Synthesis and Characterization of Novel Sprayed Ag-Doped Quaternary Cu2MgSnS4 Thin Film for Antibacterial Application
Abstract
1. Introduction
2. Materials and Methods
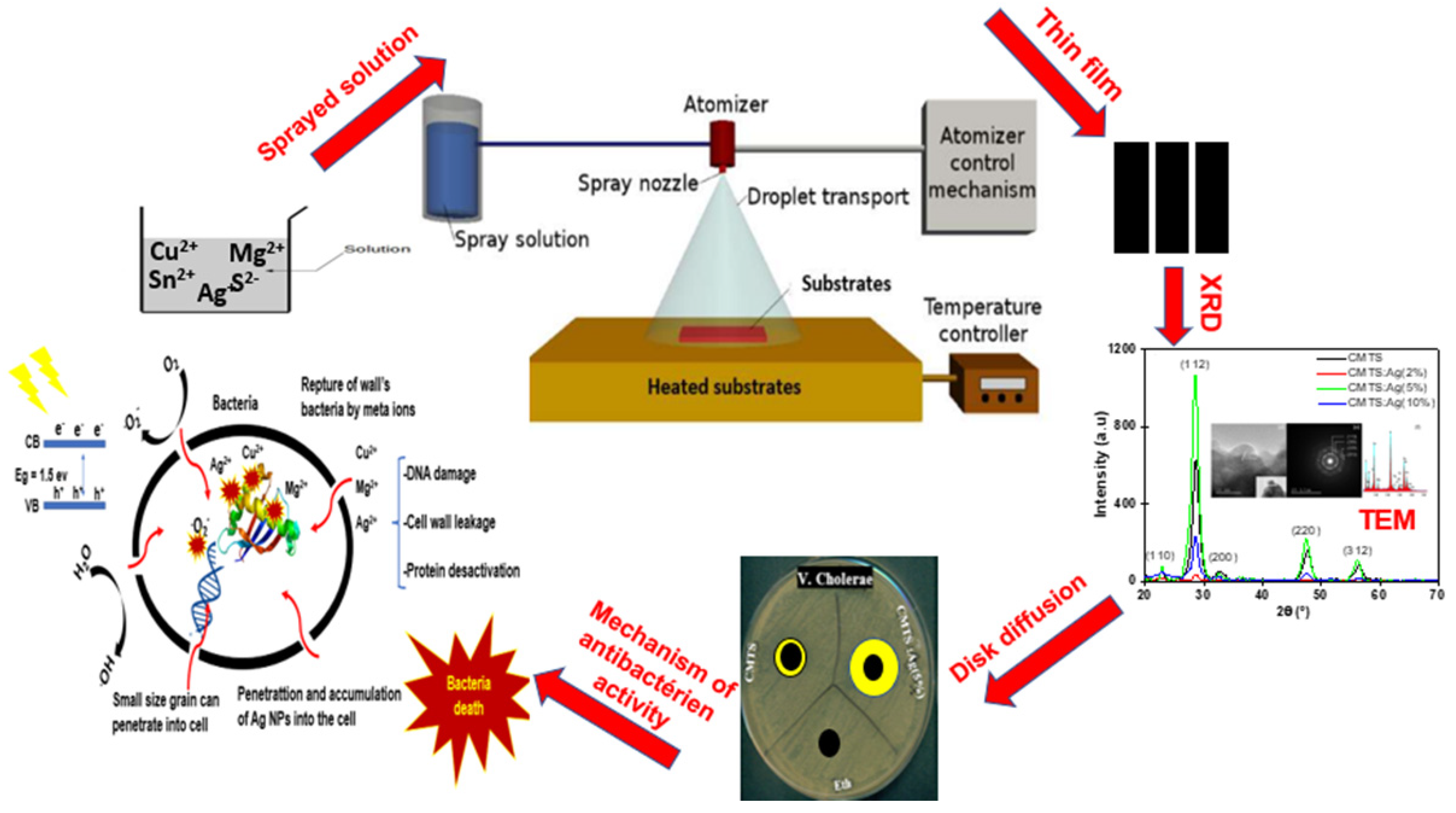
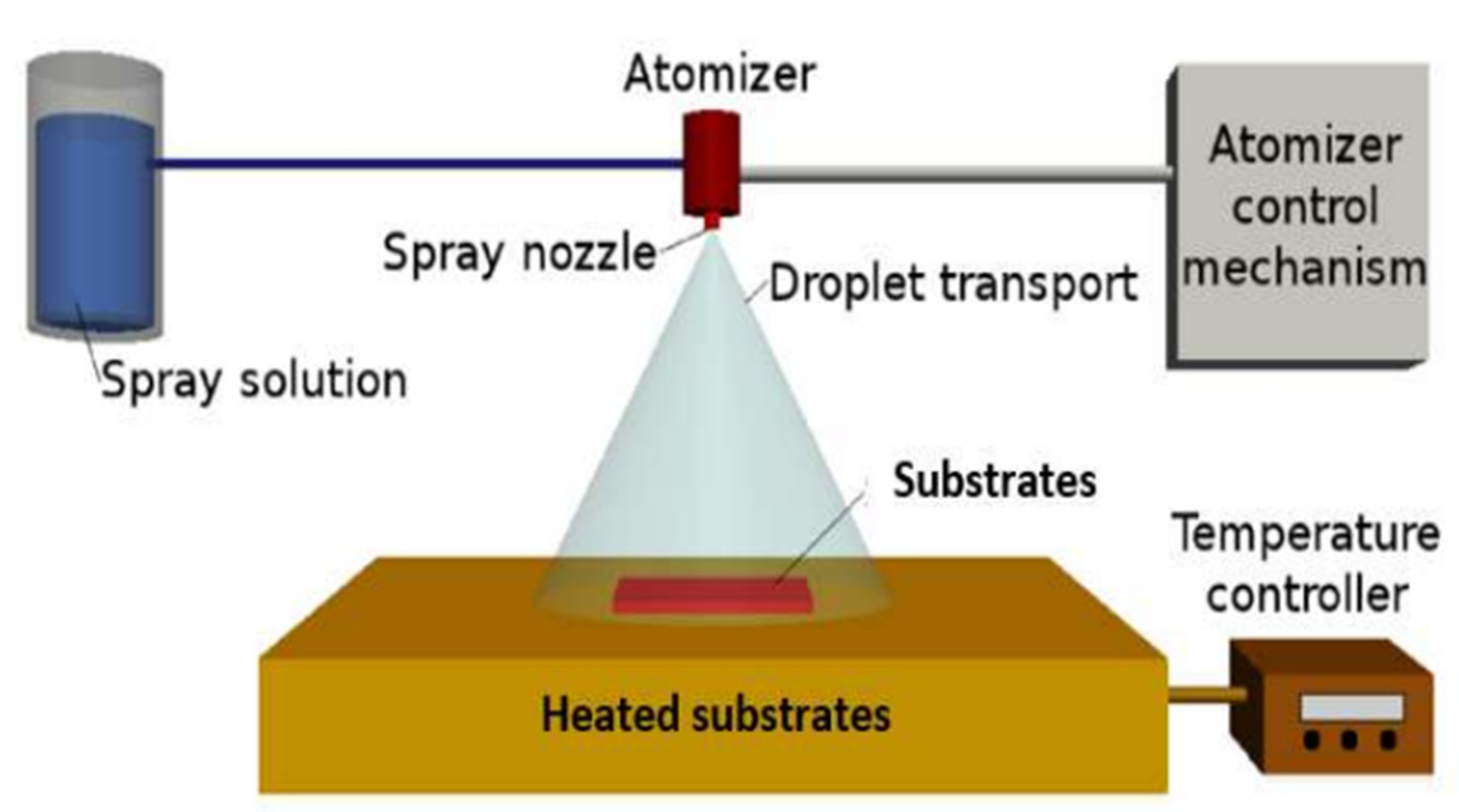
2.1. Preparation of CMTS Films
2.2. Film Characterization
2.3. Experimental Setup of Antibacterial Test
3. Results and Discussion
3.1. Structural Analysis
3.1.1. XRD Analysis
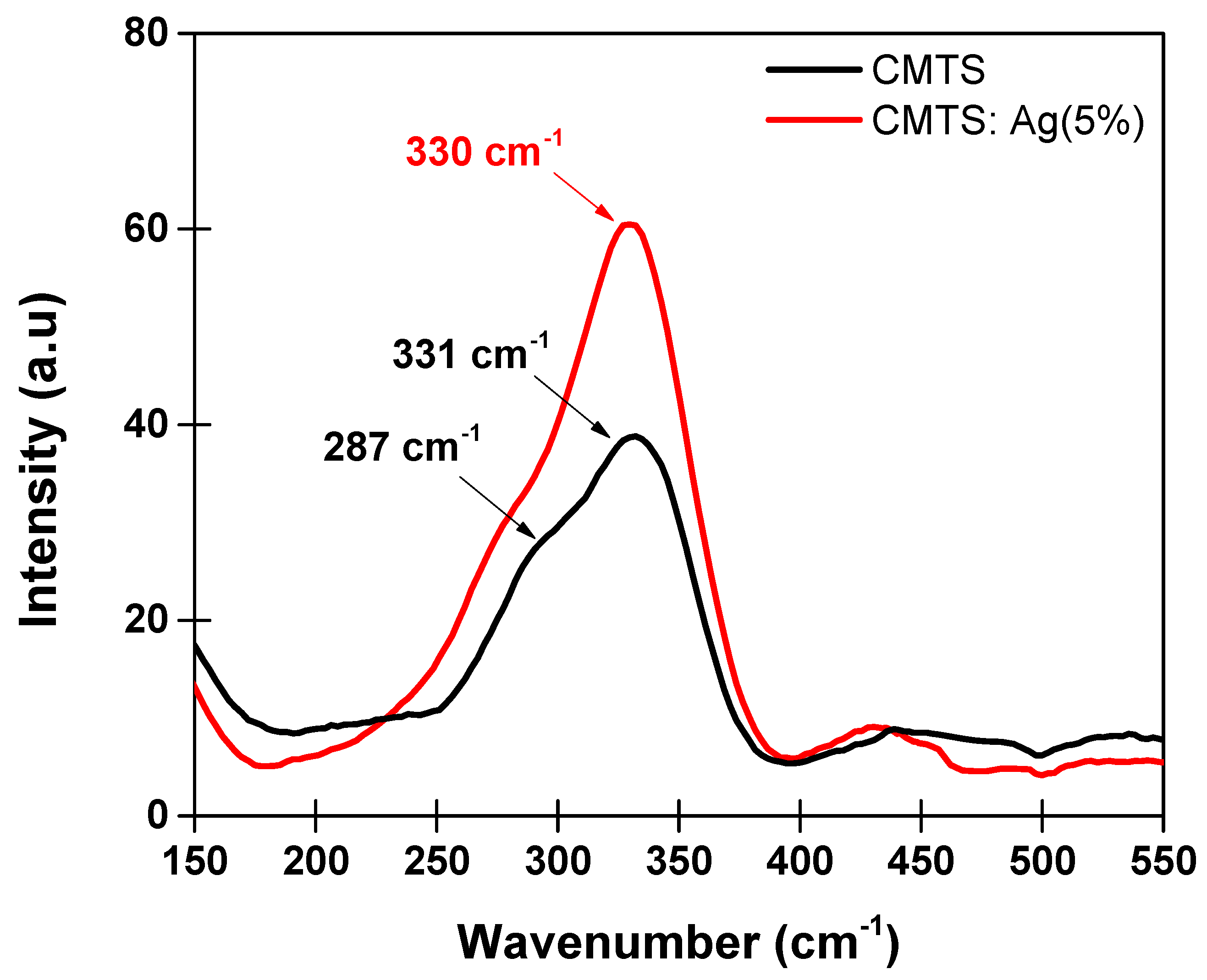
3.1.2. Raman Spectroscopy Analysis
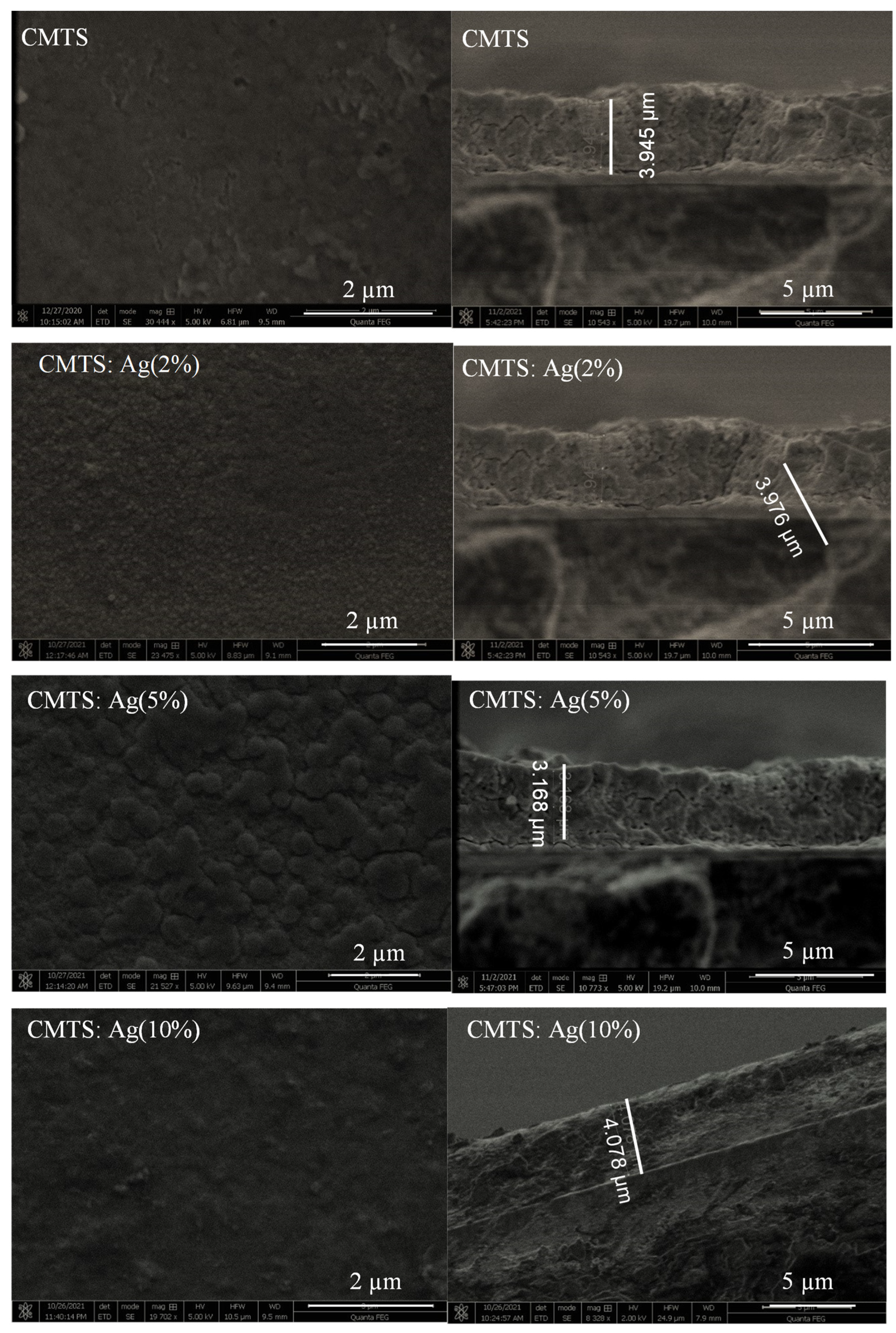
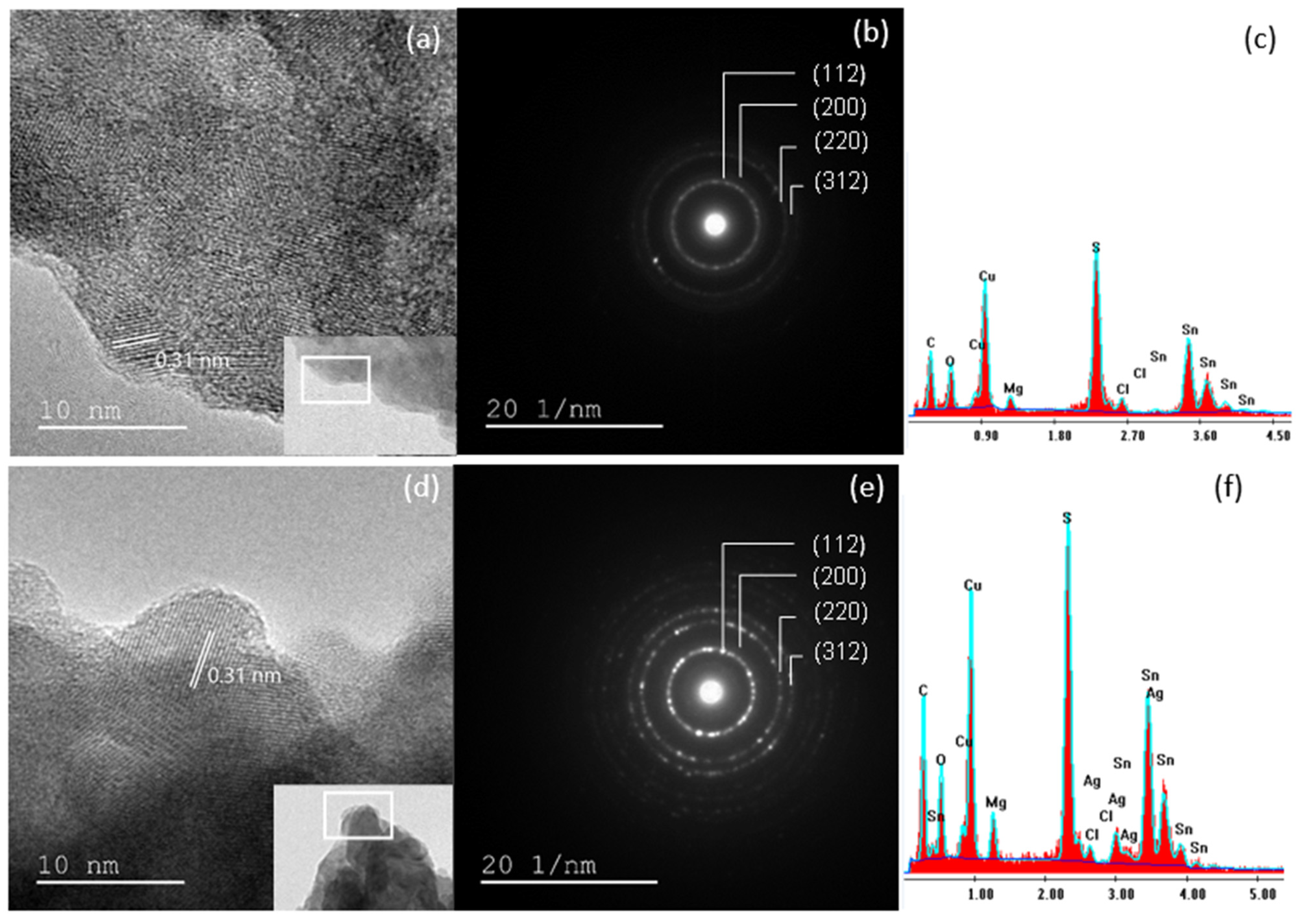
3.1.3. Morphological and Compositional Analysis
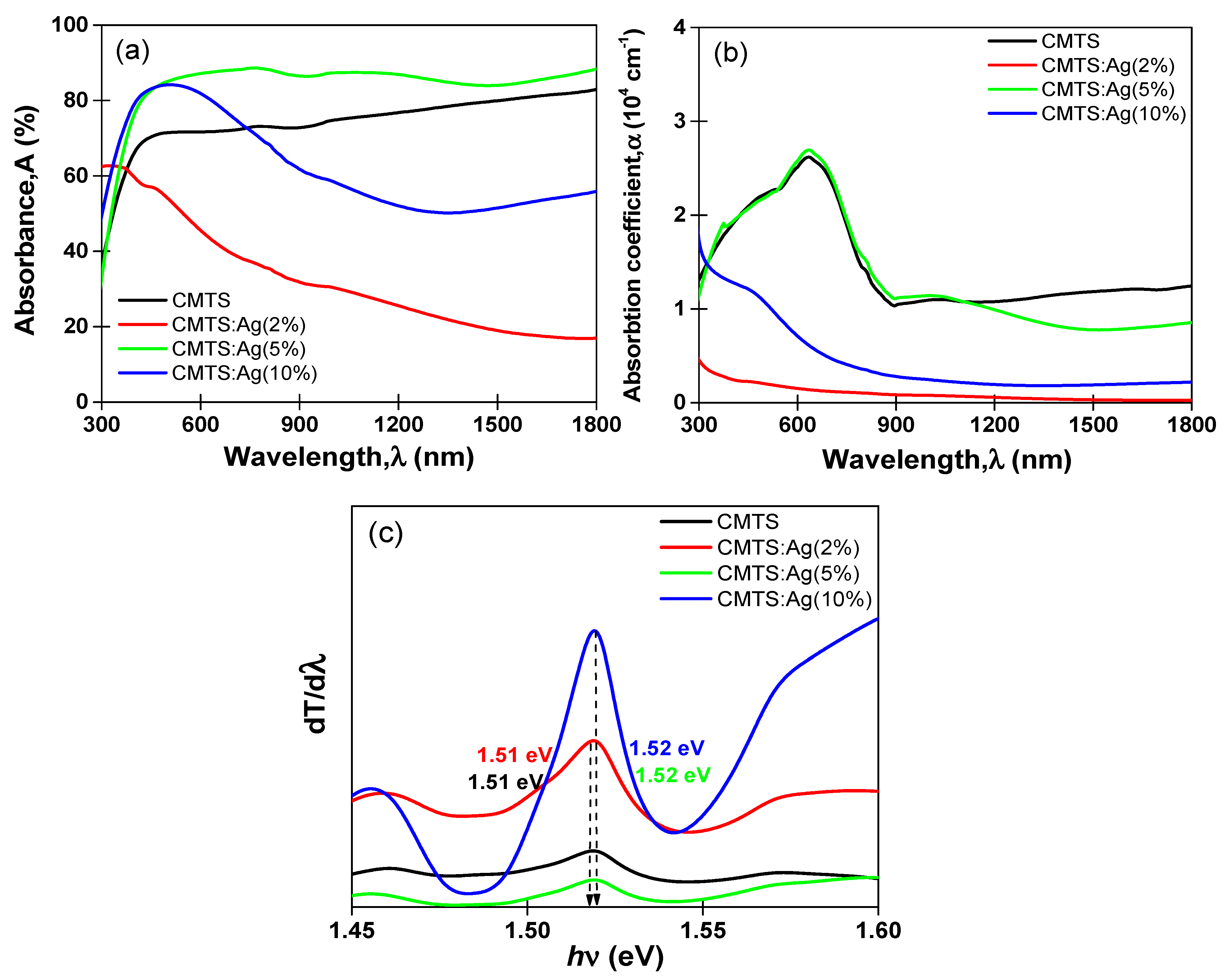
3.2. Optical Properties
3.3. Electrochemical Impedance Spectroscopy Measurements
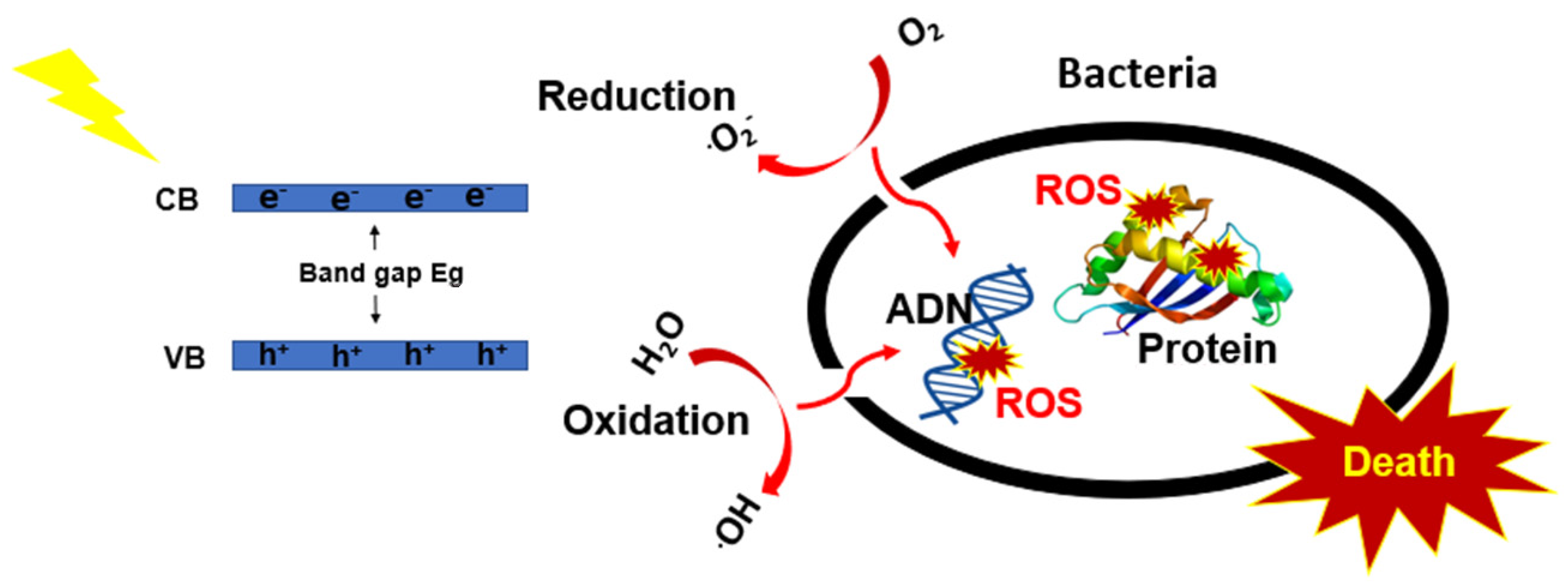
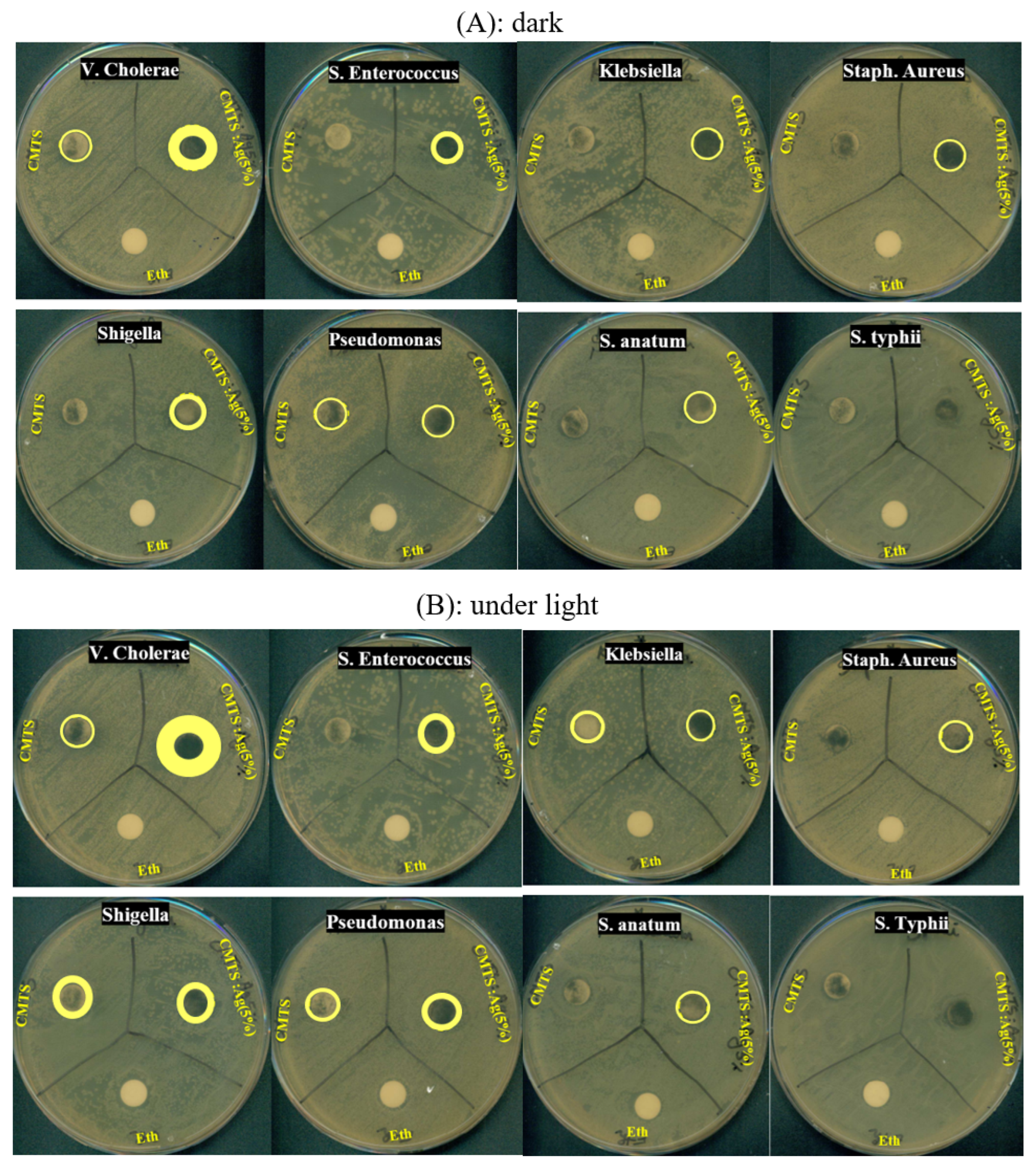
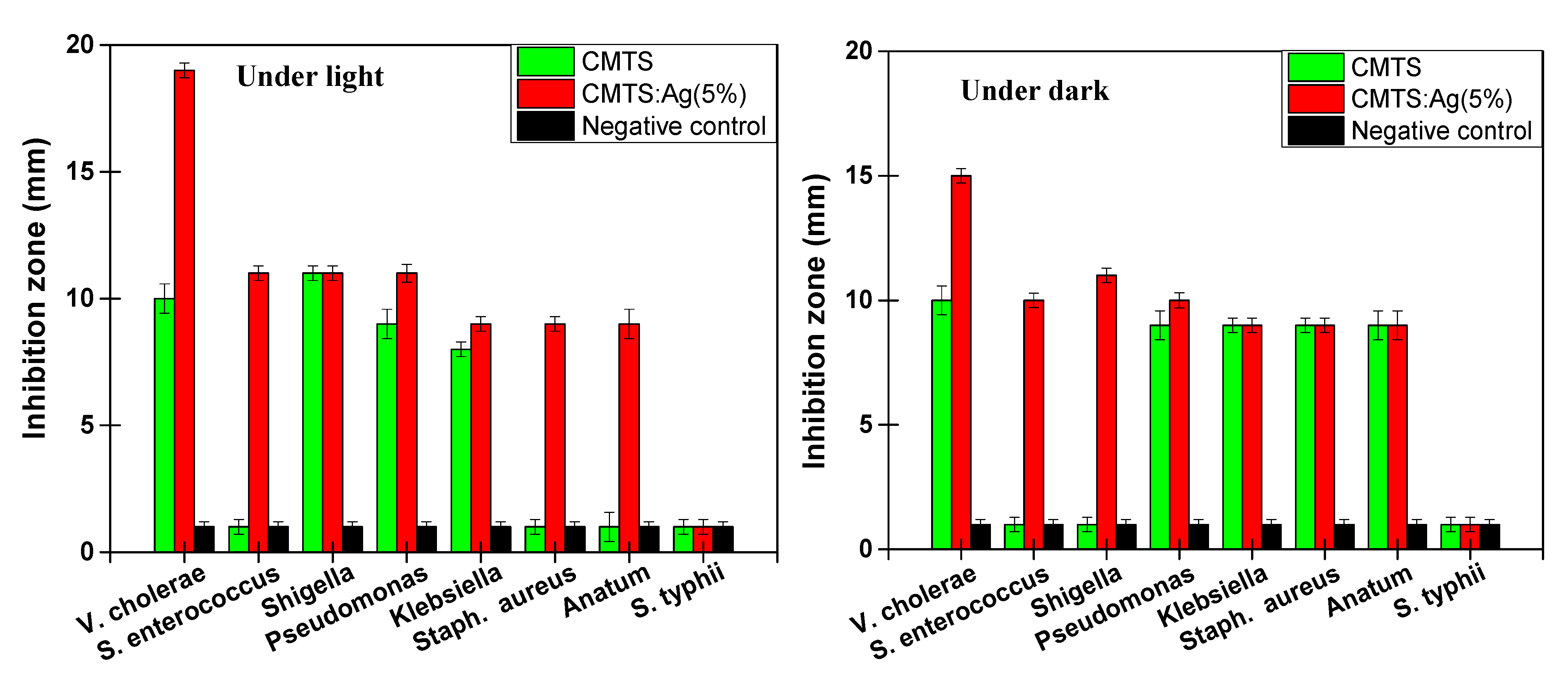
3.4. Antibacterial Study
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Syed, W.A.; Arif, W.; Ahmed, S.; Ghufran, M.A. Environment Friendly Nanoparticles of Quaternary Compound Cu0.5 Mgx SnS4 for Possible Photovoltaic and Photo-Catalytic Applications. Mater. Res. Express 2019, 6, 0950b5. [Google Scholar] [CrossRef]
- Sharma, A.; Sahoo, P.; Singha, A.; Padhan, S.; Udayabhanu, G.; Thangavel, R. Efficient Visible-Light-Driven Water Splitting Performance of Sulfidation-Free, Solution Processed Cu2MgSnS4 Thin Films: Role of Post-Drying Temperature. Solar Energy 2020, 203, 284–295. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, W.; Jiang, J.; Zuo, S.; Shi, F.; Chu, J. The Structural, Morphological and Optical–Electrical Characteristic of Cu2XSnS4 (X:Cu,Mg) Thin Films Fabricated by Novel Ultrasonic Co-Spray Pyrolysis. Mater. Lett. 2016, 172, 68–71. [Google Scholar] [CrossRef]
