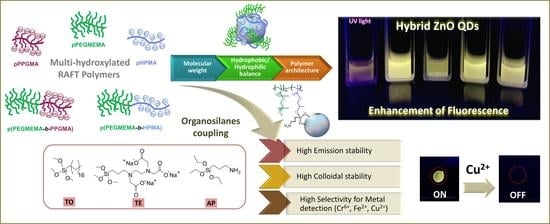
RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Homopolymers via RAFT Polymerization
2.3. Synthesis of Copolymers via RAFT Polymerization
2.4. Synthesis of Fluorescent ZnO QDs
2.5. Characterization and Properties
3. Results and Discussion
3.1. Synthesis of RAFT Hydroxylated Copolymers
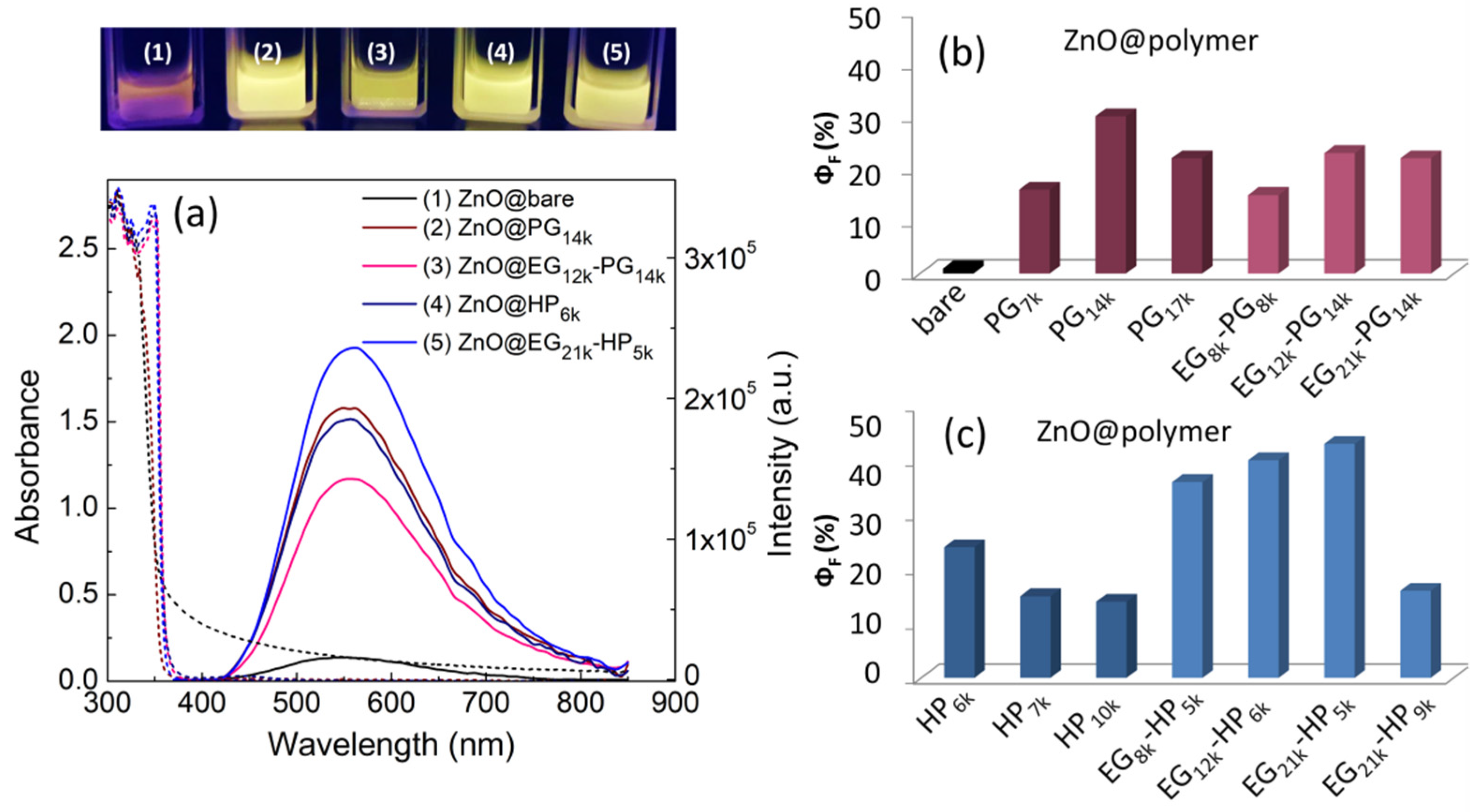
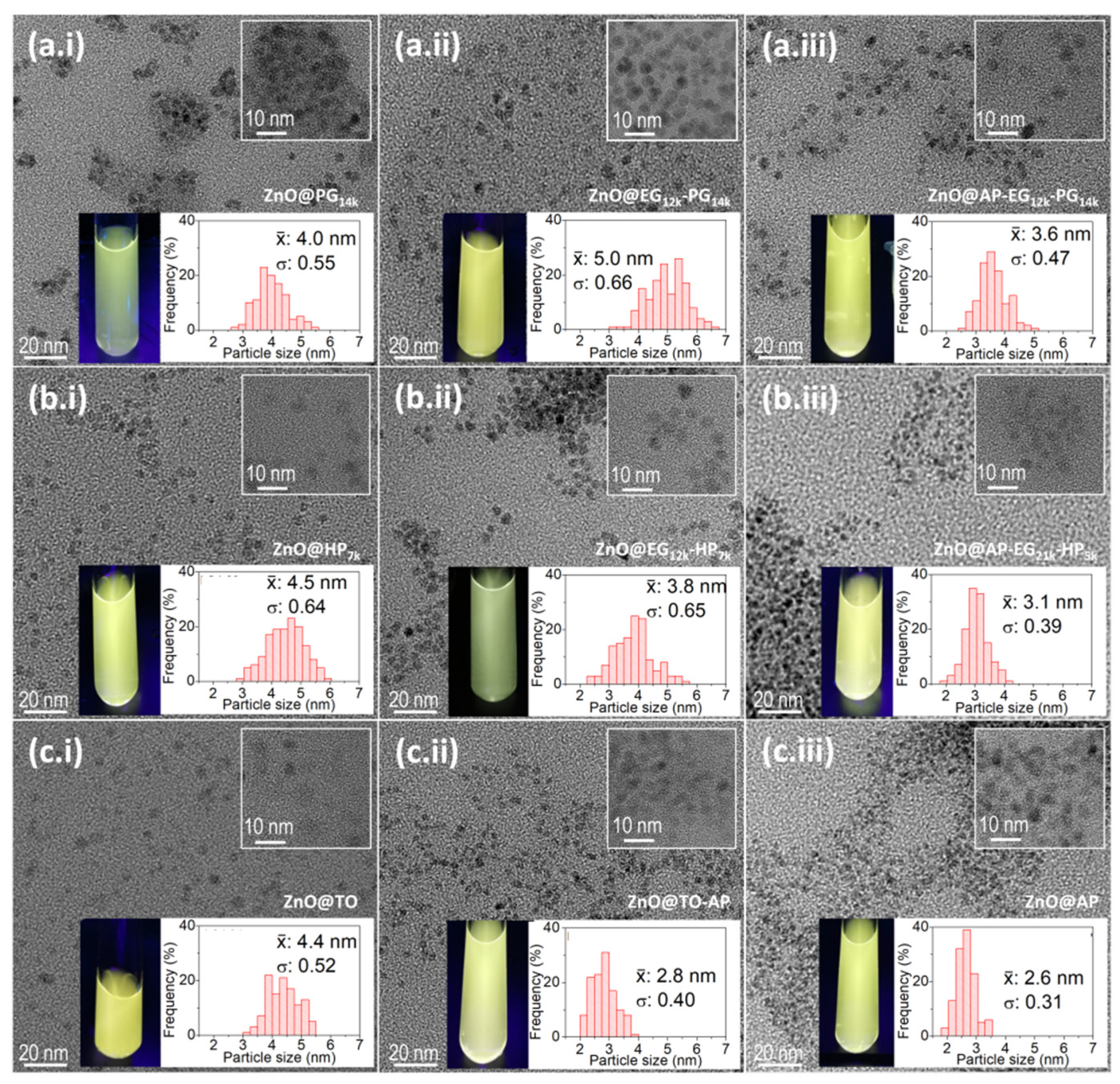
3.2. ZnO QDs with Hydroxylated Polymers as Ligands
3.3. ZnO QDs with Silanes and a Combination of APTES Silane and Hydroxylated Polymers
3.4. ZnO QDs as Sensors for Metal Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Singh, R.K.; Kumar, R. Journey of ZnO quantum dots from undoped to rare-earth and transition metal-doped and their applications. RSC Adv. 2021, 11, 2512–2545. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-M.; Xu, Y.; Ren, Q.-G.; Xia, Y.-Y. Stable Aqueous ZnO@Polymer Core−Shell Nanoparticles with Tunable Photoluminescence and Their Application in Cell Imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Guo, M.; Guo, Y.; Qi, W.; Qu, F.; Sun, F.; Zhao, H.; Zhu, G. Acid degradable ZnO quantum dots as a platform for targeted delivery of an anticancer drug. J. Mater. Chem. 2011, 21, 13406–13412. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mukherjee, S.; Mukherjee, S.; Singh, S.P.; Prasad, T. ZnO Quantum Dots: Broad Spectrum Microbicidal Agent Against Multidrug Resistant Pathogens E. coli and C. albicans. Front. Nanotechnol. 2020, 2, 576342. [Google Scholar] [CrossRef]
- Silva, B.L.D.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Yu, B.; Chen, Y.; Shen, Y.; Cong, H. ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery. Polymers 2018, 10, 1272. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Wang, X.-J.; Li, J.; Xu, G.-B. Synthesis and performance of ZnO quantum dots water-based fluorescent ink for anti-counterfeiting applications. Sci. Rep. 2021, 11, 5841. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light activation of nanocrystalline metal oxides for gas sensing: Principles, achievements, challenges. Nanomaterials 2021, 11, 892. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Laopa, P.; Vilaivan, T. Cationic-Polymer-Functionalized Zinc Oxide Quantum Dots: Preparation and Application to Iron(II) Ion Detection. ChemistrySelect 2019, 4, 4251–4257. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Mitra, T.; Sahoo, D. Metal oxide QD based ultrasensitive microsphere fluorescent sensor for copper, chromium and iron ions in water. RSC Adv. 2020, 10, 9512–9524. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Xing, X.; Yang, Y.; Wang, Z.; Wang, Z.; Zhao, R.; Zhang, X.; Wang, Y. Water-soluble ZnO quantum dots modified by (3-aminopropyl)triethoxysilane: The promising fluorescent probe for the selective detection of Cu2+ ion in drinking water. J. Alloys Compd. 2020, 825, 153904. [Google Scholar] [CrossRef]
- Willander, M.; Khun, K.; Ibupoto, Z.H. ZnO Based Potentiometric and Amperometric Nanosensors. J. Nanosci. Nanotechnol 2014, 14, 6497–6508. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiong, H.M. Photoluminescent ZnO nanoparticles and their biological applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sánchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol.-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Kumari, V.; Mittal, A.; Jindal, J.; Yadav, S.; Kumar, N. S-, N- and C-doped ZnO as semiconductor photocatalysts: A review. Front. Mater. Sci. 2019, 13, 1–22. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 39. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Lu, X.; Zhang, Q. Enhancing luminescence of ZnO quantum dots by PEG and oleic acid via a sol–gel method. J. Mater. Sci.-Mater. El. 2015, 26, 1113–1118. [Google Scholar] [CrossRef]
- Rahman, F. Zinc oxide light-emitting diodes: A review. Opt. Eng. 2019, 58, 010901. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Balandin, A.A. ZnO Quantum Dots: Physical Properties and Optoelectronic Applications. J. Nanoelectron. Optoelectron. 2006, 1, 19–38. [Google Scholar] [CrossRef]
