Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film
Abstract
:1. Introduction
2. Materials and Methods
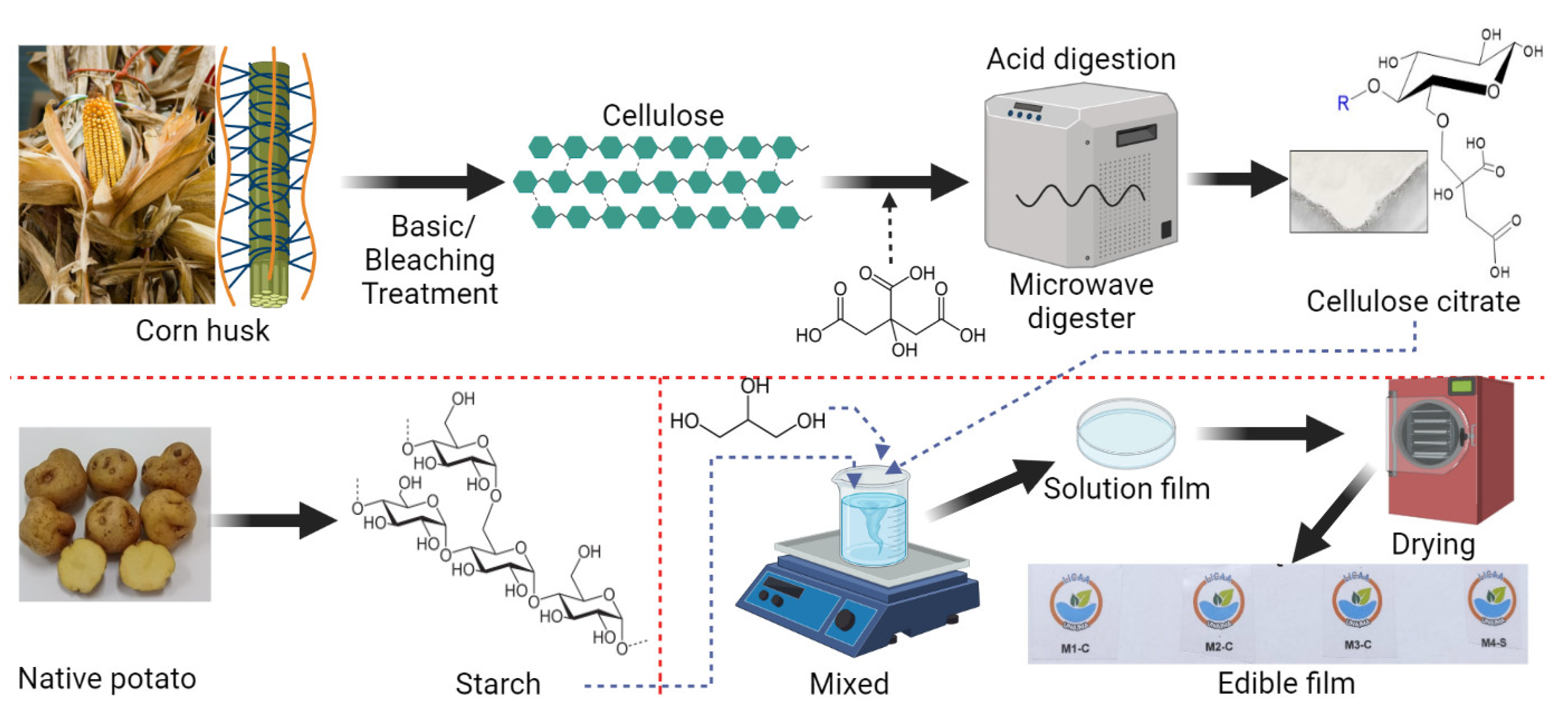
2.1. Raw Material
2.2. Extraction of Starch and Cellulose Nanocrystals
2.3. Elaboration of the Films
2.4. NCCA SEM Analysis
2.5. NCCA Particle Size
2.6. Water Activity (aw)
2.7. Color
2.8. Transparency
2.9. Solubility
2.10. Total Carbon Determination
2.11. IR Analysis
2.12. Tensile Strength Analysis
2.13. Film Thickness
2.14. Thermal Analysis
2.15. Statistical Analysis
3. Results and Discussions
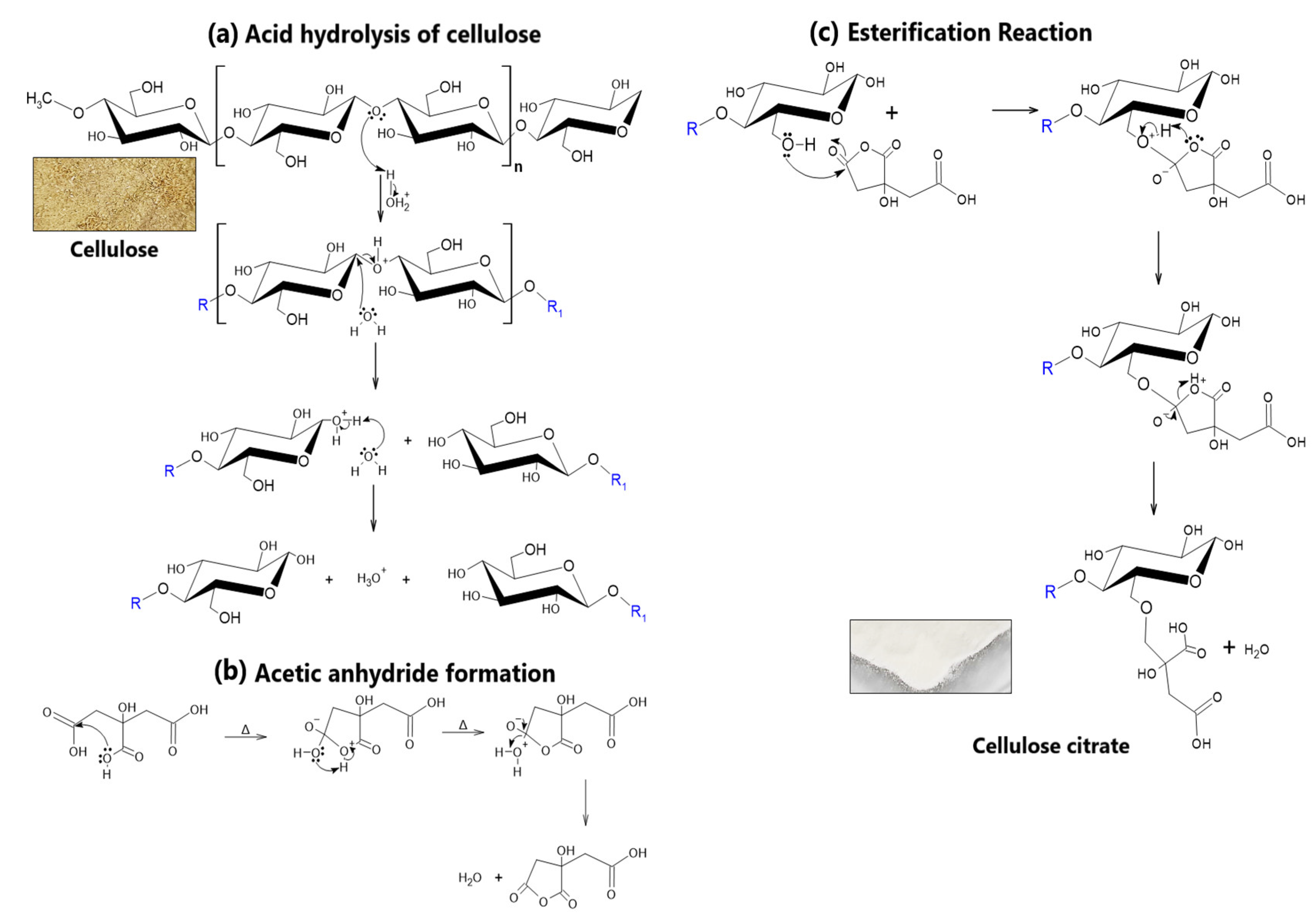
3.1. Synthesis of Activated Cellulose Nanocrystals (NCCA)
3.2. SEM Morphology of the NCCA
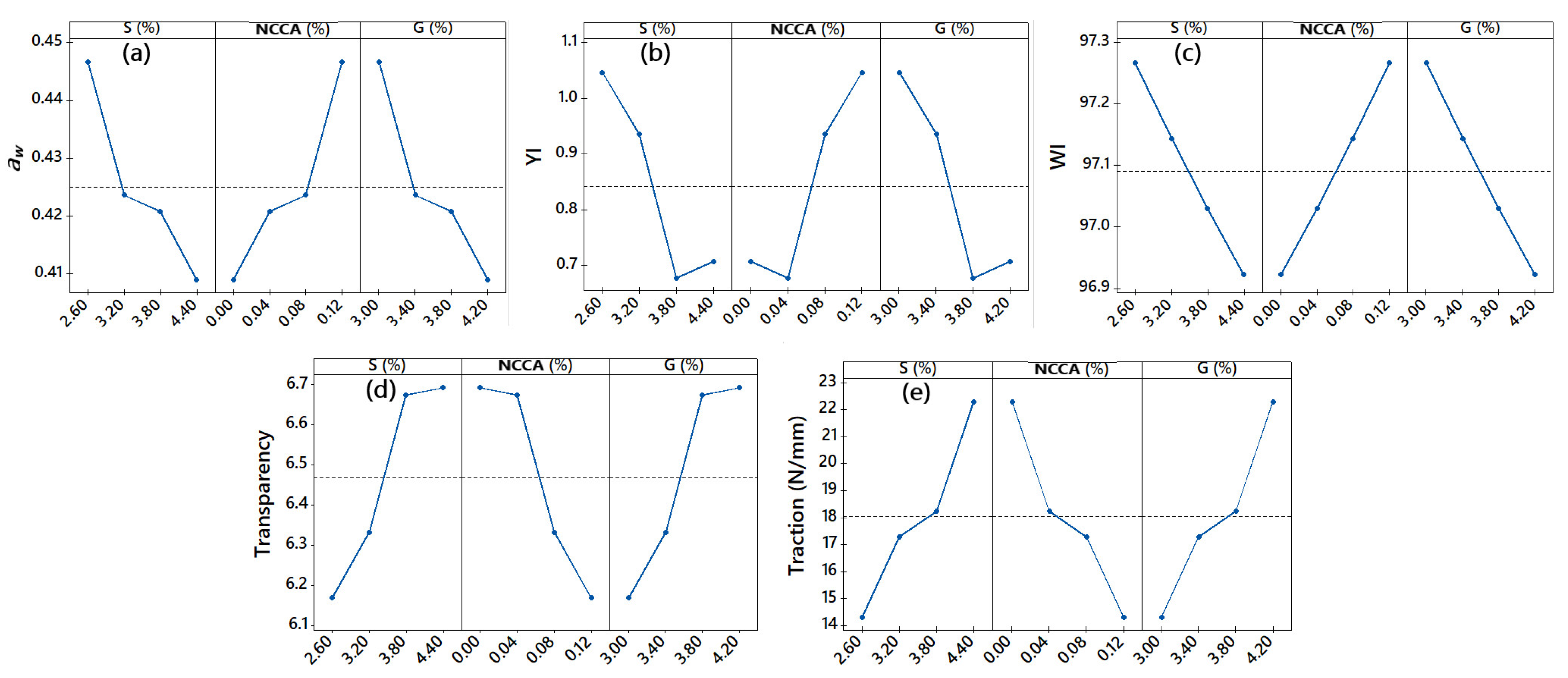
3.3. Water activity (aw)
3.4. Color
3.5. Transparency
3.6. Solubility
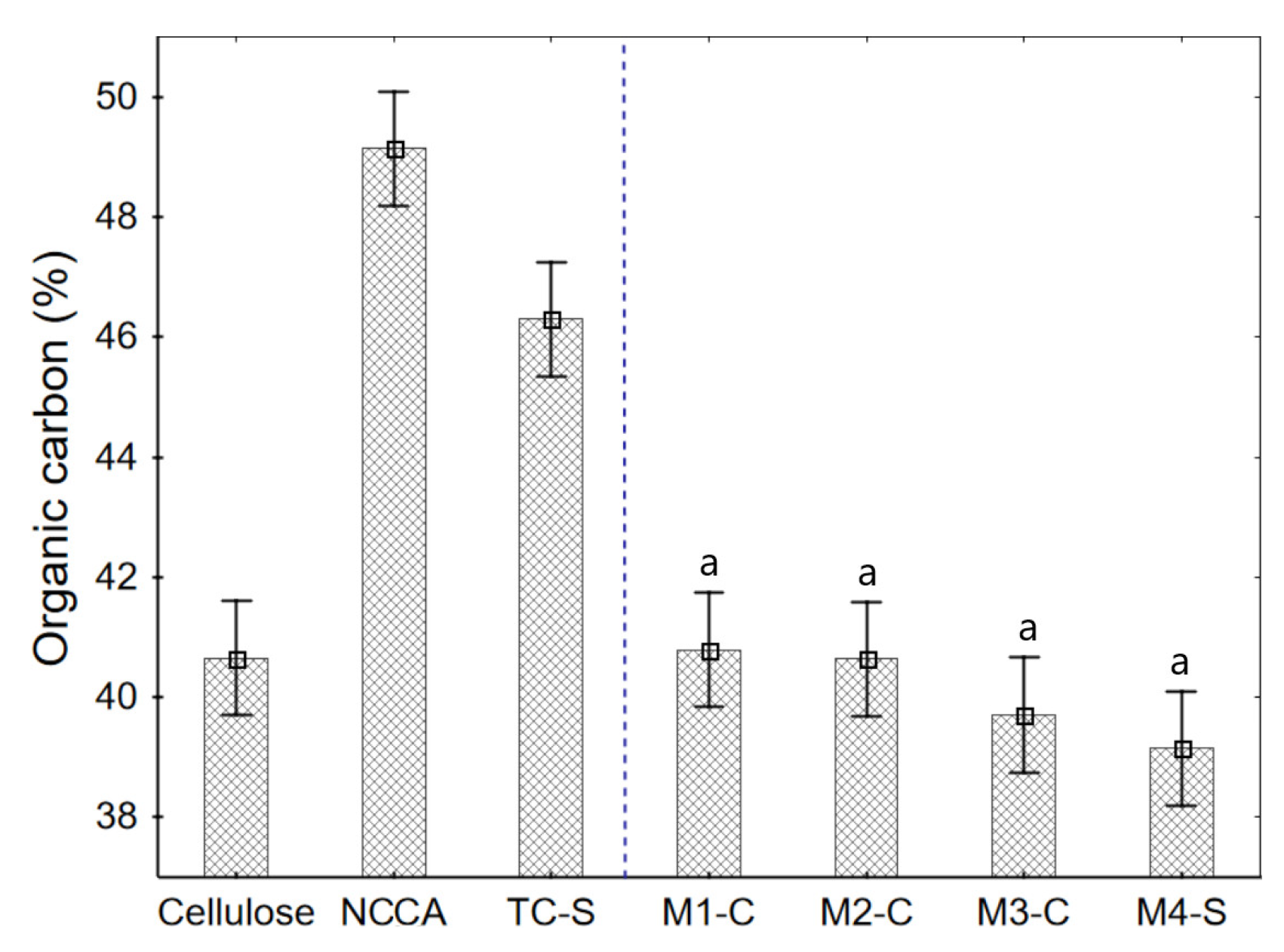
3.7. Organic Matter of Raw Materials and Films
3.8. IR Structural Analysis of Raw Materials and Films
3.9. Film Tensile Strength Analysis
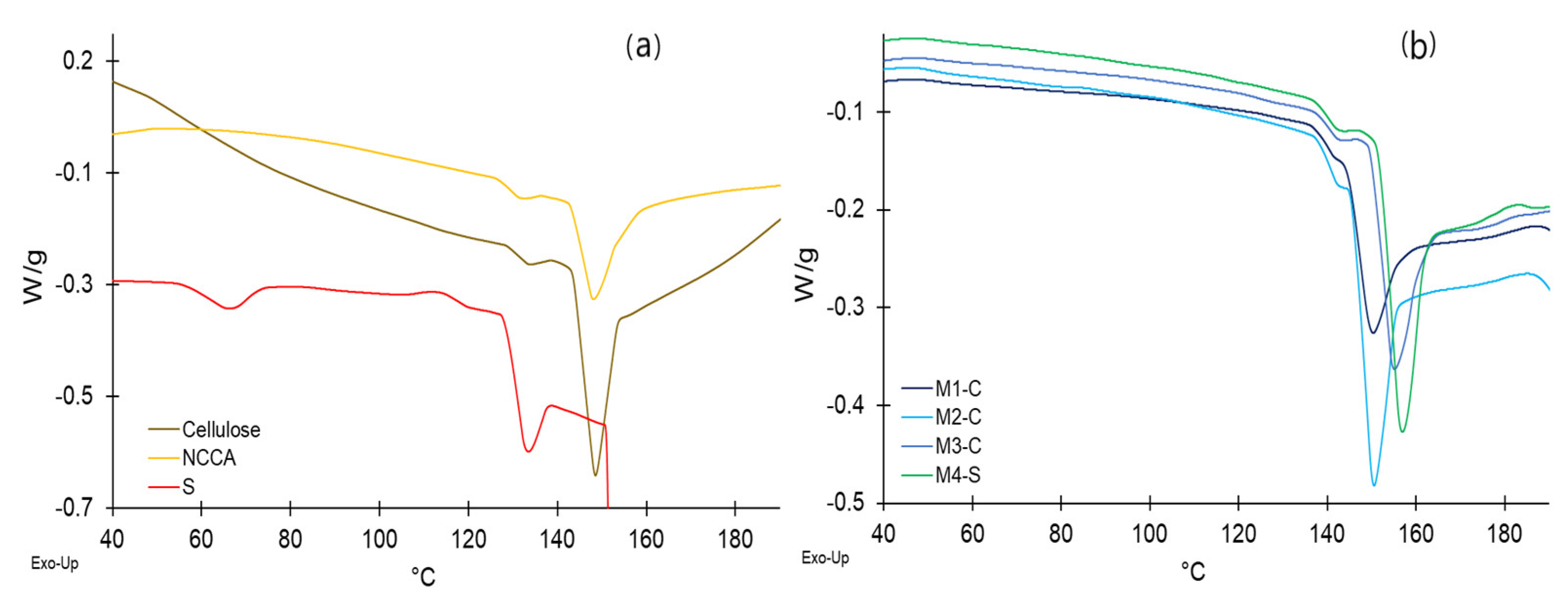
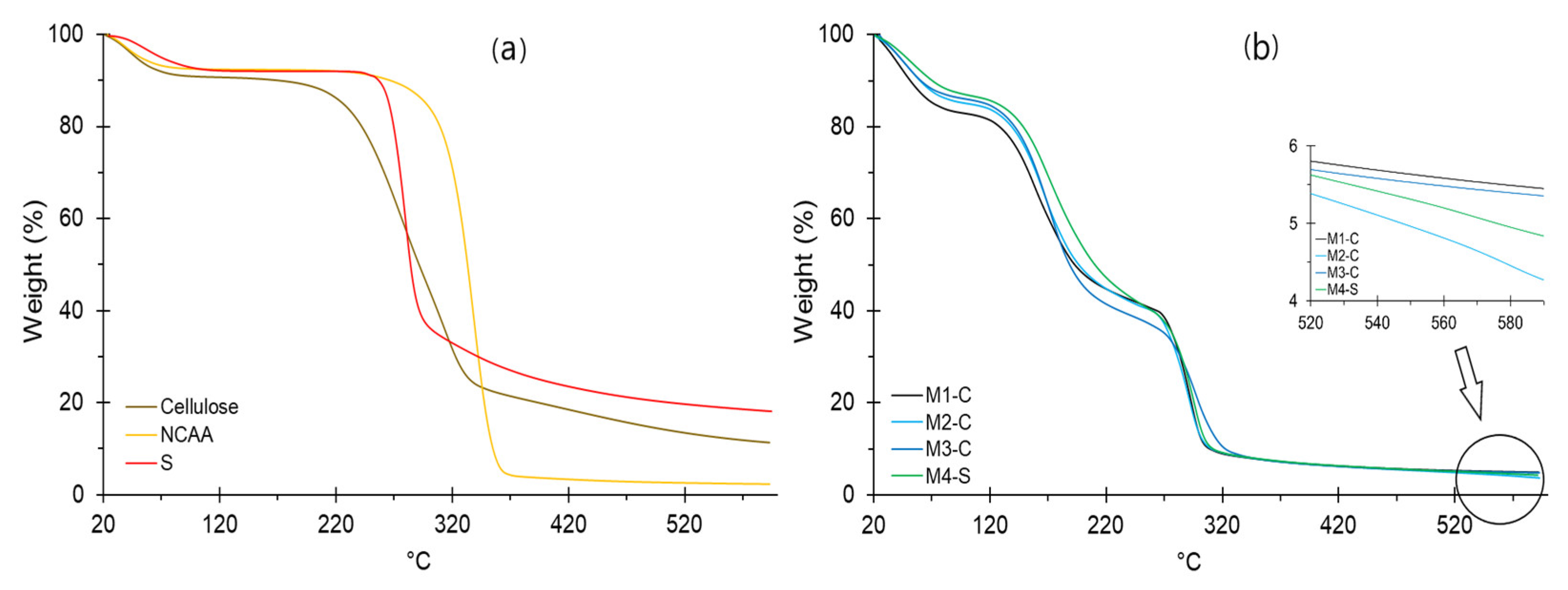
3.10. Thermal Analysis of Raw Materials and Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting polymers from cellulose nanocrystals: Synthesis, properties, and applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Liu, B. Facile and rapid one–step extraction of carboxylated cellulose nanocrystals by H2SO4/HNO3 mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Chen, Y.; Liu, J.; Ren, Z. Facile one–step extraction and oxidative carboxylation of cellulose nanocrystals through hydrothermal reaction by using mixed inorganic acids. Cellulose 2017, 24, 3243–3254. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Lu, F.; Yao, J. New approach for single–step extraction carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Xie, H.; Zou, Z.; Du, H.; Zhang, X.; Wang, X.; Yang, X.; Wang, H.; Li, G.; Li, L.; Si, C.; et al. Preparation of thermally stable and surface–functionalized cellulose nanocrystals via mixed H2SO4/Oxalic acid hydrolysis. Carbohydr. Polym. 2019, 223, 115–116. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.; Cranston, E.D. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2019, 26, 507–528. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Martakov, I.S.; Mikhaylov, V.I.; Krivoshapkin, P.V.; Tsvetkov, N.V.; Sitnikov, P.A.; Udoratina, E.V. Disk-like nanocrystals prepared by solvolysis from regenerated cellulose and colloid properties of their hydrosols. Carbohydr. Polym. 2018, 200, 162–172. [Google Scholar] [CrossRef]
