GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment
Abstract
1. Introduction
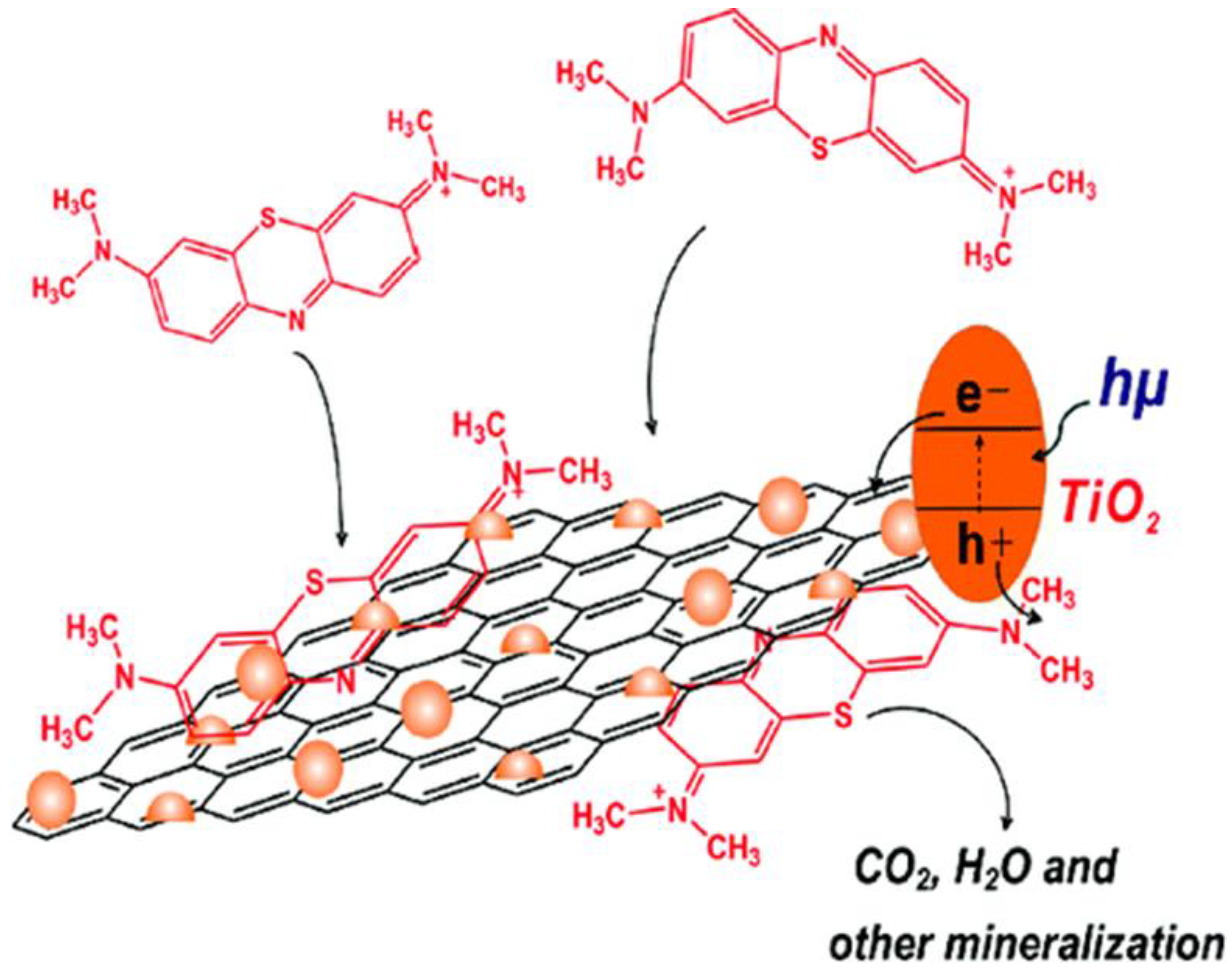
2. Dye
3. Heavy Metals
3.1. Arsenic
3.2. Mercury
3.3. Lead
3.4. Cadmium
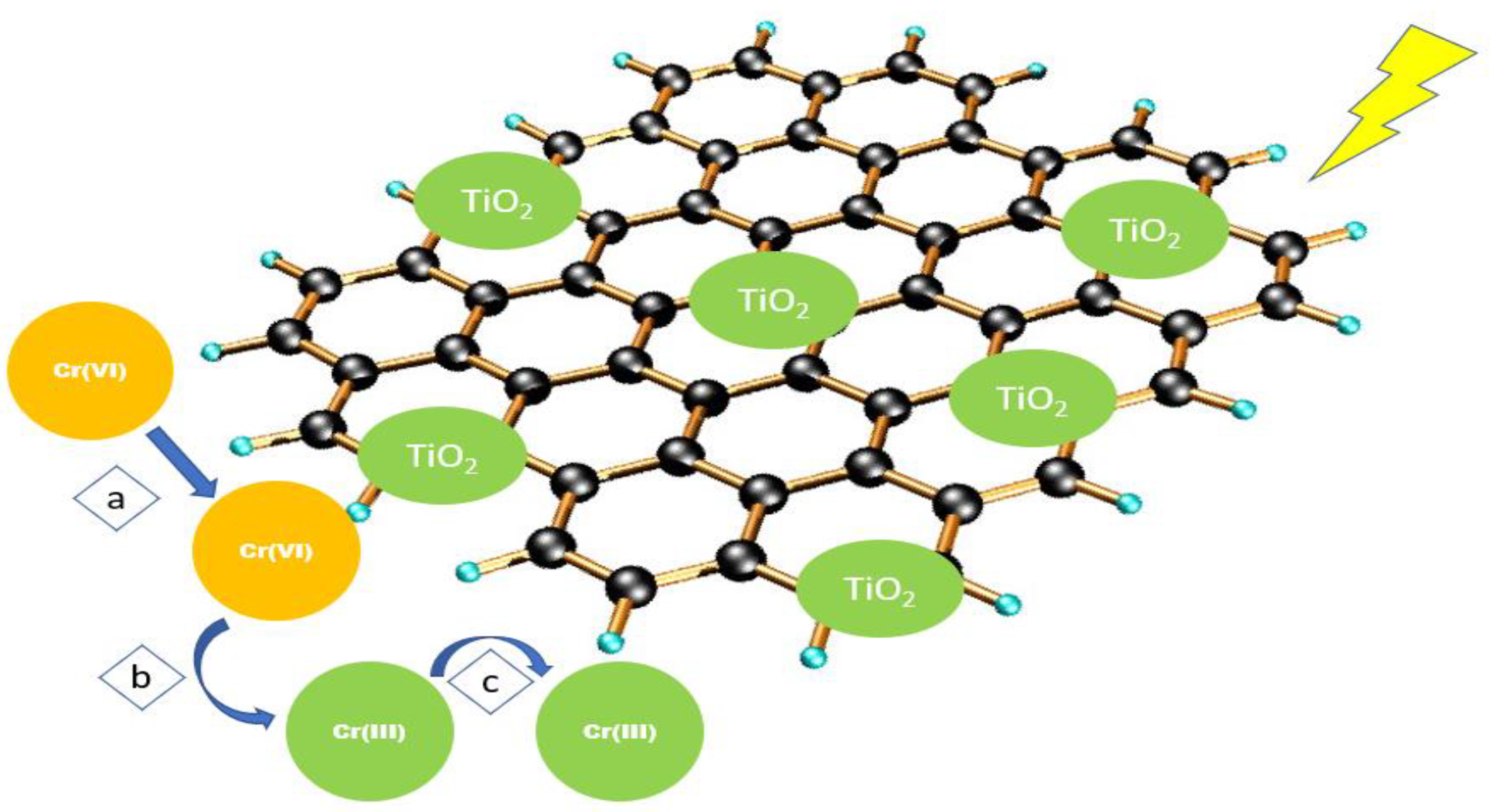
3.5. Chromium
4. Oil
5. Conventional Wastewater Treatment Methods for Waste Removal
5.1. TiO2
5.2. GO
5.3. Production of GO
5.4. Production of TiO2
5.4.1. Electrophoretic Deposition
5.4.2. Spray Pyrolysis
5.4.3. Sol Gel Method
5.4.4. Sonochemical and Microwave-Assisted Methods
5.5. Production of GO-TiO2
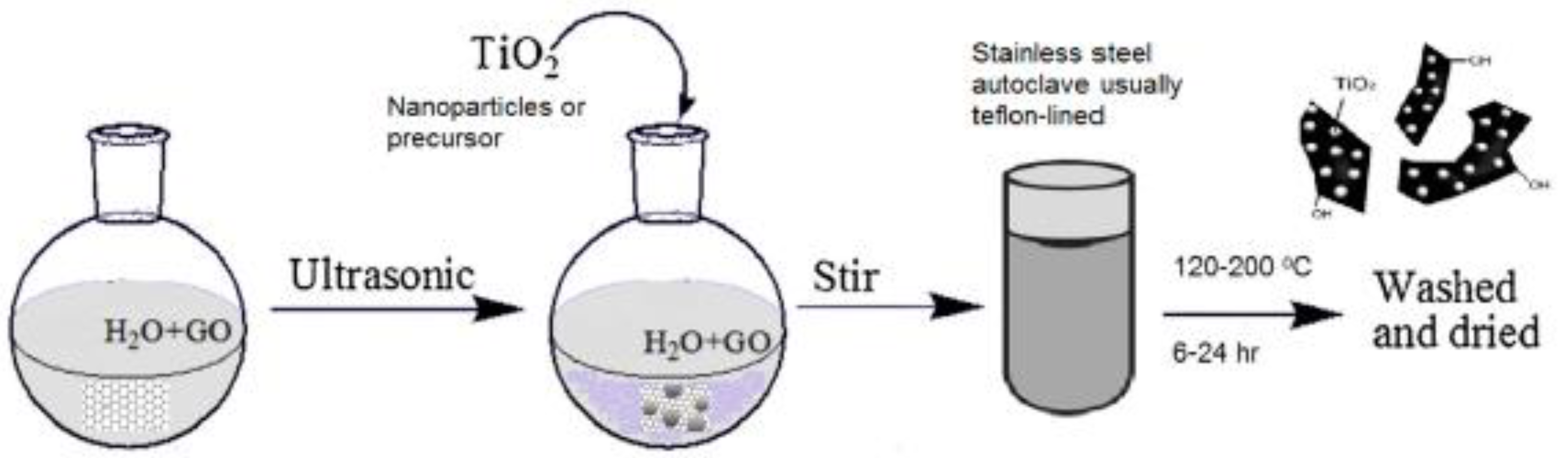
5.5.1. Hydrothermal Method
5.5.2. Solvothermal Method
5.5.3. Mechanical Mixing
6. Heterojunction of GO/TiO2 Photocatalyst
7. Advantages of GO/TiO2 Composite Structure
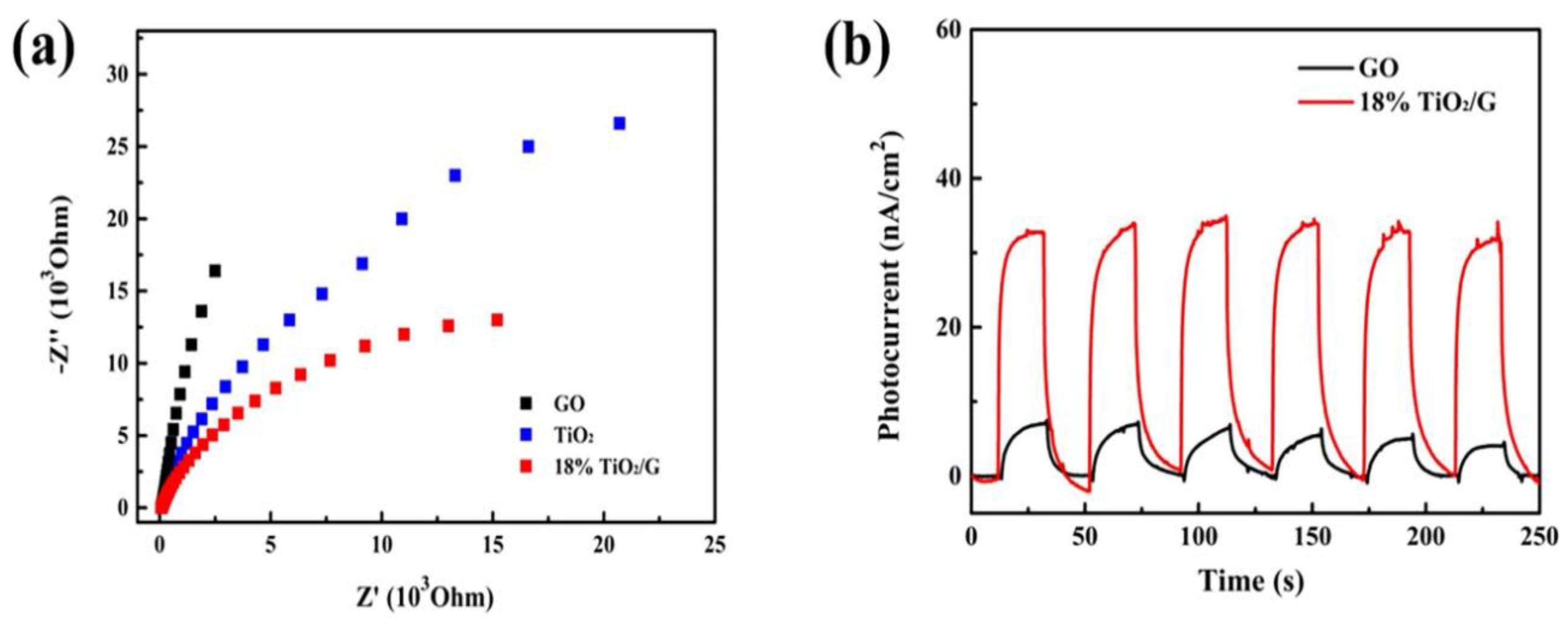
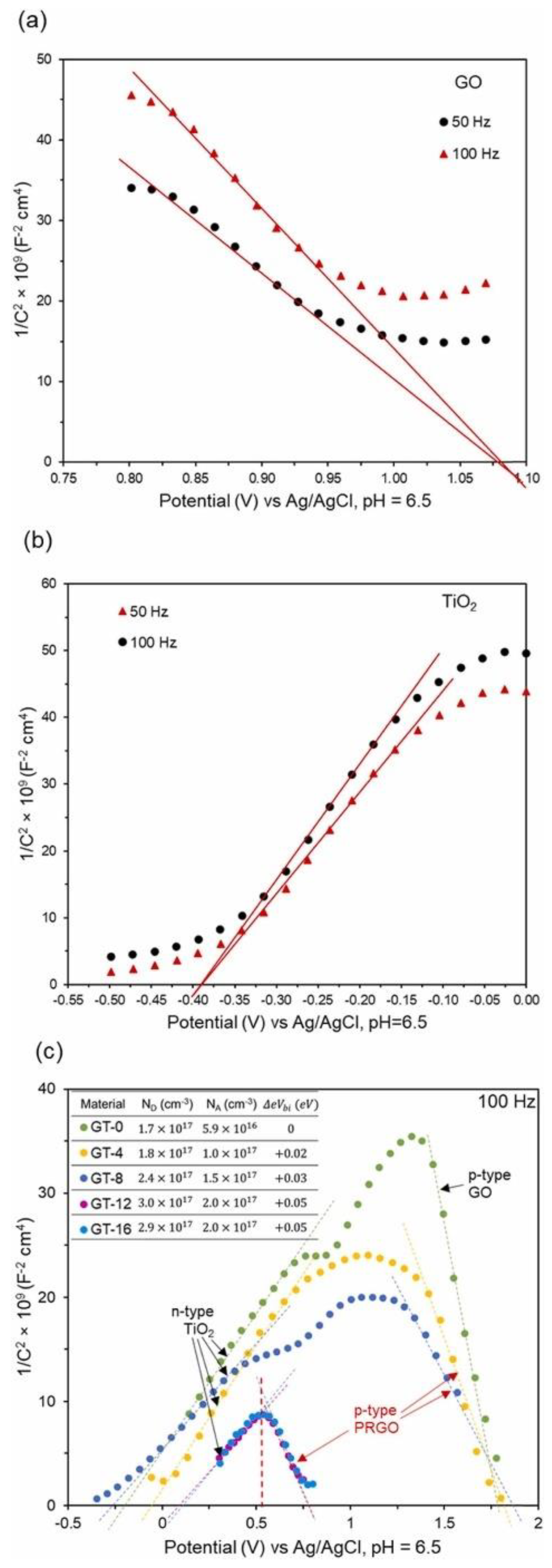
8. Recent Studies on GO-TiO2-Related Nanocomposites in Wastewater Treatment and Their Findings
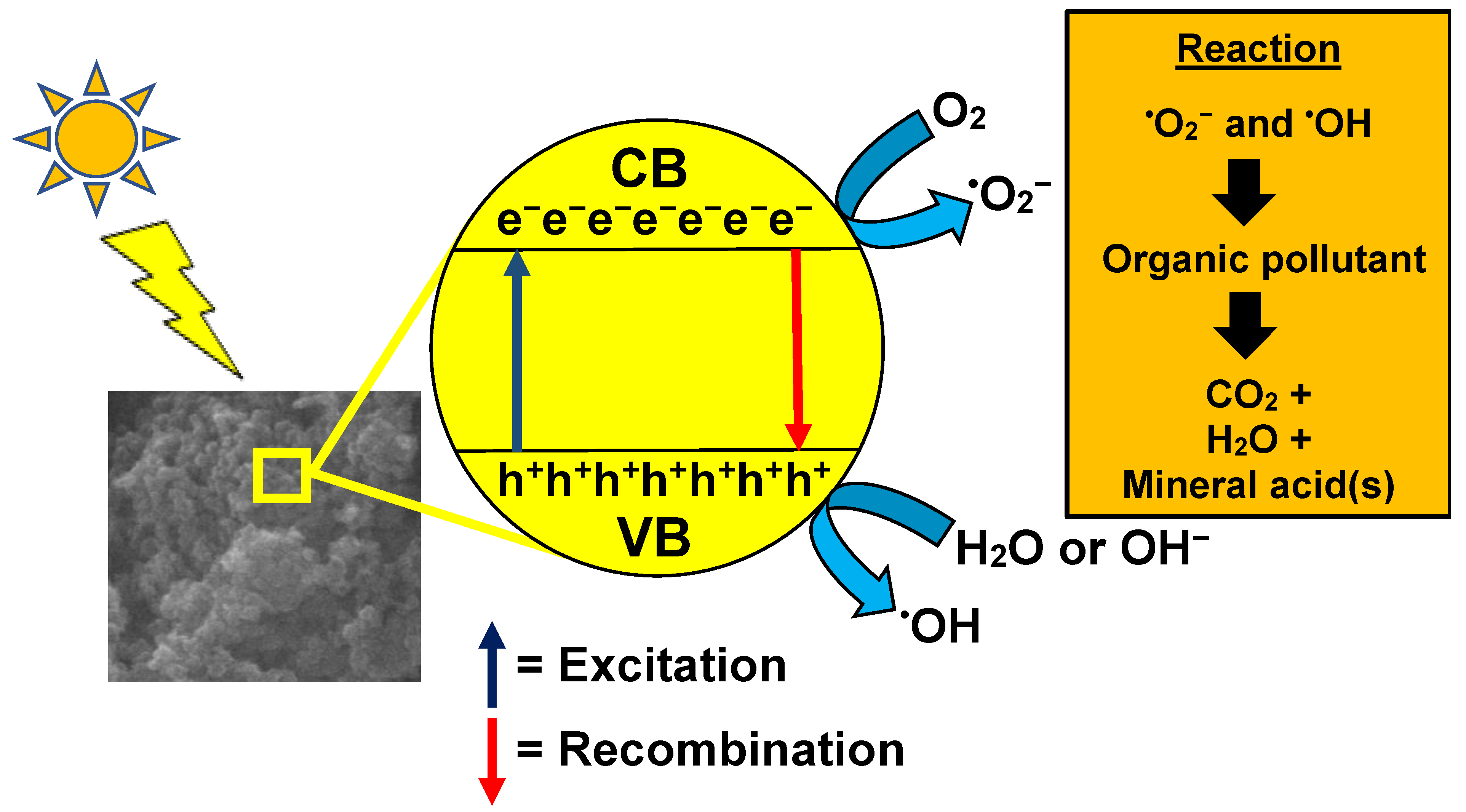
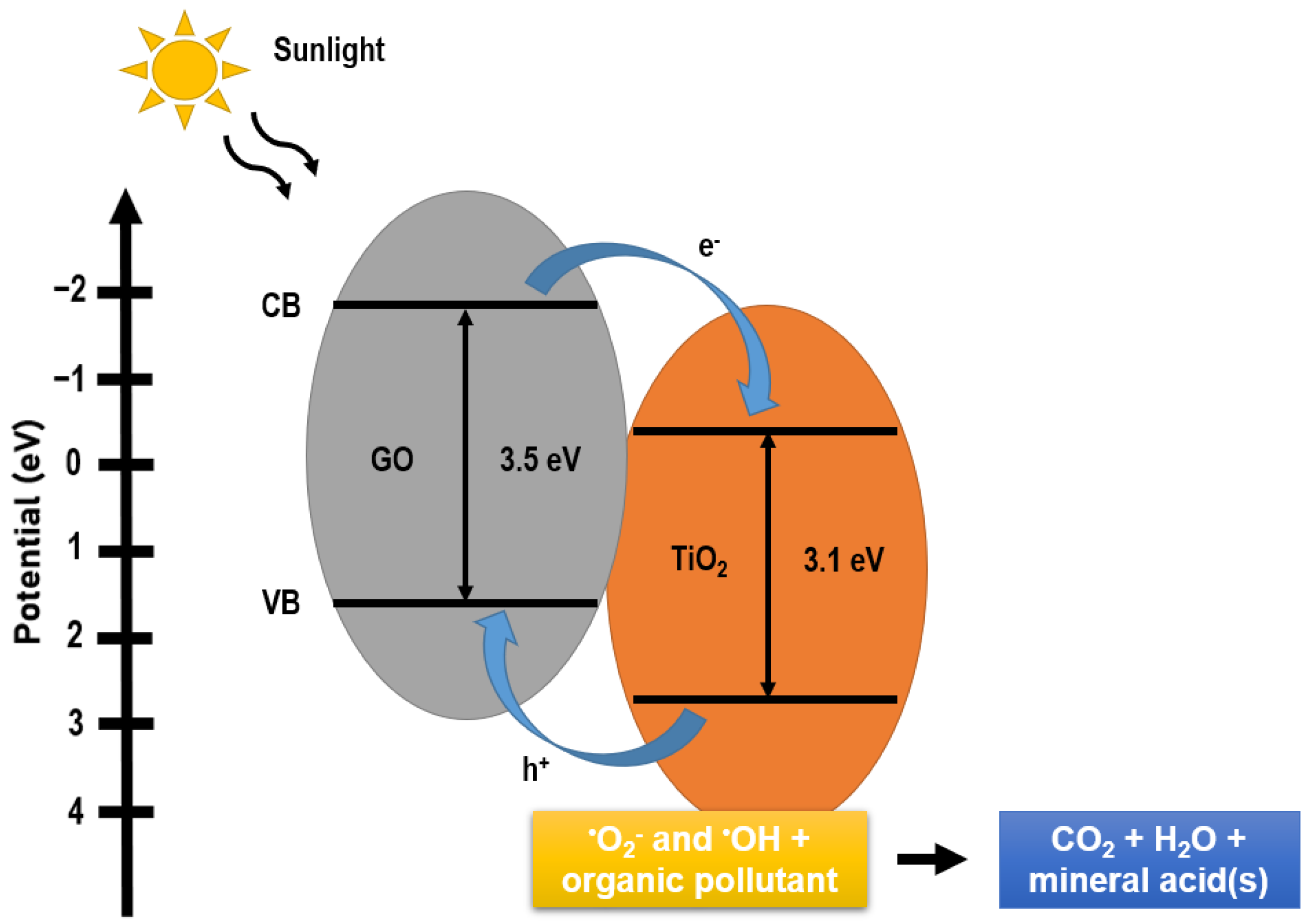
9. GO/TiO2 Photocatalytic Mechanism
10. Factors Affecting the Degradation of Organic Pollutants
10.1. Nature of Photocatalyst
10.2. Effect of pH
10.3. Initial Pollutant Concentration
10.4. Photocatalyst Concentration
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Achmad, R.T.; Auerkari, E.I. Effects of chromium on human body. Annu. Res. Rev. Biol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Adetayo, A.; Runsewe, D. Synthesis and fabrication of graphene and graphene oxide: A review. Open J. Compos. Mater. 2019, 9, 207. [Google Scholar] [CrossRef]
- Adly, M.S.; El-Dafrawy, S.M.; El-Hakam, S.A. Application of nanostructured graphene oxide/titanium dioxide composites for photocatalytic degradation of rhodamine B and acid green 25 dyes. J. Mater. Res. Technol. 2019, 8, 5610–5622. [Google Scholar] [CrossRef]
- Ahmad, A.; Van Der Wens, P.; Baken, K.; De Waal, L.; Bhattacharya, P.; Stuyfzand, P. Arsenic reduction to <1 µg/L in Dutch drinking water. Environ. Int. 2020, 134, 105253. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ahamad, T.; Ahmad, N.; Khan, M.Z. Removal enhancement of acid navy blue dye by GO—TiO2 nanocomposites synthesized using sonication method. Mater. Chem. Phys. 2019, 238, 121906. [Google Scholar] [CrossRef]
- Al Kausor, M.; Chakrabortty, D. Facile fabrication of N-TiO2/Ag3PO4@GO nanocomposite toward photodegradation of organic dye under visible light. Inorg. Chem. Commun. 2020, 116, 107907. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Kader, S.; Islam, M.; Khan, M. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Afify, A.D.; Goher, M.E. Preparation and characterization of graphene—TiO2 nanocomposite for enhanced photodegradation of Rhodamine-B dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Da Silva, J.a.L.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A. TiO2/graphene oxide immobilized in P (VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Ameta, S.; Ameta, R. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Andreoli, V.; Sprovieri, F. Genetic aspects of susceptibility to mercury toxicity: An overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective photocatalytic degradation of anthropogenic dyes using graphene oxide grafting titanium dioxide nanoparticles under UV-light irradiation. J. Photochem. Photobiol. A: Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Babatunde, A.O. Oil pollution and water conflicts in the riverine communities in Nigeria’s Niger Delta region: Challenges for and elements of problem-solving strategies. J. Contemp. Afr. Stud. 2020, 38, 274–293. [Google Scholar] [CrossRef]
- Babu, C.M.; Vinodh, R.; Sundaravel, B.; Abidov, A.; Peng, M.M.; Cha, W.S.; Jang, H.-T. Characterization of reduced graphene oxide supported mesoporous Fe2O3/TiO2 nanoparticles and adsorption of As(III) and As(V) from potable water. J. Taiwan Inst. Chem. Eng. 2016, 62, 199–208. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 2016, 123, 89–94. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Singhal, N.K.; Kim, K.-H.; Kumar, S. Copper oxide nanoblades as novel adsorbent material for cadmium removal. Ceram. Int. 2017, 43, 6075–6081. [Google Scholar] [CrossRef]