- Rezaie, M.N.; Mohammadnejad, S.; Ahadzadeh, S. Hybrid inorganic-organic light-emitting heterostructure devices based on ZnO. Opt. Laser Technol. 2021, 138, 106896. [Google Scholar] [CrossRef]
- Gao, C.; Zhong, K.; Fang, X.; Fang, D.; Zhao, H.; Wang, D.; Li, B.; Zhai, Y.; Chu, X.; Li, J.; et al. Brief review of photocatalysis and photoresponse properties of ZnO–graphene nanocomposites. Energies 2021, 14, 6403. [Google Scholar] [CrossRef]
- Wei, J.; Ji, G.; Zhang, C.; Yan, L.; Luo, Q.; Wang, C.; Chen, Q.; Yang, J.; Chen, L.; Ma, C.-Q. Silane-Capped ZnO Nanoparticles for Use as the Electron Transport Layer in Inverted Organic Solar Cells. ACS Nano 2018, 12, 5518–5529. [Google Scholar] [CrossRef] [PubMed]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Xiong, H.-M. Photoluminescent ZnO nanoparticles modified by polymers. J. Mater. Chem. 2010, 20, 4251–4262. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Ma, R.-Z.; Wang, S.-F.; Xia, Y.-Y. Photoluminescent ZnO nanoparticles synthesized at the interface between air and triethylene glycol. J. Mater. Chem. 2011, 21, 3178–3182. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Shchukin, D.G.; Möhwald, H.; Xu, Y.; Xia, Y.-Y. Sonochemical Synthesis of Highly Luminescent Zinc Oxide Nanoparticles Doped with Magnesium(II). Angew. Chem. Int. Ed. 2009, 48, 2727–2731. [Google Scholar] [CrossRef]
- Zheng, Z.; Mounsamy, M.; Lauth-de Viguerie, N.; Coppel, Y.; Harrisson, S.; Destarac, M.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Luminescent zinc oxide nanoparticles: From stabilization to slow digestion depending on the nature of polymer coating. Polym. Chem. 2019, 10, 145–154. [Google Scholar] [CrossRef]
- Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis and Applications of ZnO/Polymer Nanohybrids. ACS Mater.Lett. 2021, 3, 599–621. [Google Scholar] [CrossRef]
- Asadpour, S.; Raeisi vanani, A.; Kooravand, M.; Asfaram, A. A review on zinc oxide/poly(vinyl alcohol) nanocomposites: Synthesis, characterization and applications. J. Clean. Prod. 2022, 362, 132297. [Google Scholar] [CrossRef]
