Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Banana Peel (BP) Powder
2.3. Proximate and Chemical Composition Analyses
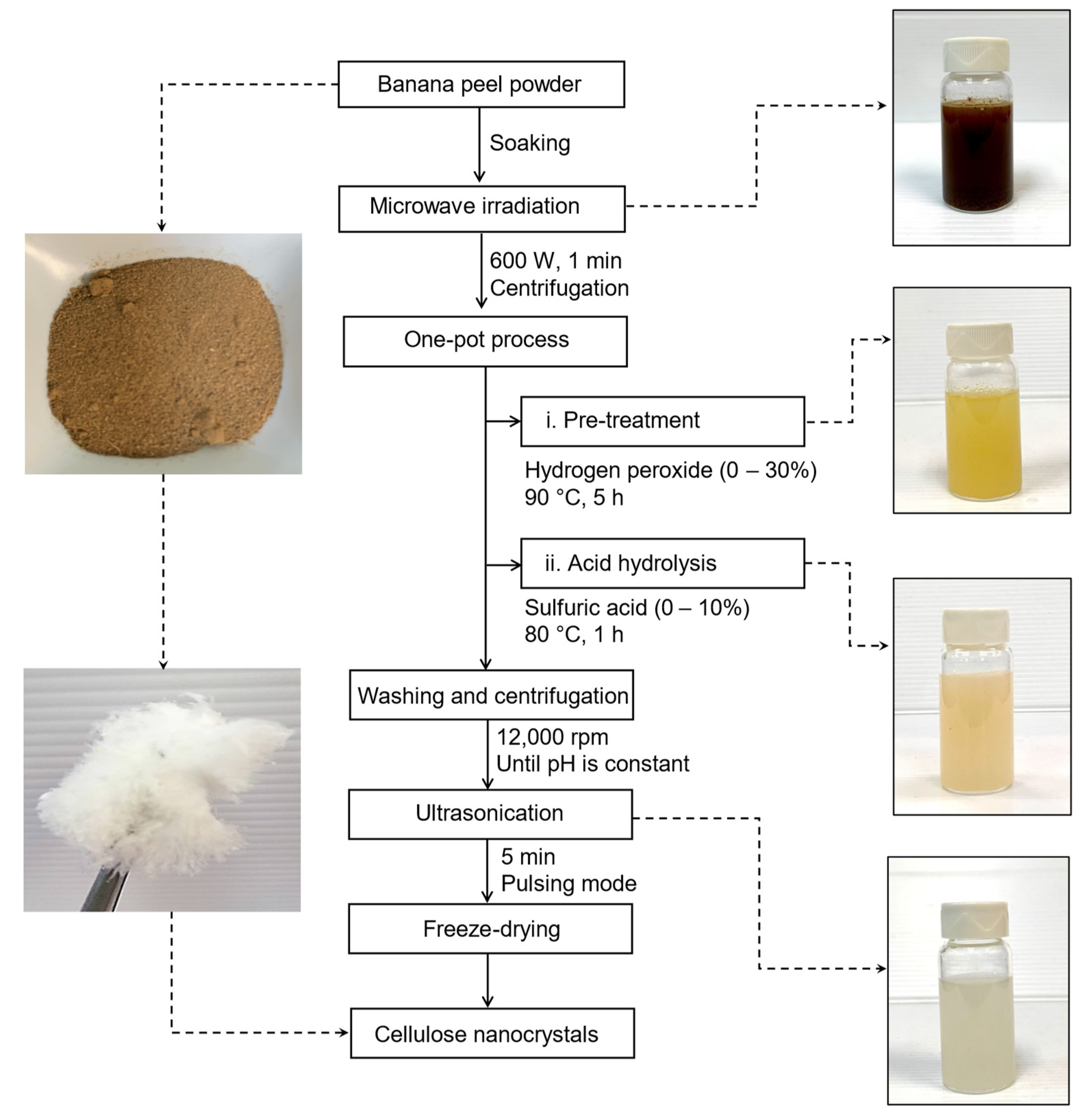
2.4. Isolation of CNCs from BP Powder by One-Pot Process
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
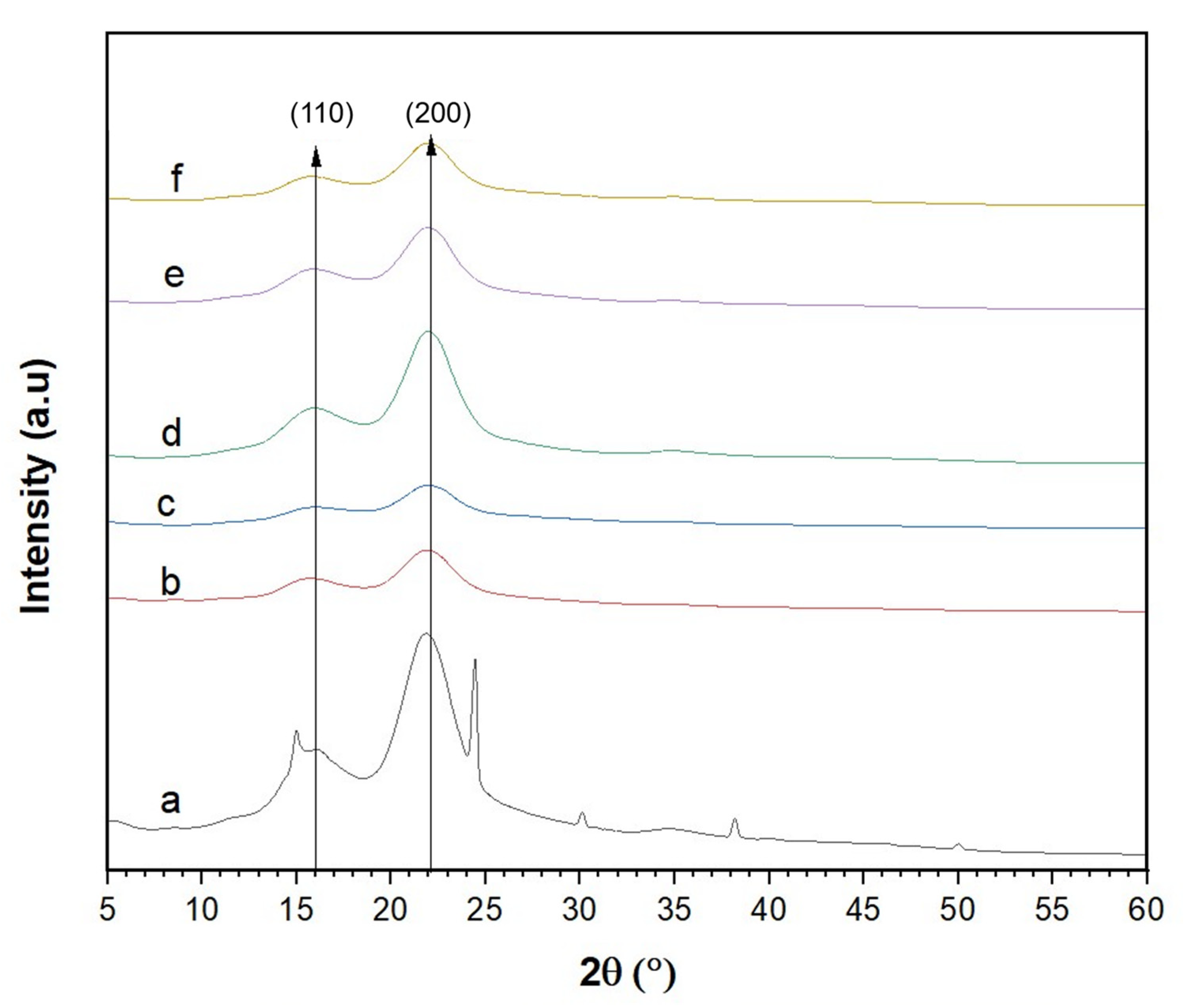
2.6. X-ray Diffraction (XRD) Analysis
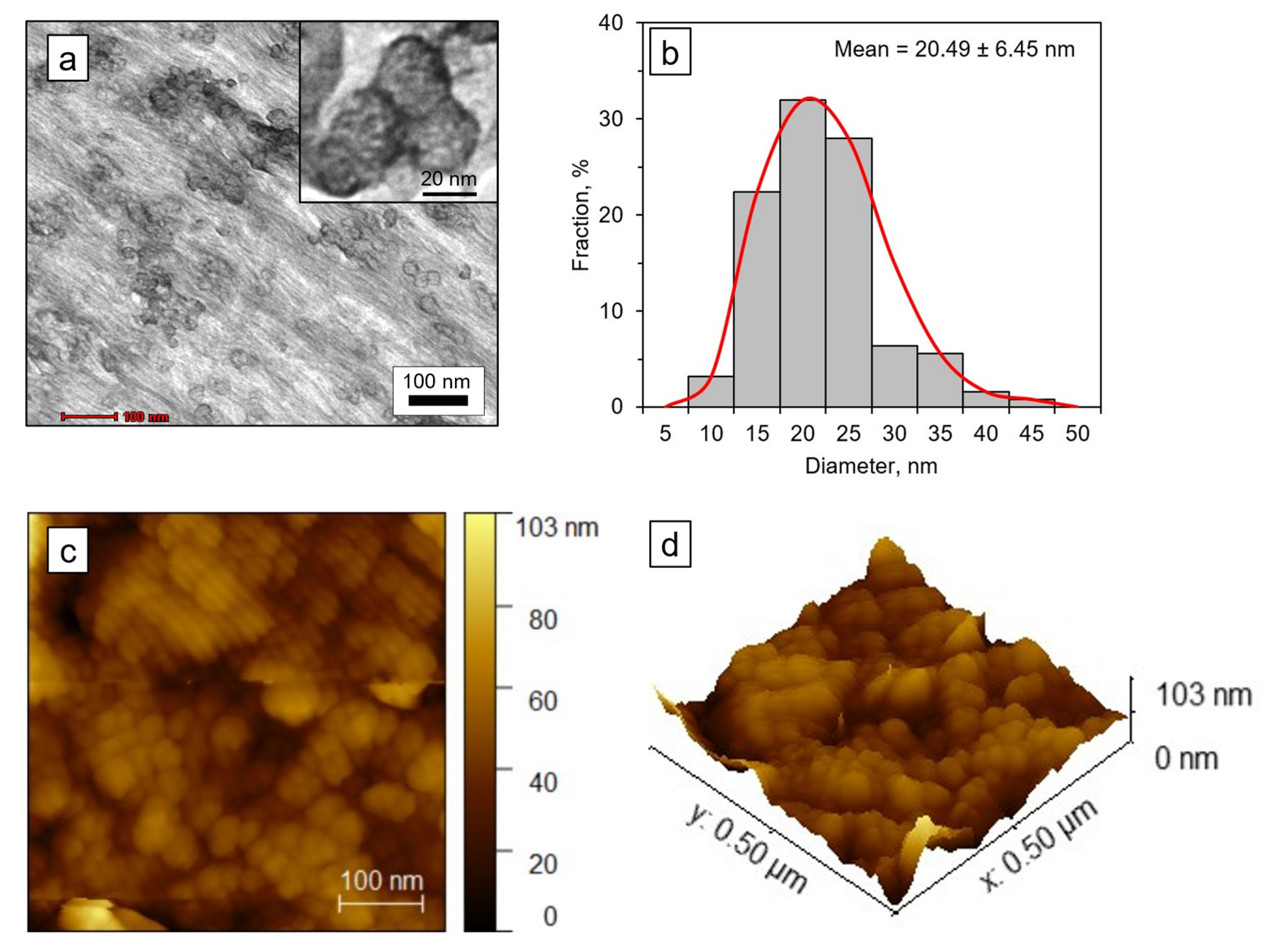
2.7. Scanning Electron Microscope (SEM) Analysis
2.8. Atomic Force Microscope (AFM) Analysis
2.9. Transmission Electron Microscopy (TEM)
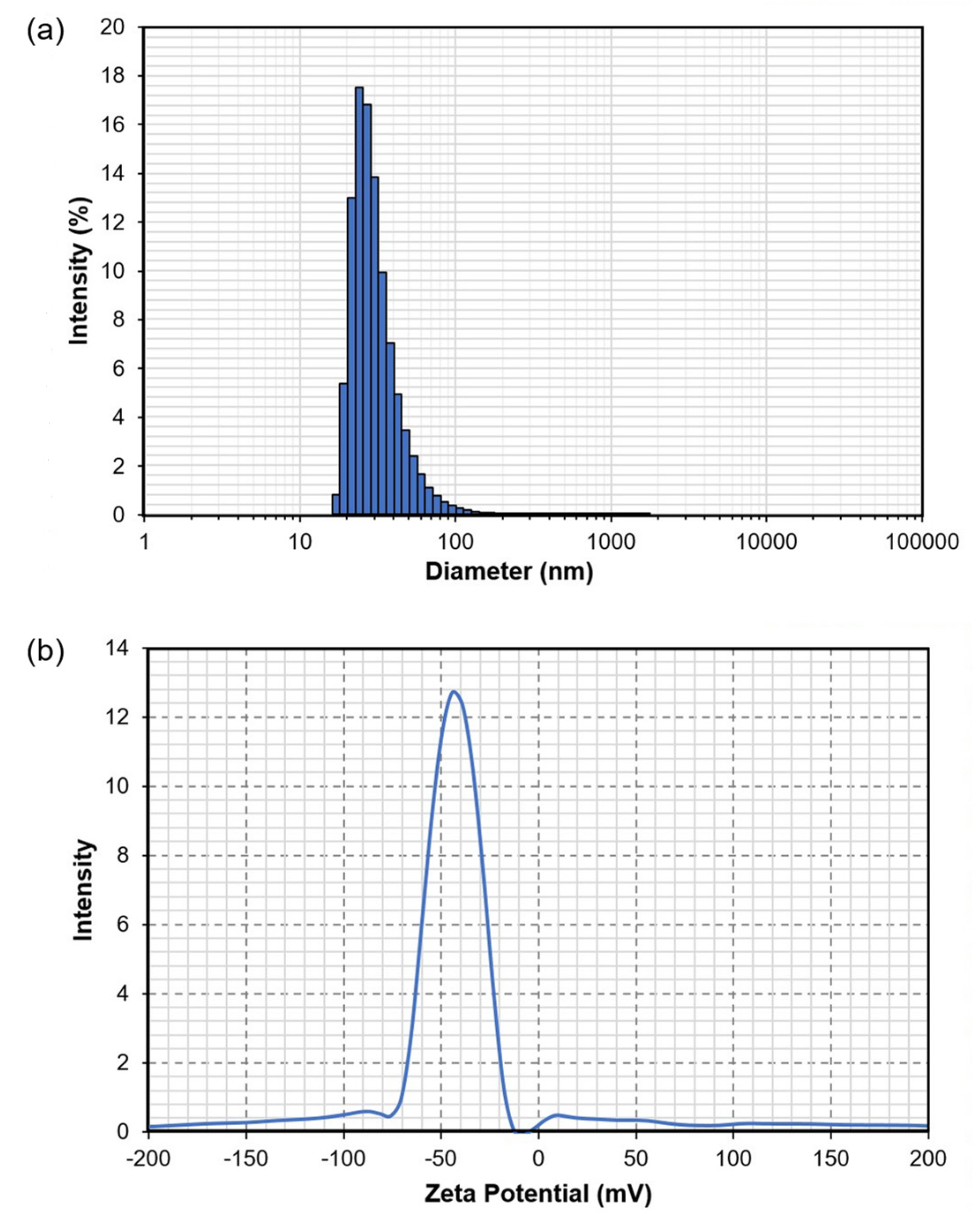
2.10. Particle Size and Zeta Potential Measurements
3. Results and Discussion
3.1. Proximate and Chemical Compositions of the BP Powder
3.2. Isolation of CNCs from BP Powder by One-Pot Process
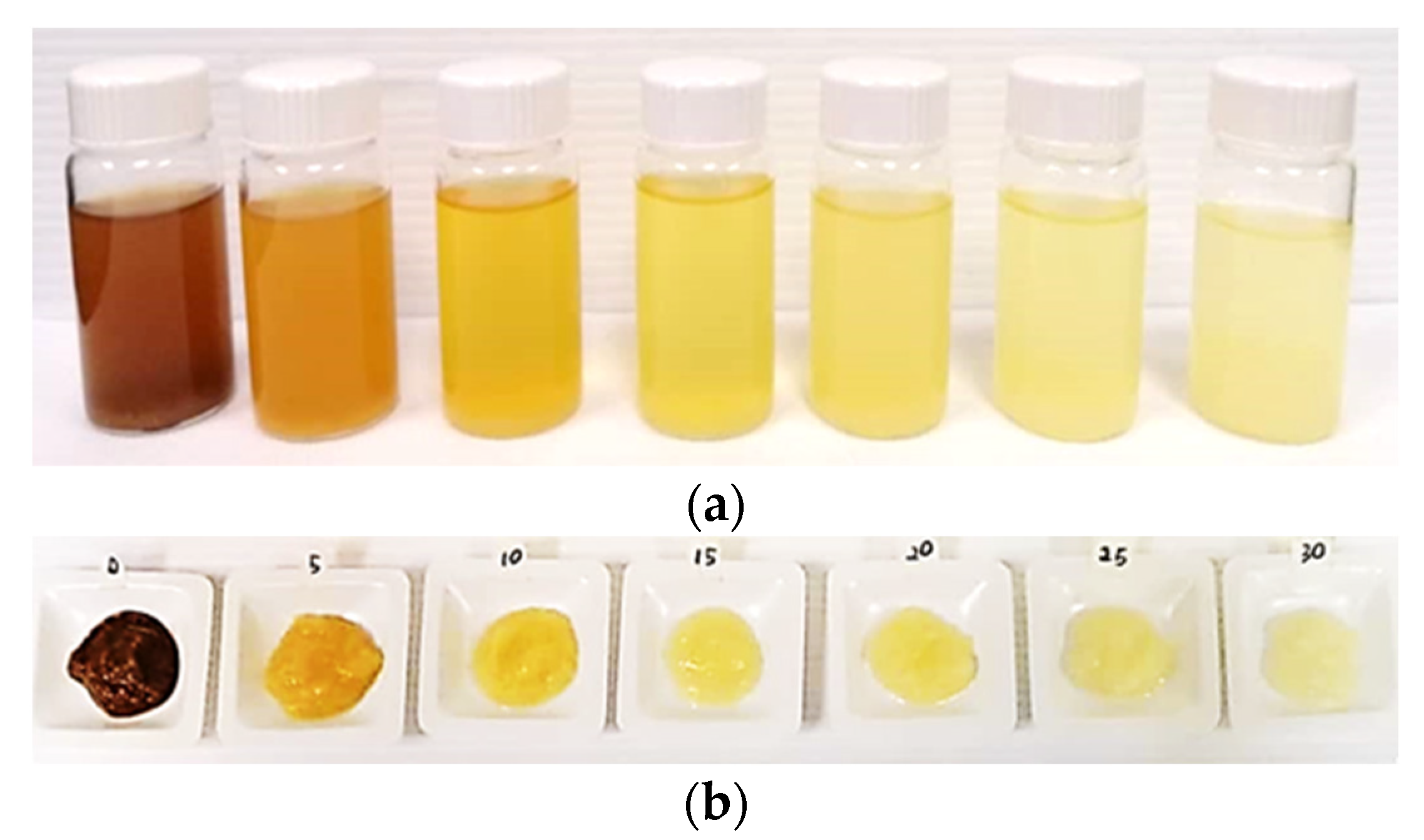
3.2.1. Effect of Hydrogen Peroxide Concentration on the Pre-Treatment of BP Powder
3.2.2. Effect of Sulfuric Acid Concentration on the Hydrolysis of BP Powder
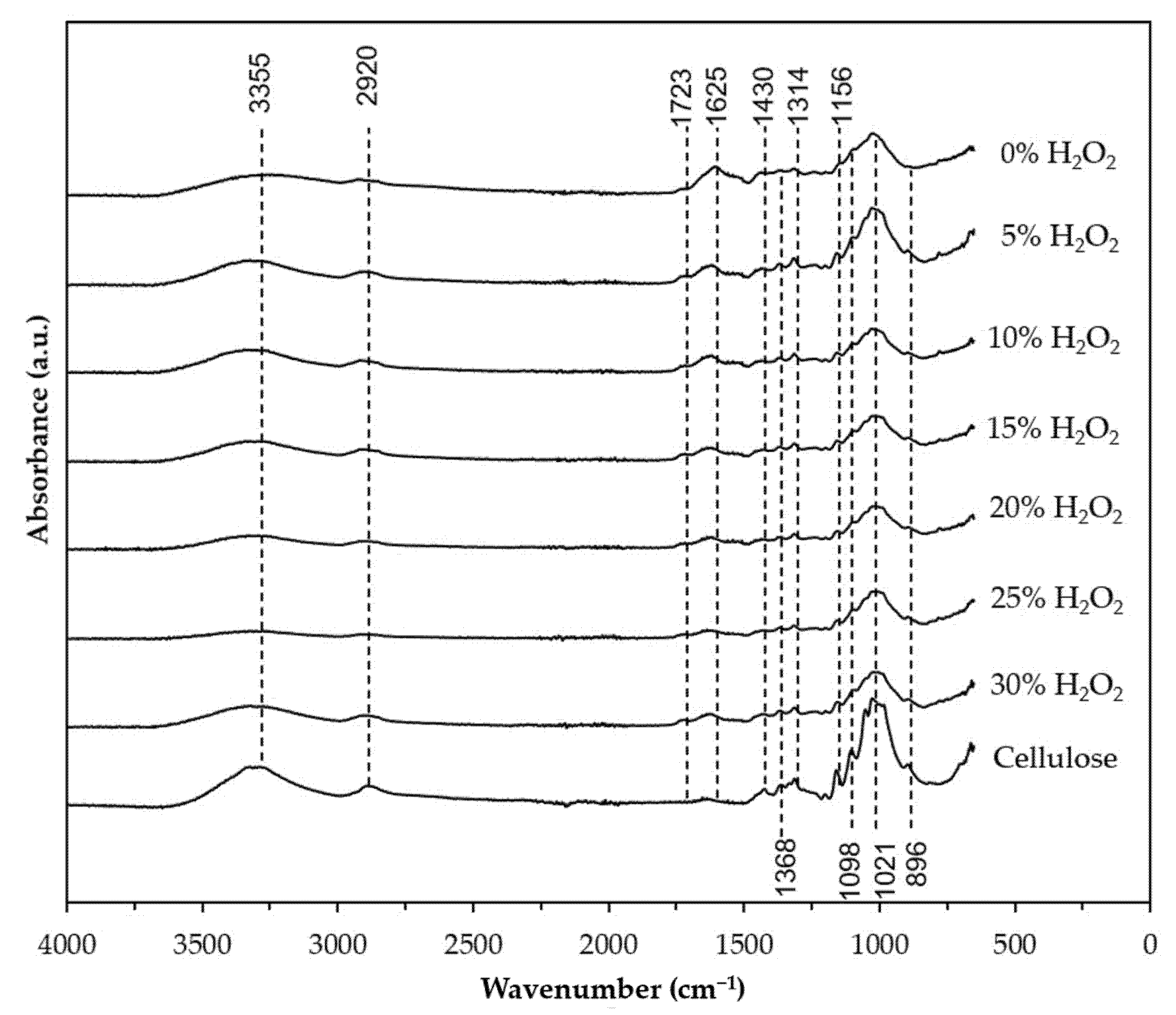
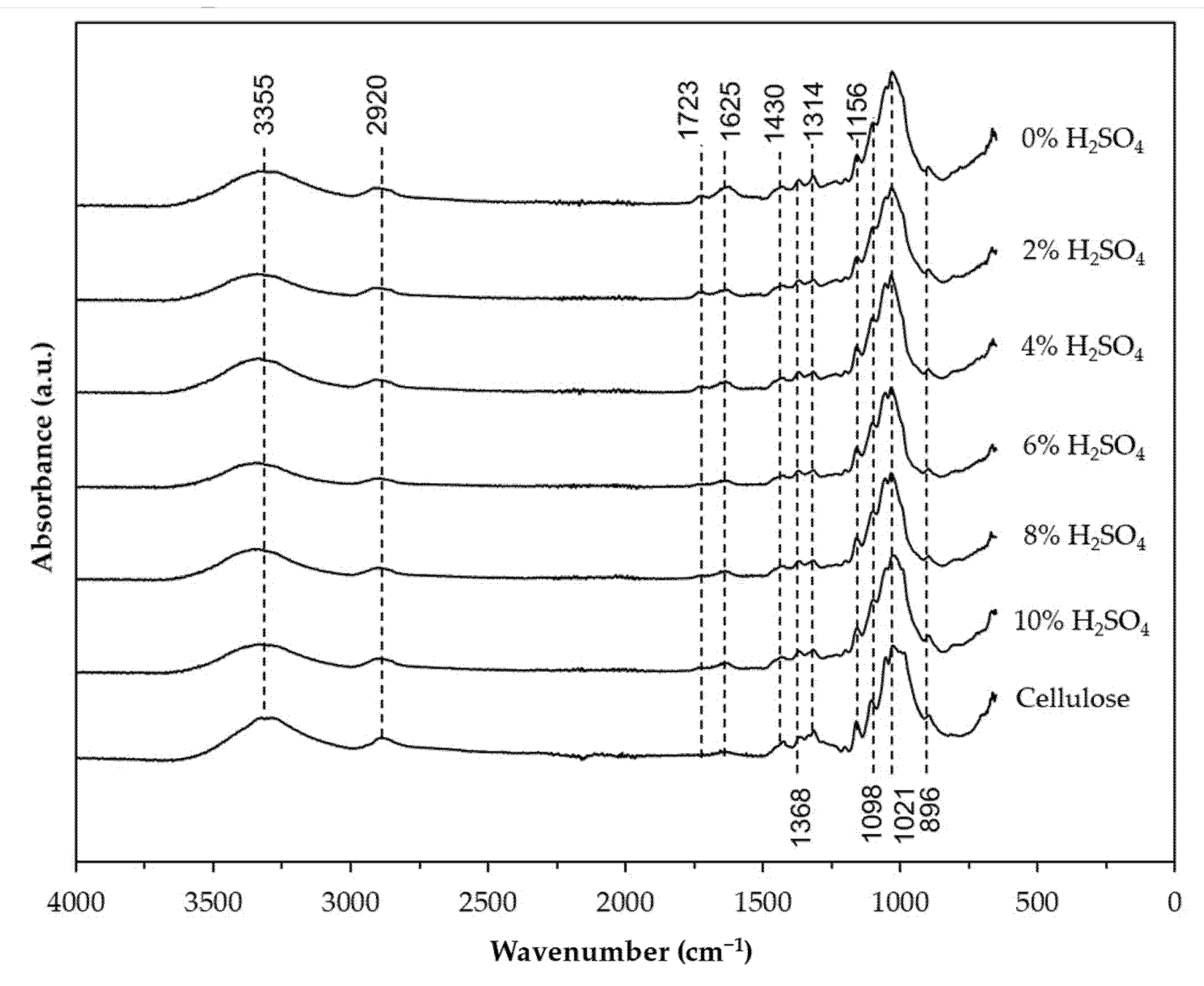
3.3. FTIR Analysis
3.4. Crystallinity of the CNCs
3.5. SEM, TEM, and AFM Analyses
3.6. Particle Size and Zeta Potential Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials Chemistry and the Futurist Eco-Friendly Applications of Nanocellulose: Status and Prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Characterization of Nanocellulose Extracted from Short, Medium and Long Grain Rice Husks. Ind. Crops Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Isolation of Oxidized Nanocellulose from Rice Straw Using the Ammonium Persulfate Method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S. Energy Efficient Process for Valorization of Corn Cob as a Source for Nanocrystalline Cellulose and Hemicellulose Production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.; Velayutham, T.S.; Chen, Y.W.; Lee, H.V. TEMPO-Oxidized Nanocellulose Films Derived from Coconut Residues: Physicochemical, Mechanical and Electrical Properties. Int. J. Biol. Macromol. 2021, 180, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Arrebola, R.I.; Bascón-Villegas, I.; Sánchez-Gutiérrez, M.; Domínguez-Robles, J.; Rodríguez, A. Industrial Application of Orange Tree Nanocellulose as Papermaking Reinforcement Agent. Cellulose 2020, 27, 10781–10797. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hasanulbasori, M.A.; Chiat, P.F.; Lee, H.V. Pyrus Pyrifolia Fruit Peel as Sustainable Source for Spherical and Porous Network Based Nanocellulose Synthesis via One-Pot Hydrolysis System. Int. J. Biol. Macromol. 2019, 123, 1305–1319. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of Nano Cellulose Fibers from the Banana Peel and Bract for Production of Acetyl and Lauroyl Cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Bardhan, P.; Mukherjee, A.K.; Deka, R.C.; Mandal, M.; Kalita, E. Integrated Pretreatment of Banana Agrowastes: Structural Characterization and Enhancement of Enzymatic Hydrolysis of Cellulose Obtained from Banana Peduncle. Int. J. Biol. Macromol. 2022, 201, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Tibolla, H.; Maria, F.; Cecilia, F. Cellulose Nanofibers Produced from Banana Peel by Chemical and Enzymatic Treatment LWT-Food Science and Technology Cellulose Nano Fi Bers Produced from Banana Peel by Chemical and Enzymatic Treatment. LWT- Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Mohd Zaini, H.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana Peels as a Bioactive Ingredient and Its Potential Application in the Food Industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Alzate Acevedo, S.; Díaz Carrillo, Á.J.; Flórez-López, E.; Grande-Tovar, C.D. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 2021, 26, 5282. [Google Scholar] [CrossRef] [PubMed]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Ying, Z.; Zhang, J.; Sun, D.; Li, X. Contrasting Effects of Banana Peels Waste and Its Biochar on Greenhouse Gas Emissions and Soil Biochemical Properties. Process Saf. Environ. Prot. 2019, 122, 366–377. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Enzymatic Treatment: Study of Process Conditions. Ind. Crops Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Vicente, A.A.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Chemical and Mechanical Treatments: Characterization and Cytotoxicity Assessment. Food Hydrocoll. 2018, 75, 192–201. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and Characterization of Cellulose Nanofibers from Banana Peels. Cellulose 2014, 21, 417–432. [Google Scholar] [CrossRef]