- Wei, M.; Du, Q.; Wang, R.; Jiang, G.; Liu, W.; Zhu, C. Synthesis of New Earth-Abundant Kesterite Cu2MgSnS4 Nanoparticles by Hot-Injection Method. Chem. Lett. 2014, 43, 1149–1151. [Google Scholar] [CrossRef]
- Yang, G.; Zhai, X.; Li, Y.; Yao, B.; Ding, Z.; Deng, R.; Zhao, H.; Zhang, L.; Zhang, Z. Synthesis and Characterizations of Cu2MgSnS4 Thin Films with Different Sulfuration Temperatures. Mater. Lett. 2019, 242, 58–61. [Google Scholar] [CrossRef]
- Agawane, G.L.; Vanalakar, S.A.; Kamble, A.S.; Moholkar, A.V.; Kim, J.H. Fabrication of Cu2(ZnxMg1-x)SnS4 Thin Films by Pulsed Laser Deposition Technique for Solar Cell Applications. Mater. Sci. Semicond. Process. 2018, 76, 50–54. [Google Scholar] [CrossRef]
- Ali, A.; Liang, Y.; Ahmed, S.; Yang, B.; Guo, B.; Yang, Y. Mutual Contaminants Relational Realization and Photocatalytic Treatment Using Cu2MgSnS4 Decorated BaTiO3. Appl. Mater. Today 2020, 18, 100534. [Google Scholar] [CrossRef]
- Souli, M.; Engazou, R.; Ajili, L.; Kamoun-Turki, N. Physical Properties Evolution of Sprayed Cu2MgSnS4 Thin Films with Growth Parameters and Vacuum Annealing. Superlattices Microstruct. 2020, 147, 106711. [Google Scholar] [CrossRef]
- Hammoud, A.; Souli, M.; Kouki, F.; Ajili, L.; Bouricha, B.; Kamoun, N. Investigation of Substrate Temperature Effect on Physical Properties of Sprayed Cu2MgSnS4 Thin Films for Solar Cells and Humidity Sensing Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 6926–6941. [Google Scholar] [CrossRef]
