Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution
Abstract
1. Introduction
2. Experiments
2.1. Chemicals
2.2. Characterization
2.3. Preparation and Chemical Composition of Catalyst
3. Results and Discussion
3.1. Cation Exchange Capacity (CEC)
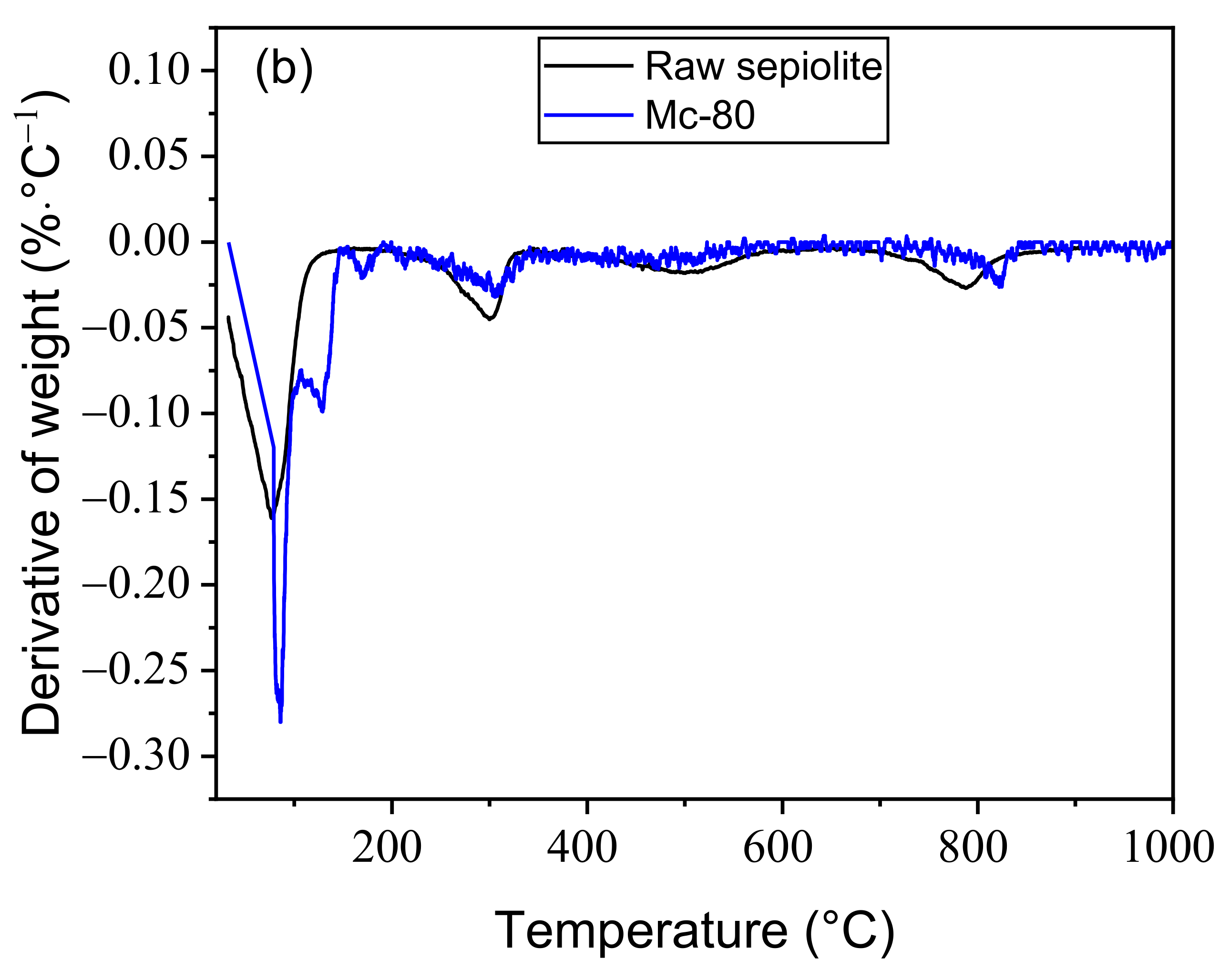
3.2. Thermogravimetric Analysis
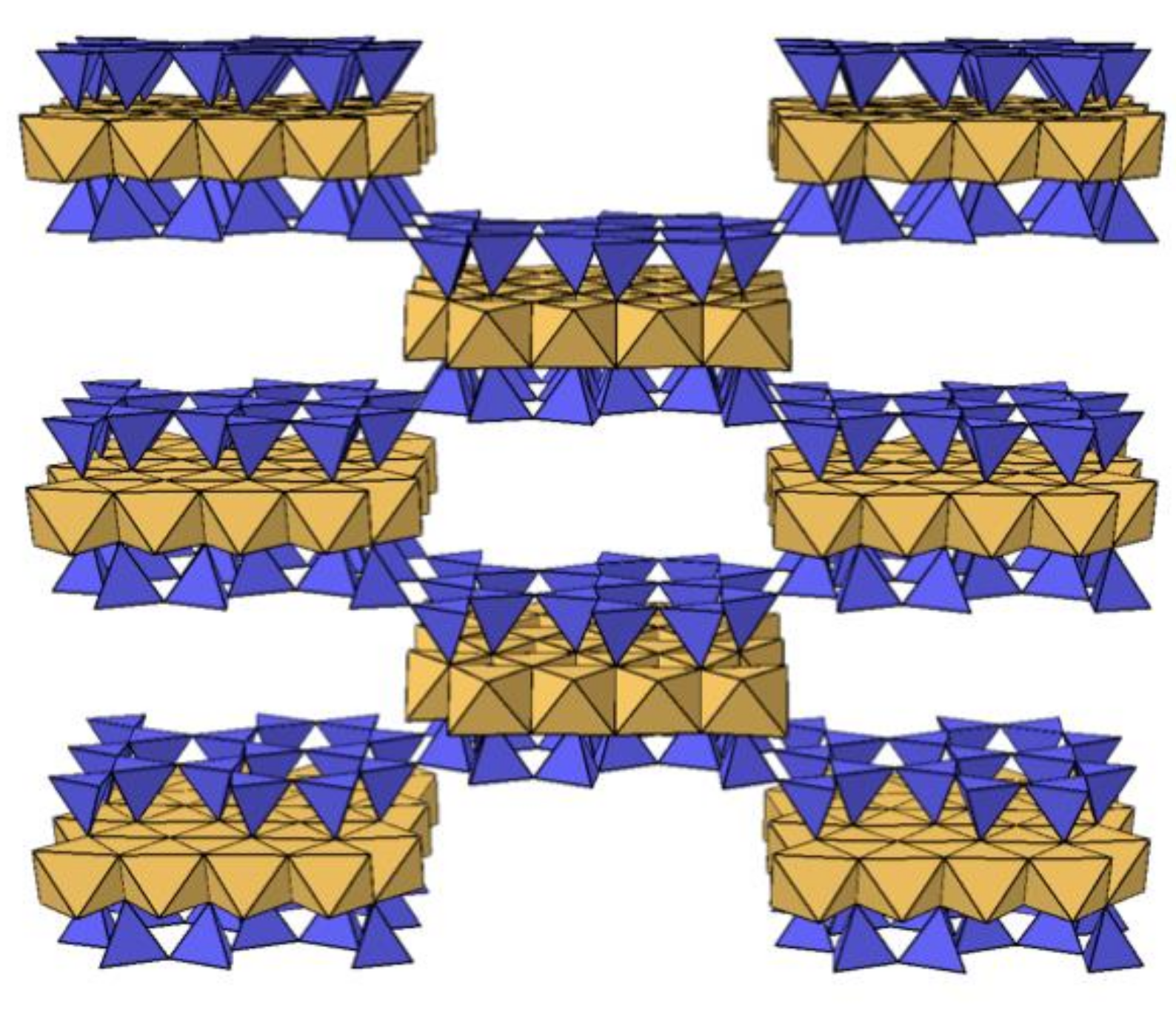
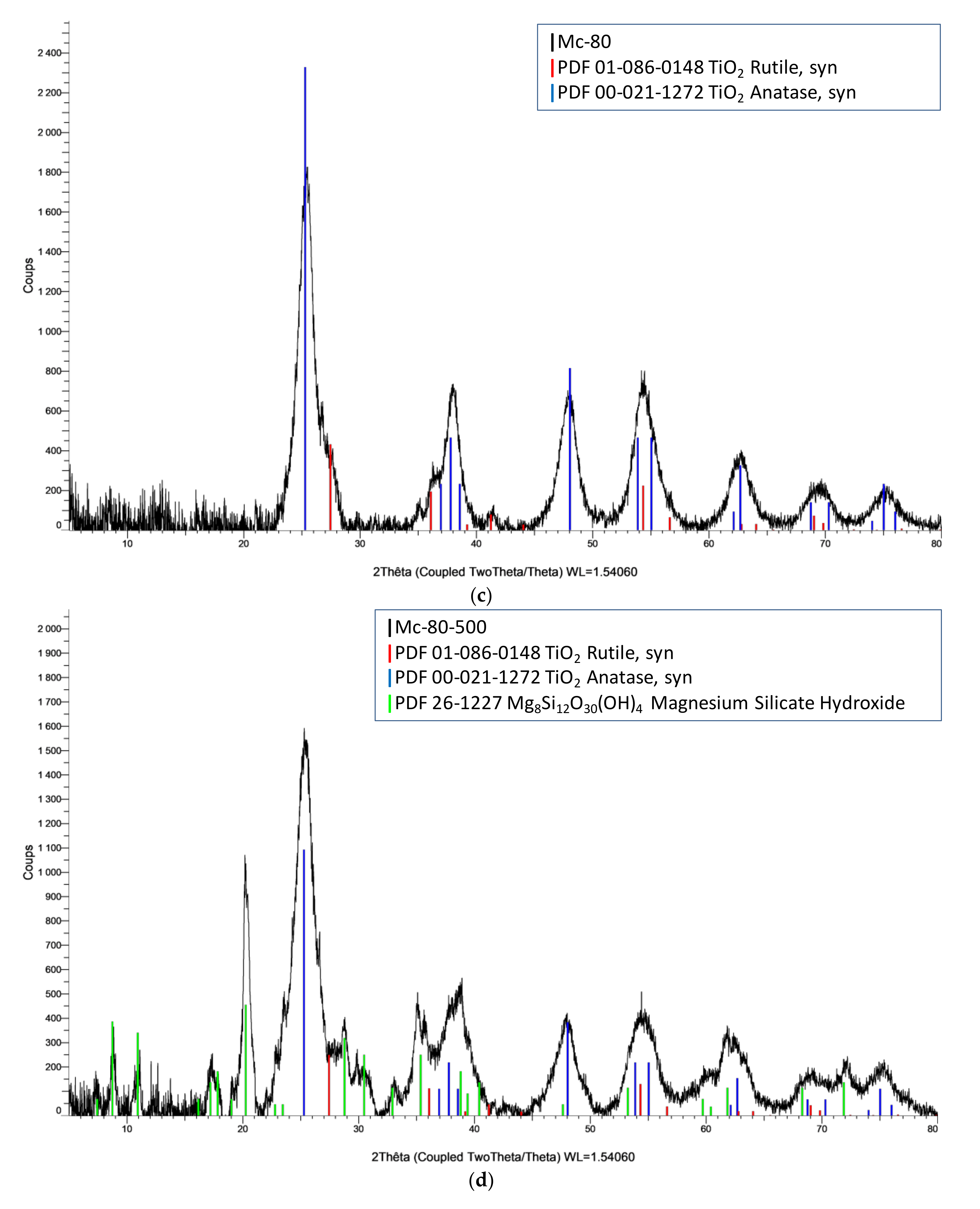
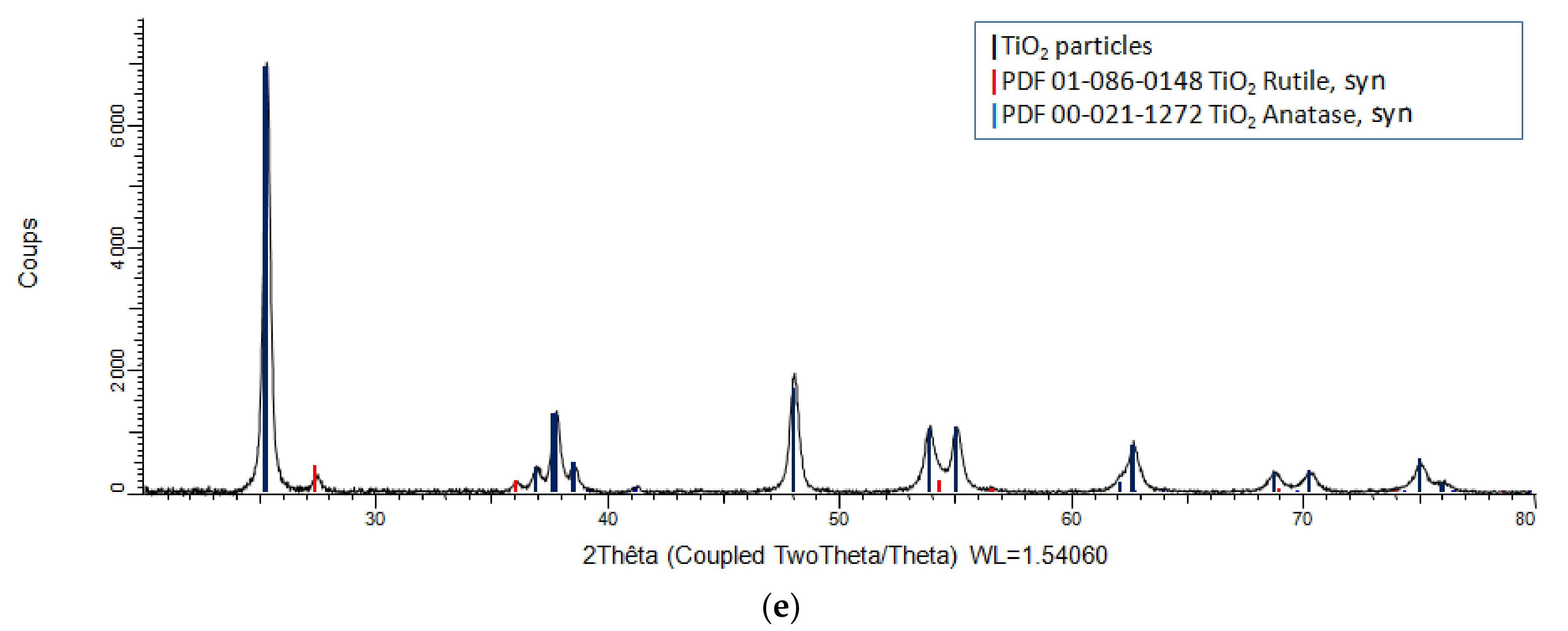
3.3. XRD Analysis
3.4. Microscopic Analysis
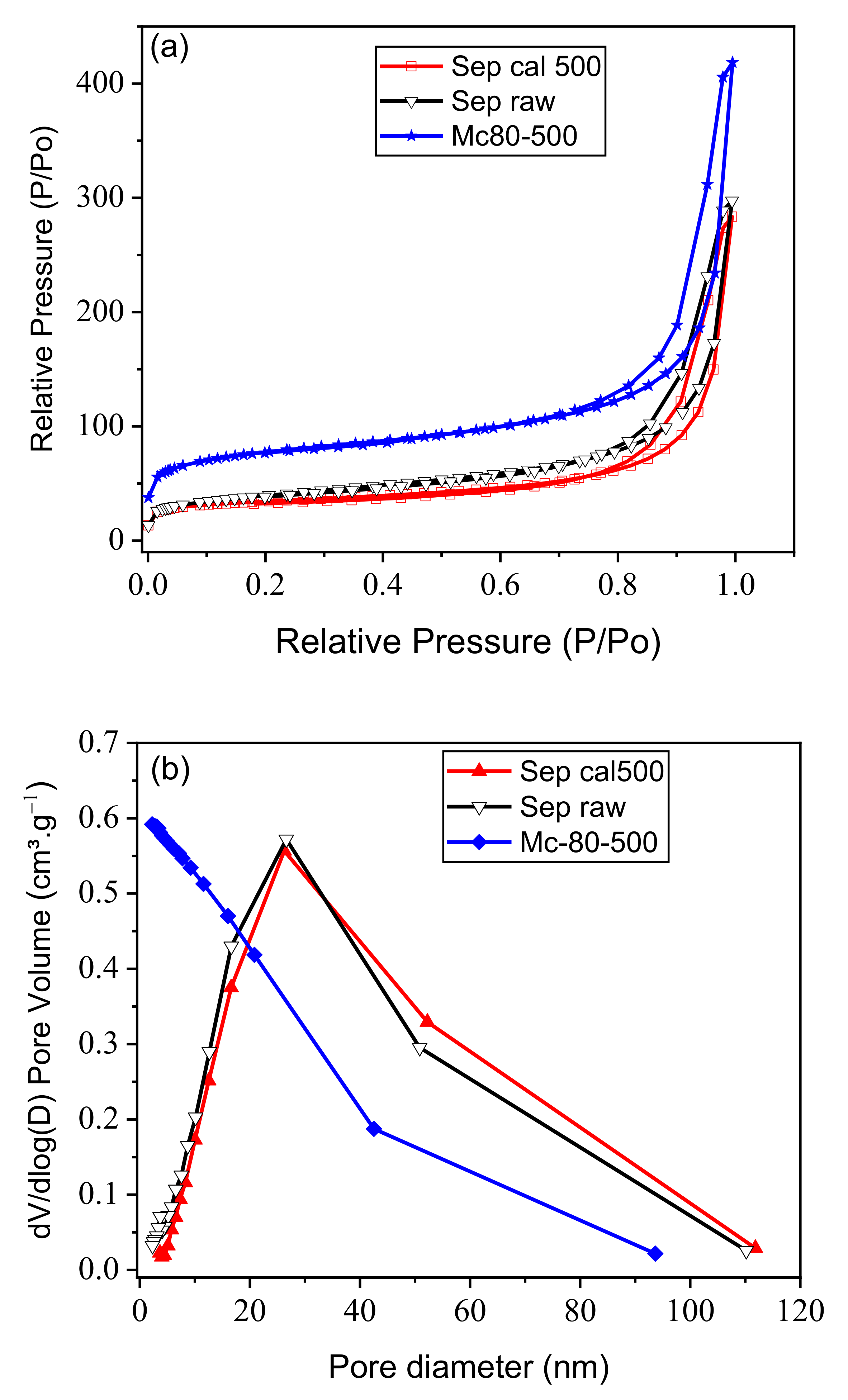
3.5. N2 Adsorption/Desorption
| (a) | ||||||
| Material | S (BET) (m2⋅g−1) | SLangmuir (m2⋅g−1) | QmBET (cm3⋅g−1) | Qm.Langmuir (cm3⋅g−1) | Micropore Area (m2⋅g−1) | Vtot-Pore (cm3⋅g−1) |
