Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
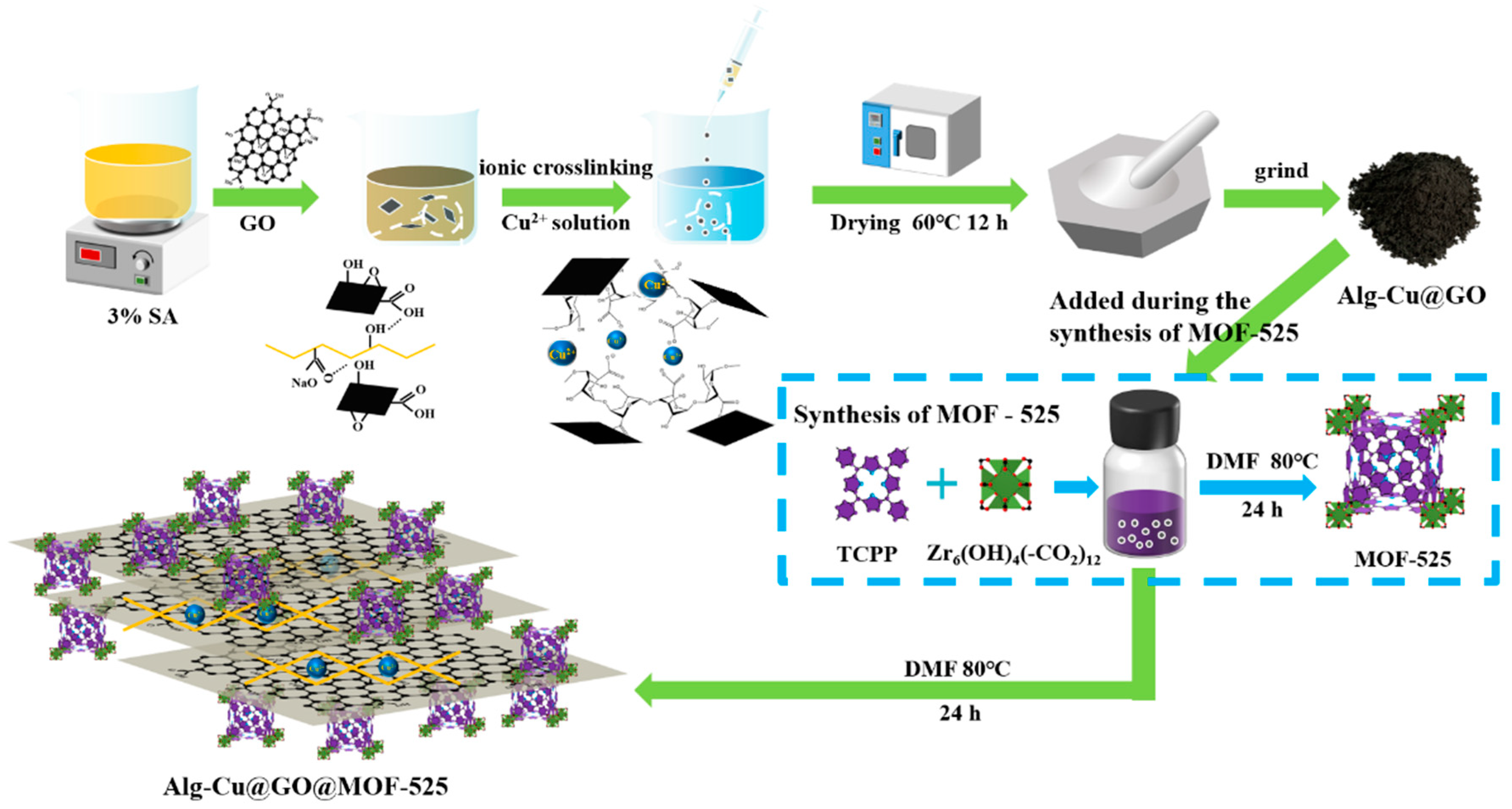
2.2. Synthesis
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
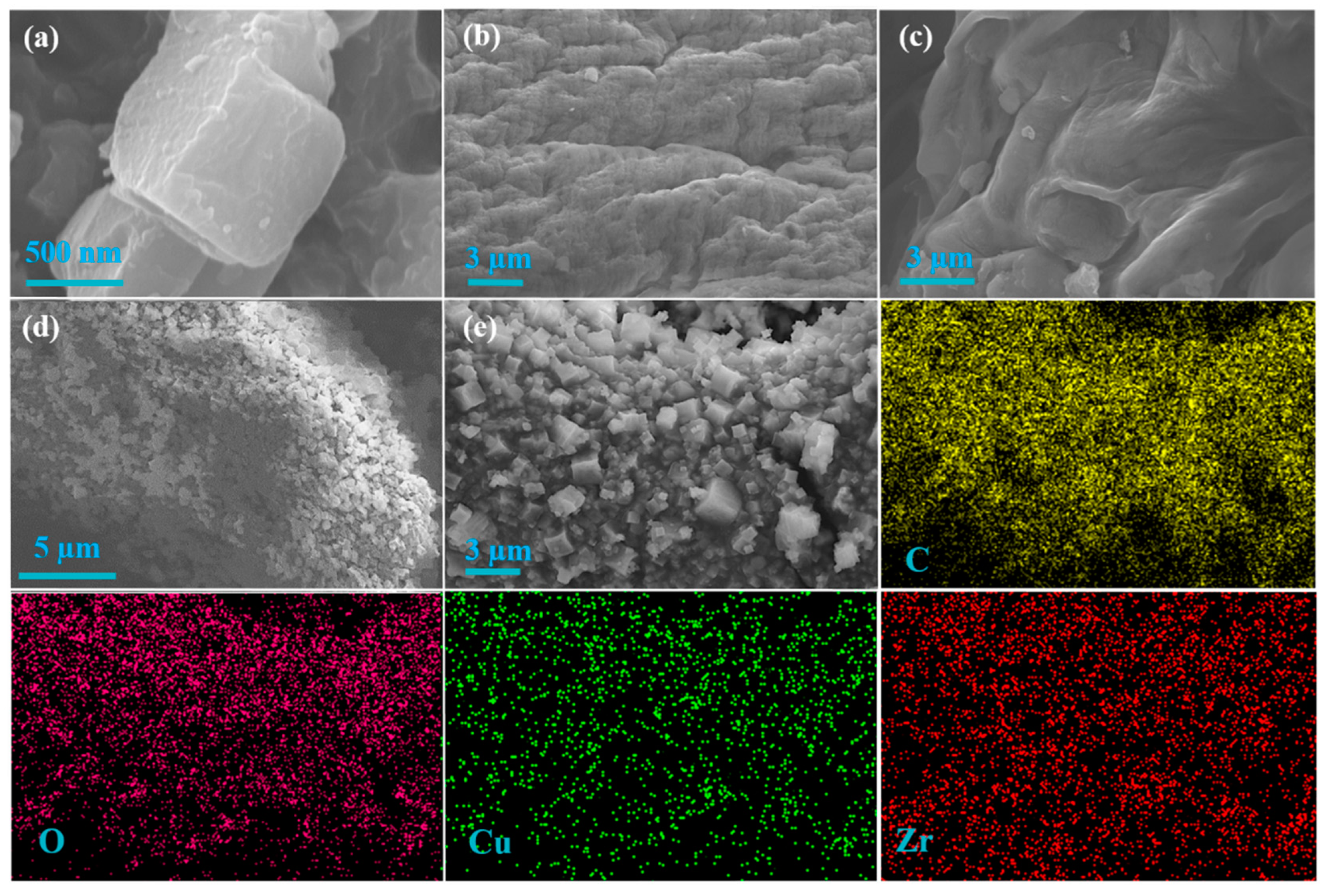
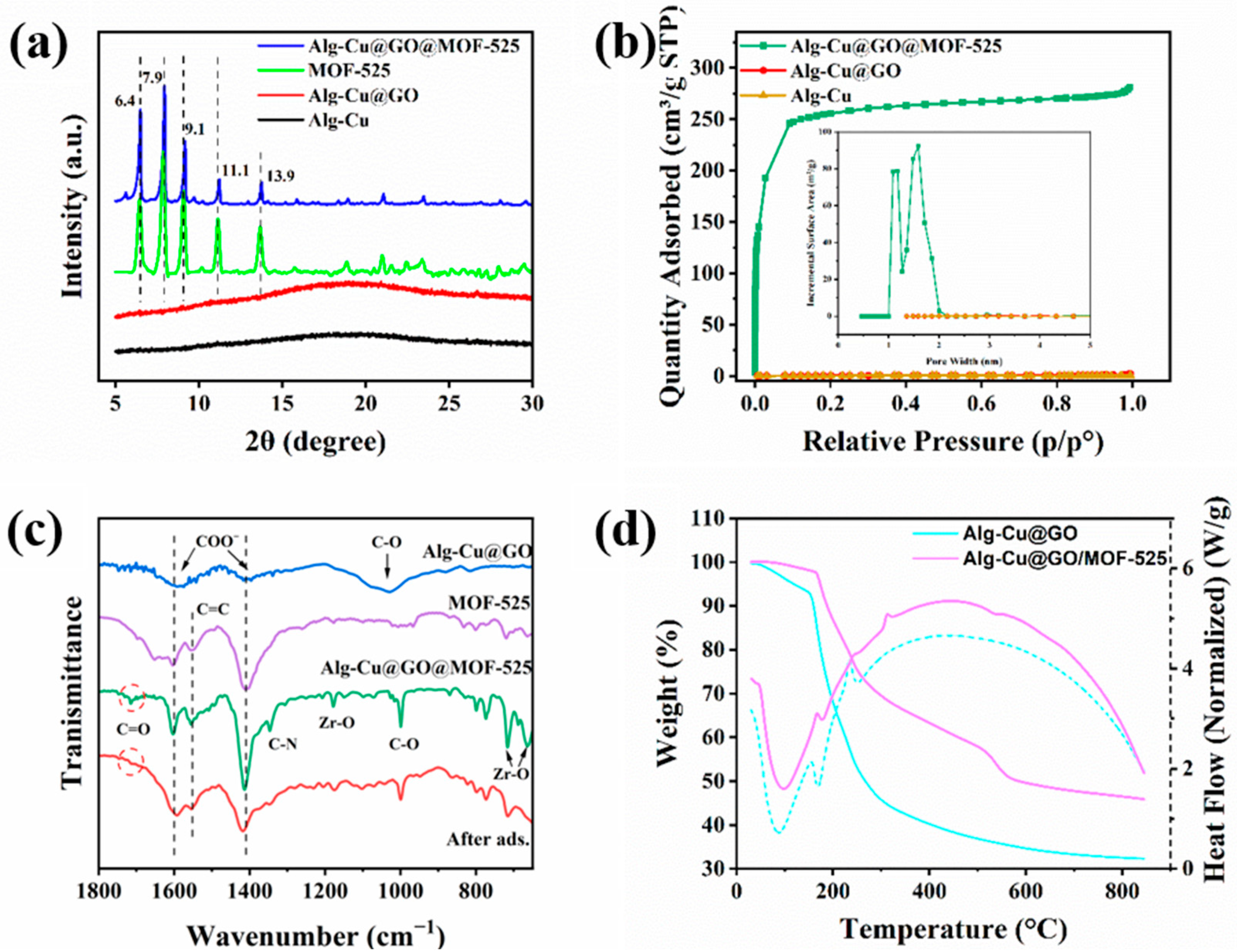
3.1. Characterization
3.2. Bath Experiment
3.2.1. Effect of GO Content on Adsorption Performance
3.2.2. Effects of pH, Dosage, Temperature, and Contact Time on Adsorption Properties
3.2.3. Alg-Cu@GO@MOF-525 Adsorption Properties of Different Dyes
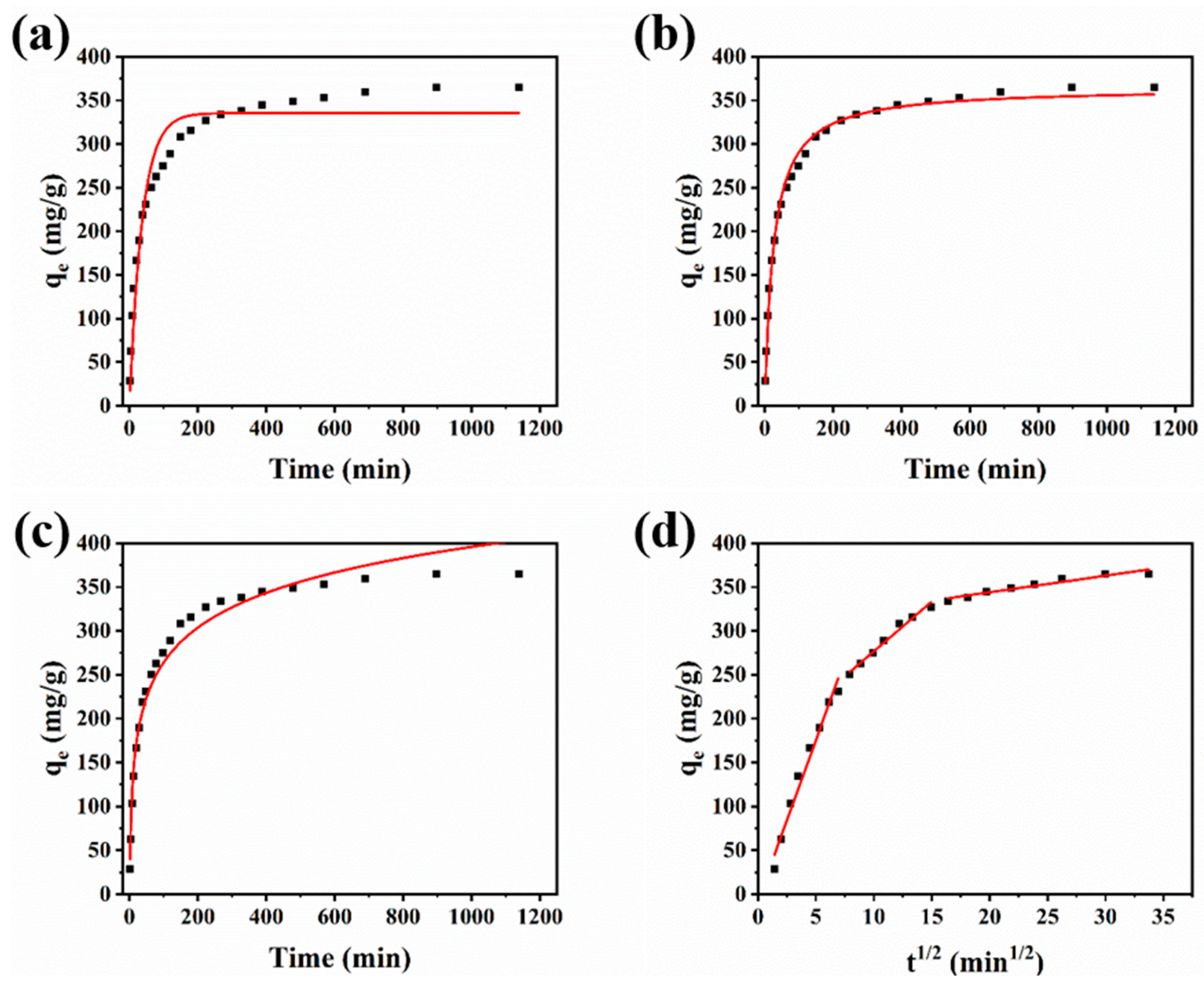
3.3. Adsorption Kinetics
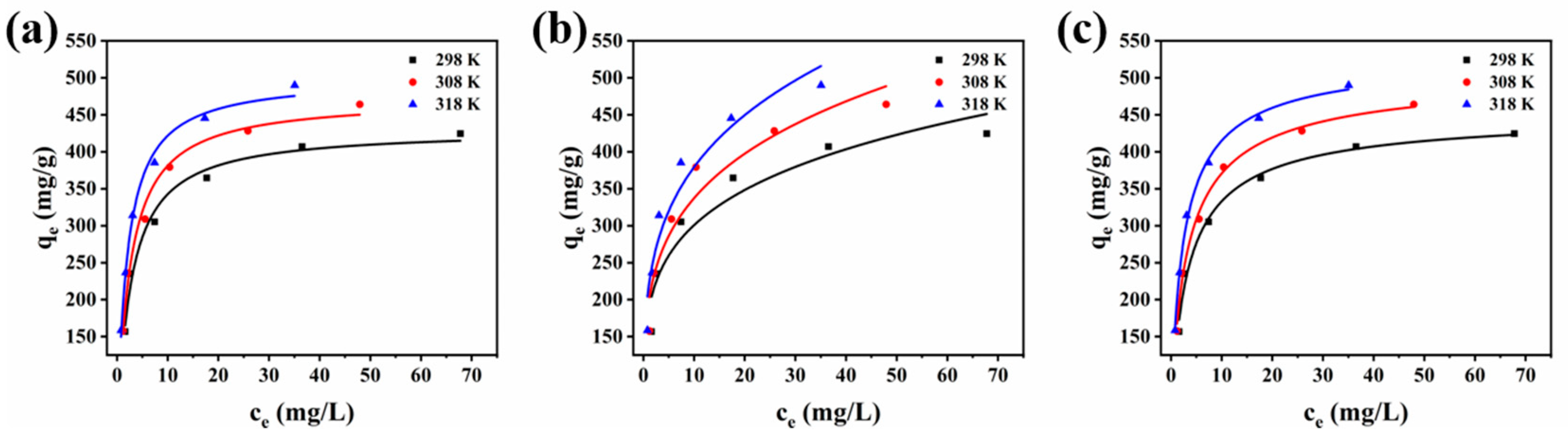
3.4. Adsorption Isotherms
3.5. Thermodynamic Study
3.6. Possible Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brillas, E. Progress of homogeneous and heterogeneous electro-Fenton treatments of antibiotics in synthetic and real wastewaters. A critical review on the period 2017–2021. Sci. Total Environ. 2022, 819, 153102. [Google Scholar] [CrossRef] [PubMed]
- El-Monaem, E.M.A.; Omer, A.M.; Khalifa, R.E.; Eltaweil, A.S. Floatable cellulose acetate beads embedded with flower-like zwitterionic binary MOF/PDA for efficient removal of tetracycline. J. Colloid Interface Sci. 2022, 620, 333–345. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, S.; Wu, P.; Wang, D.; Lu, S.; Wang, S.; Gong, F.; Wei, X.; Ye, X.; Ding, P. Recent advances in performance improvement of Metal-organic Frameworks to remove antibiotics: Mechanism and evaluation. Sci. Total Environ. 2022, 811, 152351. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393. [Google Scholar] [CrossRef]
- Song, Z.; Gao, H.; Liao, G.; Zhang, W.; Wang, D. A novel slag-based Ce/TiO2@LDH catalyst for visible light driven degradation of tetracycline: Performance and mechanism. J. Alloys Compd. 2022, 901, 163525. [Google Scholar] [CrossRef]
