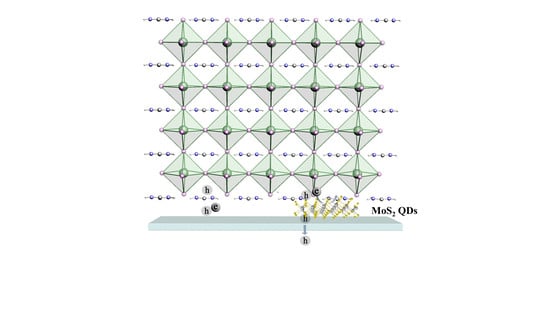
Enhancing the Efficiency of Perovskite Solar Cells through Interface Engineering with MoS2 Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
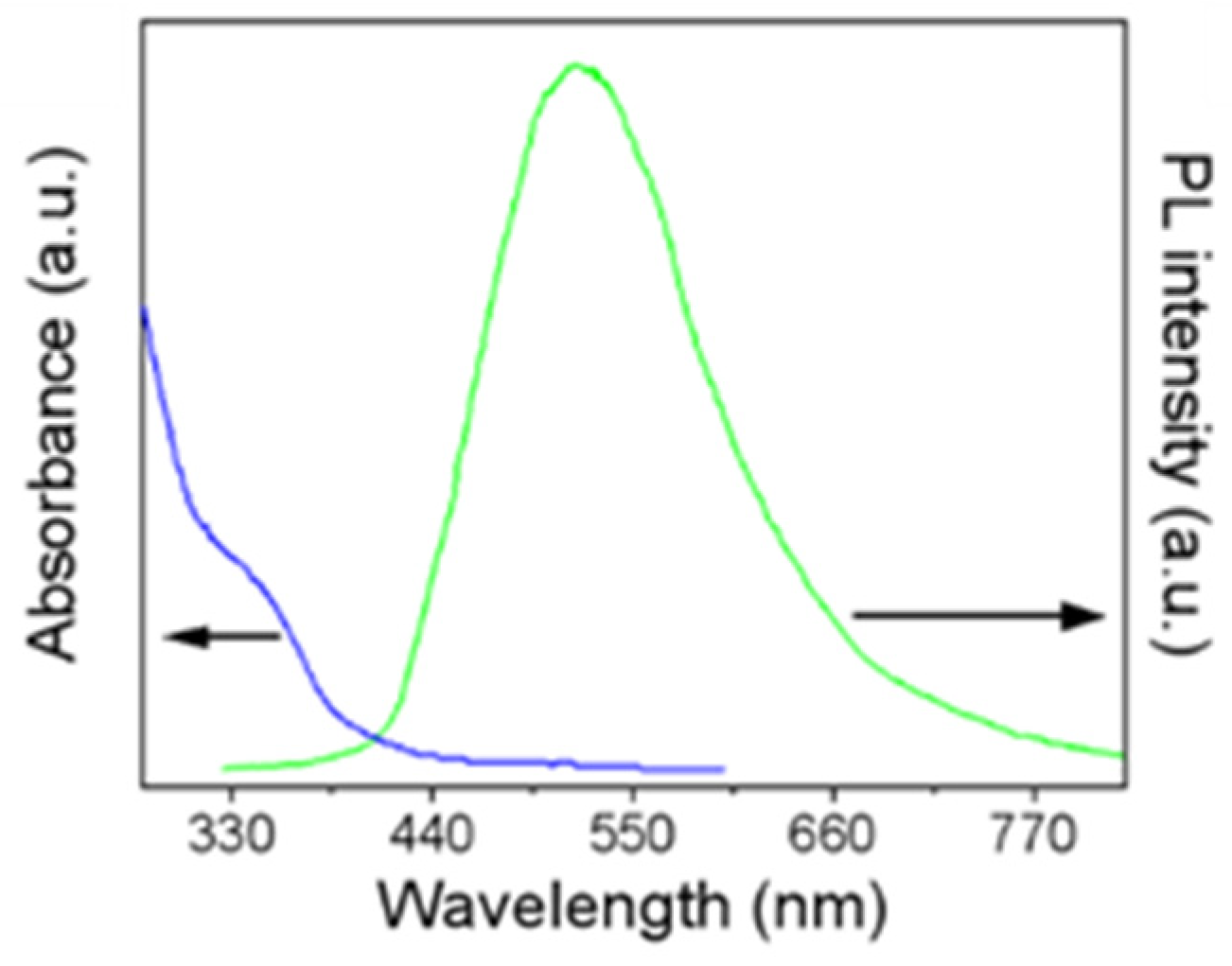
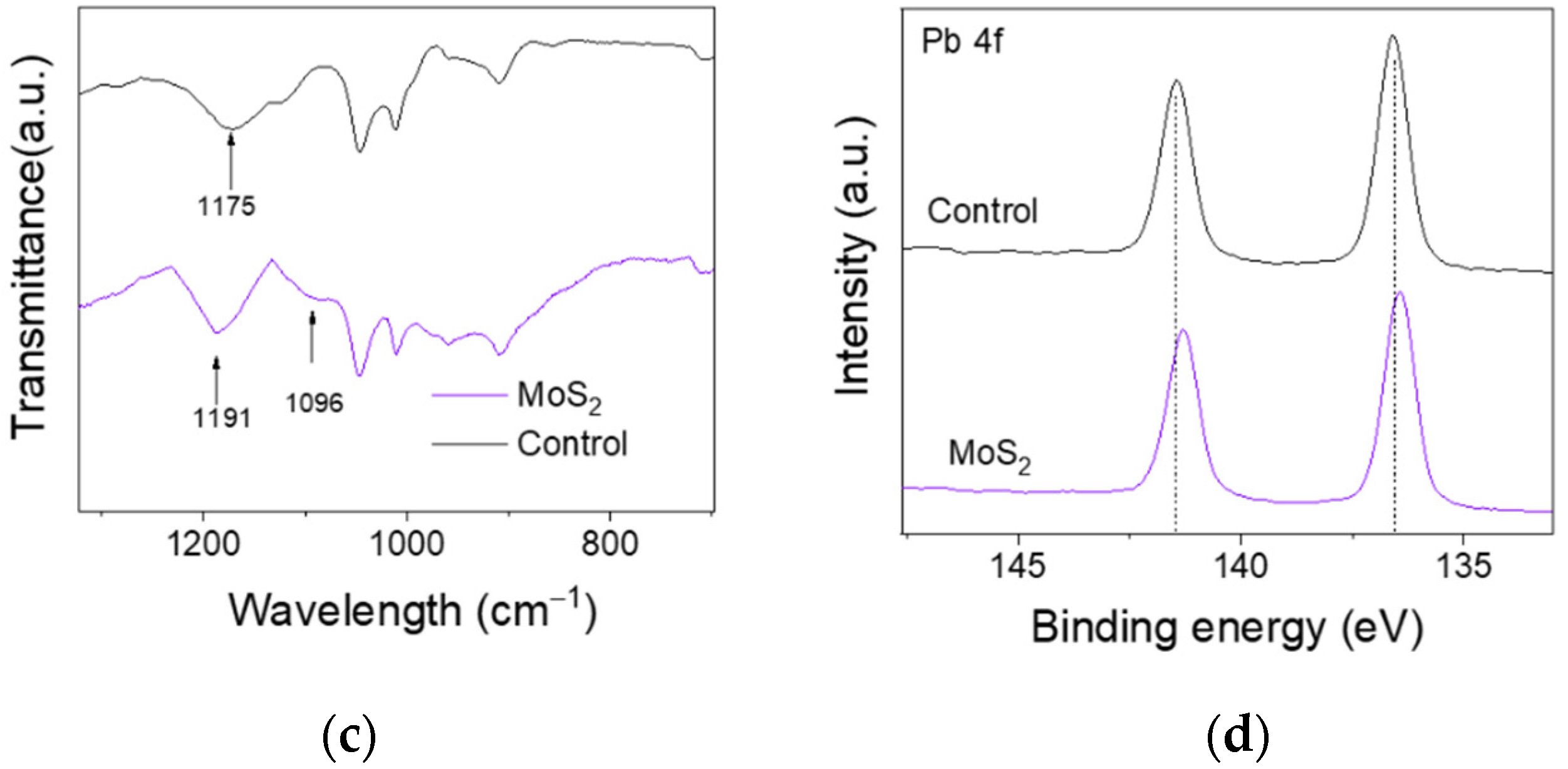
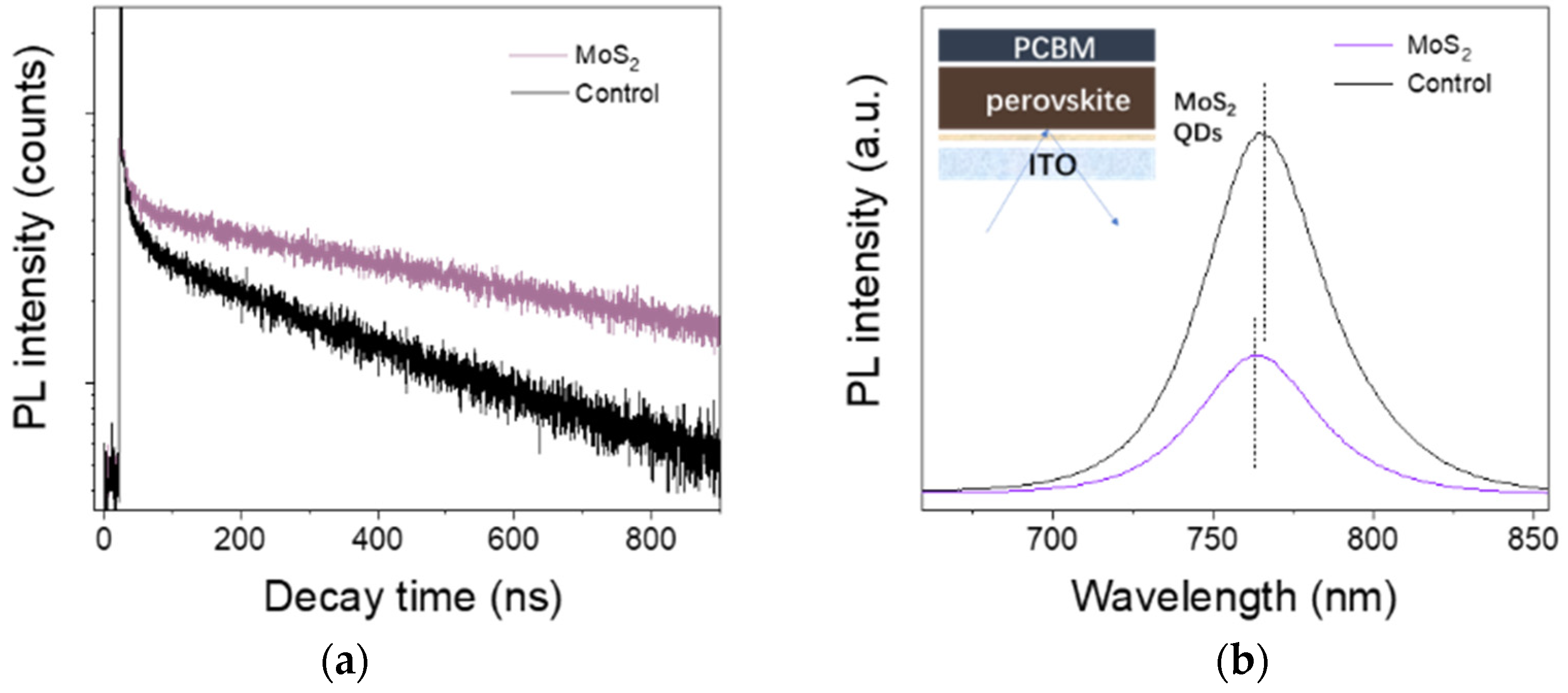
3.1. Characterization of MoS2 QDs
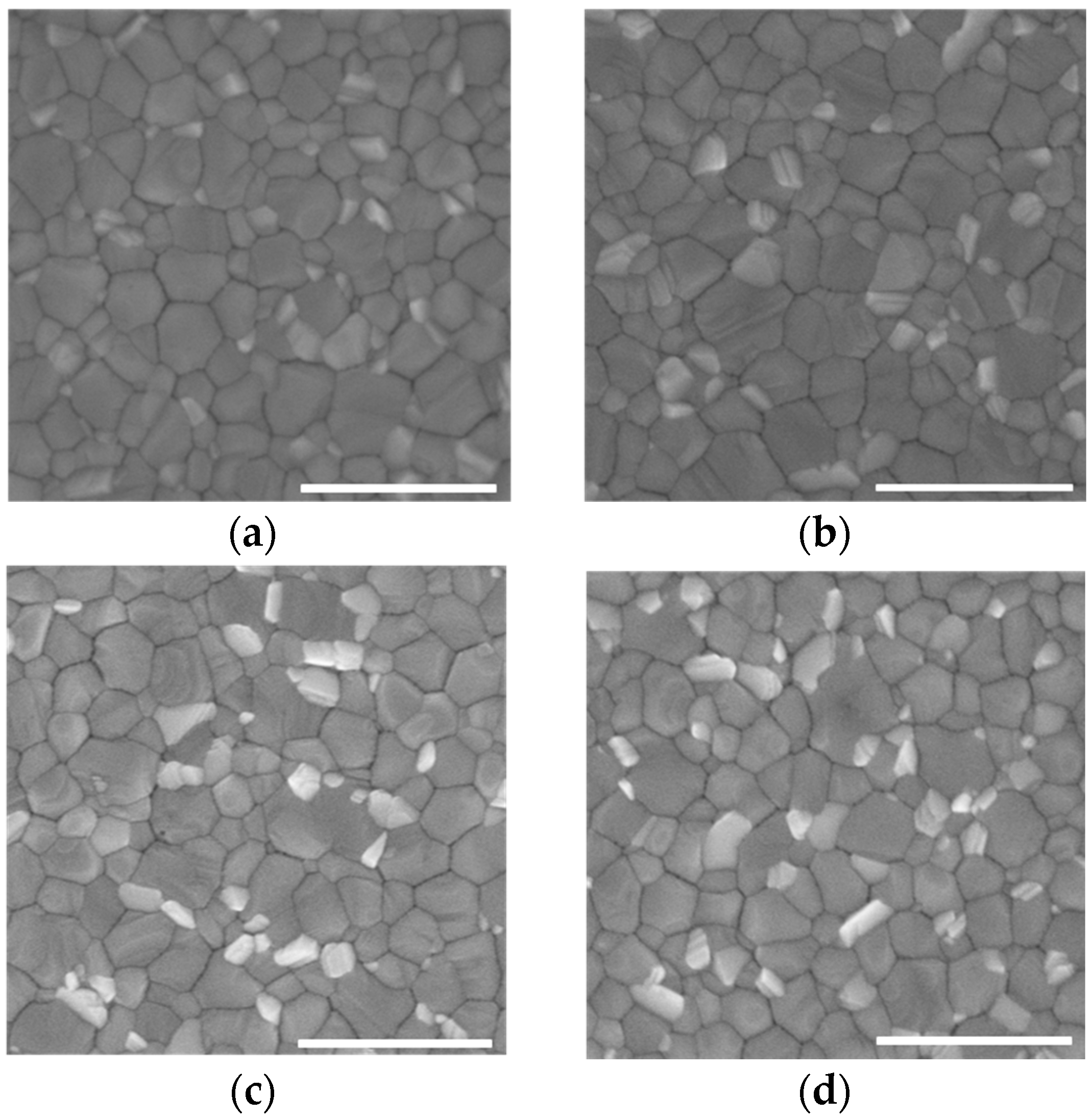
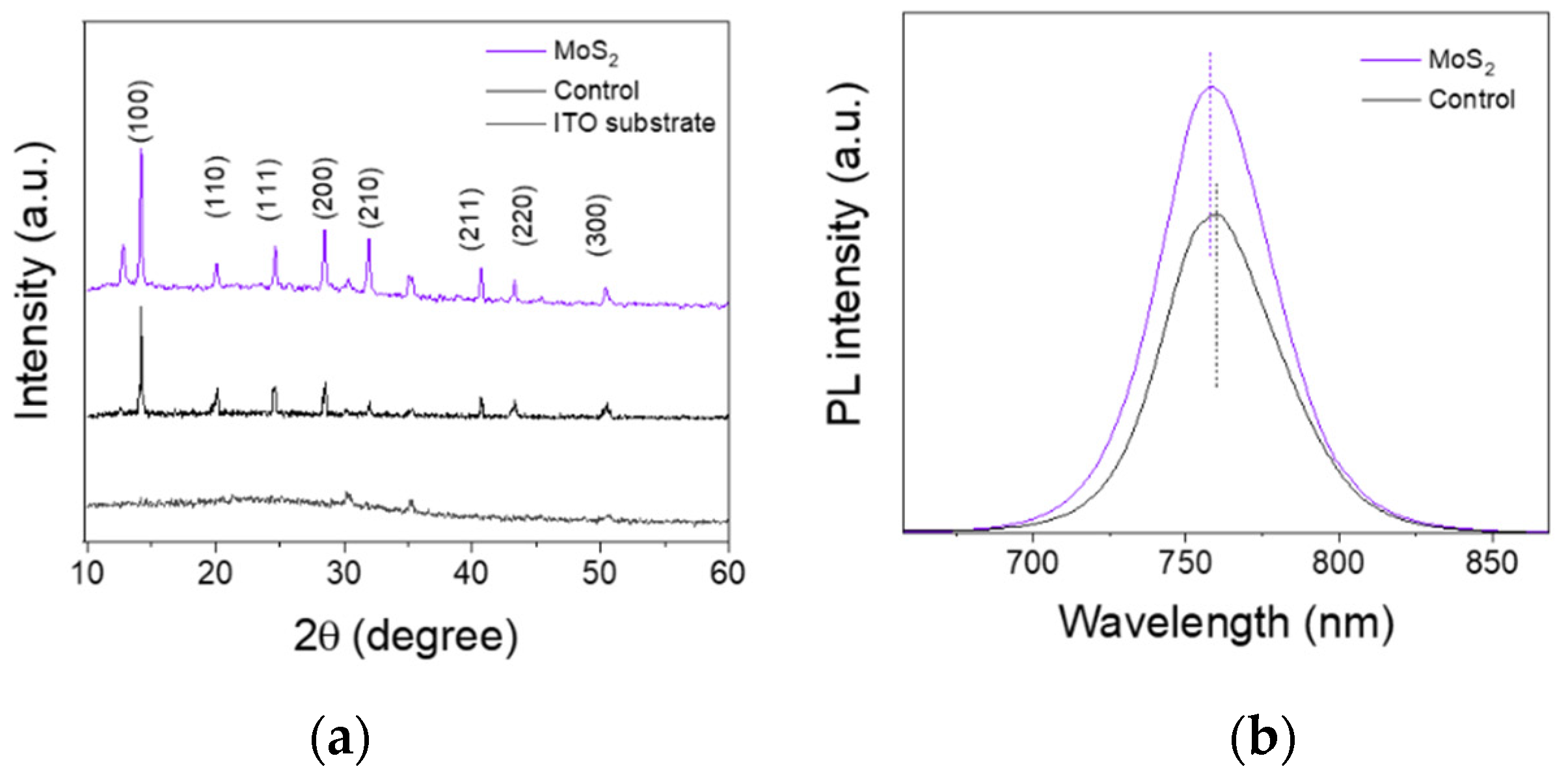
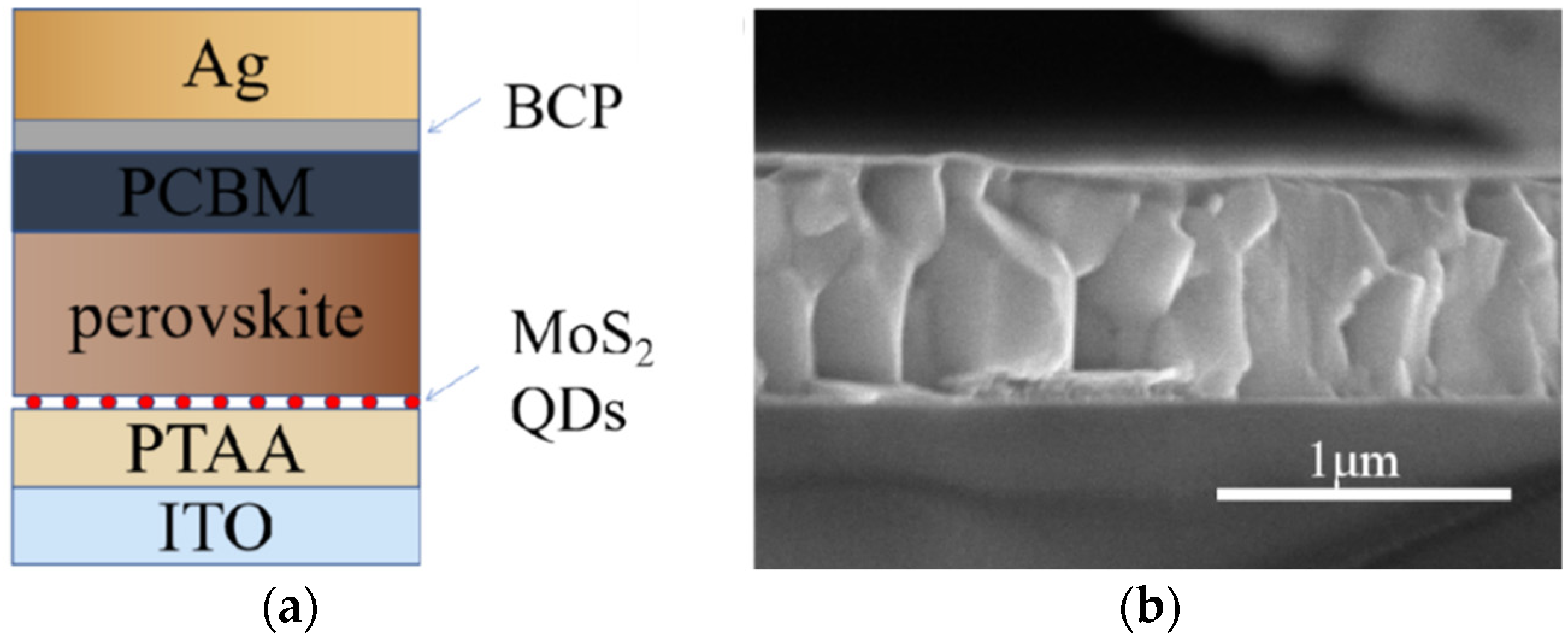
3.2. Characterization of Perovskite Films
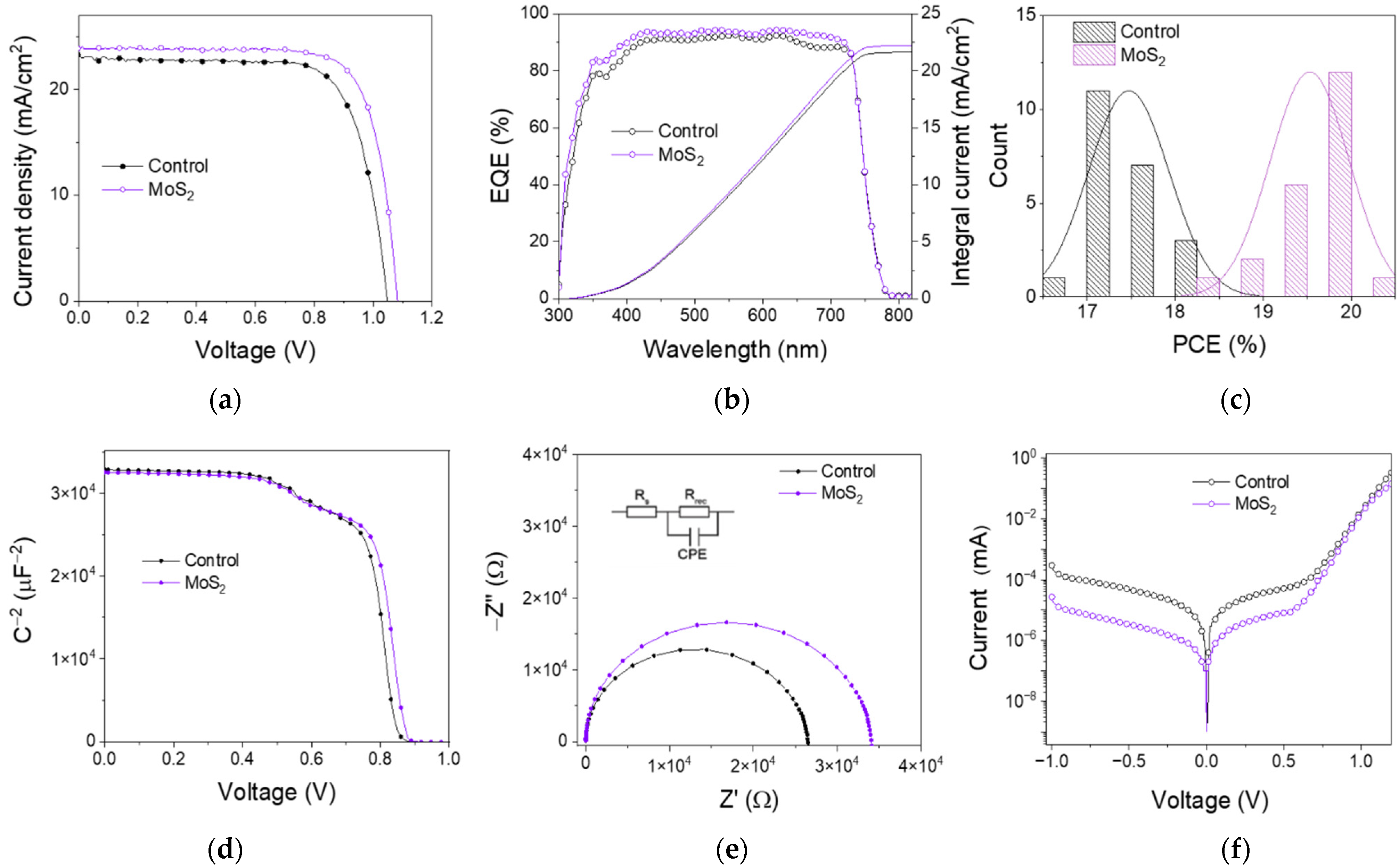
3.3. Perovskite Solar Cell Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Yu, Z.; Wei, H.; Xiao, X.; Ni, Z.; Chen, B.; Deng, Y.; Habisreutinger, S.N.; Chen, X.; Wang, K.; et al. Enhancing Electron Diffusion Length in Narrow-Bandgap Perovskites for Efficient Monolithic Perovskite Tandem Solar Cells. Nat. Commun. 2019, 10, 4498. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lee, H.B.; Hwang, S.; Kang, J.W. Large-Area, Green Solvent Spray Deposited Nickel Oxide Films for Scalable Fabrication of Triple-Cation Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 3357–3368. [Google Scholar] [CrossRef]
- Sandhu, S.; Saharan, C.; Buruga, S.K.; Kumar, S.A.; Rana, P.S.; Nagajyothi, P.C.; Mane, S.D. Micro Structurally Engineered Hysteresis-Free High Efficiency Perovskite Solar Cell Using Zr-Doped Tio2 Electron Transport Layer. Ceram. Int. 2021, 47, 14665–14672. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of Energy Disorder and Open-Circuit Voltage in Hybrid Perovskite Solar Cells. Nat. Energy 2016, 1, 15001. [Google Scholar] [CrossRef]
- Chen, S.; Dai, X.; Xu, S.; Jiao, H.; Zhao, L.; Huang, J. Stabilizing Perovskite-Substrate Interfaces for High-Performance Perovskite Modules. Science 2021, 373, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, D.; Xiang, Y.; Zhao, L.; Anaya, M.; Shen, Y.; Wu, J.; Yang, W.; Chiang, Y.H.; Tu, Y.; et al. Buried Interfaces in Halide Perovskite Photovoltaics. Adv. Mater. 