- Hammoud, A.; Yahmadi, B.; Souli, M.; Ahmed, S.A.; Ajili, L.; Kamoun-Turki, N. Effect of Sulfur Content on Improving Physical Properties of New Sprayed Cu2MgSnS4 Thin Films Compound for Optoelectronic Applications. Eur. Phys. J. Plus 2022, 137, 232. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A Green Synthesis of Copper Nanoparticles Using Native Cyclodextrins as Stabilizing Agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Raj, V.; Raorane, C.J.; Lee, J.-H.; Lee, J. Appraisal of Chitosan-Gum Arabic-Coated Bipolymeric Nanocarriers for Efficient Dye Removal and Eradication of the Plant Pathogen Botrytis Cinerea. ACS Appl. Mater. Interfaces 2021, 13, 47354–47370. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Lee, J.-H.; Shim, J.-J.; Lee, J. Recent Findings and Future Directions of Grafted Gum Karaya Polysaccharides and Their Various Applications: A Review. Carbohydr. Polym. 2021, 258, 117687. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-Gum Arabic Embedded Alizarin Nanocarriers Inhibit Biofilm Formation of Multispecies Microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Shastri, D.; Parveen, A.S.; Haldhar, R.; Raj, V.; Kim, S.-C. Grafted Chitosan-Hyaluronic Acid (CS-g-Poly (MA-Co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida Albicans Biofilm Formation. Antibiotics 2022, 11, 950. [Google Scholar] [CrossRef]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial Activity of Photocatalysts: Fundamentals, Mechanisms, Kinetics and Recent Advances. Appl. Catal. B Environ. 