- Duan, R.; Ma, S.; Xu, S.; Wang, B.; He, M.; Li, G.; Fu, H.; Zhao, P. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism. Water Res. 2022, 218, 118489. [Google Scholar] [CrossRef]
- Cui, J.Y.; Xie, A.T.; Liu, Y.; Xue, C.G.; Pan, J.M. Fabrication of multi-functional imprinted composite membrane for selective tetracycline and oil-in-water emulsion separation. Compos. Commum. 2021, 28, 100985. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Wei, F.; Ding, T.; Zhang, M.; Zhu, R. Insights into the adsorption mechanism of tetracycline on hierarchically porous carbon and the effect of nanoporous geometry. Chem. Eng. J. 2022, 437, 135454. [Google Scholar] [CrossRef]
- Lin, X.; Zeng, W.; Chen, Y.; Su, T.; Zhong, Q.; Gong, L.; Liu, Y. UiO-66-derived porous-carbon adsorbents: Synthesis, characterization and tetracycline adsorption performance. Carbon Lett. 2022, 32, 875–884. [Google Scholar] [CrossRef]
- Cheng, S.; Xie, P.; Yu, Z.; Gu, R.; Su, Y. Enhanced adsorption performance of UiO-66 via modification with functional groups and integration into hydrogels. Environ. Res. 2022, 212, 113354. [Google Scholar] [CrossRef]
- Mouchaham, G.; Cui, F.S.; Nouar, F.; Pimenta, V.; Chang, J.S.; Serre, C. Metal-Organic Frameworks and Water: ‘From Old Enemies to Friends’? Trends Chem. 2020, 2, 990–1003. [Google Scholar] [CrossRef]
- Ka, D.; Jang, S.; Kim, M.-K.; Jung, H.; Lee, J.; Jung, H.; Jin, Y. UiO-66-NH2/graphene oxide nanocomposites as reactive adsorbents for soman upon long-term exposure to high-humidity environment. Mater. Lett. 2021, 285, 129105. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhao, H.F.; Zhao, X.D.; Qu, Y.X.; Liu, D.H. Synergistic effect of electrostatic and coordination interactions for adsorption removal of cephalexin from water using a zirconium-based metal-organic framework. J. Colloid Interface Sci. 2020, 580, 256–263. [Google Scholar] [CrossRef]