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of botanic origin and amylose content on the morphology of starch nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A mixed acid methodology to produce thermally stable cellulose nanocrystal at high yield using phosphoric acid. J. Bioresour. Bioprod. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef] [PubMed]
- Guiao, K.S.; Tzoganakis, C.; Mekonnen, T.H. Thermo-mechano-chemical deconstruction of cellulose for cellulose nanocrystal production by reactive processing. Carbohydr. Polym. 2022, 291, 119543. [Google Scholar] [CrossRef] [PubMed]
- Park, N.M.; Choi, S.; Oh, J.E.; Hwang, D.Y. Facile extraction of cellulose nanocrystals. Carbohydr. Polym. 2019, 223, 115114. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Mariano, M.; Rabelo, S.C.; Gouveia, R.F.; Lona, L.M.F. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro-to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Bai, W.; Holbery, J.; Li, K. A technique for production of nanocrystalline cellulose with a narrow size distribution. Cellulose 2009, 16, 455–465. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Zheng, D.; Zang, Y.; Guo, Y.; Yue, J. Isolation and characterization of nanocellulose with a novel shape from walnut (Juglans regia L.). Polymers 2019, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lee, P. Development and applications of transparent conductive nanocellulose paper. Sci. Technol. Adv. Mater. 2017, 62, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hu, D.; Wang, H.; Wang, L. Tara gum edible film incorporated with oleic acid. Food Hydrocoll. 2016, 56, 127–133. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crop. Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT-Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Janssen, L.; Moscicki, L. Thermoplastic starch as packaging material, Acta Sci. Pol. Tech. Agrar. 2006, 5, 19–25. [Google Scholar]
- Choque-Quispe, D.; Froehner, S.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Taipe-Pardo, F.; Zamalloa-Puma, L.M.; Calla-Florez, M.; et al. Preparation and Chemical and Physical Characteristics of an Edible Film Based on Native Potato Starch and Nopal Mucilage. Polymers 2021, 13, 3719. [Google Scholar] [CrossRef] [PubMed]
- Gujral, H.; Sinhmar, A.; Nehra, M.; Nain, V.; Thory, R.; Pathera, A.K.; Chavan, P. Synthesis, characterization, and utilization of potato starch nanoparticles as a filler in nanocomposite films. Int. J. Biol. Macromol. 2021, 186, 155–162. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Abreu, S.; Oliveira, M.; de Sa, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial nanostructured starch based films for packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef]