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Meng, D.; Liu, X.; Xie, Y.; Du, Y.; Yang, Y.; Xiao, C. Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping. Adv. Mater. Sci. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.S.; Maddirevula, S.; Easwaran, M.; Dananjaya, S.H.S.; Kim, M.-D. Antibacterial Activity of Novel Cu2ZnSnS4 Nanoparticles against Pathogenic Strains. RSC Adv. 2015, 5, 106400–106405. [Google Scholar] [CrossRef]
- Tlili, M.; Jebbari, N.; Naffouti, W.; Kamoun, N.T. Effect of Precursor Nature on Physical Properties of Chemically Sprayed MgO Thin Films for Optoelectronic Application. Eur. Phys. J. Plus 2020, 135, 687. [Google Scholar] [CrossRef]
- Wang, D.; Wu, J.; Liu, X.; Wu, L.; Ao, J.; Liu, W.; Sun, Y.; Zhang, Y. Formation of the Front-Gradient Bandgap in the Ag Doped CZTSe Thin Films and Solar Cells. J. Energy Chem. 2019, 35, 188–196. [Google Scholar] [CrossRef]
- Gu, K.; Hao, R.; Guo, J.; Aierken, A.; Liu, X.; Chang, F.; Li, Y.; Wei, G.; Liu, B.; Wang, L.; et al. Influence of Ag Layer Location on the Performance of Cu2ZnSnS4 Thin Film Solar Cells. J. Electron. Mater. 2020, 49, 1819–1826. [Google Scholar] [CrossRef]
- Chandel, T.; Zaman, M.B.; Dwivedi, S.K.; Poolla, R. Structural, Morphological and Optical Properties of Sprayed Cu2ZnSnS4 Thin Films by Varying the Molar Concentration of Zn & Sn. Vacuum 2019, 159, 341–345. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kawaguchi, T.; Chantana, J.; Minemoto, T.; Harada, T.; Nakanishi, S.; Ikeda, S. Structural and Solar Cell Properties of a Ag-Containing Cu2 ZnSnS4 Thin Film Derived from Spray Pyrolysis. ACS Appl. Mater. Interfaces 2018, 10, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.; Jrad, A.; Yahmadi, B.; Souli, M.; Kouki, F.; Ajili, L.; Kamoun-Turki, N. Investigation on Cu2MgSnS4 Thin Film Prepared by Spray Pyrolysis for Photovoltaic and Humidity Sensor Applications. Opt. Mater. 2022, 127, 112296. [Google Scholar] [CrossRef]
- Gunavathy, K.V.; Tamilarasan, K.; Rangasami, C.; Arulanantham, A.M.S. Effect of Solvent on the Characteristic Properties of Nebulizer Spray Pyrolyzed Cu2ZnSnS4 Absorber Thin Films for Photovoltaic Application. Thin Solid Films 2020, 697, 137841. [Google Scholar] [CrossRef]
- Tumbul, A.; Göktaş, A.; Zarbali, M.Z.; Aslan, F. Structural, Morphological and Optical Properties of the Vacuum-Free Processed CZTS Thin Film Absorbers. Mater. Res. Express 2018, 5, 066408. [Google Scholar] [CrossRef]
- Singh, O.P.; Sharma, A.; Gour, K.S.; Husale, S.; Singh, V.N. Fast Switching Response of Na-Doped CZTS Photodetector from Visible to NIR Range. Sol. Energy Mater. Sol. Cells 2016, 157, 28–34. [Google Scholar] [CrossRef]
- Sheng, L.; Xiao, Y.; Jiao, C.; Du, B.; Li, Y.; Wu, Z.; Shao, L. Influence of Layer Number on Microstructure, Mechanical Properties and Wear Behavior of the TiN/Ti Multilayer Coatings Fabricated by High-Power Magnetron Sputtering Deposition. J. Manuf. Process. 2021, 70, 529–542. [Google Scholar] [CrossRef]