| Raw sepiolite | 137 | 195 | 31 | 44 | 24 | 0.291 |
| Sep-500 | 115 | 161 | 26 | 37 | 24 | 0.392 |
| Mc-80-500 | 261 | 366 | 59 | 84 | 118 | 0.427 |
| (b) | ||||||
| Material Parameters/Raw Sepiolite Parameters | Mc-80-500 | Sep-500 | ||||
| S BET (Material/raw sep) | 1.9 | 0.8 | ||||
| V mp (Material/raw sep) | 4.9 | 2.2 | ||||
| S mp (Material/raw sep) | 4.7 | 2.1 | ||||
| Sext (Material/raw sep) | 1.2 | 0.5 | ||||
3.6. Quantitative Inductively Coupled Plasma (ICP-AES) Analysis
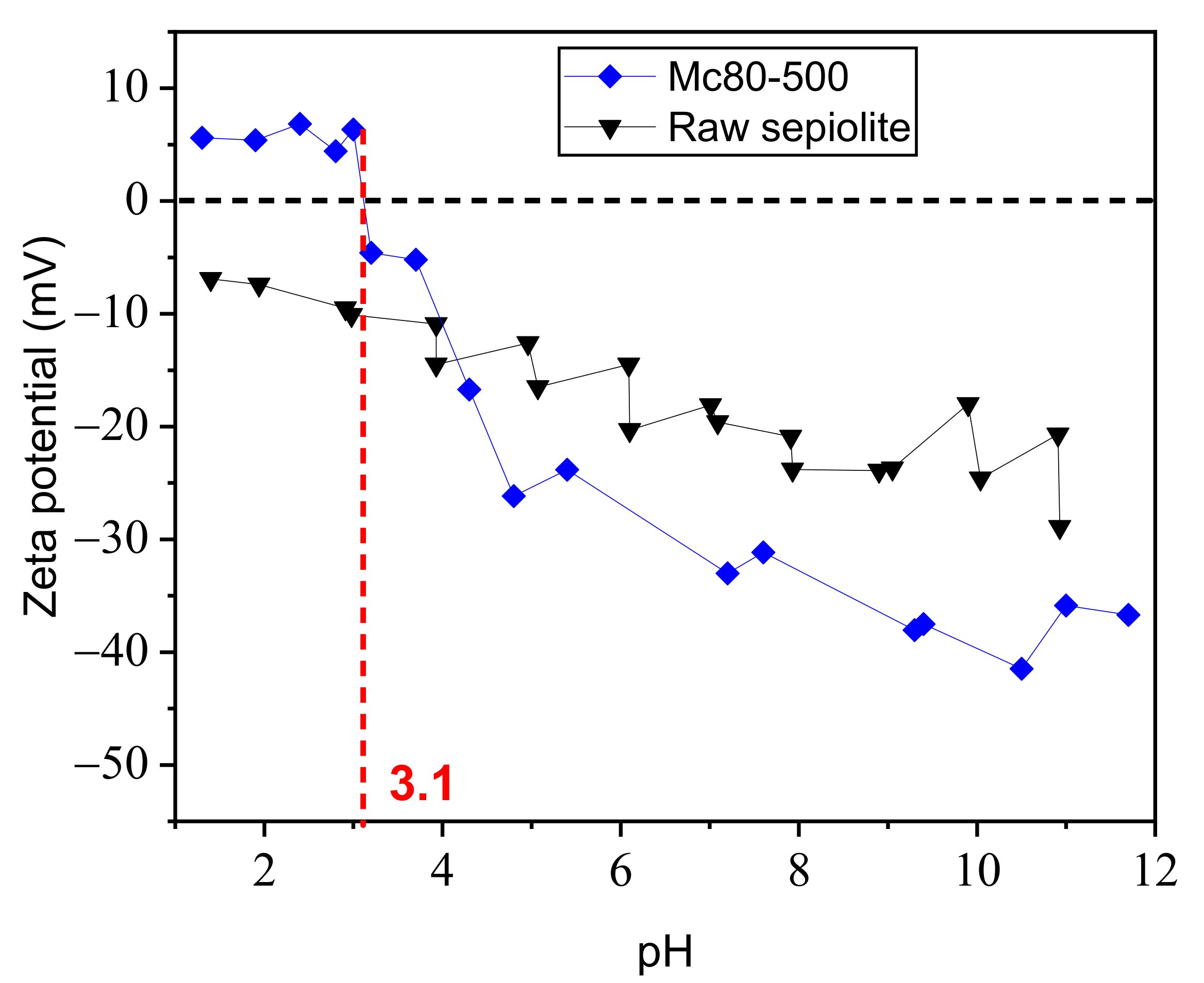
3.7. Zeta Potential
3.8. Photocatalytic Efficiency of Elaborated Materials
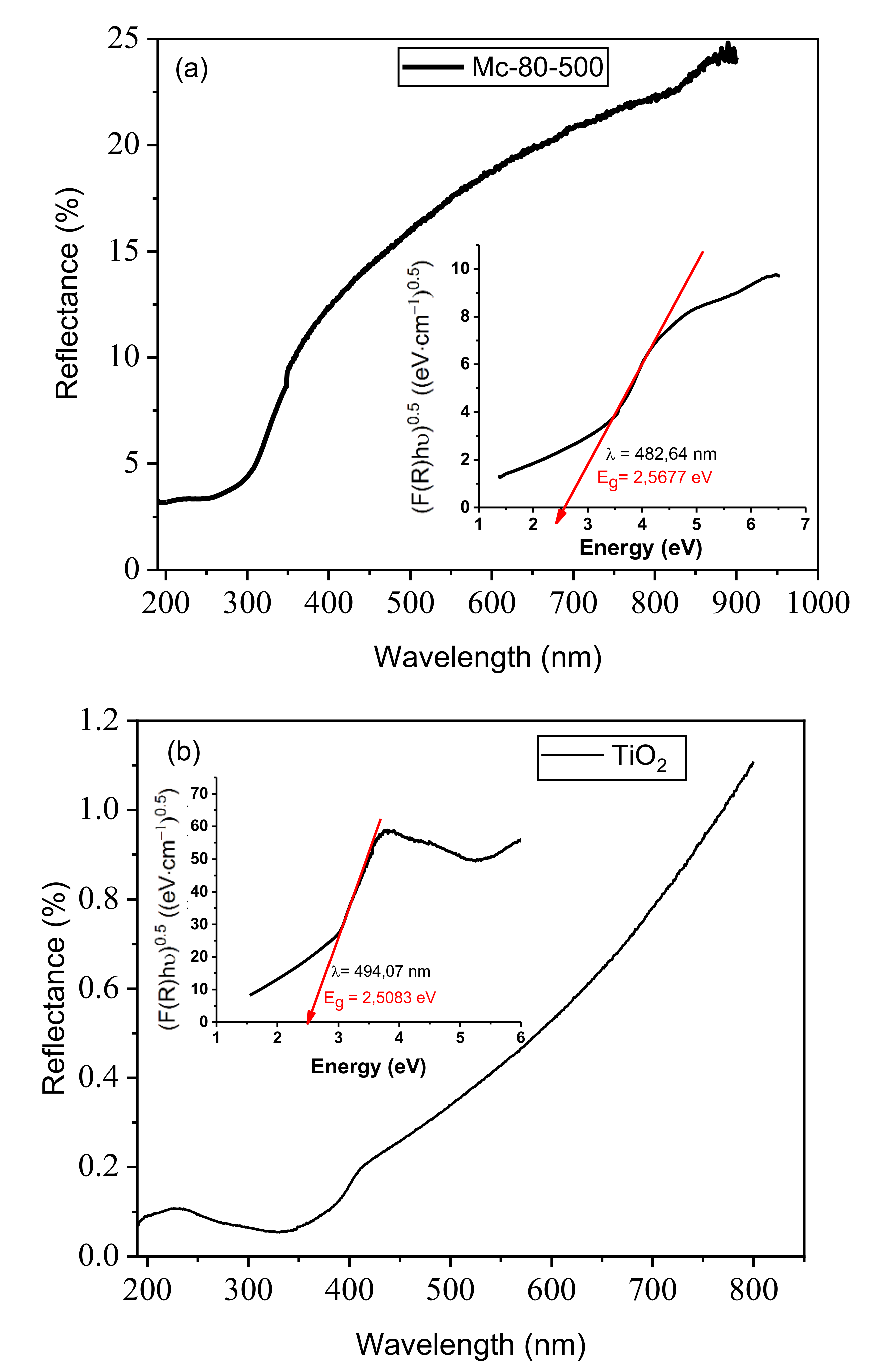
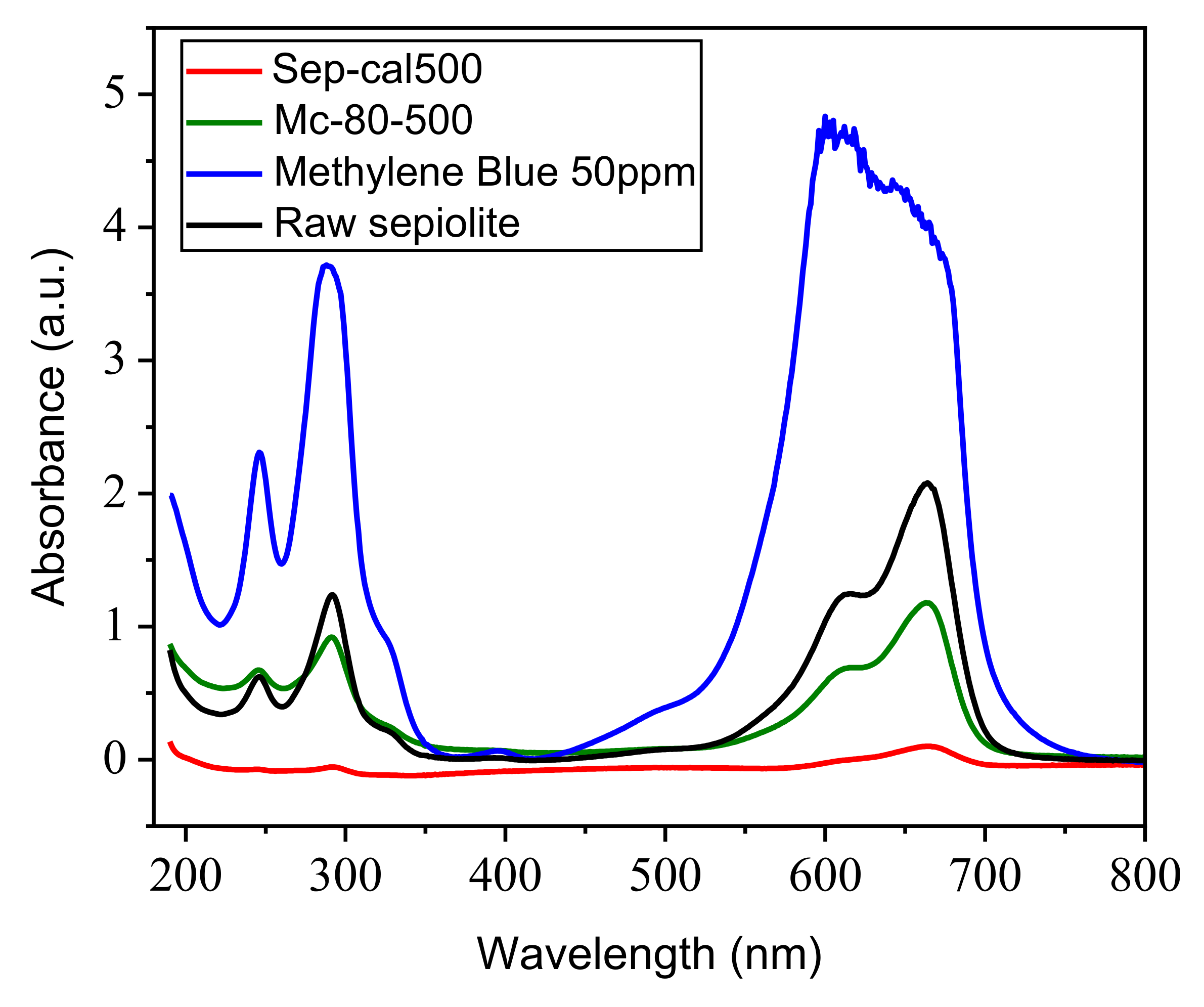