- Branco, V.; Caito, S.; Farina, M.; Teixeira Da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health Part B 2017, 20, 119–154. [Google Scholar] [CrossRef]
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef]
- Cao, X.-L.; Yan, Y.-N.; Zhou, F.-Y.; Sun, S.-P. Tailoring nanofiltration membranes for effective removing dye intermediates in complex dye-wastewater. J. Membr. Sci. 2020, 595, 117476. [Google Scholar] [CrossRef]
- Chaudhari, S.D.; Deshpande, A.; Kularkar, A.; Tandulkar, D.; Hippargi, G.; Rayalu, S.S.; Nagababu, P. Engineering of heterojunction TiO2/CaIn2S4@rGO novel nanocomposite for rapid photodegradation of toxic contaminants. J. Ind. Eng. Chem. 2022, 114, 305–316. [Google Scholar] [CrossRef]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Johan, M.R. Advanced photocatalytic degradation of acetaminophen using Cu2O/WO3/TiO2 ternary composite under solar irradiation. Catal. Commun. 2022, 163, 106396. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Y.; Yang, L.; Luo, S.; Yang, L.; Luo, X.; Luo, Y.; Li, T.; Gao, J.; Dionysiou, D.D. One–step reductive synthesis of Ti3+ self–doped elongated anatase TiO2 nanowires combined with reduced graphene oxide for adsorbing and degrading waste engine oil. J. Hazard. Mater. 2019, 378, 120752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Fu, F.; Dionysiou, D.D.; Tang, B. Adsorption, oxidation, and reduction behavior of arsenic in the removal of aqueous As(III) by mesoporous Fe/Al bimetallic particles. Water Res. 2016, 96, 22–31. [Google Scholar] [CrossRef]
- Chuntanalerg, P.; Bureekaew, S.; Klaysom, C.; Lau, W.-J.; Faungnawakij, K. Nanomaterial-incorporated nanofiltration membranes for organic solvent recovery. In Advanced Nanomaterials for Membrane Synthesis and Its Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–181. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Corda, N.C.; Kini, M.S. A review on adsorption of cationic dyes using activated carbon. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; p. 02022. [Google Scholar]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- De Silva, K.; Huang, H.-H.; Joshi, R.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Du, Y.; Qiu, S.; Zhang, X.; Nie, G. Nanoconfined hydrous titanium oxides with excellent acid stability for selective and efficient removal of As(V) from acidic wastewater. Chem. Eng. J. 2020, 400, 125907. [Google Scholar] [CrossRef]
- Ejiba, I.; Onya, S.; Adams, O. Impact of Oil Pollution on Livelihood: Evidence from the Niger Delta Region of Nigeria. J. Sci. Res. Rep. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Fallah Shojaei, A.; Shams-Nateri, A.; Ghomashpasand, M. Comparative study of photocatalytic activities of magnetically separable WO3/TiO2/Fe3O4 nanocomposites and TiO2, WO3/TiO2 and TiO2/Fe3O4 under visible light irradiation. Superlattices Microstruct. 2015, 88, 211–224. [Google Scholar] [CrossRef]
- Fausey, C.L.; Zucker, I.; Shaulsky, E.; Zimmerman, J.B.; Elimelech, M. Removal of arsenic with reduced graphene oxide-TiO2-enabled nanofibrous mats. Chem. Eng. J. 2019, 375, 122040. [Google Scholar] [CrossRef]
- Fiałkiewicz-Kozieł, B.; Vleeschouwer, F.; Mattielli, N.; Fagel, N.; Palowski, B.; Pazdur, A.; Smieja-Król, B. Record of Anthropocene pollution sources of lead in disturbed peatlands from Southern Poland. Atmos. Environ. 2018, 179, 61–68. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, X.; Dong, X.; Zhao, P.; Wang, Z. Study of adsorption property and mechanism of lead (II) and cadmium (II) onto sulfhydryl modified attapulgite. Arab. J. Chem. 2020, 14, 102960. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, S.; Fu, Z. Preparation of Multicycle GO/TiO2 Composite Photocatalyst and Study on Degradation of Methylene Blue Synthetic Wastewater. Appl. Sci. 2019, 9, 3282. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’amato, C. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Grant, L.D. LEAD AND COMPOUNDS. In Environmental Toxicants; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2020; pp. 627–675. [Google Scholar]