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of Banana Peels Wastes as Potential Slow Pyrolysis Feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of Pectin Extraction from Banana Peels with Citric Acid by Using Response Surface Methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Orozco, R.S.; Hernández, P.B.; Morales, G.R.; Núñez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramírez, N.F.; Díaz, C.E.B.; Vázquez, P.C. Characterization of Lignocellulosic Fruit Waste as an Alternative Feedstock for Bioethanol Production. BioResources 2014, 9, 1873–1885. [Google Scholar]
- Khawas, P.; Deka, S.C. Comparative Nutritional, Functional, Morphological, and Diffractogram Study on Culinary Banana (Musa ABB) Peel at Various Stages of Development. Int. J. Food Prop. 2016, 19, 2832–2853. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.C.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and Interface Engineering for Nanocellulosic Advanced Materials. Adv. Mater. 2021, 33, 2002264. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and Its Derived Composite Electrodes toward Supercapacitors: Fabrication, Properties, and Challenges. J. Bioresour. Bioprod. 2022. [Google Scholar] [CrossRef]
- Niu, Z.; Cheng, W.; Cao, M.; Wang, D.; Wang, Q.; Han, J.; Long, Y.; Han, G. Recent Advances in Cellulose-Based Flexible Triboelectric Nanogenerators. Nano Energy 2021, 87, 106175. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A Mixed Acid Methodology to Produce Thermally Stable Cellulose Nanocrystal at High Yield Using Phosphoric Acid. J. Bioresour. Bioprod. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Franco, T.S. Nanocellulose in Food Science and Technology—Potential, Advantages and Gaps of Research. Nov. Tech. Nutr. Food Sci. 2018, 1, 2016–2017. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-Stabilized Pickering Emulsions and Their Applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Singanusong, R.; Tochampa, W.; Kongbangkerd, T.; Sodchit, C. Extraction and Properties of Cellulose From Banana Peels. Suranaree J. Sci. Technol. 2013, 21, 14. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Toxqui-Terán, A.; Pérez-Camacho, O. One-Pot Isolation of Nanocellulose Using Pelagic Sargassum Spp. from the Caribbean Coastline. J. Appl. Phycol. 2022, 34, 637–645. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Pelissari, F.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Achira as a Source of Biodegradable Materials: Isolation and Characterization of Nanofibers. Carbohydr. Polym. 2015, 123, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and Characterization of Cellulose Nanocrystal Extracted from Calotropis Procera Biomass. Bioresour. Bioprocess. 2019, 6, 45. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Zakaria, N.N.; Pindi, W.; Siddiquee, S.; Misson, M.; Ongkudon, C.M.; Mohd Jamil, N.H.A.; Lenggoro, W. Isolation of Nanocellulose from Saba’ (Musa Acuminata × Balbisiana) Banana Peel by One-Pot Oxidation-Hydrolysis System. Adv. Agric. Food Res. J. 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Camacho, M.; Ureña, Y.R.C.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Vega Baudrit, J.R. Synthesis and Characterization of Nanocrystalline Cellulose Derived from Pineapple Peel Residues. J. Renew. Mater. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Ibiyinka, O.; Akinwumi Oluwafemi, A.; Adebayo, O.O.; Olugbenga Kayode, P. Comparative Study of Chemical Composition and Evaluation of the In-Vitro Antioxidant Capacity of Unripe and Ripe Banana Species (Musa Sapientum) Biowastes. Int. J. Agric. Sci. Food Technol. 2021, 7, 061–066. [Google Scholar] [CrossRef]
- Pyar, H.; Peh, K.K. Chemical Compositions of Banana Peels (Musa Sapientum) Fruits Cultivated in Malaysia Using Proximate Analysis. Res. J. Chem. Environ. 2018, 22, 108–113. [Google Scholar]
- Deb, S.; Kumar, Y.; Saxena, D.C. Functional, Thermal and Structural Properties of Fractionated Protein from Waste Banana Peel. Food Chem. X 2022, 13, 100205. [Google Scholar] [CrossRef]
- Dibanda Romelle, F.; Ashwini, R.P.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Happi Emaga, T.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the Stage of Maturation and Varieties on the Chemical Composition of Banana and Plantain Peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Tiwari, G.; Sharma, A.; Kumar, A.; Sharma, S. Assessment of Microwave-Assisted Alkali Pretreatment for the Production of Sugars from Banana Fruit Peel Waste. Biofuels 2019, 10, 3–10. [Google Scholar] [CrossRef]