3.8.1. UV Spectroscopy of Photocatalysts
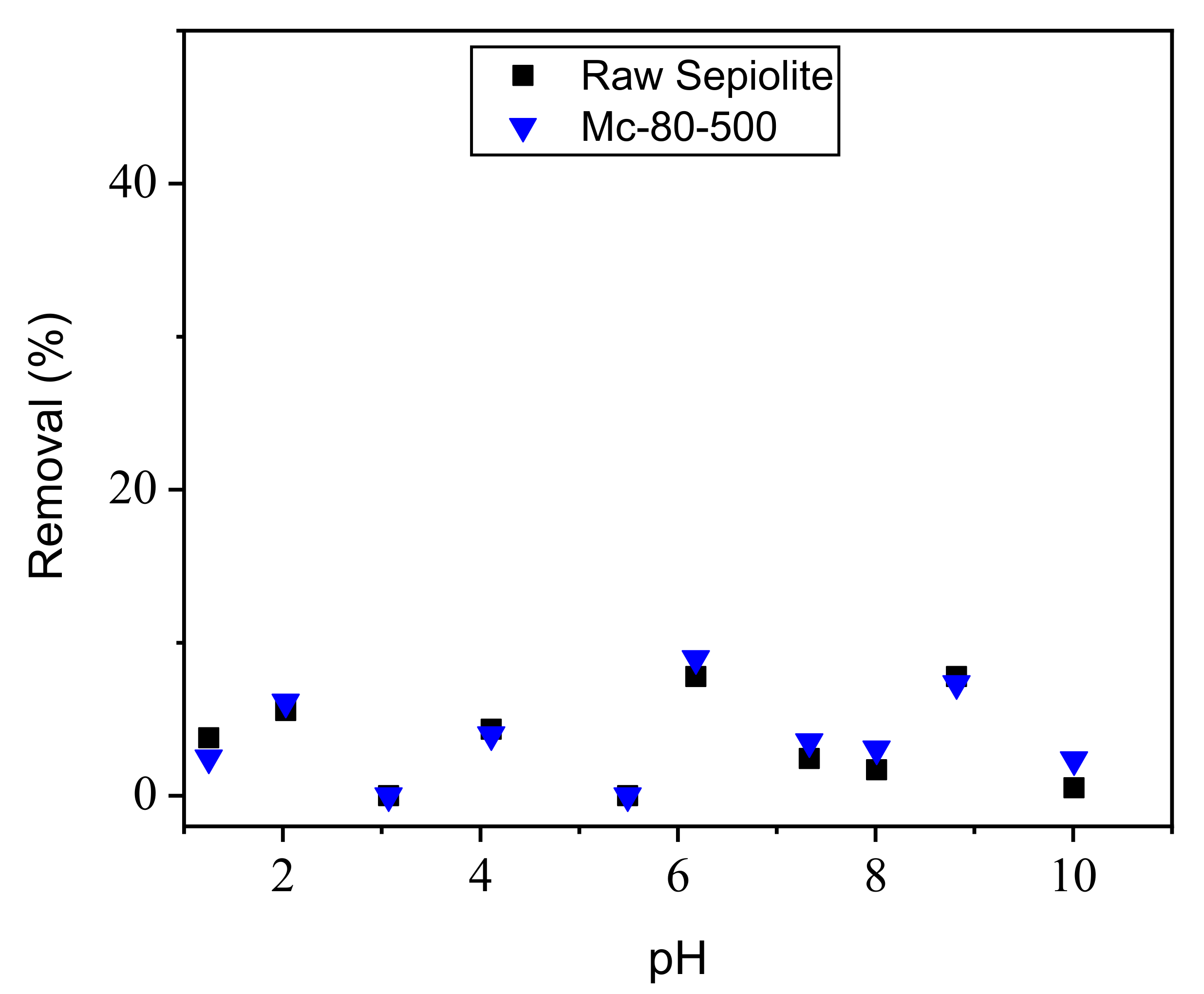
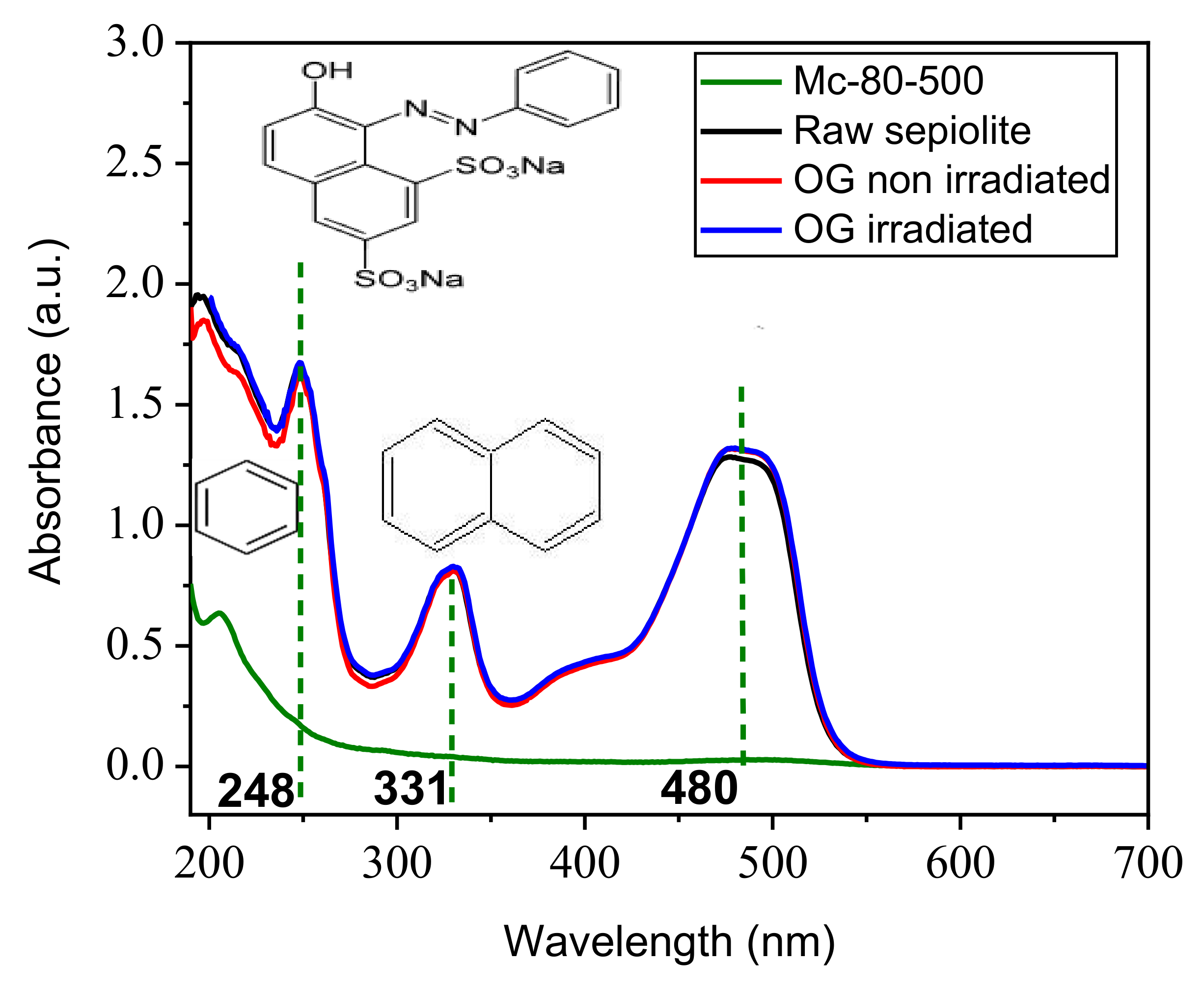
3.8.2. Study of the Effect of pH on the Adsorption of Orange G on Photocatalysts
3.8.3. Study of the Effect of TiO2 Loading in Clay on Methylene Blue Discolorization
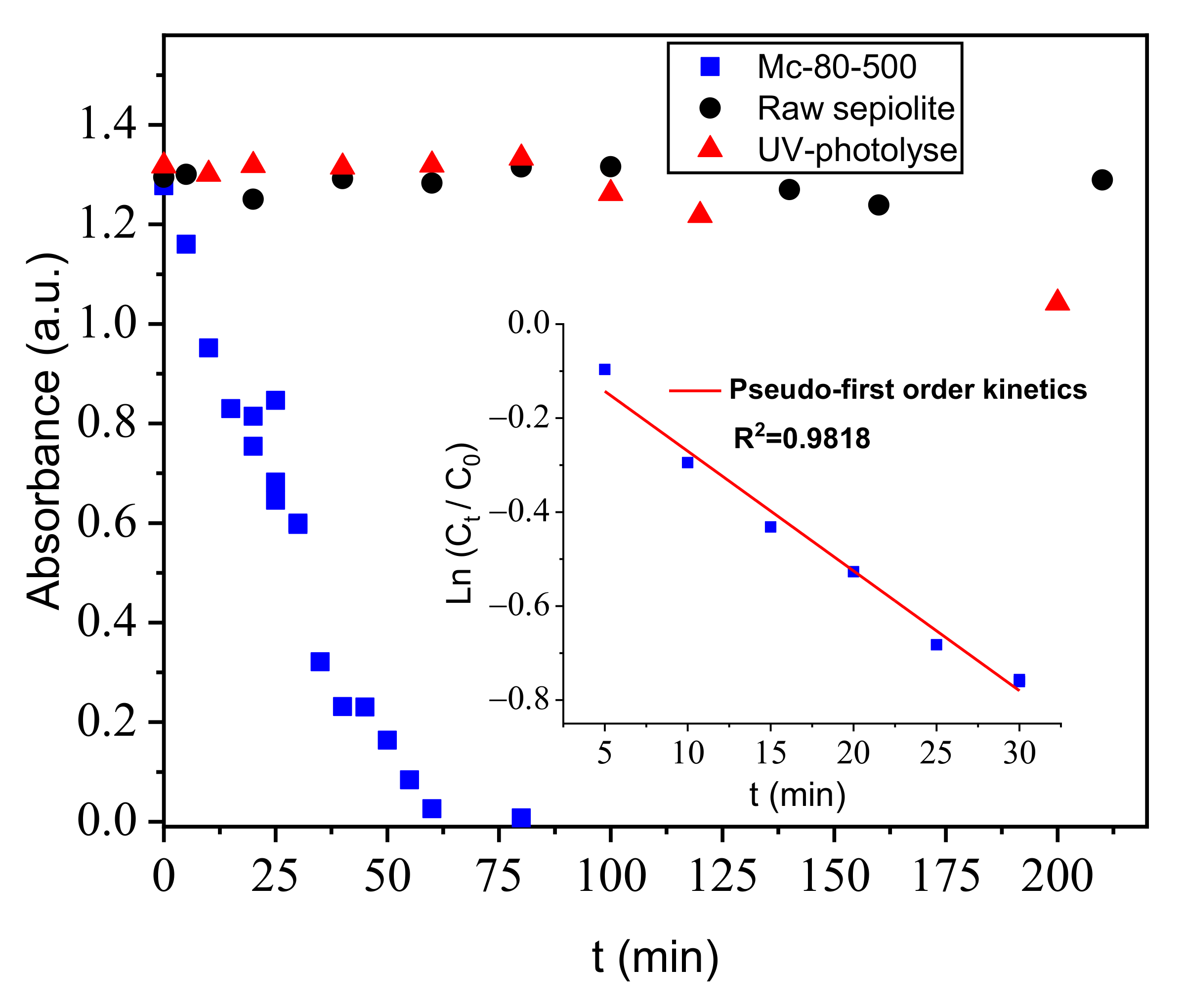
3.8.4. Discolorization of Orange G with a Heterogeneous Photocatalysis Study
Experimental Description
Comparison of the Catalytic Efficiency of the Synthesized Catalysts with Other Similar Works
Photocatalytic Mechanism
| (a) | |||||||
| Author | Clay | n Ti/g Clay (mol⋅g−1) | C0pollutant (mg⋅L−1) | Cmaterial (g⋅L−1) | Flux of Photon (mW⋅cm−2) | λirr (nm) | Timedepollution (min) |