- Khalil, M.S.; Ahmed, Z.S.; Elnawawy, A.S. Evaluation of the physicochemical properties and antimicrobial activities of bioactive biodegradable films. Jordan J. Biol. Sci. 2013, 147, 1–10. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pan, I.; Mate, J.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Nieto-Souza, L.; Acevedo-Guevara, L.; Sánchez, L.T.; Pinzón, M.I.; Villa, C.C. Characterization of Aloe vera-banana starch composite films reinforced with curcumin-loaded starch nanoparticles. Food Struct. 2019, 22, 100–131. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khaneghah, A.M. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, S.; Zhang, S.; Xi, T.; Sun, Q.; Xiong, L. Characterization of edible corn starch nanocomposite films: The effect of self-assembled starch nanoparticles. Starch-Stärke 2016, 68, 239–248. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crop. Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Djomeh, Z.E.; Ghasemlou, M.; Jouki, M. Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydr. Polym. 2014, 102, 199–206. [Google Scholar] [CrossRef]
- Lapuz, A.R.; Tsuchikawa, S.; Inagaki, T.; Ma, T.; Migo, V. Production of Nanocellulose Film from Abaca Fibers. Crystals 2022, 12, 601. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Castro-Lopez, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(lactic acid)–Poly(hydroxybutyrate) (PLA–PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of sodium caseinate on properties and ageing behaviour of corn starch based films. Food Hydrocoll. 2012, 29, 265–271. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of native starch’s properties on starch nanocrystals thermal properties. Carbohydr. Polym. 2012, 87, 658–666. [Google Scholar] [CrossRef]
- Syafiq, R.M.O.; Sapuan, S.M.; Zuhri, M.Y.M.; Othman, S.H.; Ilyas, R.A. Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films. Nanotechnol. Rev. 2022, 11, 423–437. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Nurazzi, M.N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated cellulose concentrations on morphological, mechanical and physical properties of biodegradable films based on agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Solano-Reynoso, A.M.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Quispe-Quispe, Y. Drying and color in punamuña leaves (Satureja boliviana). Dyna 2021, 88, 31–37. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT-Food Sci. Technol. 2005, 38, 617–624. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Emam-Djomeh, Z. Effect of active edible coatings made by basil seed gum and thymol on oil uptake and oxidation in shrimp during deep-fat frying. Carbohydr. Polym. 2016, 137, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT-Food Sci. Technol. 2021, 152, 112346. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Armada, M.; Gottifredi, J.C. Physicochemical characterization of starch based films. J. Food Eng. 2007, 82, 17–25. [Google Scholar] [CrossRef]