- Gustin, M.S.; Evers, D.C.; Bank, M.S.; Hammerschmidt, C.R.; Pierce, A.; Basu, N.; Blum, J.; Bustamante, P.; Chen, C.; Driscoll, C.T.; et al. Importance of Integration and Implementation of Emerging and Future Mercury Research into the Minamata Convention. Environ. Sci. Technol. 2016, 50, 2767–2770. [Google Scholar] [CrossRef]
- Hao, J.; Ji, L.; Li, C.; Hu, C.; Wu, K. Rapid, efficient and economic removal of organic dyes and heavy metals from wastewater by zinc-induced in-situ reduction and precipitation of graphene oxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 137–145. [Google Scholar] [CrossRef]
- Hasan, J.; Li, H.; Tian, G.; Qin, C. Fabrication of Cr2S3-GO-TiO2 composite with high visible-light-driven photocatalytic activity on degradation of organic dyes. Chem. Phys. 2020, 539, 110950. [Google Scholar] [CrossRef]
- Hausladen, D.M.; Alexander-Ozinskas, A.; Mcclain, C.; Fendorf, S. Hexavalent Chromium Sources and Distribution in California Groundwater. Environ. Sci. Technol. 2018, 52, 8242–8251. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, P. Surface Water Pollution in the Middle Chinese Loess Plateau with Special Focus on Hexavalent Chromium (Cr6+): Occurrence, Sources and Health Risks. Expo. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Hernández-Majalca, B.C.; Meléndez-Zaragoza, M.J.; Salinas-Gutiérrez, J.M.; López-Ortiz, A.; Collins-Martínez, V. Visible-light photo-assisted synthesis of GO-TiO2 composites for the photocatalytic hydrogen production. Int. J. Hydrog. Energy 2019, 44, 12381–12389. [Google Scholar] [CrossRef]
- Hoan, N.T.V.; Minh, N.N.; Nhi, T.T.K.; Van Thang, N.; Tuan, V.A.; Nguyen, V.T.; Thanh, N.M.; Van Hung, N.; Khieu, D.Q. TiO2/Diazonium/Graphene Oxide Composites: Synthesis and Visible-Light-Driven Photocatalytic Degradation of Methylene Blue. J. Nanomater. 2020, 2020, 4350125. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Wang, H.; Cai, X.; Hu, X.; Tang, C.-F.; Liu, Y.; Yuanxiu, Y. Decontamination of Cr(VI) by graphene oxide@TiO2 in an aerobic atmosphere: Effects of pH, ferric ions, inorganic anions, and formate. J. Chem. Technol. Biotechnol. 2018, 93, 2226–2233. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochemistry 2020, 61, 104849. [Google Scholar] [CrossRef]
- Huang, W. Chapter 1—Graphene Oxide Nanopapers. In Nanopapers; Huang, W., Ed.; William Andrew Publishing: Midland, MI, USA, 2018; pp. 1–26. [Google Scholar]
- Islam, M.; Mostafa, M. Textile dyeing effluents and environment concerns-a review. J. Environ. Sci. Nat. Resour. 2018, 11, 131–144. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Prospects and Challenges of Using Coagulation-Flocculation method in the treatment of Effluents. Adv. J. Chem.-Sect. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Jang, Y.; Somanna, Y.; Kim, H. Source, distribution, toxicity and remediation of arsenic in the environment–a review. Int. J. Appl. Env. Sci 2016, 11, 559–581. [Google Scholar]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee , A.; Klemeš, J.J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Johnston, J.E.; Lim, E.; Roh, H. Impact of upstream oil extraction and environmental public health: A review of the evidence. Sci. Total Environ. 2019, 657, 187–199. [Google Scholar] [CrossRef]
- Justh, N.; Berke, B.; László, K.; Bakos, L.P.; Szabó, A.; Hernádi, K.; Szilágyi, I.M. Preparation of graphene oxide/semiconductor oxide composites by using atomic layer deposition. Appl. Surf. Sci. 2018, 453, 245–251. [Google Scholar] [CrossRef]
- Kale, D.P.; Deshmukh, S.P.; Shirsath, S.R.; Bhanvase, B.A. Sonochemical preparation of multifunctional rGO-ZnS-TiO2 ternary nanocomposite and its application for CV dye removal. Optik 2020, 208, 164532. [Google Scholar] [CrossRef]