| Bouna (2013) [68] | Palygorskite | 0.0164 | 45 OG | 1.5 | 1 | 365 | 90 |
| Zhang et al. (2011) [35] | Sepiolite | 0.03 | 30 ARG | 1.5 | 20.102 | 253.7 | 90 |
| Du et al. (2015) [65] | Sepiolite | 0.0062 | 100 ARG | 1.5 | 30 | 420 | 20% at 40 min |
| Zhou et al. (2018) [58] | Sepiolite | 0.04 | 10 OG | 0.8 | 300.103 | 365 | 150 |
| This work (2022) | Sepiolite | 0.004 | 30 OG | 1 | 20 (distance: 3 cm) | 300–700 | 60 |
| (b) | |||||||
| Author | Photocatalyst | Cmaterial (g⋅L−1) | C0pollutant (mg⋅L−1) | Catalytic Efficiency (%) | Timedepollution (min) | ||
| Liu et al. (2017) [69] | TiO2/sepiolite | 0.15 | 6 MB | 58 | 120 | ||
| Zhou et al. (2020) [70] | TiO2/sepiolite | 0.8 | 10 OG | 10 | 540 | ||
| Hu et al. (2019) [71] | BiOCl/TiO2/sepiolite | 0.6 | 50 simulated antibiotic wastewater (TC) | 49.8 | 180 | ||
| This work (2022) | TiO2/sepiolite | 1 | 30 OG | 100 | 60 | ||
| Author | Support Nature | Tiloading (wt %) | Pollutant Nature | C0pollutant (mg⋅L−1) | Timedepollution (min) | λirr (nm) | Regeneration Method |
|---|---|---|---|---|---|---|---|
| Chun et al. (2001) [28] | silica gel | 30 | R15 azo dye | 20 | 40 | >330 | not studied |
| Sakthivel et al. (2002) [29] | TiO2 (P-25)/alumina beads TiO2 (P-25)/glass beads | ~7–8 | Acid brown 14 | 124 | 210–240 | solar light | not studied |
| Li et al. (2008) [30] | Natural mordenite (zeolite) | 5 | Methyl orange | 30 | 110 | 365 | not studied |