- Costa, L.A.; Assis, D.D.J.; Gomes, G.V.; da Silva, J.B.; Fonsêca, A.F.; Druzian, J.I. Extraction and characterization of nanocellulose from corn stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, L.; Zheng, H.; Lu, Q.; Liao, Y.; Huang, B. Preparation, characterization and optimization of nanocellulose whiskers by simultaneously ultrasonic wave and microwave assisted. Bioresour. Technol. 2013, 146, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar] [CrossRef]
- Ditzel, F.I.; Prestes, E.; Carvalho, B.M.; Demiate, I.M.; Pinheiro, L.A. Nanocrystalline cellulose 40 extracted from pine wood and corncob. Carbohydr. Polym. 2017, 157, 1577–1585. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, S.; Abu-El Magd, E.E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; El-Kashif, E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- De Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, L.; Li, D.; Cheng, Y.; Adhikari, B. Preparation and characterization of cellulose nanofibers from depectinated sugar beet pulp. Carbohyd. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (cncs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Nepomuceno, N.C.; Santos, A.S.F.; Oliveira, J.E.; Glenn, G.M.; Medeiros, E.S. Extraction and characterization of cellulose nanowhiskers from Mandacaru (Cereus jamacaru DC.) spines. Cellulose 2017, 24, 119–129. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 5, 1–25. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tan, T.H.; Lee, H.V.; Abd Hamid, S.B. Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions. Materials 2017, 10, 42. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Tang, J.; Villa-Rojas, R.; Sablani, S.; Carter, B.; Campbell, G. Influence of Water Activity on Thermal Resistance of Microorganisms in Low-Moisture Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 353–370. [Google Scholar] [CrossRef]
- Pakowski, Z.; Adamski, R.; Kokocinska, M.; Kwapisz, S. Generalized desorption equilibrium equation of lignite in a wide temperature and moisture content range. Fuel 2011, 90, 3330–3335. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Alves, A.J.; Scamparini, A.R.P. Mechanical properties, hydrophilicity and water activity of starch-gum films: Ffect of additives and deacetylated xanthan gum. Food Hydrocoll. 2005, 19, 341–349. [Google Scholar] [CrossRef]