- Nefzi, C.; Souli, M.; Cuminal, Y.; Kamoun-Turki, N. Effect of Sulfur Concentration on Structural, Optical and Electrical Properties of Cu2FeSnS4 Thin Films for Solar Cells and Photocatalysis Applications. Superlattices Microstruct. 2018, 124, 17–29. [Google Scholar] [CrossRef]
- Nefzi, C.; Souli, M.; Cuminal, Y.; Kamoun-Turki, N. Effect of Substrate Temperature on Physical Properties of Cu2FeSnS4 Thin Films for Photocatalysis Applications. Mater. Sci. Eng. B 2020, 254, 114509. [Google Scholar] [CrossRef]
- Ziti, A. Characteristics of Kesterite CZTS Thin Films Deposited by Dip-Coating Technique for Solar Cells Applications. J. Mater. Sci. 2019, 30, 13134–13143. [Google Scholar] [CrossRef]
- Dabbabi, S.; Souli, M.; Ben Nasr, T.; Garcia-Loureiro, A.; Kamoun, N. Vacuum Annealing Effect on Physical Properties and Electrical Circuit Model of ZnO:Sn/SnO2:F Bilayer Structure. Vacuum 2019, 167, 416–420. [Google Scholar] [CrossRef]
- Patil, S.J.; Lokhande, V.C.; Lee, D.-W.; Lokhande, C.D. Electrochemical Impedance Analysis of Spray Deposited CZTS Thin Film: Effect of Se Introduction. Opt. Mater. 2016, 58, 418–425. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Aziz, A.A. Sensing Mechanism of Hydrogen Gas Sensor Based on RF-Sputtered ZnO Thin Films. Int. J. Hydrog. Energy 2010, 35, 4428–4434. [Google Scholar] [CrossRef]
- Dabbabi, S.; Souli, M.; Ben Nasr, T.; Garcia-Loureiro, A.; Kamoun, N. Effects of Ni and La Dopants on the Properties of ZnO and SnO2 Thin Films: Microstructural, Optical and Impedance Spectroscopy Studies. J. Electron. Mater. 2020, 49, 1314–1321. [Google Scholar] [CrossRef]
