Graphene-Based Composites with Silver Nanowires for Electronic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphite Oxide
2.3. Simultaneous Reduction and Chemical Functionalization of GO (f-rGODBSA)
2.3.1. f-rGODBSA Synthesis
2.3.2. f-rGODBSA Scale-Up Process
2.4. Synthesis of Silver Nanowires
2.5. Synthesis of f-rGODBSA/AgNWs Nanocomposites
2.5.1. Physical Mixture Method
2.5.2. In Situ Method
2.5.3. Scale-Up of f-rGODBSA/AgNWs10% In Situ Nanocomposite
2.6. Synthesis of FLG/MWNT-f-OH/AgNWs Nanohybrids
2.6.1. Synthesis of Hybrid FLG/MWNT-f-OH
2.6.2. Physical Mixture of AgNWs with FLG/MWNT-f-OH
2.7. Preparation of f-rGODBSA/AgNWs Ink Formulations with Different Resin Content
2.8. Characterization
2.9. Inks Rheological Characterization
2.10. Gravure Printing
2.11. Electrical Measurements
3. Results and Discussion
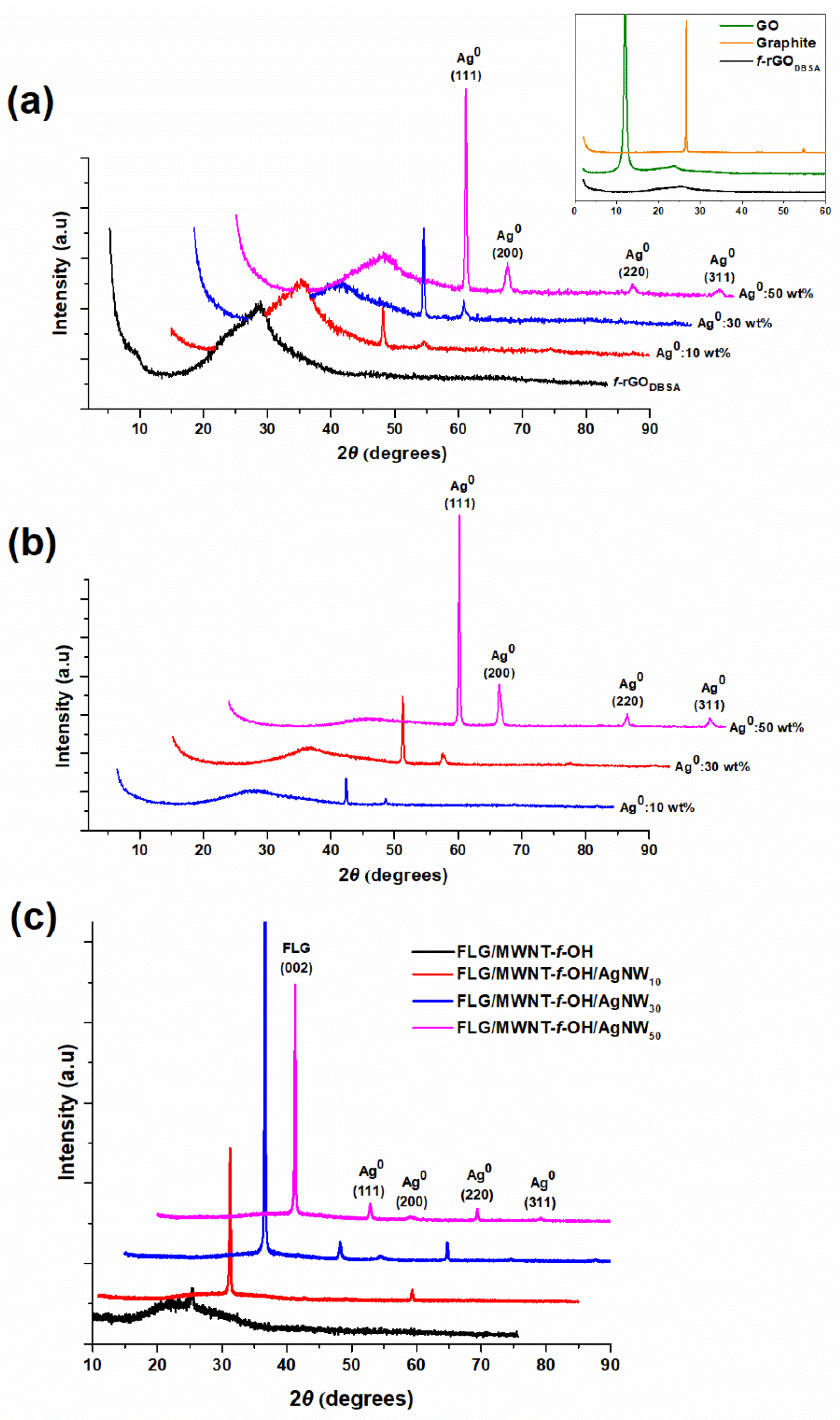
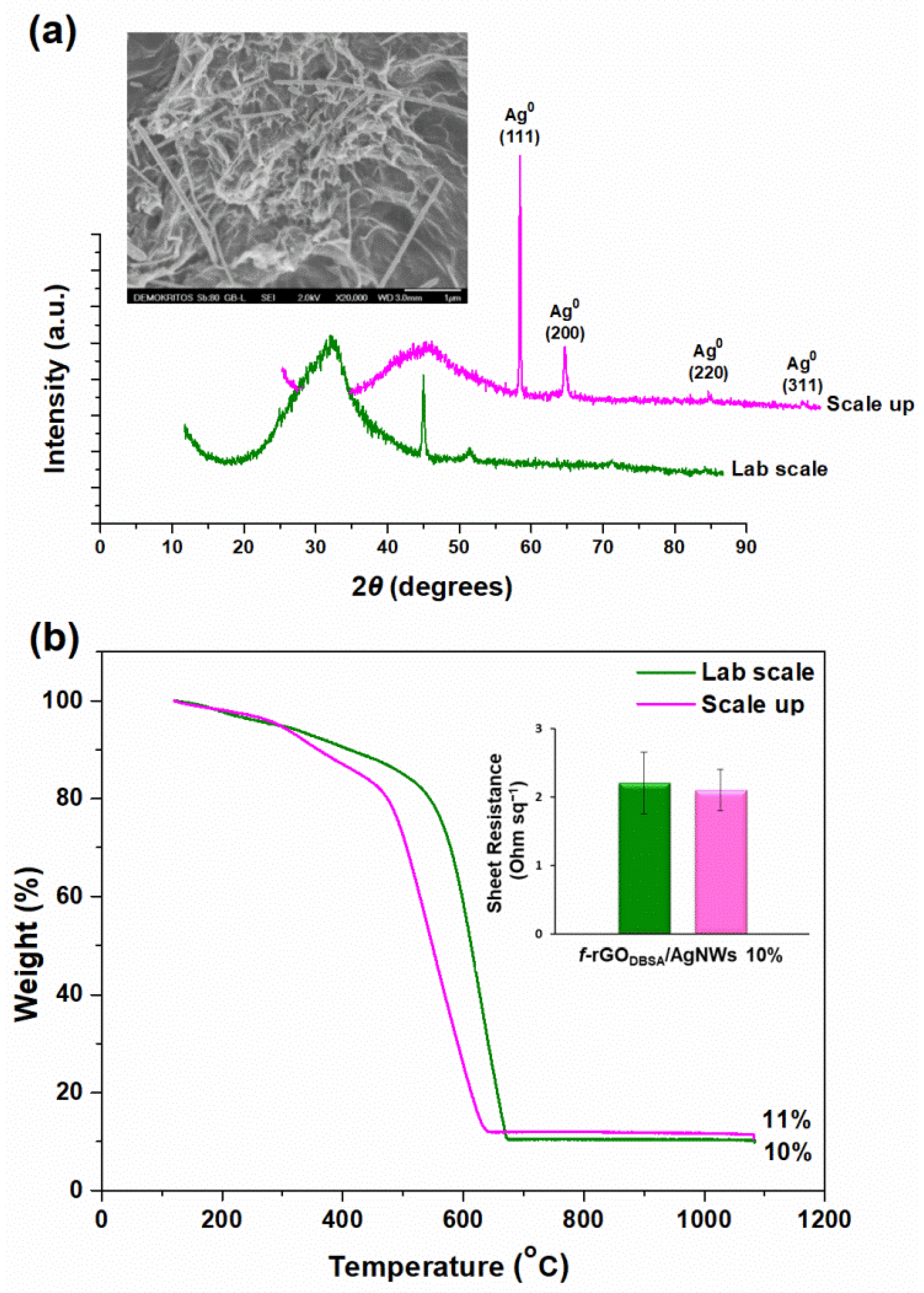
3.1. X-ray Diffraction Measurements
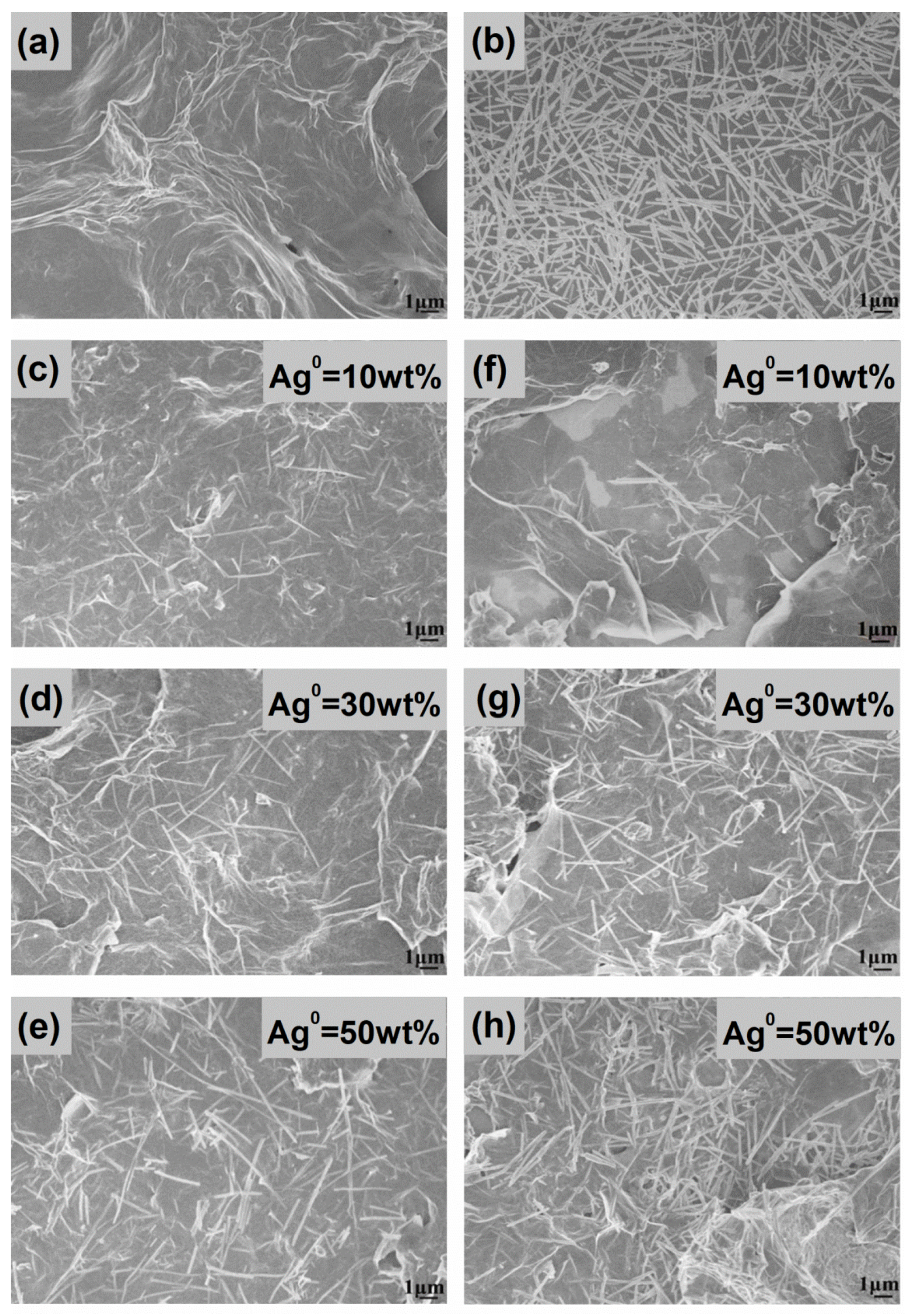
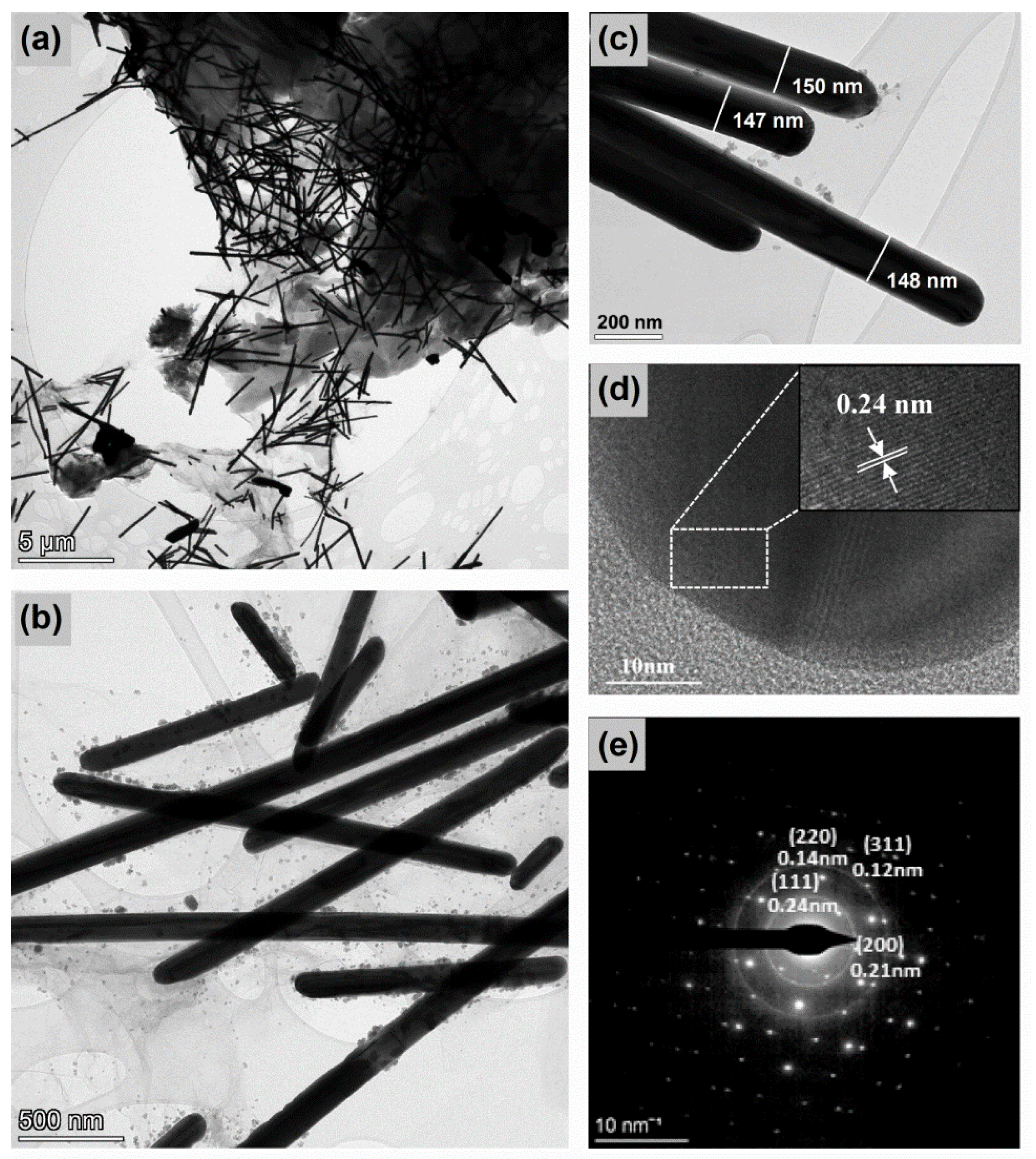
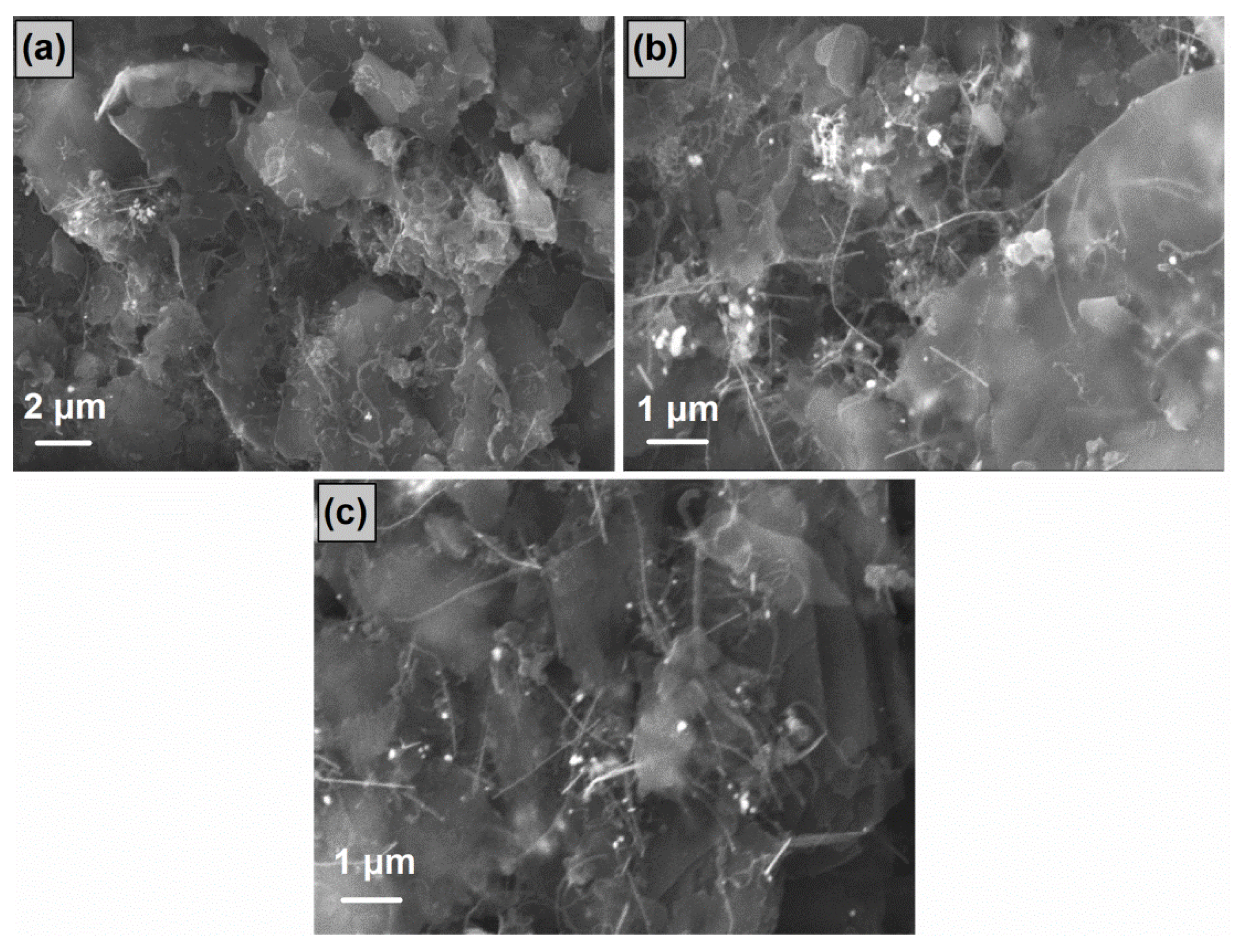
3.2. SEM/TEM Analysis
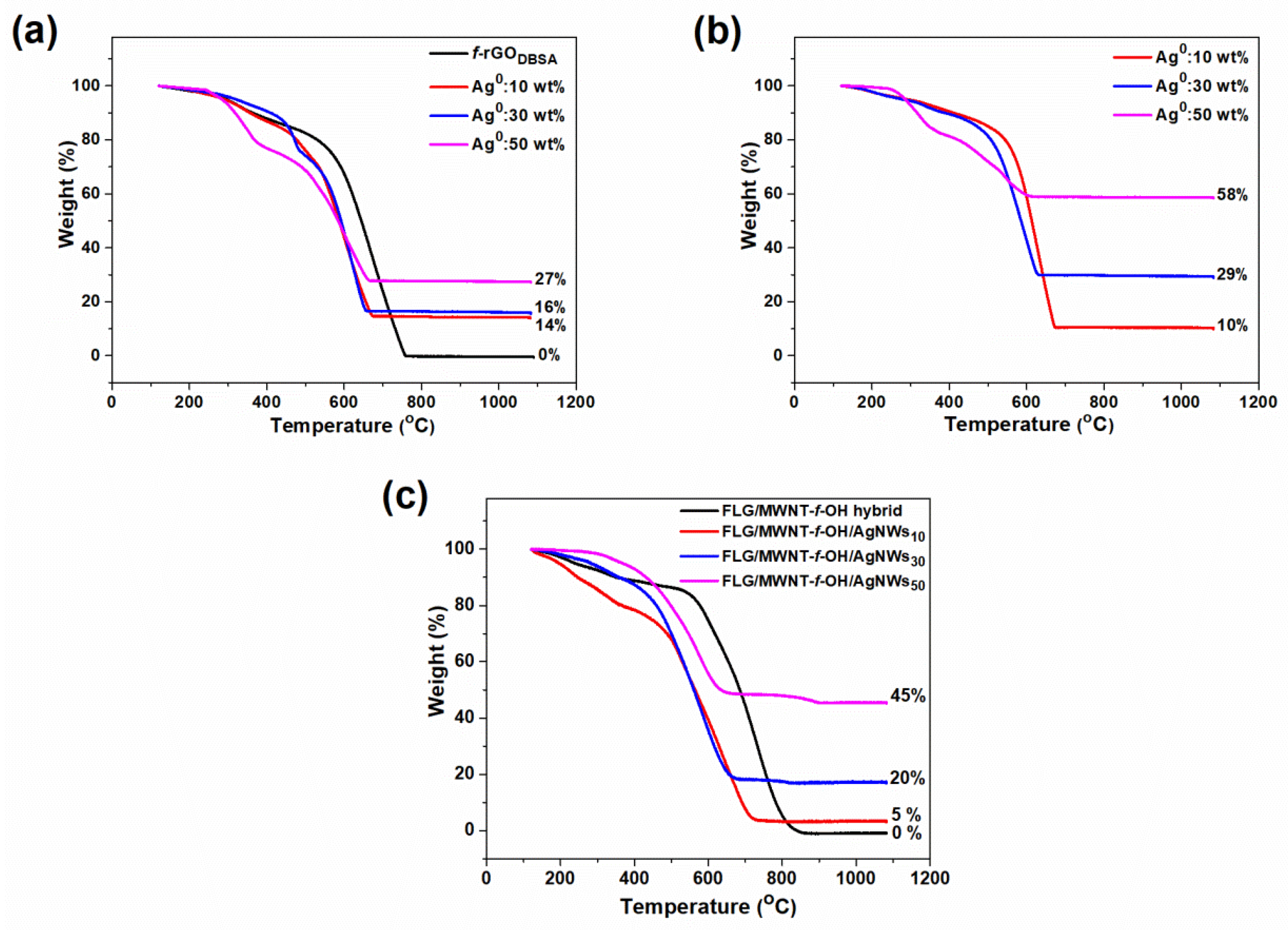
3.3. Thermogravimetric Analysis
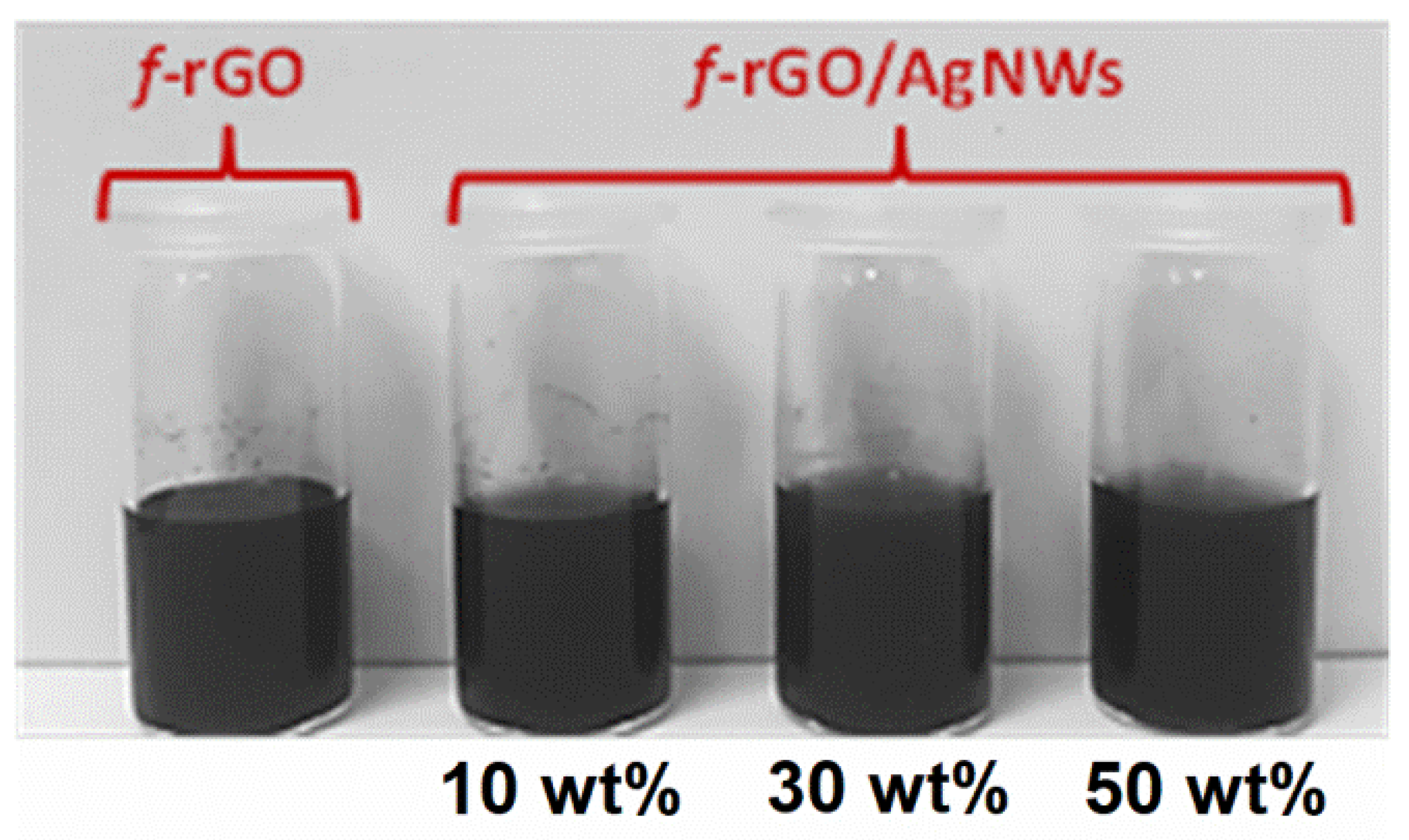
3.4. Water Dispersibility and Stability of f-rGODBSA and f-rGODBSA/AgNWs Hybrids
3.5. Electrical Measurements
3.6. Characteristics of the Up-Scaled f-rGODBSA/AgNWs10% Hybrid
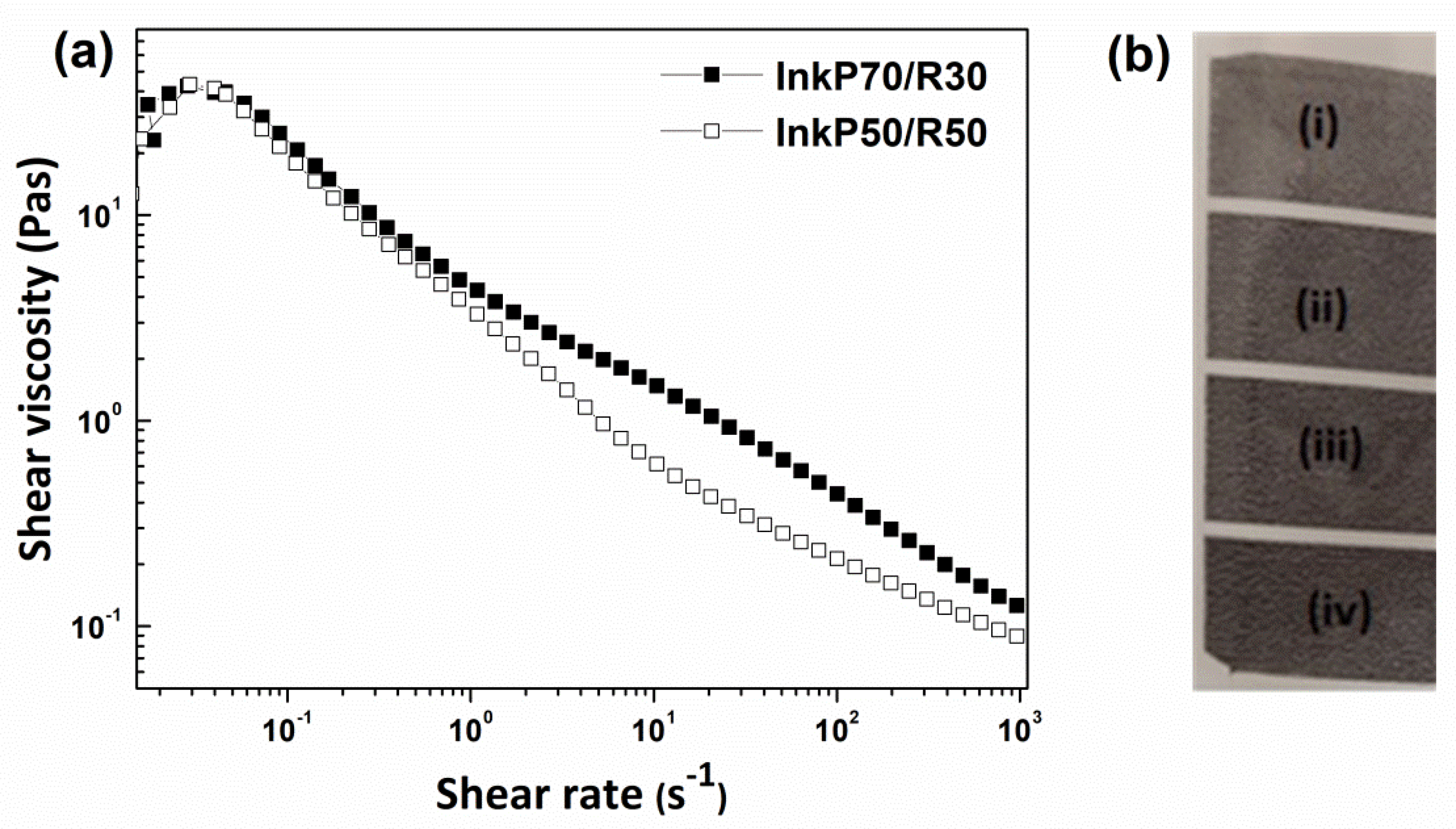
3.7. Rheological Characteristics
3.8. Printing Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiklund, J.; Karakoç, A.; Palko, T.; Yigitler, H.; Ruttik, K.; Jäntti, R.; Paltakari, J. A Review on Printed Electronics: Fabrication Methods, Inks, Substrates, Applications and Environmental Impacts. J. Manuf. Mater. Process. 2021, 5, 89. [Google Scholar] [CrossRef]