- Qiu, J.H.; Feng, Y.; Zhang, X.F.; Jia, M.M.; Yao, J.F. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Yu, K.; Ahmed, I.; Won, D.I.; Lee, W.I.; Ahn, W.S. Highly efficient adsorptive removal of sulfamethoxazole from aqueous solutions by porphyrinic MOF-525 and MOF-545. Chemosphere 2020, 250, 126133. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Xia, J.; Gao, Y.; Yu, G. Tetracycline removal from aqueous solution using zirconium-based metal-organic frameworks (Zr-MOFs) with different pore size and topology: Adsorption isotherm, kinetic and mechanism studies. J. Colloid Interface Sci. 2021, 590, 495–505. [Google Scholar] [CrossRef]
- Moghadam, G.; Abdi, J.; Banisharif, F.; Khataee, A.; Kosari, M. Nanoarchitecturing hybridized metal-organic framework/graphene nanosheet for removal of an organic pollutant. J. Mol. Liq. 2021, 341, 117323. [Google Scholar] [CrossRef]
- Tong, X.F.; Zhang, J.J.; Chen, Q.B.; Liu, H.L. Zeolitic imidazolate framework-8/graphene oxide/magnetic chitosan nanocomposites for efficient removal of Congo red from aqueous solution. New J. Chem. 2021, 45, 19416–19424. [Google Scholar] [CrossRef]
- Lentz, L.; Mayer, D.A.; Dogenski, M.; Ferreira, S.R.S. Hybrid aerogels of sodium alginate/graphene oxide as efficient adsorbents for wastewater treatment. Mater. Chem. Phys. 2022, 283, 125981. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.H.; Li, M.X.; Cui, M.F.; Xu, W.S.; Li, L.B.; Sun, Y.H.; Wang, M.Z.; Zhang, Y.; Chen, K.W. Rapid adsorption of tetracycline in aqueous solution by using MOF-525/graphene oxide composite. Microporous Mesoporous Mater. 2021, 328, 111457. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, C.; Ma, F.; Zhang, X.; Yang, M.; Nian, J.; Liu, L.; Dong, H.; Zhu, L.; Wang, Q.; et al. Growth of a mesoporous Zr-MOF on functionalized graphene oxide as an efficient adsorbent for recovering uranium (VI) from wastewater. Microporous Mesoporous Mater. 2021, 323, 111223. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yang, K.; Luan, X.; Li, M.; Cui, M.; Sun, Y.; Wang, H.; Sun, Q.; Tang, K.; et al. Removal of Methylene Blue from Water by Peach Gum Based Composite Aerogels. J. Polym. Environ. 2021, 29, 1752–1762. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.H.; Du, Q.J.; Wang, Z.H.; Xia, Y.Z.; Yedinak, E.; Lou, J.; Ci, L.J. High performance agar/graphene oxide composite aerogel for methylene blue removal. Carbohydr. Polym. 2017, 155, 345–353. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Pan, J.; Li, Y.H.; Zhang, P.F.; Li, M.X.; Zheng, H.; Zhang, X.P.; Li, H.; Du, Q.J. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.W.; Zhao, M.H.; Luo, H.Y.; Chu, Z.R.; Wang, R.D. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Luo, J.; Yan, S.; Rengel, Z.; Zhang, Z. Interaction of veterinary antibiotic tetracyclines and copper on their fates in water and water hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2014, 280, 389–398. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, G.; Zhang, Y.; Li, D.; Ling, C.; Wang, Q.; Liu, G. Superhigh co-adsorption of tetracycline and copper by the ultrathin g-C3N4 modified graphene oxide hydrogels. J. Hazard. Mater. 2022, 424, 127362. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Chang, T.H.; Young, C.; Lee, M.H.; Salunkhe, R.R.; Alshehri, S.M.; Ahamad, T.; Islam, M.T.; Wu, K.C.W.; Hossain, M.S.A.; Yamauchi, Y.; et al. Synthesis of MOF-525 Derived Nanoporous Carbons with Different Particle Sizes for Supercapacitor Application. Chem.