- Villacrés, R.A.E.; Flores, S.K.; Gerschenson, L.N. Biopolymeric antimicrobial films: Study of the influence of hydroxypropyl methylcellulose, tapioca starch and glycerol contents on physical properties. Mater. Sci. Eng. C 2014, 36, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Tee, Y.B.; Wong, J.; Tan, M.C.; Talib, R.A. Development of edible film from flaxseed mucilage. BioResources 2016, 11, 10286–10295. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A. Starch films from a novel (Pachyrhizus ahipa) and conventional sources: Development and characterization. Mater. Sci. Eng. C 2012, 32, 1931–1940. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Diaz-Barrera, Y.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Ramos-Pacheco, B.S.; Ligarda-Samanez, C.A.; Peralta-Guevara, D.E.; Martínez-Huamán, E.L.; Aguirre Landa, J.P.; Correa-Cuba, O.; et al. Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana). Polymers 2022, 14, 3421. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids. Complex World Polysacch. 2012, 14, 395–406. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef]
- Galindez, A.; Daza, L.D.; Homez-Jara, A.; Eim, V.S.; Váquiro, H.A. Characterization of ulluco starch and its potential for use in edible films prepared at low drying temperature. Carbohydr. Polym. 2019, 215, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, functional and morphological characterization of starches isolated fromthree native potatoes of the Andean region. Food Chem. X 2019, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Ayquipa-Cuellar, E.; Salcedo-Sucasaca, L.; Azamar-Barrios, J.A.; Chaquilla-Quilca, G. Assessment of Prickly Pear Peel Mucilage and Potato Husk Starch for Edible Films Production for Food Packaging Industries. Waste Biomass Valorization 2021, 12, 321–331. [Google Scholar] [CrossRef]
- Mendoza, B.; Hernandez, E.M.; Romo, L.D.; Vargas, A.; Cervantes, J.A.; Fernandez, A.G.E. Edible films based on chayotextle starch and its effect on the shelf life of apples. J. Appl. Biotechnol. Bioeng. 2021, 8, 36–40. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Ornelas-Paz, J.D.J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.C.G.; Sosa, B.L.; Martínez-Ávila, G.C.G.; Fuentes, H.R.; Abarca, V.H.A.; Rojas, R. Formulation and Characterization of Edible Films Based on Organic Mucilage fromMexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; RamosPacheco, B.S.; Solano-Reynoso, A.M.; Quispe-Marcatoma, J.; ChoqueQuispe, Y.; Peralta-Guevara, D.E.; Martínez-Huamán, E.L.; CorreaCuba, O.; Masco-Arriola, M.L. Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater. Polymers 2022, 14, 2803. [Google Scholar] [CrossRef]
- Rodríguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films fromthe cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Canteri, M.H.; Renard, C.M.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water vapour permeability, thermal andwetting properties of whey protein isolate based edible films. Int. Dairy J. 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Antares, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Han, L.; Wang, W.; Zhang, R.; Dong, H.; Liu, J.; Kong, L.; Hou, H. Effects of Preparation Method on the Physicochemical Properties of Cationic Nanocellulose and Starch Nanocomposites. Nanomaterials 2019, 9, 1702. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Yi, T.; Qi, M.; Xu, H.; Mo, Q.; Huang, C.; Wang, S.; Liu, Y. Preparation and Properties of Cassava Residue Cellulose Nanofibril/Cassava Starch Composite Films. Nanomaterials 2020, 10, 755. [Google Scholar] [CrossRef]
- Aila, S.; Palma, R.H.; Rodri, H.A.; Hernandez, U.J.; Bello, P.L. Characterization of films made with chayote tuber and potato starches blending with cellulose nanoparticles. Carbohydr. Polym. 2013, 98, 102–107. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Liu, P.; Xie, F.; Li, M.; Liu, X.; Yu, L.; Halley, P.J.; Chen, L. Phase transitions of maize starches with different amylose contents in glycerol–water systems. Carbohydr. Polym. 2011, 85, 180–187. [Google Scholar] [CrossRef]



| Formulation | Starch (S) (%) | NCCA (%) | Glycerin (G) (%) | Water (%) |
|---|---|---|---|---|
| M1-C | 2.60 | 0.12 | 3.00 | 94.28 |
| M2-C | 3.20 | 0.08 | 3.40 | 93.32 |
| M3-C | 3.80 | 0.04 | 3.80 | 92.36 |
| M4-S | 4.40 | 0.00 | 4.20 | 91.40 |
| Material | ± | SD | CV (%) | * | T (°C) | |
|---|---|---|---|---|---|---|
| Cellulose | 0.404 | ± | 0.001 | 0.21 | 20.7 | |
| NCCA | 0.398 | ± | 0.004 | 1.06 | 23.4 | |
| S | 0.252 | ± | 0.003 | 1.23 | 20.0 | |
| M1-C | 0.447 | ± | 0.003 | 0.66 | a | 20.4 |
| M2-C | 0.424 | ± | 0.004 | 0.83 | b | 20.8 |
| M3-C | 0.421 | ± | 0.005 | 1.26 | b | 20.9 |
| M4-S | 0.409 | ± | 0.007 | 1.62 | c | 21.0 |
| Material | L* | a* | b* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV (%) | * | ± | SD | CV (%) | * | ± | SD | CV (%) | * | ||||
| Cellulose | 69.46 | ± | 0.19 | 0.27 | 0.04 | ± | 0.01 | 13.32 | 10.56 | ± | 0.09 | 0.88 | |||
| NCCA | 84.10 | ± | 0.07 | 0.08 | 0.94 | ± | 0.01 | 0.61 | 4.84 | ± | 0.02 | 0.32 | |||
| S | 92.10 | ± | 0.01 | 0.01 | −0.02 | ± | 0.01 | 34.64 | 2.79 | ± | 0.01 | 0.21 | |||
| M1-C | 97.36 | ± | 0.02 | 0.02 | a | 0.11 | ± | 0.01 | 9.09 | a | 0.71 | ± | 0.02 | 2.14 | a |
| M2-C | 97.22 | ± | 0.02 | 0.02 | b | 0.08 | ± | 0.01 | 6.93 | b | 0.64 | ± | 0.01 | 1.81 | b |