- Barmpakos, D.; Belessi, V.; Schelwald, R.; Kaltsas, G.K. Evaluation of Inkjet-Printed Reduced and Functionalized Water-Dispersible Graphene Oxide and Graphene on Polymer Substrate-Application to Printed Temperature Sensors. Nanomaterials 2021, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Barmpakos, D.; Belessi, V.; Xanthopoulos, N.; Krontiras, C.A.; Kaltsas, G. Flexible Inkjet-Printed Heaters Utilizing Graphene-Based Inks. Sensors 2022, 22, 1173. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Takeda, Y.; Shiwaku, R.; Tokito, S.; Cho, K.; Jung, S. Three-Dimensional Monolithic Integration in Flexible Printed Organic Transistors. Nat. Commun. 2019, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.; Zhou, L.; Wan, X.; Zhu, C.; Yang, B.; Huang, Y.A. Laser Transfer, Printing, and Assembly Techniques for Flexible Electronics. Adv. Electron. Mater. 2019, 5, 1800900. [Google Scholar] [CrossRef]
- Choi, S.; Jo, W.; Jeon, Y.; Kwon, S.; Kwon, J.H.; Son, Y.H.; Kim, J.; Park, J.H.; Kim, H.; Lee, H.S.; et al. Multi-Directionally Wrinkle-Able Textile OLEDs for Clothing-Type Displays. Npj Flex. Electron. 2020, 4, 33. [Google Scholar] [CrossRef]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef] [Green Version]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 9, 8035. [Google Scholar] [CrossRef] [Green Version]
- Htwe, Y.Z.N.; Mariatti, M. Printed Graphene and Hybrid Conductive Inks for Flexible, Stretchable, and Wearable Electronics: Progress, Opportunities, and Challenges. J. Sci. Adv. Mater. Devices 2022, 7, 100435. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-Resolution Patterning of Graphene by Screen Printing with a Silicon Stencil for Highly Flexible Printed Electronics. Adv. Mater. 2015, 27, 109–115. [Google Scholar] [CrossRef]