- Peng, W.; Cai, Y.; Fanslau, L.; Vana, P. Nanoengineering with RAFT polymers: From nanocomposite design to applications. Polym. Chem. 2021, 12, 6198–6229. [Google Scholar] [CrossRef]
- Quijada-Garrido, I.; García, O. How a family of nanostructured amphiphilic block copolymers synthesized by RAFT-PISA take advantage of thiol groups to direct the in situ assembly of high luminescent CuNCs within their thermo-responsive core. Eur. Polym. J. 2021, 160, 110806. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Xiong, H.-M.; Ren, Q.-G.; Xia, Y.-Y.; Kong, J.-L. ZnO@silica core–shell nanoparticles with remarkable luminescence and stability in cell imaging. J. Mater. Chem. 2012, 22, 13159–13165. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Li, R.; Dong, M.; Zhang, L.; Qi, W.; Zhang, X.; Wang, H. ZnO Nanocomposites Modified by Hydrophobic and Hydrophilic Silanes with Dramatically Enhanced Tunable Fluorescence and Aqueous Ultrastability toward Biological Imaging Applications. Sci. Rep. 2015, 5, 8475. [Google Scholar] [CrossRef]
- Moussodia, R.-O.; Balan, L.; Schneider, R. Synthesis and characterization of water-soluble ZnO quantum dots prepared through PEG-siloxane coating. New J. Chem. 2008, 32, 1388–1393. [Google Scholar] [CrossRef]
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Regonia, P.R.; Pelicano, C.M.; Tani, R.; Ishizumi, A.; Yanagi, H.; Ikeda, K. Predicting the band gap of ZnO quantum dots via supervised machine learning models. Optik 2020, 207, 164469. [Google Scholar] [CrossRef]
- Abdullah, M.; Lenggoro, I.W.; Okuyama, K.; Shi, F.G. In situ synthesis of polymer nanocomposite electrolytes emitting a high luminescence with a tunable wavelength. J. Phys. Chem. B 2003, 107, 1957–1961. [Google Scholar] [CrossRef]
- Muşat, V.; Tăbăcaru, A.; Vasile, B.Ş.; Surdu, V.-A. Size-dependent photoluminescence of zinc oxide quantum dots through organosilane functionalization. RSC Adv. 2014, 4, 63128–63136. [Google Scholar] [CrossRef]