| Trabelsi et al. (2016) [32] | TiO2 PC500/synthetic fibers (mainly cellulose, with some polyester) | PC500-sheet | Methyl orange | 35 | 240 | solar light | not studied |
| Saqib et al. (2021) [31] | Aluminosilicate (synthetic zeolite) | 80 | Methylene Blue | 150 | 180 | 400–750 | high temperature combustion and Fentonoxidation method |
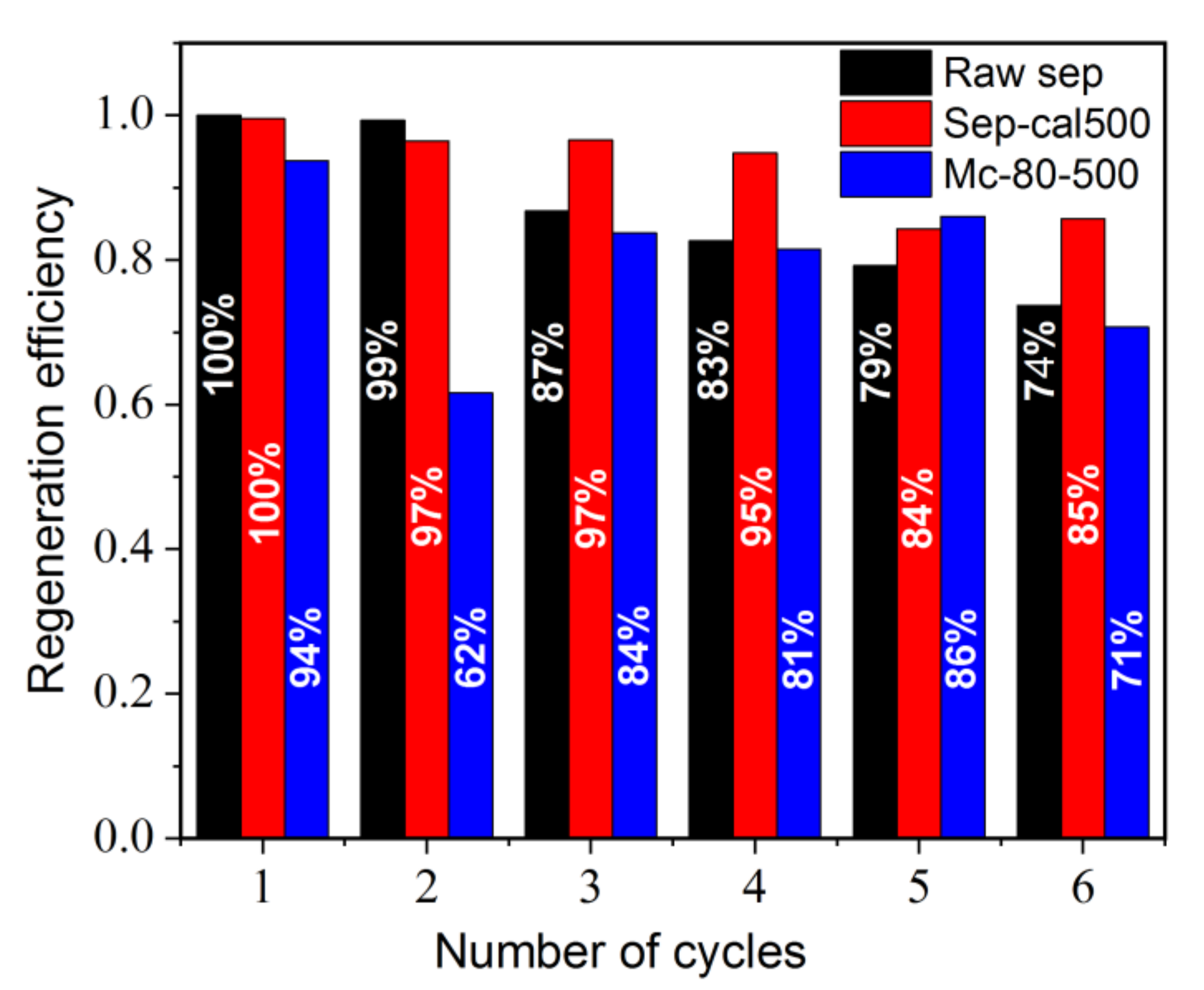
3.9. Regeneration of Functional Materials after MB Adsorption and after Orange G Photodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. United Nations Secretary-General’s Plan: Water Action Decade 2018–2028. 2018. Available online: https://www.un.org/sustainabledevelopment/water-action-decade/ (accessed on 12 February 2020).
