google Scholar] [CrossRef]
- Ye, G.; Fang, L.; Zhou, X.; Xia, H.; Song, H.; Chen, B. Original KGd2F7 nanocrystals in fluoro-oxide glass ceramics by Dy3+/Sm3+ co-doped for white light emission. J. Alloys Compd. 2022, 912, 165126. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pietrasik, E.; Pisarski, W.A. Luminescence of SiO2-BaF2:Tb3+,Eu3+ Nano-Glass-Ceramics Made from Sol–Gel Method at Low Temperature. Nanomaterials 2022, 12, 259. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Xia, W.; Wang, Y.; Ling, F.; Jiang, S.; Xiang, G.; Zhou, X.; Li, D.; Hua, Y. Investigation on the optical sensing behaviors in single Eu3+-activated Sr2InSbO6 phosphors under green light excitation. J. Alloys Compd. 2022, 905, 164322. [Google Scholar] [CrossRef]
- Du, P.; Wan, X.; Luo, L.; Li, W.; Li, L. Thermally Stable Tb3+/Eu3+-Codoped K0.3Bi0.7F2.4 Nanoparticles with Multicolor Luminescence for White-Light-Emitting Diodes. ACS Appl. Nano Mater. 2021, 4, 7062–7071. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Auzel, F. A fundamental self-generated quenching center for lanthanide-doped high-purity solids. J. Lumin. 2002, 100, 125–130. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, B.; Hua, R.; Sun, J.; Cheng, L.; Zhong, H.; Li, X.; Zhang, J.; Zheng, Y.; Yu, T.; et al. Optical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor. J. Appl. Phys. 2011, 109, 053511. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Tian, Y.; Huang, P.; Shi, Q.; Cui, C. High luminescent brightness and thermal stability of red emitting Li3Ba2Y3(WO4)8:Eu3+ phosphor. Ceram. Int. 2016, 42, 13648–13653. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Du, J.; Pan, X.; Liu, Z.; Jing, Y.; Wang, B.; Luo, L.; Wang, J.; Du, P. Highly efficient Eu3+-activated Ca2Gd8Si6O26 red-emitting phosphors: A bifunctional platform towards white light-emitting diode and ratiometric optical thermometer applications. J. Alloys Compd. 2021, 859, 157843. [Google Scholar] [CrossRef]
- Huang, X.; Liang, J.; Rtimi, S.; Devakumar, B.; Zhang, Z. Ultra-high color rendering warm-white light-emitting diodes based on an efficient green-emitting garnet phosphor for solid-state lighting. Chem. Eng. J. 2021, 405, 126950. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.; Yang, T.; Li, J.; Yang, S.; Zhu, J. Bi4BPO10:Dy3+: A single-phase white-emitting phosphor for light-emitting diodes. Mater. Today Chem. 2022, 26, 101199. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, D.; Zhang, R.; Jia, L.; Yao, Q. Enhancing luminescence intensity and improving thermostability of red phosphors Li3Ba2La3(WO4)8:Eu3+ by co-doping with Sm3+ ions. J. Alloys Compd. 2022, 891, 161973. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Wu, Q.; Zhu, J.; Cai, P.; Du, P. Room-Temperature Synthesis of Highly-Efficient Eu3+-Activated KGd2F7 Red-Emitting Nanoparticles for White Light-Emitting Diode. Nanomaterials 2022, 12, 4397. https://doi.org/10.3390/nano12244397
Zhong Y, Wu Q, Zhu J, Cai P, Du P. Room-Temperature Synthesis of Highly-Efficient Eu3+-Activated KGd2F7 Red-Emitting Nanoparticles for White Light-Emitting Diode. Nanomaterials. 2022; 12(24):4397. https://doi.org/10.3390/nano12244397
Chicago/Turabian StyleZhong, Yongqiang, Qian Wu, Jiujun Zhu, Peiqing Cai, and Peng Du. 2022. "Room-Temperature Synthesis of Highly-Efficient Eu3+-Activated KGd2F7 Red-Emitting Nanoparticles for White Light-Emitting Diode" Nanomaterials 12, no. 24: 4397. https://doi.org/10.3390/nano12244397
APA StyleZhong, Y., Wu, Q., Zhu, J., Cai, P., & Du, P. (2022). Room-Temperature Synthesis of Highly-Efficient Eu3+-Activated KGd2F7 Red-Emitting Nanoparticles for White Light-Emitting Diode. Nanomaterials, 12(24), 4397. https://doi.org/10.3390/nano12244397