—Asian J. 2017, 12, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.W.; Chang, T.H.; Chou, L.Y.; Hupp, J.T.; Farha, O.K.; Ho, K.C. Porphyrin-based metal-organic framework thin films for electrochemical nitrite detection. Electrochem. Commun. 2015, 58, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, J.; Durance, T.D. Water sorption and physical properties of locust bean gum-pectin-starch composite gel dried using different drying methods. Food Hydrocoll. 2008, 22, 1352–1361. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.Y.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. 2007, 17, 3678–3680. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Usov, P.M.; Zhu, J.; Cai, M.; Alatis, J.; Morris, A.J. Synthesis and Defect Characterization of Phase-Pure Zr-MOFs Based on Meso-tetracarboxyphenylporphyrin. Inorg. Chem. 2019, 58, 5145–5153. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lin, T.R.; Li, S.X.; Chen, X.B.; Que, X.Y.; Sheng, L.; Hu, Y.; Peng, J.; Ma, H.L.; Li, J.Q.; et al. Polyacrylamide/Copper-Alginate Double Network Hydrogel Electrolyte with Excellent Mechanical Properties and Strain-Sensitivity. Macromol. Biosci. 2022, 22, 2100361. [Google Scholar] [CrossRef]
- Boughrara, L.; Sebba, F.Z.; Sebti, H.; Choukchou-Braham, E.; Bounaceur, B.; Kada, S.O.; Zaoui, F. Removal of Zn(II) and Ni(II) heavy metal ions by new alginic acid-ester derivatives materials. Carbohydr. Polym. 2021, 272, 118439. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G.; Sibley, S.D.; Pedersen, J.A. Complexation of the antibiotic tetracycline with humic acid. Chemosphere 2007, 66, 1494–1501. [Google Scholar] [CrossRef]