- He, P.; Cao, J.; Ding, H.; Liu, C.; Li, Z.; Kinloch, I.A.; Derby, B. Screen Printing of a Highly Conductive Graphene Ink for Flexible Printed Electronics. Appl. Mater. Interfaces 2019, 11, 32225–32234. [Google Scholar] [CrossRef] [PubMed]
- Koutsioukis, A.; Philippakopoulou, T.; Anastasopoulou, M.; Giasafaki, D.; Mitzithra, C.; Theodore, S.; Charalambopoulou, G.; Georgakilas, V.; Belessi, V. Carbon Black and Reduced Graphene Oxide Water-Based Conductive Inks. Adv. Print. Media Technol. 2021, 47, 85–96. [Google Scholar] [CrossRef]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Kang, J.; Ng, L.W.T.; Zhu, X.; Howe, R.C.T.; Jones, C.G.; Hersam, M.C.; Hasan, T. Functional Inks and Printing of Two-Dimensional Materials. Chem. Soc. Rev. 2018, 47, 3265–3300. [Google Scholar] [CrossRef] [Green Version]
- Secor, E.B.; Hersam, M.C. Emerging Carbon and Post-Carbon Nanomaterial Inks for Printed Electronics. J. Phys. Chem. Lett. 2015, 6, 620–626. [Google Scholar] [CrossRef]
- Belessi, V.; Petridis, D.; Steriotis, T.; Spyrou, K.; Manolis, G.K.; Psycharis, V.; Georgakilas, V. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Highly Conductive and Water Dispersible Graphene Derivatives. SN Appl. Sci. 2019, 1, 77. [Google Scholar] [CrossRef] [Green Version]
- Koutsioukis, A.; Belessi, V.; Georgakilas, V. Solid Phase Functionalization of MWNTs: An Eco-Friendly Approach for Carbon-Based Conductive Inks. Green Chem. 2021, 23, 5442–5448. [Google Scholar] [CrossRef]
- Koutsioukis, A.; Georgakilas, V.; Belessi, V.; Zboril, R. Highly Conductive Water-Based Polymer/Graphene Nanocomposites for Printed Electronics. Chem. A Eur. J. 2017, 23, 8268–8274. [Google Scholar] [CrossRef]