| M3-C | 97.07 | ± | 0.02 | 0.02 | c | 0.04 | ± | 0.01 | 13.32 | c | 0.46 | ± | 0.01 | 2.17 | c |
| M4-S | 96.96 | ± | 0.01 | 0.01 | d | 0.03 | ± | 0.01 | 17.32 | c | 0.48 | ± | 0.01 | 2.08 | c |
| YI | WI | ΔE* | |||||||||||||
| ± | SD | CV (%) | * | ± | SD | CV (%) | * | ± | SD | CV (%) | * | ||||
| Cellulose | 21.73 | ± | 0.24 | 1.10 | 67.69 | ± | 0.20 | 0.30 | 24.14 | ± | 0.20 | 0.84 | |||
| NCCA | 8.22 | ± | 0.03 | 0.37 | 83.36 | ± | 0.07 | 0.08 | 8.57 | ± | 0.06 | 0.73 | |||
| S | 4.32 | ± | 0.01 | 0.21 | 91.63 | ± | 0.01 | 0.01 | 1.48 | ± | 0.00 | 0.14 | |||
| M1-C | 1.05 | ± | 0.02 | 2.13 | a | 97.27 | ± | 0.01 | 0.01 | a | 5.65 | ± | 0.01 | 0.21 | a |
| M2-C | 0.94 | ± | 0.02 | 1.82 | b | 97.14 | ± | 0.02 | 0.02 | b | 5.52 | ± | 0.02 | 0.39 | b |
| M3-C | 0.69 | ± | 0.01 | 2.17 | c | 97.03 | ± | 0.02 | 0.02 | c | 5.40 | ± | 0.02 | 0.38 | c |
| M4-S | 0.71 | ± | 0.01 | 2.09 | c | 96.92 | ± | 0.01 | 0.01 | d | 5.29 | ± | 0.01 | 0.22 | d |
| Film | Transmittance (%) | Transparency (nm/mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV (%) | * | ± | SD | CV (%) | * | |||
| M1-C | 77.75 | ± | 0.55 | 0.70 | a | 6.17 | ± | 0.04 | 0.70 | a |
| M2-C | 79.80 | ± | 0.66 | 0.83 | a | 6.33 | ± | 0.05 | 0.83 | a |
| M3-C | 83.43 | ± | 0.59 | 0.70 | b | 6.67 | ± | 0.05 | 0.70 | b |
| M4-S | 84.33 | ± | 0.39 | 0.46 | c | 6.69 | ± | 0.03 | 0.46 | c |
| Film | Ultrapure Water | Ethanol (96% v/v) | Sodium Hydroxide (0.10 M) | Acetic Acid (0.18 M) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV (%) | * | ± | SD | CV (%) | * | ± | SD | CV (%) | * | ± | SD | CV (%) | * | |||||
| M1-C | 38.06 | ± | 1.00 | 2.63 | a | 39.48 | ± | 0.65 | 1.64 | a | 42.34 | ± | 0.40 | 0.94 | a | 9.53 | ± | 0.24 | 2.55 | a |
| M2-C | 38.78 | ± | 1.12 | 2.89 | a,b | 39.53 | ± | 0.65 | 1.65 | a | 38.27 | ± | 0.25 | 0.67 | b | 8.87 | ± | 0.39 | 4.38 | b |
| M3-C | 40.64 | ± | 0.65 | 1.59 | b | 42.25 | ± | 0.47 | 1.10 | b | 38.51 | ± | 0.46 | 1.19 | b | 8.91 | ± | 0.05 | 0.58 | a,b |
| M4-S | 40.11 | ± | 0.41 | 1.01 | a,b | 42.36 | ± | 0.38 | 0.89 | b | 37.70 | ± | 0.46 | 1.22 | b | 9.02 | ± | 0.20 | 2.17 | a,b |
| Film | Thickness (mm) | Traction (N/mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV (%) | * | ± | SD | CV (%) | * | |||
| M1-C | 0.12 | ± | 0.01 | 4.68 | a | 14.33 | ± | 0.50 | 3.49 | a |
| M2-C | 0.12 | ± | 0.01 | 4.68 | a | 17.30 | ± | 0.17 | 0.98 | b |
| M3-C | 0.15 | ± | 0.01 | 6.67 | a | 18.24 | ± | 0.89 | 4.88 | b |
| M4-S | 0.14 | ± | 0.01 | 4.22 | a | 22.29 | ± | 0.96 | 4.32 | c |
| Material | Tp (°C) | ΔH (J/g) | Tg (°C) | ΔH (J/g) | Tm (°C) | ΔH (J/g) |
|---|---|---|---|---|---|---|