2021, 33, e2006435. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Yang, F.; Zeng, Q.; Yin, W.; Zhang, W.; Wang, H.; Yang, X.; Kershaw, S.V.; Yang, B.; et al. Amine-Terminated Carbon Dots Linking Hole Transport Layer and Vertically Oriented Quasi-2d Perovskites through Hydrogen Bonds Enable Efficient Leds. ACS Nano 2022, 16, 9679–9690. [Google Scholar] [CrossRef]
- Kim, K.M.; Ahn, S.; Jang, W.; Park, S.; Park, O.O.; Wang, D.H. Work Function Optimization of Vacuum Free Top-Electrode by Pedot:Pss/Pei Interaction for Efficient Semi-Transparent Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 176, 435–440. [Google Scholar] [CrossRef]
- Jia, X.; Wu, N.; Wei, J.; Zhang, L.; Luo, Q.; Bao, Z.; Li, Y.-Q.; Yang, Y.; Liu, X.; Ma, C.-Q. A Low-Cost and Low-Temperature Processable Zinc Oxide-Polyethylenimine (Zno:Pei) Nano-Composite as Cathode Buffer Layer for Organic and Perovskite Solar Cells. Org. Electron. 2016, 38, 150–157. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Huang, H.; Reckmeier, C.; Zhang, Y.; Choy, W.C.; Rogach, A.L. Enhancing the Brightness of Cesium Lead Halide Perovskite Nanocrystal Based Green Light-Emitting Devices through the Interface Engineering with Perfluorinated Ionomer. Nano Lett. 2016, 16, 1415–1420. [Google Scholar] [CrossRef]
- Degani, M.; An, Q.; Albaladejo-Siguan, M.; Hofstetter, Y.J.; Cho, C.; Paulus, F.; Grancini, G.; Vaynzof, Y. 23.7% Efficient Inverted Perovskite Solar Cells by Dual Interfacial Modification. Sci. Adv. 2021, 7, eabj7930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, C.; Zhang, Y.; Wu, H.; Ji, C.; Chuai, Y.; Wang, P.; Wen, S.; Zhang, C.; Yu, W.W. Bright Perovskite Nanocrystal Films for Efficient Light-Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Abdu-Aguye, M.; Qiu, L.; Lai, L.-H.; Liu, J.; Adjokatse, S.; Jahani, F.; Kamminga, M.E.; Brink, G.H.T.; Palstra, T.T.M.; et al. Elimination of the Light Soaking Effect and Performance Enhancement in Perovskite Solar Cells Using a Fullerene Derivative. Energy Environ. Sci. 2016, 9, 2444–2452. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Barbaud, J.; Kong, W.; Cui, D.; Chen, H.; Yang, X.; Han, L. Stabilizing Heterostructures of Soft Perovskite Semiconductors. Science 2019, 365, 687–691. [Google Scholar] [CrossRef]
- Litvin, A.P.; Zhang, X.; Ushakova, E.V.; Rogach, A.L. Carbon Nanoparticles as Versatile Auxiliary Components of Perovskite-Based Optoelectronic Devices. Adv. Funct. Mater. 2021, 31, 2010768. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Xiong, Y.; Ji, T.; Wang, C.; Shen, X.; Lu, M.; Wang, H.; Wen, S.; Zhang, Y.; et al. Energy Level Modification with Carbon Dot Interlayers Enables Efficient Perovskite Solar Cells and Quantum Dot Based Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 1910530. [Google Scholar] [CrossRef]
- Zheng, X.; Troughton, J.; Gasparini, N.; Lin, Y.; Wei, M.; Hou, Y.; Liu, J.; Song, K.; Chen, Z.; Yang, C.; et al. Quantum Dots Supply Bulk- and Surface-Passivation Agents for Efficient and Stable Perovskite Solar Cells. Joule 2019, 3, 1963–1976. [Google Scholar] [CrossRef]
- Tang, G.; You, P.; Tai, Q.; Yang, A.; Cao, J.; Zheng, F.; Zhou, Z.; Zhao, J.; Chan, P.K.L.; Yan, F. Solution-Phase Epitaxial Growth of Perovskite Films on 2d Material Flakes for High-Performance Solar Cells. Adv. Mater. 2019, 31, e1807689. [Google Scholar] [CrossRef]
- Hu, W.; Jin, X.; Li, A.; Liu, C.L.; Wang, X.F. Efficiency Improvement of Inverted Perovskite Solar Cells Enabled by Ptaa/Mos2 Double Hole Transporters. Nanotechnology 2022, 33, 335202. [Google Scholar] [CrossRef]
- Capasso, A.; Matteocci, F.; Najafi, L.; Prato, M.; Buha, J.; Cinà, L.; Pellegrini, V.; di Carlo, A.; Bonaccorso, F. Few-Layer Mos2flakes as Active Buffer Layer for Stable Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600920. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Paradisanos, I.; Paci, B.; Generosi, A.; Papachatzakis, M.; Maksudov, T.; Najafi, L.; Del Rio Castillo, A.E.; Kioseoglou, G.; Stratakis, E.; et al. Extending the Continuous Operating Lifetime of Perovskite Solar Cells with a Molybdenum Disulfide Hole Extraction Interlayer. Adv. Energy Mater. 2018, 8, 1702287. [Google Scholar] [CrossRef]