- Liu, D.; Huang, Z.; Li, M.; Li, X.; Sun, P.; Zhou, L. Construction of magnetic bifunctional β-cyclodextrin nanocomposites for adsorption and degradation of persistent organic pollutants. Carbohydr. Polym. 2020, 230, 115564. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. J. Chem. Eng. Sci. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Li, T.; Abdelhaleem, A.; Chu, W.; Pu, S.; Qi, F.; Zou, J. S-doped TiO2 photocatalyst for visible LED mediated oxone activation: Kinetics and mechanism study for the photocatalytic degradation of pyrimethanil fungicide. Chem. Eng. J. 2021, 411, 128450. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Hua, T. Removal of organic matter from landfill leachate by advanced oxidation processes: A review. Int. J. Chem. Eng. 2010, 2010, 270532. [Google Scholar] [CrossRef]
- Bruner, L.; Kozak, J. Information on the photocatalysis I the light reaction in uranium salt plus oxalic acid mixtures. Z. Elektrochem. Angew. Phys. Chem. 1911, 17, 354–360. [Google Scholar]
- Serpone, N.; Emeline, A.; Horikoshi, S.; Kuznetsov, V.; Ryabchuk, V.K. On the genesis of heterogeneous photocatalysis: A brief historical perspective in the period 1910 to the mid-1980s. Photochem. Photobiol. Sci. 2012, 11, 1121–1150. [Google Scholar] [CrossRef]
- Autin, O.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. The impact of background organic matter and alkalinity on the degradation of the pesticide metaldehyde by two advanced oxidation processes: UV/H2O2 and UV/TiO2. Water Res. 2013, 47, 2041–2049. [Google Scholar] [CrossRef]
- Miron, S.M.; Brendlé, J.; Josien, L.; Fourcade, F.; Rojas, F.; Amrane, A.; Limousy, L.J. Development of a new cathode for the electro-Fenton process combining carbon felt and iron-containing organic–inorganic hybrids. Comptes Rendus Chim. 2019, 22, 238–249. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Yasmina, M.; Mourad, K.; Mohammed, S.H.; Khaoula, C. Treatment heterogeneous photocatalysis; factors influencing the photocatalytic degradation by TiO2. Energy Procedia 2014, 50, 559–566. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of Industrial Effluents—Available Methods and Emerging Technologies—A Review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Jardim, W.; Moraes, S.; Takiyama, M. Photocatalytic degradation of aromatic chlorinated compounds using TiO2: Toxicity of intermediates. Water Res. 1997, 31, 1728–1732. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Lin, C. Photocatalytic decolorization of methylene blue in aqueous solutions using coupled ZnO/SnO2 photocatalysts. Powder Technol. 2013, 246, 137–143. [Google Scholar] [CrossRef]
- Wang, G.; Chang, J.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Xu, Z.; Zhang, B.; Liu, H.; Lin, Y.; Li, S.; Li, G. InGaN nanorods decorated with Au nanoparticles for enhanced water splitting based on surface plasmon resonance effects. Nanomaterials 2020, 10, 912. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xie, W.; Tang, Q.; Wang, Y.; Guo, H.; Gao, P.; Dang, S.; Chang, J. Type-II CdS/PtSSe heterostructures used as highly efficient water-splitting photocatalysts. J. Appl. Surf. Sci. 2022, 589, 152931. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Ravindranadh, K.; Reddy, K.R.; Kim, D.; Shim, J. Ni-dopant concentration effect of ZrO2 photocatalyst on photoelectrochemical water splitting and efficient removal of toxic organic pollutants. Sep. Purif. Technol. 2020, 252, 117352. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.; Bayramoglu, M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl. Catal. B. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. A simple mechanical mixing method for preparation of visible-light-sensitive NiO–CaO composite photocatalysts with high photocatalytic activity. J. Hazard. Mater. 2010, 174, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Gao, F.; Luo, D. g-C3N4-Stabilised Organic–Inorganic Halide Perovskites for Efficient Photocatalytic Selective Oxidation of Benzyl Alcohol. J. Catal. 2021, 11, 505. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Borges, M.; Sierra, M.; Cuevas, E.; García, R.; Esparza, P. Photocatalysis with solar energy: Sunlight-responsive photocatalyst based on TiO2 loaded on a natural material for wastewater treatment. Sol. Energy 2016, 135, 527–535. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Li, Y.; Zhao, W.; Kuang, A.; Li, Y.; Xia, L.; Li, Y.; Xiao, S. Biaxial strain tunable photocatalytic properties of 2D ZnO/GeC heterostructure. J. Phys. D Appl. Phys. 2020, 53, 015104. [Google Scholar] [CrossRef]
- Chun, H.; Yizhong, W.; Hongxiao, T. Preparation and characterization of surface bond-conjugated TiO2/SiO2 and photocatalysis for azo dyes. Appl. Catal. B. 2001, 30, 277–285. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Photocatalytic decomposition of leather dye: Comparative study of TiO2 supported on alumina and glass beads. J. Photochem. Photobiol. 2002, 148, 153–159. [Google Scholar] [CrossRef]
- Li, F.; Sun, S.; Jiang, Y.; Xia, M.; Sun, M.; Xue, B. Photodegradation of an azo dye using immobilized nanoparticles of TiO2 supported by natural porous mineral. J. Hazard. Mater. 2008, 152, 1037–1044. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I.; Hussain, I. Sequential uptake of Cadmium and Methylene Blue from Binary Solution using Zeolite-TiO2 Modified Porous CaCu3Ti4O12 Photocatalyst. Environ. Prog. Sustain. Energy 2021, e13595. [Google Scholar]
- Trabelsi, H.; Atheba, G.P.; Hentati, O.; Mariette, Y.D.; Robert, D.; Drogui, P.; Ksibi, M. Solar photocatalytic decolorization and degradation of methyl orange using supported TiO2. J. Adv. Oxid. Technol. 2016, 19, 79–84. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Dai, M.; Guo, J.; Wang, G.; Wang, S.; He, Z. Stable Ti3+ in B-TiO2/BN based hybrids for efficient photocatalytic reduction. Chem. Eng. J. Adv. 2022, 11, 100333. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Kajitvichyanukul, P.; Chaveanghong, S.; Trakulmututa, J.; Amornsakchai, T.; Smith, S.M. Photocatalytic Activity of TiO2/g-C3N4 Nanocomposites for Removal of Monochlorophenols from Water. Nanomaterials 2022, 12, 2852. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, G. Photocatalytic degradation of organic contaminants by TiO2/sepiolite composites prepared at low temperature. Chem. Eng. J. 2011, 173, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Huang, R.; Zhong, L.; Yan, P.; Zhang, S.; Zhao, Q.; Jiang, D.; Tang, A.; Yang, H. A heterogeneous Fenton reaction system of N-doped TiO2 anchored on sepiolite activates peroxymonosulfate under visible light irradiation. Chem. Eng. J. 2020, 383, 123142. [Google Scholar] [CrossRef]
- Morjène, L.; Tasbihi, M.; Schwarze, M.; Schomäcker, R.; Aloulou, F.; Seffen, M. A composite of clay, cement, and wood as natural support material for the immobilization of commercial titania (P25, P90, PC500, C-TiO2) towards photocatalytic phenol degradation. Water Sci. Technol. 2020, 81, 1882–1893. [Google Scholar] [CrossRef]
- Pohan, L.A.G.; Kambiré, O.; Nasir, M.; Ouattara, L. Photocatalytic and Antimicrobial Properties of [AgTiO2]:[Clay] Nanocomposite Prepared with Clay Different Ratios. Mod. Reas. Catal. 2020, 9, 47. [Google Scholar] [CrossRef]