- Su, J.; Zhu, H.; Wang, L.; Liu, X.; Nie, S.; Xiong, J. Optimization of Microwave-Hydrogen Peroxide Pretreatment of Cellulose. BioResources 2016, 11, 7416–7430. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Li, F.; Xie, J.; Hse, C.Y.; Qi, J.; Jiang, Y.; Chen, Y. Dilute Alkali and Hydrogen Peroxide Treatment of Microwave Liquefied Rape Straw Residue for the Extraction of Cellulose Nanocrystals. J. Nanomater. 2017, 2017, 4049061. [Google Scholar] [CrossRef]
- Koshani, R.; Van De Ven, T.G.M.; Madadlou, A. Characterization of Carboxylated Cellulose Nanocrytals Isolated through Catalyst-Assisted H2O2 Oxidation in a One-Step Procedure. J. Agric. Food Chem. 2018, 66, 7692–7700. [Google Scholar] [CrossRef]
- Andrade, D.R.M.; Mendonça, M.H.; Helm, C.V.; Magalhães, W.L.E.; de Muniz, G.I.B.; Kestur, S.G. Assessment of Nano Cellulose from Peach Palm Residue as Potential Food Additive: Part II: Preliminary Studies. J. Food Sci. Technol. 2015, 52, 5641–5650. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Tao, H.; Zhao, M.; Yang, B.; Wen, D.; Yuan, Q.; Rao, G. Effect of Hydrogen Peroxide Pretreatment on the Enzymatic Hydrolysis of Cellulose. J. Food Process Eng. 2011, 34, 905–921. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of Alkaline Hydrogen Peroxide Pretreatment and Tween 80 to Enhance Enzymatic Hydrolysis of Sugarcane Bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef] [PubMed]
- Pavalaydon, K.; Ramasawmy, H.; Surroop, D. Comparative Evaluation of Cellulose Nanocrystals from Bagasse and Coir Agro-Wastes for Reinforcing PVA-Based Composites. Environ. Dev. Sustain. 2021, 24, 9963–9984. [Google Scholar] [CrossRef] [PubMed]
- Beyan, S.M.; Amibo, T.A.; Prabhu, S.V.; Ayalew, A.G. Production of Nanocellulose Crystal Derived from Enset Fiber Using Acid Hydrolysis Coupled with Ultrasonication, Isolation, Statistical Modeling, Optimization, and Characterizations. J. Nanomater. 