- Kalikeri, S.; Shetty Kodialbail, V. Solar light-driven photocatalysis using mixed-phase bismuth ferrite (BiFeO3/Bi25FeO40) nanoparticles for remediation of dye-contaminated water: Kinetics and comparison with artificial UV and visible light-mediated photocatalysis. Environ. Sci. Pollut. Res. 2018, 25, 13881–13893. [Google Scholar] [CrossRef]
- Kam, C.S.; Leung, T.L.; Liu, F.; Djurišić, A.B.; Xie, M.H.; Chan, W.-K.; Zhou, Y.; Shih, K. Lead removal from water—Dependence on the form of carbon and surface functionalization. RSC Adv. 2018, 8, 18355–18362. [Google Scholar] [CrossRef]
- Kang, H.; Shi, J.; Liu, L.; Shan, M.; Xu, Z.; Li, N.; Li, J.; Lv, H.; Qian, X.; Zhao, L. Sandwich morphology and superior dye-removal performances for nanofiltration membranes self-assemblied via graphene oxide and carbon nanotubes. Appl. Surf. Sci. 2017, 428, 990–999. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, A.; Bahuguna, A.; Sharma, V.; Krishnan, V. Two-dimensional carbon-based nanocomposites for photocatalytic energy generation and environmental remediation applications. Beilstein J. Nanotechnol. 2017, 8, 1571–1600. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, V.; Sudhagar, P.; Konga, A.K.; Ponniah, G. Photocatalytic Degradation of Synthetic Organic Reactive Dye Wastewater Using GO-TiO2. Nanocomposite 2020, 29, 1683–1690. [Google Scholar]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Facile synthesis of xanthan biopolymer integrated 3D hierarchical graphene oxide/titanium dioxide composite for adsorptive lead removal in wastewater. Bioresour. Technol. 2020, 309, 123296. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.D.P.; Romero, A.; Garrido, J.; Sanchez-Silva, L.; Valverde, J.L. Influence of Different Improved Hummers Method Modifications on the Characteristics of Graphite Oxide in Order to Make a More Easily Scalable Method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [Google Scholar] [CrossRef]
- Li, D.; Dai, S.; Li, J.; Zhang, C.; Richard-Plouet, M.; Goullet, A.; Granier, A. Microstructure and Photocatalytic Properties of TiO2–Reduced Graphene Oxide Nanocomposites Prepared by Solvothermal Method. J. Electron. Mater. 2018, 47, 7372–7379. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Springer: Cham, Switzerland, 2016; p. 12. [Google Scholar]
- Liang, J.; Ning, X.-A.; Sun, J.; Song, J.; Hong, Y.; Cai, H. An integrated permanganate and ozone process for the treatment of textile dyeing wastewater: Efficiency and mechanism. J. Clean. Prod. 2018, 204, 12–19. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Y.; Kang, K.; Zhang, Y.; Cai, Z.; Pan, J. Synthesis and characterization of porous TiO2-NS/Pt/GO aerogel: A novel three-dimensional composite with enhanced visible-light photoactivity in degradation of chlortetracycline. J. Photochem. Photobiol. A Chem. 2017, 346, 1–9. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Y.; Zhang, J.; Xue, D.; Fang, H.; Tian, J.; Zhou, C.; Zhang, C.; Li, Y.; Li, H. GO/TiO2 Composites as a highly active photocatalyst for the Degradation of Methyl Orange. J. Mater. Res. 2020, 35, 1307–1315. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; He, Y.; Li, B.; Dong, Y.; Wang, L. Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour. Technol. 2019, 284, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Li, Y.; Huang, Z.; Li, T.; Ye, S.; Dionysiou, D.D.; Song, X. Synthesis of GO/TiO2/Bi2WO6 nanocomposites with enhanced visible light photocatalytic degradation of ethylene. Appl. Catal. B Environ. 2019, 246, 303–311. [Google Scholar] [CrossRef]
- Makertihartha, I.; Rizki, Z.; Zunita, M.; Dharmawijaya, P. Dyes removal from textile wastewater using graphene based nanofiltration. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; p. 110006. [Google Scholar]
- Mani, S.; Chowdhary, P.; Bharagava, R.N. Textile wastewater dyes: Toxicity profile and treatment approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 219–244. [Google Scholar]