- Belessi, V.; Manolis, G.K.; Vlahopoulos, G.; Philippakopoulou, T.; Steriotis, T.; Koutsioukis, A.; Georgakilas, V. Gravure and Flexography Printing of Highly Conductive Reduced Graphene Oxide Inks. In Proceedings of the 3rd International Printing Technologies Symposium, Instabul, Turkey, 10–12 October 2019; pp. 180–188. [Google Scholar]
- Liu, P.; Tang, Q.; Liu, H.; Lu, A. Low Electrical Resistivity of a Graphene-AgNHPs Based Ink with a New Processing Method. RSC Adv. 2017, 7, 15228–15235. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Zhu, Y. Printing Conductive Nanomaterials for Flexible and Stretchable Electronics: A Review of Materials, Processes, and Applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Liu, P.; He, W.; Lu, A. Preparation of Low-Temperature Sintered High Conductivity Inks Based on Nanosilver Self-Assembled on Surface of Graphene. J. Cent. South Univ. 2019, 26, 2953–2960. [Google Scholar] [CrossRef]
- Kumar, A.; Shaikh, M.O.; Chuang, C.H. Silver Nanowire Synthesis and Strategies for Fabricating Transparent Conducting Electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.; Van Helvert, M.; Hamans, R.F.; Verbroekken, R.; Sinha, R.; Bieberle-Hütter, A.; Baldi, A. Simple and Fast High-Yield Synthesis of Silver Nanowires. Nano Lett. 2020, 20, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Lahane, T.K.; Agrawal, J.; Singh, V. Optimization of Polyol Synthesized Silver Nanowires for Transparent Conducting Electrodes Application. Mater. Today Proc. 2021, 59, 257–263. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Chen, X.; Wan, J.; Qian, Y. A Simple Hydrothermal Route to Large-Scale Synthesis of Uniform Silver Nanowires. Chem. A Eur. J. 2005, 11, 160–163. [Google Scholar] [CrossRef]