- Chen, C.; Wu, W.; Xu, W.Z.; Charpentier, P. The effect of silica thickness on nano TiO2 particles for functional polyurethane nanocomposites. Nanotechnology 2017, 28, 115709. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Miyata, M. Inclusion polymerization of isoprene in the channels of sepiolite. Res. Chem. Intermed. 1995, 21, 167–180. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Medici, L.; Poppi, L. Sepiolite and industrial waste-water purification: Removal of Zn2+ and Pb2+ from aqueous solutions. Appl. Clay. Sci. 1996, 11, 43–54. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Santos, S.C.; Boaventura, R. Adsorption of cationic and anionic azo dyes on sepiolite clay: Equilibrium and kinetic studies in batch mode. J. Environ. Chem. Eng. 2016, 4, 1473–1483. [Google Scholar] [CrossRef]
- Tian, G.; Han, G.; Wang, F.; Liang, J. Sepiolite nanomaterials: Structure, properties and functional applications. In Nanomaterials from Clay Minerals, a New Approach to Green Functional Materials; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–201. [Google Scholar]
- Aranda, P.; Kun, R.; Martín-Luengo, M.A.; Letaïef, S.; Dékány, I.; Ruiz-Hitzky, E. Titania–sepiolite nanocomposites prepared by a surfactant templating colloidal route. Chem. Mater. 2008, 20, 84–91. [Google Scholar] [CrossRef]
- Ökte, A.N.; Sayınsöz, E. Characterization and photocatalytic activity of TiO2 supported sepiolite catalysts. Sep. Purif. Technol. 2008, 62, 535–543. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Doi, H.; Kamigaito, O. Pore size distribution and adsorption selectivity of sepiolite. Clay Miner. 1990, 25, 99–105. [Google Scholar] [CrossRef]
- Mahyar, A.; Amani-Ghadim, A.J.M.; Letters, N. Influence of solvent type on the characteristics and photocatalytic activity of TiO2 nanoparticles prepared by the sol–gel method. Micro. Nano Lett. 2011, 6, 244–248. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol–gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, C.; Yeng, M.; Chiu, H. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Wang, W.; Gu, B.; Liang, L.; Hamilton, W.A.; Wesolowski, D. Synthesis of rutile (α-TiO2) nanocrystals with controlled size and shape by low-temperature hydrolysis: Effects of solvent composition. J. Phys. Chem. 2004, 108, 14789–14792. [Google Scholar] [CrossRef]
- Dandy, A.J. Zeolitic water content and adsorptive capacity for ammonia of microporous sepiolite. J. Chem. Soc. A 1971, 2383–2387. [Google Scholar] [CrossRef]
- Ovarlez, S.; Chaze, A.-M.; Giulieri, F.; Delamare, F. Indigo chemisorption in sepiolite. Application to Maya blue formation. Comptes Rendus Chim. 2006, 9, 1243–1248. [Google Scholar] [CrossRef]
- Fernandes, F.M.; Manjubala, I.; Ruiz-Hitzky, E. Gelatin renaturation and the interfacial role of fillers in bionanocomposites. Phys. Chem. Chem. Phys. 2011, 13, 4901–4910. [Google Scholar] [CrossRef]
- Veira de Oliveira, W.; Sousa Morais, A.Í.; Castro Honorio, L.M.; Aragão Trigueiro, P.; Costa Almeida, L.; Peña Garcia, R.R.; Cruz Viana, B.; Furtini, M.B.; Silva-Filho, E.C.; Anteveli Osajima, J. TiO2 immobilized on fibrous clay as strategies to photocatalytic activity. Mater. Res. 2020, 23, e20190463. [Google Scholar] [CrossRef]
- Haro-Poniatowski, E.; Rodriguez-Talavera, R.; Heredia, M.; Cano-Corona, O.; Arroyo-Murillo, R. Crystallization of nanosized titania particles prepared by the sol-gel process. J. Mater. Res. 1994, 9, 2102–2108. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Liang, T.; Sun, Q.; Wang, H. Photocatalytic degradation of Orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]
- Valentín, J.; López-Manchado, M.; Rodríguez, A.; Posadas, P.; Ibarra, L. Novel anhydrous unfolded structure by heating of acid pre-treated sepiolite. Appl. Clay Sci. 2007, 36, 245–255. [Google Scholar] [CrossRef]
- Yebra-Rodriguez, A.; Martin-Ramos, J.; del Rey, F.; Viseras, C.; Lopez-Galindo, A. Effect of acid treatment on the structure of sepiolite. Clay. Miner. 2003, 38, 353–360. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A.; MacKenzie, K. Effect of silica additive on the anatase to rutile phase transition. J. Am. Ceram. Soc. 2001, 84, 1591–1596. [Google Scholar] [CrossRef]
- Nilchi, A.; Janitabar-Darzi, S.; Rasouli-Garmarodi, S. Sol-gel preparation of nanoscale TiO2/SiO2 composite for eliminating of Con Red azo dye. Mater. Sci. Appl. 2011, 2, 476–480. [Google Scholar]
- Aguado, J.; van Grieken, R.; López-Muñoz, M.-J.; Marugán, J. A comprehensive study of the synthesis, characterization and activity of TiO2 and mixed TiO2/SiO2 photocatalysts. Appl. Catal. A 2006, 312, 202–212. [Google Scholar] [CrossRef]
- Gaya, U. Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer: Dordrecht, The Netherlands, 2014; pp. 43–71. [Google Scholar]
- Du, Y.; Tang, D.; Zhang, G.; Wu, X. Facile synthesis of Ag2O-TiO2/sepiolite composites with enhanced visible-light photocatalytic properties. Chin. J. Catal. 2015, 36, 2219–2228. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Titania–clay heterostructures with solar photocatalytic applications. J. Appl. Catal. B Environ. 2015, 176, 278–287. [Google Scholar] [CrossRef]
- Parks, G.A. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Bouna, L.; Rhouta, B.; Maury, F. Physicochemical Study of Photocatalytic Activity of TiO2 supported Palygorskite Clay Mineral. Int. J. Photoenergy 2013, 815473. [Google Scholar]
- Liu, S.; Zhu, D.; Zhu, J.; Yang, Q.; Wu, H. Preparation of Ag@AgCl-doped TiO2/sepiolite and its photocatalytic mechanism under visible light. J. Environ. Sci. 2017, 60, 43–52. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, H.; Zhou, S.; Liu, Y.; Yan, C. Fabrication of europium-nitrogen co-doped TiO2/Sepiolite nanocomposites and its improved photocatalytic activity in real wastewater treatment. J. Appl. Clay Sci. 2020, 197, 105791. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Song, J.; Zhang, G.; Li, C.; Zheng, S. Synthesis of novel ternary heterogeneous BiOCl/TiO2/sepiolite composite with enhanced visible-light-induced photocatalytic activity towards tetracycline. J. Colloid Interface Sci. 2019, 533, 238–250. [Google Scholar] [CrossRef]
- Kacha, S.; Derriche, Z. Equilibrium and kinetics of color removal from dye solutions with bentonite and polyaluminum hydroxide. Water. Environ. Res. 2003, 75, 15–20. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Amal, R. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration. J. Chem. Eng. Sci. 2014, 105, 46–52. [Google Scholar] [CrossRef]




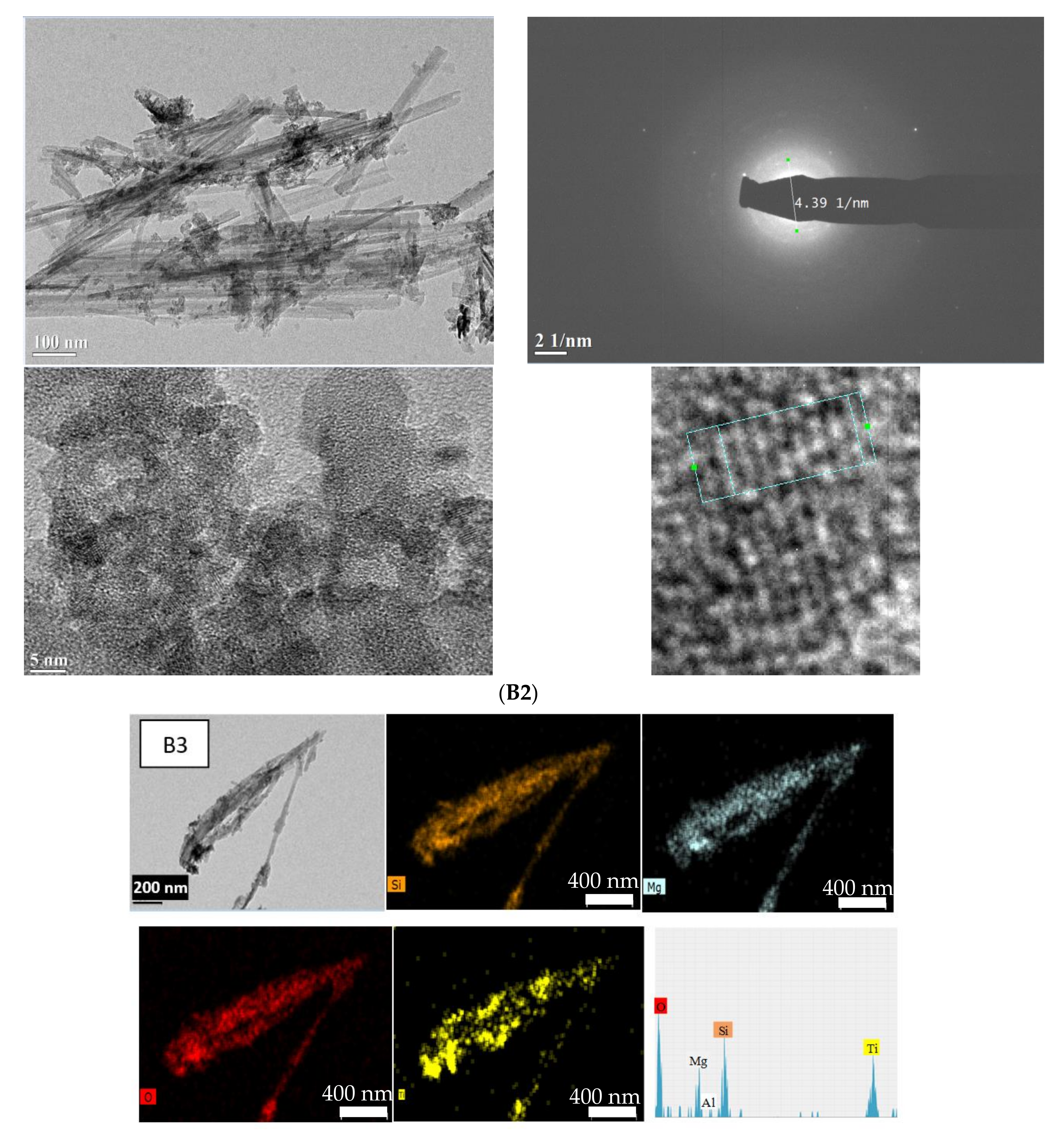

| Concentration (mg⋅g−1) | Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Mg | Fe | K | Ti/Mg | Ti/Al | |
| Raw sepiolite | 2.29 | 0.61 | 13.79 | 2.86 | 7.88 | 0.04 | 0.26 |
| Mc-80-500 | 3.78 | 191 | 0.93 | 0.44 | 4.29 | 205.4 | 50.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtiar, A.; Bouberka, Z.; Roussel, P.; Volkringer, C.; Addad, A.; Ouddane, B.; Pierlot, C.; Maschke, U. Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution. Nanomaterials 2022, 12, 3313. https://doi.org/10.3390/nano12193313
Bakhtiar A, Bouberka Z, Roussel P, Volkringer C, Addad A, Ouddane B, Pierlot C, Maschke U. Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution. Nanomaterials. 2022; 12(19):3313. https://doi.org/10.3390/nano12193313
Chicago/Turabian StyleBakhtiar, Amina, Zohra Bouberka, Pascal Roussel, Christophe Volkringer, Ahmed Addad, Baghdad Ouddane, Christel Pierlot, and Ulrich Maschke. 2022. "Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution" Nanomaterials 12, no. 19: 3313. https://doi.org/10.3390/nano12193313
APA StyleBakhtiar, A., Bouberka, Z., Roussel, P., Volkringer, C., Addad, A., Ouddane, B., Pierlot, C., & Maschke, U. (2022). Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution. Nanomaterials, 12(19), 3313. https://doi.org/10.3390/nano12193313