- Mao, B.; Sidhureddy, B.; Thiruppathi, A.R.; Wood, P.C.; Chen, A. Efficient dye removal and separation based on graphene oxide nanomaterials. New J. Chem. 2020, 44, 4519–4528. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–125. [Google Scholar]
- Molla, A.; Li, Y.; Mandal, B.; Kang, S.G.; Hur, S.H.; Chung, J.S. Selective adsorption of organic dyes on graphene oxide: Theoretical and experimental analysis. Appl. Surf. Sci. 2019, 464, 170–177. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Akbarzadeh Pivehzhani, O.; Johan, M.R. Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Mousa, K.; Hadi, H. Coagulation/Flocculation Process for Produced Water Treatment. Int. J. Curr. Eng. Technol. 2016, 6, 551–555. [Google Scholar]
- Nadimi, M.; Ziarati Saravani, A.; Aroon, M.A.; Ebrahimian Pirbazari, A. Photodegradation of methylene blue by a ternary magnetic TiO2/Fe3O4/graphene oxide nanocomposite under visible light. Mater. Chem. Phys. 2019, 225, 464–474. [Google Scholar] [CrossRef]
- Nasar, A.; Mashkoor, F. Application of polyaniline-based adsorbents for dye removal from water and wastewater—A review. Environ. Sci. Pollut. Res. 2019, 26, 5333–5356. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Nriagu, J.; Udofia, E.A.; Ekong, I.; Ebuk, G. Health Risks Associated with Oil Pollution in the Niger Delta, Nigeria. Int. J. Environ. Res. Public Health 2016, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic methods for titanium dioxide nanoparticles: A review. In Titanium Dioxide: Material for a Sustainable Environment; IntechOpen: London, UK, 2018; pp. 151–1755. [Google Scholar]
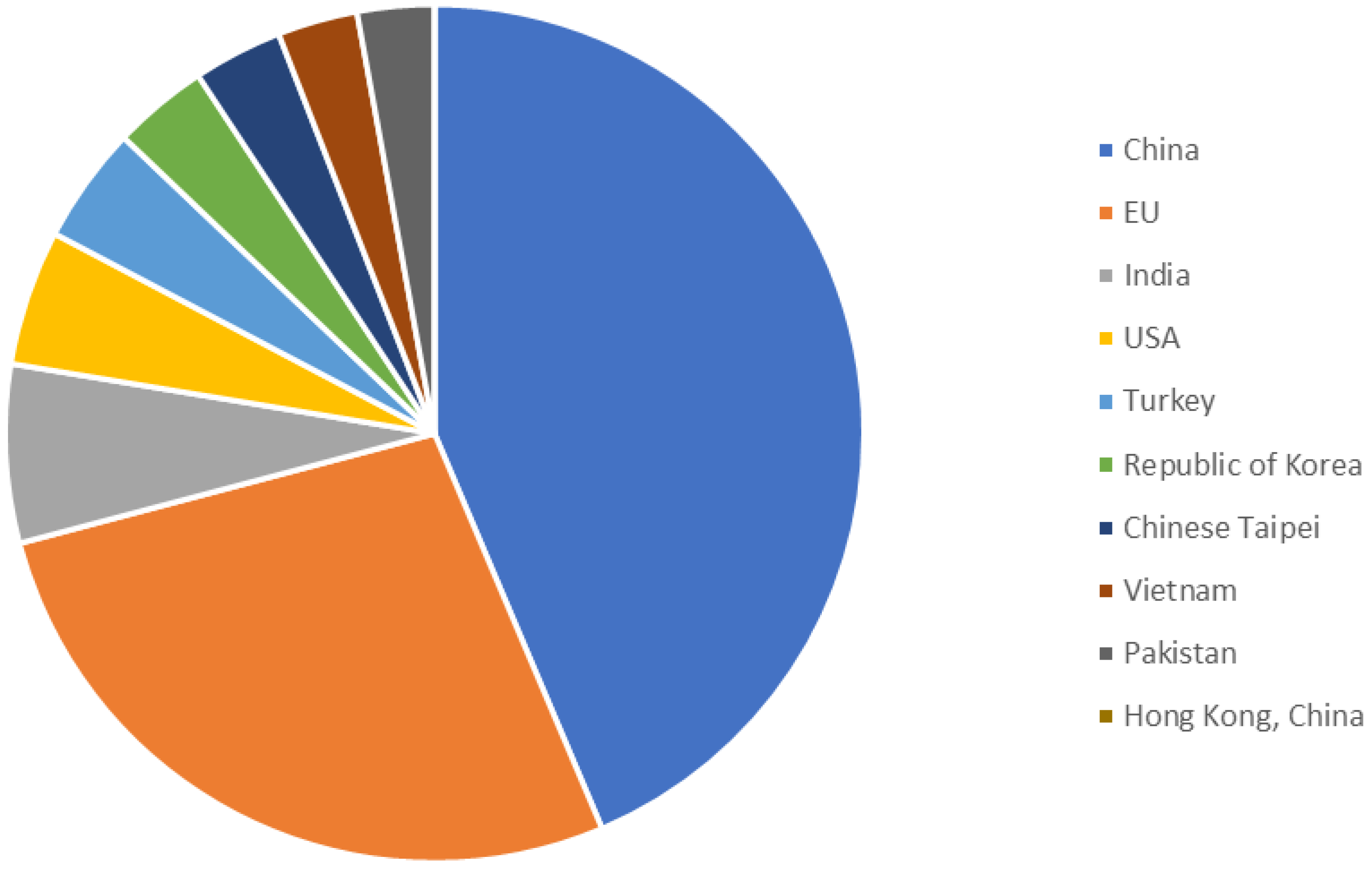
- World Trade Organization. World Trade Statistical Review 2019; World Trade Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Palanivel, C.; Prabhakaran, N.R.; Selvakumar, G. Morphological expedient flower like nanostructures WO3–TiO2 nanocomposite material and its multi applications. OpenNano 2019, 4, 100026. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. Adsorption of methylene blue on graphene oxide prepared from amorphous graphite: Effects of pH and foreign ions. J. Mol. Liq. 2016, 221, 82–87. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal Synthesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Pichtel, J. Oil and gas production wastewater: Soil contamination and pollution prevention. Appl. Environ. Soil Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Price, R.J.; Ladislaus, P.I.; Smith, G.C.; Davies, T.J. A novel ‘bottom-up’ synthesis of few- and multi-layer graphene platelets with partial oxidation via cavitation. Ultrason. Sonochem. 2019, 56, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, M.D.; Sil, S.; Singh, N.; Ray, P.P.; Darbha, G.K.; Bhattacharyya, S.; Majumder, T.P. Sonochemical synthesis of nanospherical TiO2 within graphene oxide nanosheets and its application as a photocatalyst and a Schottky diode. FlatChem 2020, 22, 100180. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Qian, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. TiO2/sulfonated graphene oxide/Ag nanoparticle membrane: In situ separation and photodegradation of oil/water emulsions. J. Membr. Sci. 2018, 554, 16–25. [Google Scholar] [CrossRef]
- Qu, C.; Wang, S.; Ding, L.; Zhang, M.; Wang, D.; Giesy, J.P. Spatial distribution, risk and potential sources of lead in soils in the vicinity of a historic industrial site. Chemosphere 2018, 205, 244–252. [Google Scholar] [CrossRef]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediat ion of mercury: An essence review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Ran, R.; Meng, X.; Zhang, Z. Facile Preparation of Novel Graphene Oxide-Modified Ag2O/Ag3VO4/AgVO3 Composites with High Photocatalytic Activities under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 196, 1–15. [Google Scholar] [CrossRef]