- Hwang, J.; Shim, Y.; Yoon, S.M.; Lee, S.H.; Park, S.H. Influence of Polyvinylpyrrolidone (PVP) Capping Layer on Silver Nanowire Networks: Theoretical and Experimental Studies. RSC Adv. 2016, 6, 30972–30977. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Luo, Z.; Cai, Z.; Wang, Y.; Wang, Y.; Wang, B. In Situ Growth of Silver Nanowires on Reduced Graphene Oxide Sheets for Transparent Electrically Conductive Films. RSC Adv. 2016, 6, 37124–37129. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Wu, Y.; Wang, Z.; Wu, L.; Zheng, L. A One-Step Approach to Green and Scalable Production of Graphene Inks for Printed Flexible Film Heaters. Mater. Chem. Front. 2021, 5, 1895–1905. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Mariatti, M. Surfactant-Assisted Water-Based Graphene Conductive Inks for Flexible Electronic Applications. J. Taiwan Inst. Chem. Eng. 2021, 125, 402–412. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Park, S.H.; Joo, S.J.; Kim, H.S. UV-Assisted Flash Light Welding Process to Fabricate Silver Nanowire/Graphene on a PET Substrate for Transparent Electrodes. Nano Res. 2018, 11, 2190–2203. [Google Scholar] [CrossRef]
- Li, X.; Kim, N.; Youn, S.; An, T.K.; Kim, J.; Lim, S.; Kim, S.H. Sol-Gel-Processed Organic-Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene. Polymers 2019, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Lee, K.Y.; Kim, S.W. Ultra-Bendable and Durable Graphene–Urethane Composite/Silver Nanowire Film for Flexible Transparent Electrodes and Electromagnetic-Interference Shielding. Compos. Part B Eng. 2019, 177, 107406. [Google Scholar] [CrossRef]
- Staudenmaier, L. Method for the Preparation of the Graphite Acid. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar]