2021, 2021, 7492532. [Google Scholar] [CrossRef]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and Kinetics Study of Spherical Cellulose Nanocrystal Extracted from Cotton Cloth Waste by Acid Hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum Spruceanum (Benth.) K. Schum. And Guazuma Crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Akinjokun, A.I.; Petrik, L.F.; Ogunfowokan, A.O.; Ajao, J.; Ojumu, T.V. Isolation and Characterization of Nanocrystalline Cellulose from Cocoa Pod Husk (CPH) Biomass Wastes. Heliyon 2021, 7, e06680. [Google Scholar] [CrossRef]
- Lapuz, A.R.; Tsuchikawa, S.; Inagaki, T.; Ma, T.; Migo, V. Production of Nanocellulose Film from Abaca Fibers. Crystals 2022, 12, 601. [Google Scholar] [CrossRef]
- Bassyouni, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Mosly, I. Extraction of Nanocellulose for Eco-Friendly Biocomposite Adsorbent for Wastewater Treatment. Polymers 2022, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, A.; Kassab, Z.; El Bouchti, M.; Hannache, H.; Qaiss, A.E.K.; Oumam, M.; El Achaby, M. Micro- and Nano-Structures of Cellulose from Eggplant Plant (Solanum Melongena L.) Agricultural Residue. Carbohydr. Polym. 2021, 253, 117311. [Google Scholar] [CrossRef]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and Characterization of Carboxymethyl Cellulose-Based Composite Films Reinforced by Cellulose Nanocrystals Derived from Pea Hull Waste for Food Packaging Applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef]
- Guimarães, J.L.; Frollini, E.; da Silva, C.G.; Wypych, F.; Satyanarayana, K.G. Characterization of Banana, Sugarcane Bagasse and Sponge Gourd Fibers of Brazil. Ind. Crops Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Nang An, V.; Chi Nhan, H.T.; Tap, T.D.; Van, T.T.T.; Van Viet, P.; Van Hieu, L. Extraction of High Crystalline Nanocellulose from Biorenewable Sources of Vietnamese Agricultural Wastes. J. Polym. Environ. 2020, 28, 1465–1474. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and Characterization of Alpha and Nanocrystalline Cellulose from Date Palm (Phoenix Dactylifera L.) Trunk Mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, S.; Abu-El Magd, E.E.; Sorbara, S.; Navarro, J.; Gil-San-millan, R. Spanish Poplar Biomass as a Precursor for Nanocellulose Extraction. Appl. Sci. 2021, 11, 6863. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and Characterization of Cellulose Nanofibers from Bamboo Using Microwave Liquefaction Combined with Chemical Treatment and Ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Burhani, D.; Septevani, A.A.; Setiawan, R.; Djannah, L.M.; Putra, M.A.; Kusumah, S.S.; Sondari, D. Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of Cnc/Cnf from Oil Palm Empty Fruit Bunches. Polymers 2021, 13, 2649. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of Nanocellulose with a Novel Shape from Walnut (Juglans Regia L.) Shell Agricultural Waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef]
- Doh, H.; Lee, M.H.; Whiteside, W.S. Physicochemical Characteristics of Cellulose Nanocrystals Isolated from Seaweed Biomass. Food Hydrocoll. 2020, 102, 105542. [Google Scholar] [CrossRef]
- Maciel, M.M.Á.D.; de Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Obtainment and Characterization of Nanocellulose from an Unwoven Industrial Textile Cotton Waste: Effect of Acid Hydrolysis Conditions. Int. J. Biol. Macromol. 2019, 126, 496–506. [Google Scholar] [CrossRef]
- Fattahi Meyabadi, T.; Dadashian, F.; Mir Mohamad Sadeghi, G.; Ebrahimi Zanjani Asl, H. Spherical Cellulose Nanoparticles Preparation from Waste Cotton Using a Green Method. Powder Technol. 2014, 261, 232–240. [Google Scholar] [CrossRef]
- Zianor Azrina, Z.A.; Beg, M.D.H.; Rosli, M.Y.; Ramli, R.; Junadi, N.; Alam, A.K.M.M. Spherical Nanocrystalline Cellulose (NCC) from Oil Palm Empty Fruit Bunch Pulp via Ultrasound Assisted Hydrolysis. Carbohydr. Polym. 2017, 162, 115–120. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering Emulsions Stabilized by Spherical Cellulose Nanocrystals. Carbohydr. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef]
- Sinquefield, S.; Ciesielski, P.N.; Li, K.; Gardner, D.J.; Ozcan, S. Nanocellulose Dewatering and Drying: Current State and Future Perspectives. ACS Sustain. Chem. Eng. 2020, 8, 9601–9615. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of Different Characterization Methods for Nanoparticle Dispersions before and after Aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Moreno, G.; Ramirez, K.; Esquivel, M.; Jimenez, G. Isolation and Characterization of Nanocellulose Obtained from Industrial Crop Waste Resources by Using Mild Acid Hydrolysis. J. Renew. Mater. 2018, 6, 362–369. [Google Scholar] [CrossRef]