- Rosu, M.-C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Datta, R.; Sarkar, D. Chapter 3.10—Heavy Metal Pollution and Remediation. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–373. [Google Scholar]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Schöche, S.; Hong, N.; Khorasaninejad, M.; Ambrosio, A.; Orabona, E.; Maddalena, P.; Capasso, F. Optical properties of graphene oxide and reduced graphene oxide determined by spectroscopic ellipsometry. Appl. Surf. Sci. 2017, 421, 778–782. [Google Scholar] [CrossRef]
- Sharma, M.; Behl, K.; Nigam, S.; Joshi, M. TiO2-GO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 2018, 156, 434–439. [Google Scholar] [CrossRef]
- Sheshmani, S.; Nayebi, M. Modification of TiO2 with graphene oxide and reduced graphene oxide; enhancing photocatalytic activity of TiO2 for removal of remazol Black B. Polym. Compos. 2019, 40, 210–216. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Lead: Its Effects on Environment and Health; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2017. [Google Scholar]
- Sun, X.; Ji, S.; Wang, M.; Dou, J.; Yang, Z.; Qiu, H.; Wang, H. Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J. Alloy. Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Arsenic in Rice Soils and Potential Agronomic Mitigation Strategies to Reduce Arsenic Bioavailability: A Review. Pedosphere 2018, 28, 363–382. [Google Scholar] [CrossRef]
- Sykam, N.; Madhavi, V.; Rao, G.M. Rapid and efficient green reduction of graphene oxide for outstanding supercapacitors and dye adsorption applications. J. Environ. Chem. Eng. 2018, 6, 3223–3232. [Google Scholar] [CrossRef]
- Tahir, M.B.; Rafique, M.; Rafique, M.S.; Nawaz, T.; Rizwan, M.; Tanveer, M. Chapter 8—Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. In Nanotechnology and Photocatalysis for Environmental Applications; Tahir, M.B., Rafique, M., Rafique, M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–138. [Google Scholar]
- Tai, X.H.; Lai, C.W.; Yang, T.C.K.; Johan, M.R.; Lee, K.M.; Chen, C.-Y.; Juan, J.C. Highly effective removal of volatile organic pollutants with p-n heterojunction photoreduced graphene oxide-TiO2 photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107304. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Tayel, A.; Ramadan, A.R.; El Seoud, O.A. Titanium dioxide/graphene and titanium dioxide/graphene oxide nanocomposites: Synthesis, characterization and photocatalytic applications for water decontamination. Catalysts 2018, 8, 491. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Gritsenko, V.A.; Skalnaya, M.G.; Cherkasov, S.V.; Aaseth, J.; Skalny, A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018, 235, 429–434. [Google Scholar] [CrossRef]
- Tran, D.T.; Nguyen, M.T.; Le, T.T.T.; Ha, M.N.; Nguyen, M.V.; Pham, T.D. Enhanced photocatalytic degradation of methyl orange using ZnO/graphene oxide nanocomposites. Res. Chem. Intermed. 2018, 44, 3081–3095. [Google Scholar]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. Sol-Gel Synthesis of Titanium Dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Pillai, S.C., Hehir, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 271–283. [Google Scholar]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- W Lai, C.; W Low, F.; W Chong, S.; Pp Wong, C.; Binti Mohamed Siddick, S.Z.; C Juan, J.; B Abdul Hamid, S. An overview: Recent development of titanium dioxide loaded graphene nanocomposite film for solar application. Curr. Org. Chem. 2015, 19, 1882–1895. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; He, X.; Du, T.; Wang, Y.; Li, Y.; Hao, T. Facile prepared ball-like TiO2 at GO composites for oxytetracycline removal under solar and visible lights. Water Res. 2019, 160, 197–205. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction A. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Anion-Exchange Resins for C.I. Direct Blue 71 Removal from Aqueous Solutions and Wastewaters: Effects of Basicity and Matrix Composition and Structure. Ind. Eng. Chem. Res. 2014, 53, 11838–11849. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Zeng, G.; Huang, D.; Zhang, J.; Yin, L.; Tan, S.; Huang, L.; Chen, H.; Yu, G. A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater. Chemosphere 2018, 200, 173–179. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.G.; Takada, H.; Hosoda, J.; Kondo, A.; Yamashita, R.; Saha, M.; Maes, T. Polycyclic Aromatic Hydrocarbons (PAHs) and Hopanes in Plastic Resin Pellets as Markers of Oil Pollution via International Pellet Watch Monitoring. Arch. Environ. Contam. Toxicol. 2017, 73, 196–206. [Google Scholar] [CrossRef]