- Cao, L.; Huang, Q.; Cui, J.; Lin, H.; Li, W.; Lin, Z.; Zhang, P. Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films. Nanomaterials 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Wang, Y.S.; Hsiao, S.T.; Liao, W.H.; Lin, C.W.; Yang, S.Y.; Tien, H.W.; Ma, C.C.M.; Hu, C.C. Fabrication of a Silver Nanowire-Reduced Graphene Oxide-Based Electrochemical Biosensor and Its Enhanced Sensitivity in the Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid. J. Mater. Chem. C 2015, 3, 9444–9453. [Google Scholar] [CrossRef]
- Ain, Q.T.; Haq, S.H.; Alshammari, A.; Al-Mutlaq, M.A.; Anjum, M.N. The Systemic Effect of PEG-NGO-Induced Oxidative Stress in vivo in a Rodent Model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Dhaliwal, A.S. Electrochemical and Photocatalytic Degradation of Methylene Blue by Using RGO/AgNWs Nanocomposite Synthesized by Electroplating on Stainless Steel. J. Phys. Chem. Solids 2022, 160, 110358. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zhang, B.; Su, X.; Huo, S.; Chen, W.; Wang, X.; Wang, J. 3D-Structured Assembly of RGO and Ag Nanowires for Enhanced Microwave Absorption Performance Epoxy Composites. J. Mater. Sci. Mater. Electron. 2019, 30, 10321–10331. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Green and Facile Production of High-Quality Graphene from Graphite by the Combination of Hydroxyl Radicals and Electrical Exfoliation in Different Electrolyte Systems. RSC Adv. 2019, 9, 3693–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Khan, A.N.; Shakir, M.F. Strengthening of β Polymorph in PVDF/FLG and PVDF/GO Nanocomposites. Mater. Res. Express 2020, 7, 015017. [Google Scholar] [CrossRef] [Green Version]
- Staden, R.-I.S.; Popa-Tudor, I.; Ionescu-Tirgoviste, C.; Stoica, R.-A.; Magerusan, L. Molecular Enantiorecognition of D- and L-Glucose in Urine and Whole Blood Samples. J. Electrochem. Soc. 2019, 166, B3109–B3115. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Molaee Tavana, M.; Boukherroub, R. Sulfonated Reduced Graphene Oxide as a Highly Efficient Catalyst for Direct Amidation of Carboxylic Acids with Amines Using Ultrasonic Irradiation. Ultrason. Sonochem. 2016, 29, 371–379. [Google Scholar] [CrossRef]