- Najafi, L.; Taheri, B.; Martin-Garcia, B.; Bellani, S.; di Girolamo, D.; Agresti, A.; Oropesa-Nunez, R.; Pescetelli, S.; Vesce, L.; Calabro, E.; et al. Mos2 Quantum Dot/Graphene Hybrids for Advanced Interface Engineering of a Ch3nh3pbi3 Perovskite Solar Cell with an Efficiency of over 20. ACS Nano 2018, 12, 10736–10754. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, A.; Nepal, D.; Park, K.; Jespersen, M.; Qualley, A.; Mirau, P.; Drummy, L.F.; Vaia, R.A. Mechanism for Liquid Phase Exfoliation of Mos2. Chem. Mater. 2016, 28, 337–348. [Google Scholar] [CrossRef]
- Zhu, X.; Xiang, J.; Li, J.; Feng, C.; Liu, P.; Xiang, B. Tunable Photoluminescence of Mos2 Quantum Dots Passivated by Different Functional Groups. J. Colloid Interface Sci. 2018, 511, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Ma, T.; Wu, D.; Zhao, Y.; Wang, C.; Zhao, D.; Li, X. Managing Lead Leakage in Efficient Perovskite Solar Cells with Phosphate Interlayers. Adv. Mater. Interfaces 2022, 9, 2200570. [Google Scholar] [CrossRef]
- Lu, X.; Korgel, A.B.A.; Johnston, K.P. High Yield of Germanium Nanocrystals Synthesized from Germanium Diiodide in Solution. Chem. Mater. 2005, 17, 6479–6485. [Google Scholar] [CrossRef]
- Chen, S.T.; Zhang, X.L.; Zhang, Q.H.; Tan, W.H. Trioctylphosphine as Both Solvent and Stabilizer to Synthesize Cds Nanorods. Nanoscale Res. Lett. 2009, 4, 1159–1165. [Google Scholar] [CrossRef]
- Dong, H.F.; Tang, S.S.; Hao, Y.S.; Yu, H.Z.; Dai, W.H.; Zhao, G.F.; Cao, Y.; Lu, H.T.; Zhang, X.J.; Ju, H.X. Fluorescent Mos2 Quantum Dots: Ultrasonic Preparation, up-Conversion and Down-Conversion Bioimaging, and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef]