- Ying, X.-L.; Gao, Z.-Y.; Yan, J.; Zhang, M.; Wang, J.; Xu, J.; Markowitz, M.; Yan, C.-H. Sources, symptoms and characteristics of childhood lead poisoning: Experience from a lead specialty clinic in China. Clin. Toxicol. 2017, 56, 397–403. [Google Scholar] [CrossRef]
- Yunarti, R.T.; Safitri, T.N.; Dimonti, L.C.C.; Aulia, G.; Khalil, M.; Ridwan, M. Facile synthesis of composite between titania nanoparticles with highly exposed (001) facet and coconut shell-derived graphene oxide for photodegradation of methylene blue. J. Phys. Chem. Solids 2022, 160, 110357. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Zamri, M.S.F.A.; Sapawe, N. Effect of pH on phenol degradation using green synthesized titanium dioxide nanoparticles. Mater. Today Proc. 2019, 19, 1321–1326. [Google Scholar]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO 2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Mechanism of photocatalysis. In Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–15. [Google Scholar]
- Zhang, Q.; Hao, F.; Li, J.; Zhou, Y.; Wei, Y.; Lin, H. Perovskite solar cells: Must lead be replaced—and can it be done? Sci. Technol. Adv. Mater. 2018, 19, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Env. Pollut 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, Q.; Yu, D.; Sun, J.; Liu, B. A novel pn heterojunction of BiVO4/TiO2/GO composite for enhanced visible-light-driven photocatalytic activity. Mater. Lett. 2017, 209, 379–383. [Google Scholar] [CrossRef]

| Dye | Chemical Structures |
|---|---|
| Methyl Orange | 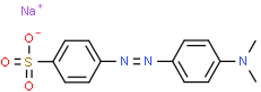 |
| Congo Red | 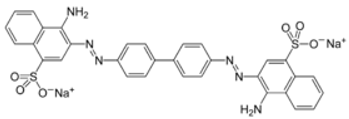 |
| Direct Blue 1 | 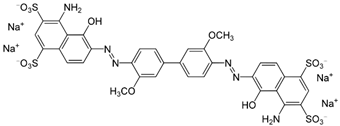 |
| Remazol Brilliant Blue R | 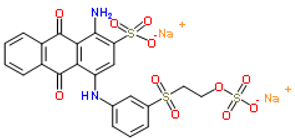 |
| Alizarin | 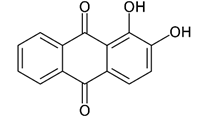 |
| Metal Ion | Adverse Effects on Human Health | Ref. |
|---|---|---|
| Hg+ | Neurological alterations, motor dysfunction, and premature death | [18,34,35] |
| Pb2+ | Impairment of brain and nervous functions, reproductive system, and miscarriage | [36,37,38] |
| Cd2+ | Hypertension, teratogenic towards liver, kidney, and lungs | [39,40] |
| As3+ | Skin lesions, diabetes, and cancers (e.g., skin, lung, kidney, bladder) | [41,42,43] |
| Cr6+ | Damage hearing, skin problems, cancer | [31,44,45] |
| Method | Strength | Weakness | Ref. |
|---|---|---|---|
| Coagulation–flocculation | Simple Economically friendly | Handling and disposal problems due to high sludge production High cost of chemical reagent High operational cost | [52] |
| Ozonation | Effective decolorization | High operational cost | [53] |
| Ion exchange | No loss of sorbents | Economically unattractive Not applicable to certain organic wastes | [54] |
| Biological treatment | Ecofriendly Energy saving | Time-consuming Occupy a certain area of land | [55] |
| Adsorption | High removal efficiency Low-cost Simple | Some adsorbents can be costly | [56,57] |
| Nanofiltration | Efficient Low energy consumption | Membrane fouling Membrane pore size restricted to nanopore size | [58,59,60] |
| Photocatalysis | Complete degradation of organic pollutant Production of harmless end products Usage of renewable sunlight energy Stable Inexpensive | Photocatalysts need to be activated by UV light Fast recombination of charge carrier | [61,62,63] |
| Phases | Crystal Structure | Band Gap (eV) | Space Group | Density (g/cm3) | Refractive Index | Structure Geometry |
|---|---|---|---|---|---|---|
| Rutile | Tetragonal | 3.05 | P42/mnm | 4.25 | 2.609 |  |