- Jiang, D.D.; Yao, Q.; McKinney, M.A.; Wilkie, C.A. TGA/FTIR Studies on the Thermal Degradation of Some Polymeric Sulfonic and Phosphonic Acids and Their Sodium Salts. Polym. Degrad. Stab. 1999, 63, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Jia, Y.; Jia, D.; Li, S.; Ji, S.; Ye, C. Formulation of Concentrated and Stable Ink of Silver Nanowires with Applications in Transparent Conductive Films. RSC Adv. 2017, 7, 1936–1942. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, W.; Wang, X.; Liu, W.; Chen, K.; Gan, W. Low Effective Content of Reduced Graphene Oxide/Silver Nanowire Hybrids in Epoxy Composites with Enhanced Conductive Properties. J. Mater. Sci. Mater. Electron. 2019, 30, 7384–7392. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Z.; Li, H.; Zou, W. Functionalization of AgNWs with Amino Groups and Its Application in Epoxy Matrix in Antistatic and Thermal Conductivity Nanocomposites. RCS Adv. 2015, 5, 91516–91523. [Google Scholar] [CrossRef]
- Duffy, J. Analytical Strategies for Ink Formulation; White Pap. Malvern Instruments Ltd.: Worcestershire, UK, 2015; pp. 1–4. [Google Scholar]
- Zhang, Y.; Sullivan, J.P.; Bose, A. Rheological and Microstructural Characterization of Aqueous Suspensions of Carbon Black and Reduced Graphene Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124591. [Google Scholar] [CrossRef]
- Kippax, P. Why Particle Sizing? Paint. Coat. Ind. Mag. 01 March 2005, pp. 1–3. Available online: https://www.pcimag.com/articles/83460-why-particle-sizing (accessed on 31 August 2022).
- Bluvol, G.; Kässberger, M.; Reichhart, F. Optimizing Solids and Rheology in Blade Coating Using Pigment Blends—Part 1. O Pap. 2011, 72, 55–59. [Google Scholar]