| Banana Variety | % Dry Weight | Reference | |||
|---|---|---|---|---|---|
| Moisture | Ash | Fat | Protein | ||
| Saba (Musa acuminata × balbisiana) | 8.2 ± 0.2 | 13.8 ± 0.3 | 12.0 ± 0.2 | 5.4 ± 0.1 | Present study * |
| Musa acuminata | 10.0 ± 0.9 | 15.6 ± 0.3 | 10.4 ± 0.4 | 10.6 ± 0.2 | [37] |
| Musa sapientum | 15.5–17.8 | 11.3–14.7 | 2.4–3.3 | 2.2–2.7 | [35] |
| Musa sapientum Linn. cv. Mali-On | 7.7 | 12.6 | 4.3 | 3.7 | [29] |
| Musa sapientum | 50.5 ± 2.7 | 8.8 ± 0.5 | 1.6 ± 0.1 | 5.3 ± 0.02 | [36] |
| Banana Variety | % Dry Weight | Reference | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| Saba (Musa acuminata × balbisiana) | 12.1 ± 0.3 | 14.8 ± 0.9 | 15.7 ± 2.1 | Present study * |
| n.a. | 11.5 | 25.5 | 9.8 | [19] |
| Terra (Musa paradisiaca) | 12.1 | 10.2 | 2.9 | [15] |
| Terra (Musa paradisiaca) | 7.5 | - | 7.9 | [14] |
| Mpologoma, Kisansa and Kibuzi | 9.9 ± 0.1 | 41.4 ± 0.8 | 8.9 ± 1.3 | [17] |
| Cavendish | 18.7 ± 3.2 | 20.3 ± 7.4 | 16.8 ± 4.6 | [18] |
| Musa ABB | 15.4 ± 1.5 | 4.0 ± 1.0 | 3.2 ± 0.8 | [20] |
| Sulfuric Acid Concentration (%) | Crystallinity Index (CrI) |
|---|---|
| BP powder | 58.1 |
| 0 | 64.0 |
| 2 | 65.6 |
| 4 | 64.1 |
| 6 | 85.2 |
| 8 | 77.8 |
| 10 | 71.7 |
| Avicel® PH-101 | 88.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Jamil, N.A.; Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials 2022, 12, 3537. https://doi.org/10.3390/nano12193537
Mohd Jamil NA, Jaffar SS, Saallah S, Misson M, Siddiquee S, Roslan J, Lenggoro W. Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials. 2022; 12(19):3537. https://doi.org/10.3390/nano12193537
Chicago/Turabian StyleMohd Jamil, Nurhidayah Azmirah, Syafiqah Syazwani Jaffar, Suryani Saallah, Mailin Misson, Shafiquzzaman Siddiquee, Jumardi Roslan, and Wuled Lenggoro. 2022. "Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System" Nanomaterials 12, no. 19: 3537. https://doi.org/10.3390/nano12193537
APA StyleMohd Jamil, N. A., Jaffar, S. S., Saallah, S., Misson, M., Siddiquee, S., Roslan, J., & Lenggoro, W. (2022). Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials, 12(19), 3537. https://doi.org/10.3390/nano12193537