- Sadhu, A.; Rai, M.; Salim, T.; Jin, X.; Tan, J.M.R.; Leow, S.W.; Ahmed, M.G.; Magdassi, S.; Mhaisalkar, S.G.; Wong, L.H. Dual Role of Cu-Chalcogenide as Hole-Transporting Layer and Interface Passivator for P–I–N Architecture Perovskite Solar Cell. Adv. Funct. Mater. 2021, 31, 2103807. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Y.; Eickemeyer, F.T.; Pan, L.; Ren, D.; Ruiz-Preciado, M.A.; Carlsen, B.; Yang, B.; Dong, X.; Wang, Z.; et al. Tailored Amphiphilic Molecular Mitigators for Stable Perovskite Solar Cells with 23.5% Efficiency. Adv. Mater. 2020, 32, 1907757. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Ahmad, S.; Tu, D.; Liu, X.; Jia, G.; Guo, X.; Li, C. Dion-Jacobson 2d-3d Perovskite Solar Cells with Improved Efficiency and Stability. Nano Energy 2020, 75, 104892. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, Y.; Wang, X.; Gao, F.; Zhao, Q. Self-Induced Type-I Band Alignment at Surface Grain Boundaries for Highly Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2021, 33, e2103231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hou, Y.; Bao, C.; Yin, J.; Yuan, F.; Huang, Z.; Song, K.; Liu, J.; Troughton, J.; Gasparini, N.; et al. Managing Grains and Interfaces Via Ligand Anchoring Enables 22.3%-Efficiency Inverted Perovskite Solar Cells. Nat. Energy 2020, 5, 131–140. [Google Scholar] [CrossRef]
- Leng, X.; Wan, K.; Wang, H.; Wu, G.; Zhang, X.; Liu, Y.; Han, B.; Wang, B.; Zhang, Y.; Zhou, H.; et al. Adenosine Triphosphate Disodium Modified Hole Transport Layer for Efficient Inverted Perovskite Solar Cells. ChemNanoMat 2022, 8, e202200041. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in Ch3nh3pbi3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Liu, X.; Wang, Z.; Guo, X.; Li, C. Deeper Insight into the Role of Organic Ammonium Cations in Reducing Surface Defects of the Perovskite Film. Angew. Chem. 2022, 134, e202115663. [Google Scholar]