- Shelke, H.D.; Lokhande, A.C.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Cu2SnS3 Thin Film: Structural, Morphological, Optical and Photoelectrochemical Studies. Surf. Interfaces 2017, 9, 238–244. [Google Scholar] [CrossRef]
- Sankır, N.D.; Aydın, E.; Sankır, M. Impedance Spectroscopy and Dielectric Properties of Silver Incorporated Indium Sulfide Thin Films. Int. J. Electrochem. Sci. 2014, 9, 12. [Google Scholar]
- Paraye, A.; Manivannan, R.; Victoria, S.N. CZTS Nanoparticles by One-Pot Sonochemical Route—Effect of Power Density, PH and Bath Temperature. J. Indian Chem. Soc. 2021, 98, 100094. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An Enhancement of Antimicrobial Efficacy of Biogenic and Ceftriaxone-Conjugated Silver Nanoparticles: Green Approach. Environ. Sci. Pollut. Res. 2018, 25, 10362–10370. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K.; Ighalo, J.O. Multifunctional CuO Nanoparticles with Enhanced Photocatalytic Dye Degradation and Antibacterial Activity. Sustain. Environ. Res. 2022, 32, 2. [Google Scholar] [CrossRef]
- Zhao, X.; Wan, P.; Wang, H.; Zhang, S.; Liu, J.; Chang, C.; Yang, K.; Pan, Y. An Antibacterial Strategy of Mg-Cu Bone Grafting in Infection-Mediated Periodontics. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal Activity of Biosynthesized Silver Nanoparticles: Effect on Growth, Cell Morphology, and Key Virulence Attributes of Candida Species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
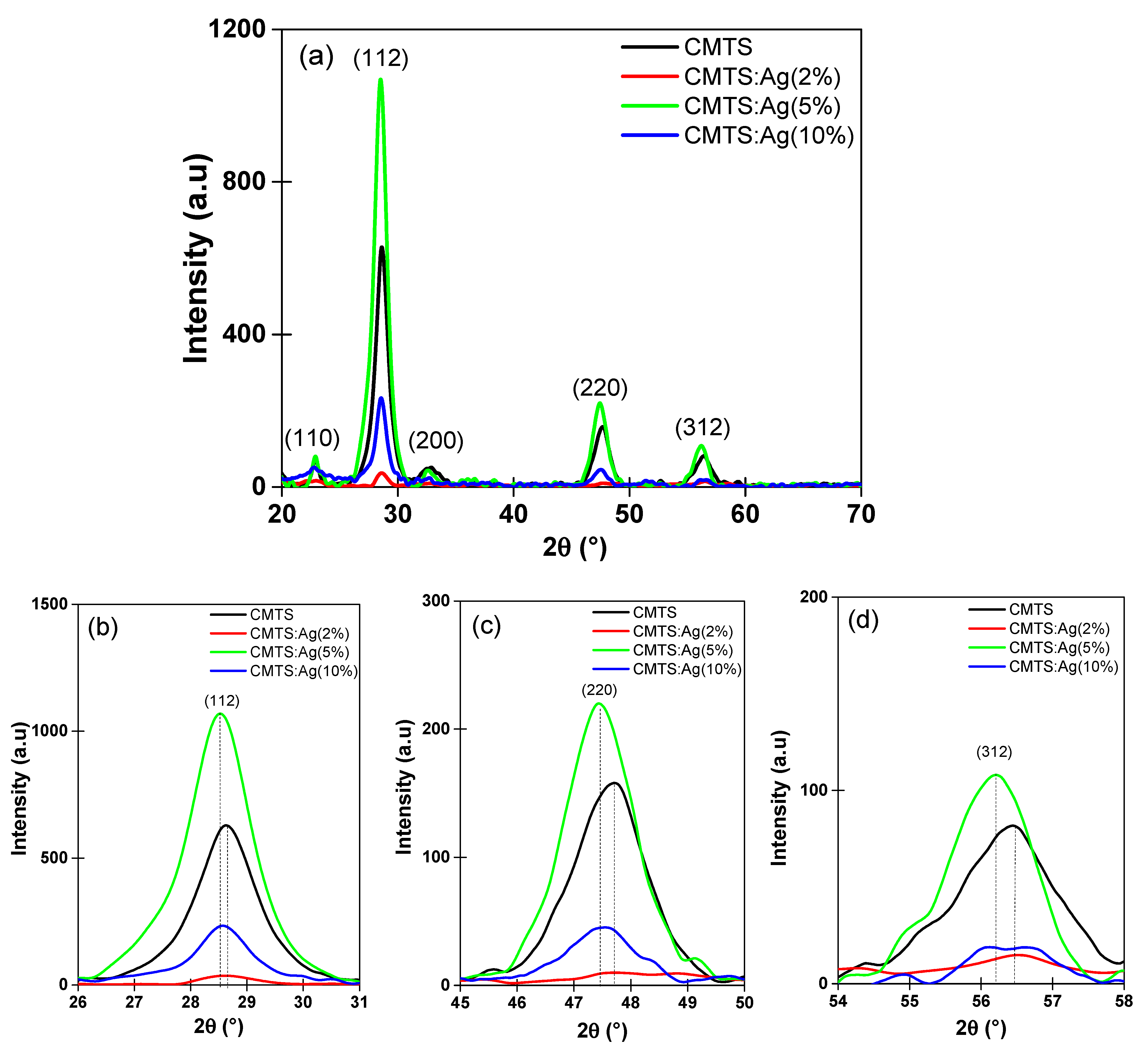


| Samples | 2θ (°) | D (nm) | δ (1011 Lines/m2) | (10−3%) |
|---|---|---|---|---|
| CMTS | 28.6 | 14.8 | 4.5 | 2.1 |