- Zhang, L.T.; Dan, H.B.; Bukasa, O.T.; Song, L.L.; Liu, Y.; Wang, L.; Li, J.J. Low-Cost Efficient Magnetic Adsorbent for Phosphorus Removal from Water. ACS Omega 2020, 5, 25326–25333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Du, Q.J.; Liu, T.H.; Sun, J.K.; Wang, Y.H.; Wu, S.L.; Wang, Z.H.; Xia, Y.Z.; Xia, L.H. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Song, G.; Li, A.; Wang, J.; Wang, H.; Sun, Y.; Ding, G. Graphene oxide-chitosan composite aerogel for adsorption of methyl orange and methylene blue: Effect of pH in single and binary systems. Colloids Surf. A 2022, 641, 128595. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yang, K.; Li, M.; Luan, X.; Sun, Y.; Wang, H.; Sun, Q.; Tang, K.; Zheng, H.; et al. Adsorption of methylene blue by Nicandra physaloides (L.) Gaertn seed gum/graphene oxide aerogel. Environ. Technol. 2021, 43, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Du, Q.; Li, Q. Adsorption of Congo Red from Aqueous Solutions by Porous Soybean Curd Xerogels. Pol. J. Chem. Technol. 2018, 20, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, Q.; Liu, T.; Qi, Y.; Zhang, P.; Wang, Z.; Xia, Y. Preparation of activated carbon from Enteromorpha prolifera and its use on cationic red X-GRL removal. Appl. Surf. Sci. 2011, 25, 10621–10627. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Zhang, X.; Zheng, H.; Zhang, A.; Chen, T.; Liu, W.; Yu, Y.; Liu, J.; Du, Q.; et al. One-step generation of S and N co-doped reduced graphene oxide for high-efficiency adsorption towards methylene blue. RSC Adv. 2020, 10, 37757–37765. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, M.; Zheng, H.; Du, Q.; Li, H.; Wang, Y.; Wang, D.; Wang, C.; Sui, K.; et al. Preparation of improved gluten material and its adsorption behavior for congo red from aqueous solution. J. Colloid Interface Sci. 2019, 556, 249–257. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Miao, J.; Wang, F.; Chen, Y.; Zhu, Y.; Zhou, Y.; Zhang, S. The adsorption performance of tetracyclines on magnetic graphene oxide: A novel antibiotics absorbent. Appl. Surf. Sci. 2019, 475, 549–558. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.; Zhu, M.; Zheng, Y.-Z.; Tao, X. Highly effective pH-universal removal of tetracycline hydrochloride antibiotics by UiO-66-(COOH)2/GO metal–organic framework composites. J. Solid State Chem. 2020, 284, 121200. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of aqueous pharmaceuticals by magnetically functionalized Zr-MOFs: Adsorption Kinetics, Isotherms, and regeneration. J. Colloid Interface Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Namazi, H. Magnetic alginate/glycodendrimer beads for efficient removal of tetracycline and amoxicillin from aqueous solutions. Int. J. Biol. Macromol. 2022, 205, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Yönten, V.; Sanyürek, N.K.; Kivanç, M.R. A thermodynamic and kinetic approach to adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Vitis vinifera L. Surf. Interfaces 2020, 20, 100529. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Luo, X. Phenolic hydroxyl derived copper alginate microspheres as superior adsorbent for effective adsorption of tetracycline. Int. J. Biol. Macromol. 2019, 136, 445–459. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Cao, J.; Chen, Y.-P.; Li, X.; Xiong, W.-P.; Zhou, Y.-Y.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr(VI) from aqueous solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]

| Kinetic Model | Parameters | Values |
|---|---|---|
| Pseudo-first-order model | k (min−1) | 0.03 |
| qe (mg·g−1) | 335.4 | |
| R2 | 0.940 | |
| Pseudo-second-order model | v0 (mg·g−1·min−1) | 14.31 |
| qe (mg·g−1) | 364.8 | |
| R2 | 0.992 | |
| Elovich model | α | 57.38 |