- Liu, X.; Zhang, Y.; Shi, L.; Liu, Z.; Huang, J.; Yun, J.S.; Zeng, Y.; Pu, A.; Sun, K.; Hameiri, Z.; et al. Exploring Inorganic Binary Alkaline Halide to Passivate Defects in Low-Temperature-Processed Planar-Structure Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800138. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Kim, S.; Park, N. Multifunctional Chemical Linker Imidazoleacetic Acid Hydrochloride for 21% Efficient and Stable Planar Perovskite Solar Cells. Adv. Mater. 2019, 31, 1902902. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, M.; Liu, Z.; Jamshaid, A.; Ono, L.K.; Qi, Y. Highly Efficient Perovskite Solar Cells Enabled by Multiple Ligand Passivation. Adv. Energy Mater. 2020, 10, 1903696. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Song, T.-B.; Luo, S.; Hong, Z.; Duan, H.-S.; Dou, L.; Liu, Y.; Yang, Y. Controllable Self-Induced Passivation of Hybrid Lead Iodide Perovskites toward High Performance Solar Cells. Nano Lett. 2014, 14, 4158–4163. [Google Scholar] [CrossRef]
- Wang, L.; McCleese, C.; Kovalsky, A.; Zhao, Y.; Burda, C. Femtosecond Time-Resolved Transient Absorption Spectroscopy of Ch3nh3pbi3 Perovskite Films: Evidence for Passivation Effect of Pbi2. J. Am. Chem. Soc. 2014, 136, 12205–12208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-Structure Perovskite Solar Cells with Efficiency Beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Xu, Y.; Li, R.; Yu, T.; Lin, Q. Interfacial Engineering of Perovskite Solar Cells with Evaporated Pbi2 Ultrathin Layers. ACS Appl. Mater. Interfaces 2021, 13, 53282–53288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guang, S.; Ye, M.; Xu, K.; Guo, W.; Liu, X.Y.; Xu, H. Chemical Decoration of Perovskites by Nickel Oxide Doping for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 36841–36850. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Z.; Qiu, J.; Wu, J.; Zhen, C.; Tai, K.; Jiang, X.; Yang, S. Interface Regulation Enables Hysteresis Free Wide-Bandgap Perovskite Solar Cells with Low Voc Deficit and High Stability. Nano Energy 2021, 90, 106537. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Zhang, H.; Yang, J.; Han, J.; Xu, T.; Wang, S.; Wang, Z.; Gao, B.; Zhao, J.; et al. Efficient and Stable Inverted Perovskite Solar Cells with Very High Fill Factors Via Incorporation of Star-Shaped Polymer. Science 2021, 7, eabg0633. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Yang, X.; Wang, Y.; He, B.; Tang, Q. Lanthanide Ions Doped Cspbbr3 Halides for Htm-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V. Adv. Energy Mater. 2018, 8, 1802346. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, W.S.; Yu, W.; Yu, S.W.; Wu, Y.L.; Guo, X.; Liu, S.Z.; Li, C. A Two-Stage Annealing Strategy for Crystallization Control of Ch3nh3pbi3 Films toward Highly Reproducible Perovskite Solar Cells. Small 2018, 14, 1800181. [Google Scholar] [CrossRef]
- Sandhu, S.; Singh, R.; Yoo, K.; Kumar, M.; Lee, J.-J. Effect of Binary Additives in Mixed 2d/3d Sn-Based Perovskite Solar Cells. J. Power Sources 2021, 491, 229574. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Xiao, X.; Yu, Z.; Deng, Y.; Dai, X.; Ni, Z.; Huang, J. Identifying the Soft Nature of Defective Perovskite Surface Layer and Its Removal Using a Facile Mechanical Approach. Joule 2020, 12, 2661–2674. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J.; Tan, F.; Dong, C.; Liu, K.; Li, X.; Zhang, L.; Wu, H.; Wang, H.-L.; Qu, S.; et al. Quantum Dot Interface-Mediated Cspbibr2 Film Growth and Passivation for Efficient Carbon-Based Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 55338–55346. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Z.; Zhou, Y.; Liu, H.; Qin, Q.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; Liu, J. Enhancing the Efficiency of Low-Temperature Planar Perovskite Solar Cells by Modifying the Interface between Perovskite and Hole Transport Layer with Polymers. Electrochim. Acta 2018, 261, 445–453. [Google Scholar] [CrossRef]

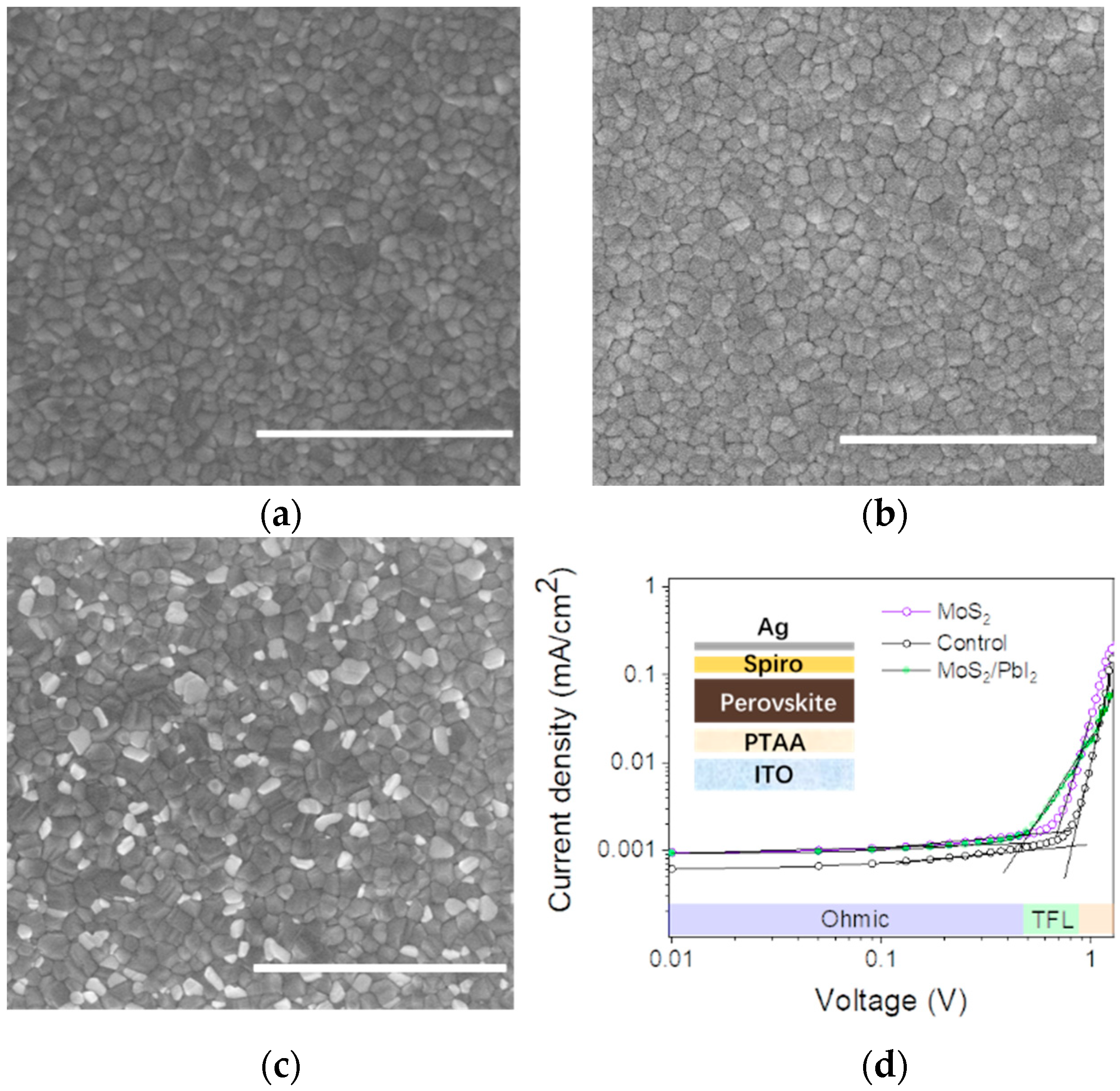