- ASTM, D 1647–89; Standard Test Methods for Resistance of Dried Films of Varnishes to Water and Alkali. ASTM Int.: Pennsylvania, PA, USA, 1996; pp. 1–2.
- ASTM, D 2248–01a; Standard Practice for Detergent Resistance of Organic Finishes. ASTM Int.: Pennsylvania, PA, USA, 2007; pp. 1–3.


| Element % by Mass 1 | ||
|---|---|---|
| Samples | C | Ag |
| FLG/MWNT-f-OH/AgNWs10 | 94.42 | 5.58 |
| FLG/MWNT-f-OH/AgNWs30 | 80.84 | 19.16 |
| FLG/MWNT-f-OH/AgNWs50 | 74.79 | 25.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giasafaki, D.; Mitzithra, C.; Belessi, V.; Filippakopoulou, T.; Koutsioukis, A.; Georgakilas, V.; Charalambopoulou, G.; Steriotis, T. Graphene-Based Composites with Silver Nanowires for Electronic Applications. Nanomaterials 2022, 12, 3443. https://doi.org/10.3390/nano12193443
Giasafaki D, Mitzithra C, Belessi V, Filippakopoulou T, Koutsioukis A, Georgakilas V, Charalambopoulou G, Steriotis T. Graphene-Based Composites with Silver Nanowires for Electronic Applications. Nanomaterials. 2022; 12(19):3443. https://doi.org/10.3390/nano12193443
Chicago/Turabian StyleGiasafaki, Dimitra, Christina Mitzithra, Vassiliki Belessi, Theodora Filippakopoulou, Apostolos Koutsioukis, Vasilios Georgakilas, Georgia Charalambopoulou, and Theodore Steriotis. 2022. "Graphene-Based Composites with Silver Nanowires for Electronic Applications" Nanomaterials 12, no. 19: 3443. https://doi.org/10.3390/nano12193443
APA StyleGiasafaki, D., Mitzithra, C., Belessi, V., Filippakopoulou, T., Koutsioukis, A., Georgakilas, V., Charalambopoulou, G., & Steriotis, T. (2022). Graphene-Based Composites with Silver Nanowires for Electronic Applications. Nanomaterials, 12(19), 3443. https://doi.org/10.3390/nano12193443