| Cellulose | --- | --- | 131.4 | 1.11 | 148.1 | 16.03 |
| NCCA | --- | --- | 133.4 | 1.21 | 148.5 | 21.40 |
| S | 66.1 | 5.31 | 132.9 | 9.66 | 153.4 | 2804.90 |
| M1-C | --- | --- | 141.3 | 0.07 | 150.1 | 11.49 |
| M2-C | --- | --- | 142.1 | 0.45 | 150.4 | 16.07 |
| M3-C | --- | --- | 142.3 | 0.71 | 154.9 | 17.41 |
| M4-S | --- | --- | 142.3 | 0.84 | 156.7 | 19.06 |
| Material | First Stage | Second Stage | Third Stage | Fourth Stage | Residue (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weight Loss (%) | T (°C) | Weight Loss (%) | T (°C) | Weight Loss (%) | T (°C) | Weight Loss (%) | T (°C) | ||
| Cellulose | 11.23 | 80.0 | 65.77 | 352 | 11.55 | 590 | 11.45 | ||
| NCCA | 7.784 | 80.0 | 85.83 | 362 | 4.07 | 590 | 2.316 | ||
| Starch | 5.72 | 110 | 55.95 | 312 | 17.12 | 590 | 21.21 | ||
| M1-C | 17.17 | 99 | 38.06 | 220 | 35.78 | 320 | 4.04 | 590 | 4.94 |
| M2-C | 14.87 | 100 | 40.23 | 220 | 35.68 | 319 | 5.41 | 590 | 3.81 |
| M3-C | 14.01 | 98 | 44.50 | 220 | 30.93 | 318 | 5.71 | 590 | 4.85 |
| M4-S | 13.08 | 99 | 39.67 | 220 | 38.03 | 320 | 4.91 | 590 | 4.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Choque-Quispe, Y.; Ligarda-Samanez, C.A.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Ramos-Pacheco, B.S.; Taipe-Pardo, F.; Martínez-Huamán, E.L.; Aguirre Landa, J.P.; Agreda Cerna, H.W.; et al. Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film. Nanomaterials 2022, 12, 3421. https://doi.org/10.3390/nano12193421
Choque-Quispe D, Choque-Quispe Y, Ligarda-Samanez CA, Peralta-Guevara DE, Solano-Reynoso AM, Ramos-Pacheco BS, Taipe-Pardo F, Martínez-Huamán EL, Aguirre Landa JP, Agreda Cerna HW, et al. Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film. Nanomaterials. 2022; 12(19):3421. https://doi.org/10.3390/nano12193421
Chicago/Turabian StyleChoque-Quispe, David, Yudith Choque-Quispe, Carlos A. Ligarda-Samanez, Diego E. Peralta-Guevara, Aydeé M. Solano-Reynoso, Betsy S. Ramos-Pacheco, Fredy Taipe-Pardo, Edgar L. Martínez-Huamán, John Peter Aguirre Landa, Henrry W. Agreda Cerna, and et al. 2022. "Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film" Nanomaterials 12, no. 19: 3421. https://doi.org/10.3390/nano12193421
APA StyleChoque-Quispe, D., Choque-Quispe, Y., Ligarda-Samanez, C. A., Peralta-Guevara, D. E., Solano-Reynoso, A. M., Ramos-Pacheco, B. S., Taipe-Pardo, F., Martínez-Huamán, E. L., Aguirre Landa, J. P., Agreda Cerna, H. W., Loayza-Céspedes, J. C., Zamalloa-Puma, M. M., Álvarez-López, G. J., Zamalloa-Puma, A., Moscoso-Moscoso, E., & Quispe-Quispe, Y. (2022). Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film. Nanomaterials, 12(19), 3421. https://doi.org/10.3390/nano12193421