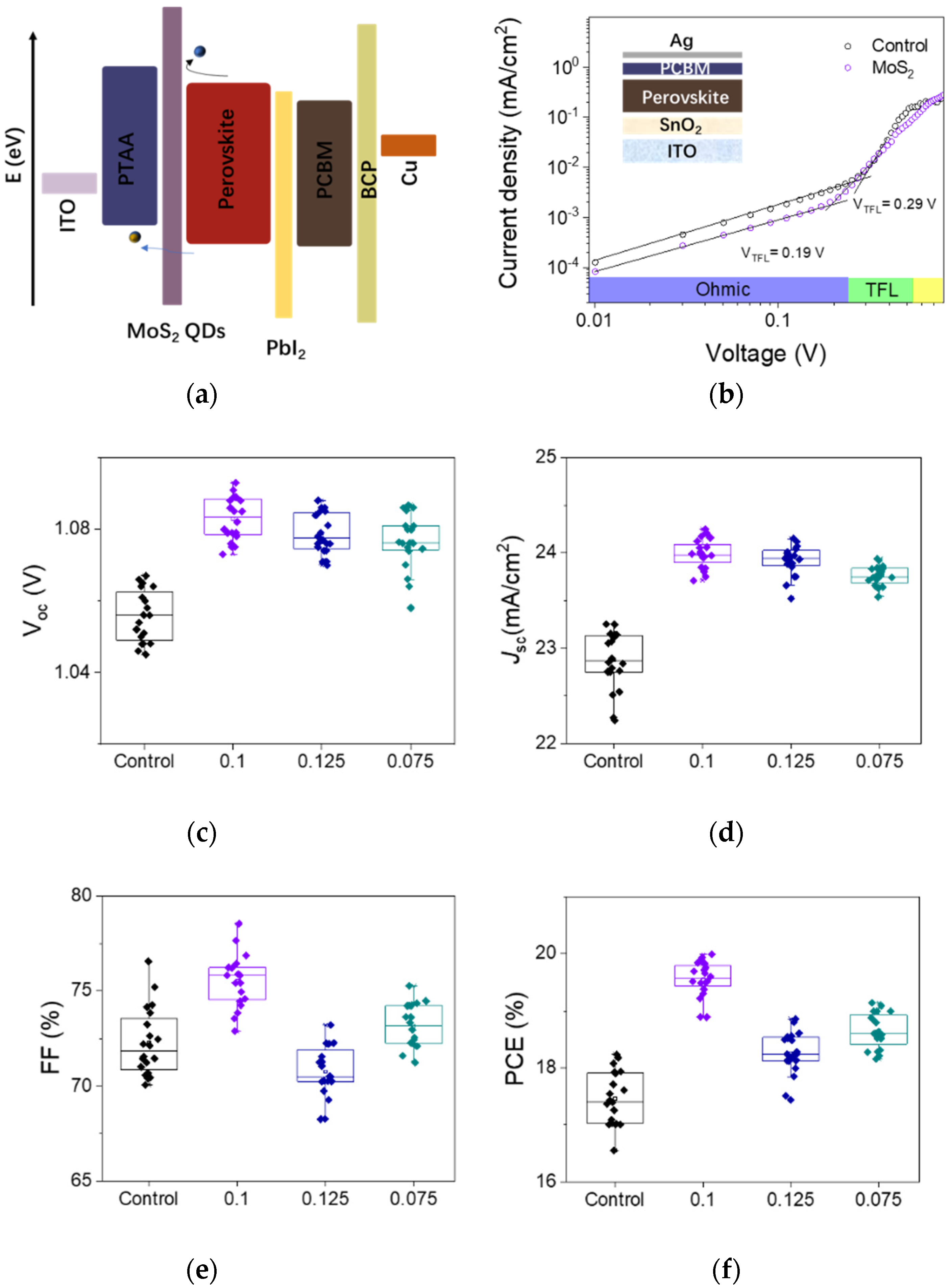
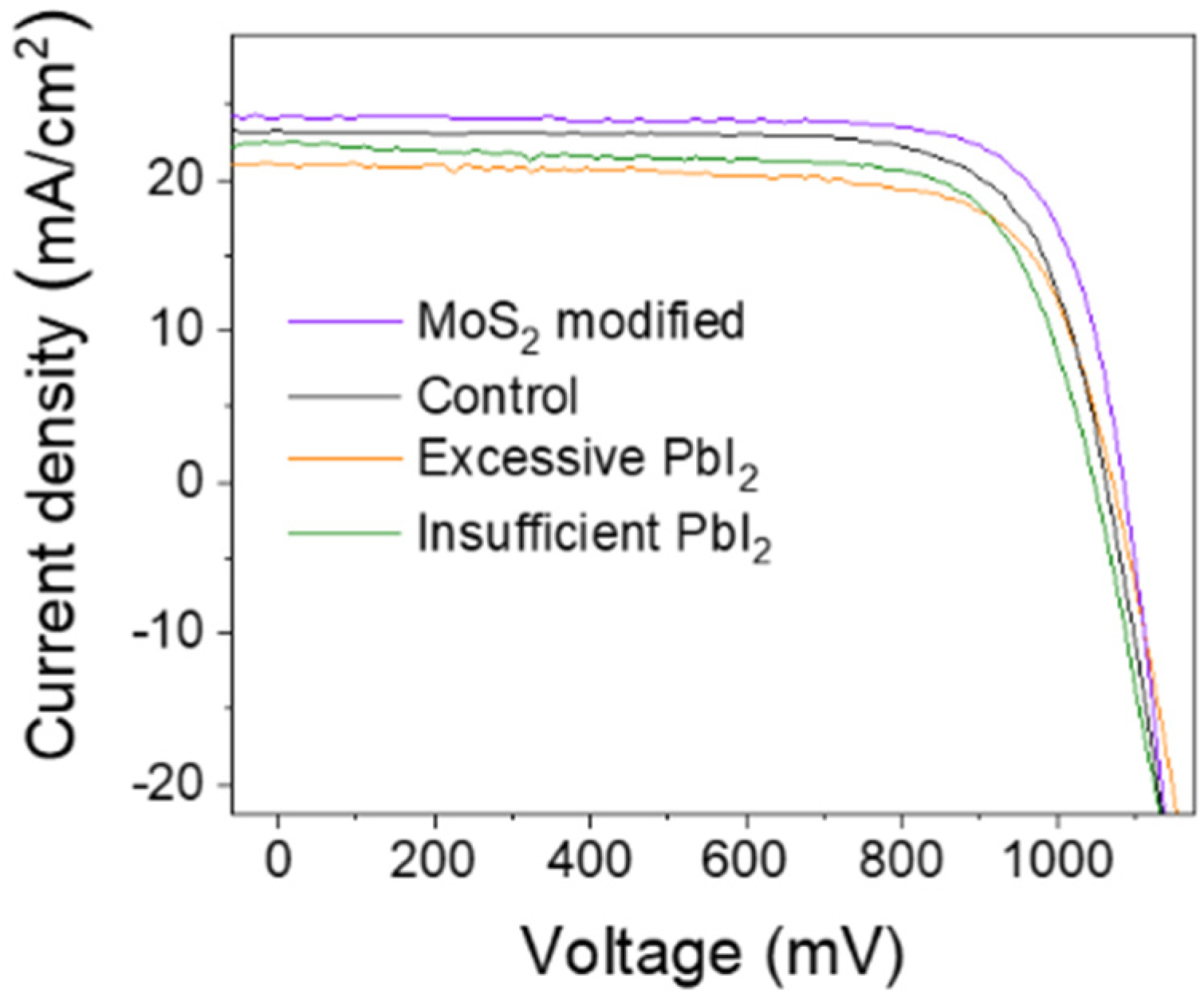
| Samples | τ1 (ns) | f1 (%) | τ2 (ns) | f2 (%) | τave (ns) |
|---|---|---|---|---|---|
| Without MoS2 | 11.7 | 52.6 | 354.6 | 47.4 | 175.3 |
| With MoS2 | 11.5 | 40.5 | 662.5 | 59.5 | 398.8 |
| Samples | Jcs (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0 mg/mL MoS2 | 22.89 | 1.05 | 74.35 | 17.87 |
| 0.075 mg/mL MoS2 | 23.30 | 1.06 | 73.81 | 18.23 |
| 0.100 mg/mL MoS2 | 23.79 | 1.08 | 77.64 | 19.95 |
| 0.125 mg/mL MoS2 | 23.99 | 1.07 | 72.07 | 18.50 |
| Samples | Jcs (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0 mg/mL MoS2 | 24.07 | 1.085 | 76.39 | 19.95 |
| 0.075 mg/mL MoS2 | 23.25 | 1.063 | 73.64 | 18.20 |
| 0.100 mg/mL MoS2 | 22.35 | 1.049 | 71.01 | 17.29 |
| 0.125 mg/mL MoS2 | 21.42 | 1.072 | 72.51 | 16.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Guo, T.; Wang, C.; Zou, J.; Wang, J.; Dong, W.; Li, J.; Zhang, W.; Zhang, X.; Zheng, W. Enhancing the Efficiency of Perovskite Solar Cells through Interface Engineering with MoS2 Quantum Dots. Nanomaterials 2022, 12, 3079. https://doi.org/10.3390/nano12173079
Luo Z, Guo T, Wang C, Zou J, Wang J, Dong W, Li J, Zhang W, Zhang X, Zheng W. Enhancing the Efficiency of Perovskite Solar Cells through Interface Engineering with MoS2 Quantum Dots. Nanomaterials. 2022; 12(17):3079. https://doi.org/10.3390/nano12173079
Chicago/Turabian StyleLuo, Zhao, Tan Guo, Chen Wang, Jifan Zou, Jianxun Wang, Wei Dong, Jing Li, Wei Zhang, Xiaoyu Zhang, and Weitao Zheng. 2022. "Enhancing the Efficiency of Perovskite Solar Cells through Interface Engineering with MoS2 Quantum Dots" Nanomaterials 12, no. 17: 3079. https://doi.org/10.3390/nano12173079
APA StyleLuo, Z., Guo, T., Wang, C., Zou, J., Wang, J., Dong, W., Li, J., Zhang, W., Zhang, X., & Zheng, W. (2022). Enhancing the Efficiency of Perovskite Solar Cells through Interface Engineering with MoS2 Quantum Dots. Nanomaterials, 12(17), 3079. https://doi.org/10.3390/nano12173079