| Anatase | Tetragonal | 3.23 | I41/amd | 3.894 | 2.488 | 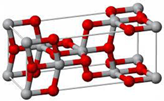 |
| Brookite | Orthorhombic | 3.26 | Pbca | 4.12 | 2.583 |  |
| Photocatalyst | Light Source/Pollutants | Experimental Conditions | Photodegradation Efficiency (%) | Ref. |
|---|---|---|---|---|
| TiO2/GO/Ag | Solar irradiation Methyl Orange (MO) | Catalyst = 0.1 g [MO] = 15 mg/L Irradiation time = 180 min | ~98 | [86] |
| Ag/GO-TMCs | 300 W Xe lamp Rhodamine B (RhB) | Catalyst = 0.5 g/L [RhB] = 20 mg/L Irradiation time = 180 min | ~100 | [121] |
| GO/TiO2 | UV lamp Rhodamine B (RhB) and Acid Green 25 (AG-25) | Catalyst = 1.0 g/L [RhB] = 10 mg/L [AG-25] = 40 mg/L Irradiation time = 180 min | RhB = 100 AG-25 = 96 | [122] |
| BiVO4/TiO2/GO | 1000 W Xe lamp C.I. Reactive Blue 19 (RB-19) | Catalyst = 6 g/L [RB-19] = 0.05 mg/L Irradiation time = 90 min | 95.87 | [125] |
| GO/TiO2 | 40 W UV lamp Methylene Blue (MB) and Methyl Orange (MO) | Catalyst = 0.4 g/L Irradiation time = 25 min (MB) and 240 min (MO) | MB = 100 MO = 84 | [126] |
| TiO2/Diazonium-GO | 75 V filament lamps Methylene Blue (MB) | Catalyst = 0.2 g/L Irradiation time = 420 min | 95 | [127] |
| TiO2-Pt/GO | 15W UV lamp Natural sunlight Amaranth Sunset yellow Tartrazine | Catalyst = 0.2 g/L [Amaranth] = 2 × 10−5 M [Sunset yellow] = 2 × 10−5 M [Tartrazine] = 2 × 10−5 M | Amaranth = 99.56 Sunset yellow = 99.15 Tartrazine = 96.23 | [128] |
| TiO2/CaIn2S4@rGO | Visible light Methylene Blue (MB) and Congo Red (CR) | Catalyst = 1 mg [MB] = 35 mg/L Irradiation time = 30 min | ~100 | [129] |
| N-TiO2/Ag3PO4@GO | Visible light Acid Blue 25 dye (AB25) | Catalyst = 1 g/L [AB25] = 18 µM Irradiation time = 20 min | 98 | [130] |
| Cr2S3-GO/TiO2 | 500 W Xe lamp Methyl Blue (MB), Rhodamine B (RhB), and Methyl Orange (MO) | Catalyst = 0.4 g/L [MB] = 10 mg/L [RhB] = 10 mg/L [MO] = 10 mg/L Irradiation time = 120 min | ~98 | [131] |
| TiO2@rGO | Rhodamine-B dye (RhB) | Catalyst = 0.3 g/L [RhB] = 30 mg/L Irradiation time = 60 min | 97 | [132] |
| GO-TiO2 | 125 W medium pressure mercury lamps Acid Navy Blue dye (ANB) | Catalyst = 0.3 g/L [ANB] = 30 mg/L Irradiation time = 90 min | 95 | [133] |
| TiO2-RGO | 150 W Xe lamp Rhodamine-B dye (RhB) | Catalyst = 0.4 g/L [RhB] = 0.001 mg/L Irradiation time = 180 min | ~85 | [134] |
| TiO2/GO | 450 W lamp Methylene Blue (MB) | Catalyst = 1 g/L [MB] = 0.01 mM Irradiation time = 60 min | ~51 | [135] |
| TiO2/Fe3O4/GO | 400 W UV lamp Methylene Blue (MB) | Catalyst = 0.1 g/L [MB] = 10mg/L Irradiation time = 90 min | ~82 | [136] |
| rGO-ZnS-TiO2 | Crystal Violet dye (CV) | Catalyst = 0.4 g/L [CV] = 500 ppm Irradiation time = 50 min | ~ 97 | [137] |
| Photocatalyst | Light Source/Pollutants | Experimental Conditions | Photodegradation Efficiency (%) | Ref. |
|---|---|---|---|---|
| rGO-TiO2@fibers | As(V) | Catalyst = 9.3 mg/40 mL [As(V)] = 1 mg/L | 97.0 | [138] |
| Biochar TiO2 | As(V) | Catalyst = 1 g/L [As(V)] = 50–300 mg/L | 118.1 | [139] |
| Hydrous TiO2 | As(V) | Catalyst = 0.5 g/L [As(V)] = 20 mg/L | 44.0 | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, E.D.H.; Chau, J.H.F.; Lai, C.W.; Khe, C.S.; Sharma, G.; Kumar, A.; Siengchin, S.; Sanjay, M.R. GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials 2022, 12, 3536. https://doi.org/10.3390/nano12193536
Kong EDH, Chau JHF, Lai CW, Khe CS, Sharma G, Kumar A, Siengchin S, Sanjay MR. GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials. 2022; 12(19):3536. https://doi.org/10.3390/nano12193536
Chicago/Turabian StyleKong, Ethan Dern Huang, Jenny Hui Foong Chau, Chin Wei Lai, Cheng Seong Khe, Gaurav Sharma, Amit Kumar, Suchart Siengchin, and Mavinkere Rangappa Sanjay. 2022. "GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment" Nanomaterials 12, no. 19: 3536. https://doi.org/10.3390/nano12193536
APA StyleKong, E. D. H., Chau, J. H. F., Lai, C. W., Khe, C. S., Sharma, G., Kumar, A., Siengchin, S., & Sanjay, M. R. (2022). GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials, 12(19), 3536. https://doi.org/10.3390/nano12193536