- Pérez-Cuapio, R.; Alvarado, J.A.; Pacio, M.; Arce-Plaza, A.; Santoyo-Salazar, J.; Liang, L.H.; Sue, H.J. Enhanced green photoluminescence and dispersion of ZnO quantum dots shelled by a silica shell. J. Nanopart. Res. 2020, 22, 254. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO quantum dots for fluorescent detection of environmental contaminants. J. Environ. Chem. Eng. 2021, 9, 106800. [Google Scholar] [CrossRef]






| Homopolymer a | [M]0/[CDTPA] | Time (h) | Mw b (g mol−1) | Mn b (g mol−1) | Ð b | Conv. c (%) | Mn (theor.) d (g mol−1) |
|---|---|---|---|---|---|---|---|
| PG7k e | 25 | 5 | 14,941 | 11,113 | 1.34 | 75 | 7435 |
| PG14k | 50 | 5 | 25,852 | 18,678 | 1.38 | 73 | 14,091 |
| PG17k | 75 | 5 | 28,156 | 20,451 | 1.38 | 58 | 16,716 |
| HP6k | 50 | 3 | 13,352 | 11,735 | 1.14 | 76 | 5882 |
| HP7k | 50 | 5 | 16,190 | 14,064 | 1.15 | 86 | 6603 |
| HP10k | 75 | 5 | 20,619 | 17,906 | 1.15 | 87 | 9811 |
| EG8k | 30 | 2 | 13,104 | 11,568 | 1.13 | 49 | 7754 |
| EG12k | 50 | 2 | 18,634 | 15,899 | 1.17 | 47 | 12,154 |
| EG21k | 50 | 4 | 25,068 | 21,494 | 1.17 | 81 | 20,654 |
| Block copolymer a | [M]0/[EG]0 | Time (h) | Mw b (g mol−1) | Mn b (g mol−1) | Ð b | Conv. c (%) | Mn (theor.) d (g mol−1) |
| EG8k-PG8k | 50 | 3 | 19,591 | 16,451 | 1.19 | 42 | 15,629 |
| EG12k-PG14k | 50 | 3 | 39,189 | 30,383 | 1.29 | 77 | 26,591 |
| EG21k-PG14k | 50 | 3 | 43,736 | 33,850 | 1.29 | 72 | 33,904 |
| EG8k-HP5k | 50 | 3 | 21,543 | 17,715 | 1.22 | 75 | 13,160 |
| EG12k-HP6k | 50 | 3 | 27,621 | 22,635 | 1.22 | 82 | 18,065 |
| EG21k-HP5k | 50 | 3 | 32,373 | 27,407 | 1.18 | 71 | 25,522 |
| EG21k-HP9k | 75 | 5 | 37,167 | 32,021 | 1.16 | 80 | 29,054 |
| Sample | OH-/Zn2+ Ratio | Size a (nm) | λem. max. (EtOH) (nm) | ΦF (EtOH) (%) |
|---|---|---|---|---|
| ZnO@bare | - | 837 | 554 | 1 |
| ZnO@PG7k | 1:1 | 8.0 | 556 | 16 |
| ZnO@PG14k | 1:1 | 7.6 | 549 | 30 |
| ZnO@PG17k | 1:1 | 5.9 | 547 | 22 |
| ZnO@EG8k-PG8k | 0.33:1 | 9.0 | 555 | 15 |
| ZnO@EG12k-PG14k | 0.33:1 | 6.7 | 557 | 23 |
| ZnO@EG21k-PG14k | 0.33:1 | 6.2 | 559 | 22 |
| ZnO@HP6k | 1:1 | 7.7 | 558 | 24 |