| Ag (2%)-CMTS | 28.55 | 13.5 | 5.4 | 2.4 |
| Ag (5%)-CMTS | 28.55 | 19.6 | 2.6 | 1.6 |
| Ag (10%)-CMTS | 28.55 | 13.9 | 5.1 | 2.2 |
| Samples | R1 (10−8 Ω) | R2 (105 Ω) | CPE-T (10−9 F) | CPE-P (F) | |
|---|---|---|---|---|---|
| CMTS | Dark | 1 | 6.1 | 4 | 0.75 |
| Light | 1 | 3.5 | 4 | 0.70 | |
| CMTS:Ag (5%) | Dark | 1 | 6.5 | 1 | 0.75 |
| Light | 1 | 3.7 | 0.1 | 0.68 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, A.; Souli, M.; Diouani, M.F.; Alhalaili, B.; Vidu, R.; Kamoun-Turki, N. Synthesis and Characterization of Novel Sprayed Ag-Doped Quaternary Cu2MgSnS4 Thin Film for Antibacterial Application. Nanomaterials 2022, 12, 3459. https://doi.org/10.3390/nano12193459
Hammoud A, Souli M, Diouani MF, Alhalaili B, Vidu R, Kamoun-Turki N. Synthesis and Characterization of Novel Sprayed Ag-Doped Quaternary Cu2MgSnS4 Thin Film for Antibacterial Application. Nanomaterials. 2022; 12(19):3459. https://doi.org/10.3390/nano12193459
Chicago/Turabian StyleHammoud, Amal, Mehdi Souli, Mohamed Fethi Diouani, Badriyah Alhalaili, Ruxandra Vidu, and Najoua Kamoun-Turki. 2022. "Synthesis and Characterization of Novel Sprayed Ag-Doped Quaternary Cu2MgSnS4 Thin Film for Antibacterial Application" Nanomaterials 12, no. 19: 3459. https://doi.org/10.3390/nano12193459
APA StyleHammoud, A., Souli, M., Diouani, M. F., Alhalaili, B., Vidu, R., & Kamoun-Turki, N. (2022). Synthesis and Characterization of Novel Sprayed Ag-Doped Quaternary Cu2MgSnS4 Thin Film for Antibacterial Application. Nanomaterials, 12(19), 3459. https://doi.org/10.3390/nano12193459