| ZnO@HP7k | 1:1 | 5.9 | 552 | 15 |
| ZnO@HP10k | 1:1 | 7.5 | 549 | 14 |
| ZnO@EG8k-HP5k | 0.35:1 | 5.1 | 563 | 36 |
| ZnO@EG12k-HP6k | 0.3:1 | 6.4 | 564 | 40 |
| ZnO@EG21k-HP5k | 0.2:1 | 6.7 | 557 | 43 |
| ZnO@EG21k-HP9k | 0.33:1 | 6.5 | 561 | 16 |
| ZnO@silane | Silane a (% mol) | Size b (nm) | λem. max. (H2O) (nm) | λem. max. (EtOH) (nm) | ΦF (EtOH) (%) | ||
|---|---|---|---|---|---|---|---|
| TE | TO | AP | |||||
| ZnO@TE3.5 | 3.5 | 9.4 | 557 | 551 | 29 | ||
| ZnO@TE10 | 10 | 16.0 | 558 | 554 | 13 | ||
| ZnO@TO10 | 10 | 7.0 | 571 | 550 | 30 | ||
| ZnO@TO10@TE3.5 | 3.5 | 10 | 7.5 | 556 | 551 | 26 | |
| ZnO@ AP3.5 | 3.5 | 5.7 | 561 | 554 | 38 | ||
| ZnO@ AP10 | 10 | 6.1 | 560 | 556 | 33 | ||
| ZnO@ TO10@AP3.5 | 10 | 3.5 | 7.5 | 547 | 550 | 31 | |
| ZnO@ TO10@AP10 | 10 | 10 | 8.0 | 557 | 561 | 22 | |
| Sample | OH-/Zn2+ ratio | Size a (nm) | λem. max.(H2O) (nm) | λem. max.(EtOH) (nm) | ΦF (EtOH) (%) |
|---|---|---|---|---|---|
| ZnO@AP-PG14k | 1:1 | 4.7 | 564 | 549 | 28 |
| ZnO@AP-EG12k-PG14k | 0.33:1 | 5.9 | 567 | 551 | 41 |
| ZnO@AP-HP6k | 1:1 | 6.1 | 564 | 537 | 13 |
| ZnO@AP-EG21k-HP5k | 0.2:1 | 5.8 | 558 | 545 | 30 |
| ZnO@AP-EG21k-HP9k | 0.33:1 | 5.2 | 559 | 552 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San José, L.; García, O.; Quijada-Garrido, I.; López-González, M. RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots. Nanomaterials 2022, 12, 3441. https://doi.org/10.3390/nano12193441
San José L, García O, Quijada-Garrido I, López-González M. RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots. Nanomaterials. 2022; 12(19):3441. https://doi.org/10.3390/nano12193441
Chicago/Turabian StyleSan José, Leire, Olga García, Isabel Quijada-Garrido, and Mar López-González. 2022. "RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots" Nanomaterials 12, no. 19: 3441. https://doi.org/10.3390/nano12193441
APA StyleSan José, L., García, O., Quijada-Garrido, I., & López-González, M. (2022). RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots. Nanomaterials, 12(19), 3441. https://doi.org/10.3390/nano12193441