| β | 0.020 | |
| R2 | 0.975 | |
| Intra-particle diffusion model | k1 (mg·g−1·min−1/2) | 36.47 |
| C1 | −6.685 | |
| R2 | 0.973 | |
| k2 (mg·g−1·min−1/2) | 11.37 | |
| C2 | 162.9 | |
| R2 | 0.974 | |
| k3 (mg·g−1·min−1/2) | 1.896 | |
| C3 | 306.0 | |
| R2 | 0.913 |
| Models | Parameters | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| Langmuir isotherm | qm (mg·g−1) | 430.7 | 472.7 | 501.1 |
| b (L·mg−1) | 0.39 | 0.42 | 0.53 | |
| R2 | 0.977 | 0.983 | 0.992 | |
| Freundlich isotherm | Kf (mg1−1/n L1/n·g−1) | 195.6 | 215.7 | 184.7 |
| n | 4.23 | 4.08 | 4.72 | |
| R2 | 0.919 | 0.923 | 0.901 | |
| Sips isotherm | qm (mg·g−1) | 455.9 | 511.6 | 533.2 |
| bs (mg−1/n·L−1/n) | 0.340 | 0.340 | 0.460 | |
| ns | 0.810 | 0.790 | 0.830 | |
| R2 | 0.977 | 0.988 | 0.996 |
| Adsorbents | Dosage (mg·mL−1) | c0 (mg·mL−1) | qm (mg·g−1) | T (K) | Ref. |
|---|---|---|---|---|---|
| MGO a | 0.067 | 5–50 | 106.6 | 313 | [51] |
| UiO-66-(COOH)2/GO | 0.5 | 10–100 | 165.0 | 298 | [52] |
| Fe3O4@MOF-525 | 0.1 | 10–200 | 277.0 | 328 | [53] |
| Alg/Fe3O4@C@TD b | 1.7 | 20–100 | 476.2 | 298 | [54] |
| MOF-525 | 0.5 | 100–200 | 372 | 303 | [22] |
| MOF-525/GO | 0.5 | 100–200 | 436 | 303 | [22] |
| Alg-Cu@GO@MOF-525 | 0.5 | 80–240 | 533.2 | 318 | This work |
| T (K) | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·mol−1 K−1) |
|---|---|---|---|
| 298 | −4.6 | 31.6 | 121.5 |
| 308 | −5.8 | - | - |
| 318 | −7.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Li, Y.; Du, Q.; Pi, X.; Wang, Y.; Sun, Y.; Wang, M.; Zhang, Y.; Chen, K.; Zhu, J. Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms. Nanomaterials 2022, 12, 2897. https://doi.org/10.3390/nano12172897
Chen B, Li Y, Du Q, Pi X, Wang Y, Sun Y, Wang M, Zhang Y, Chen K, Zhu J. Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms. Nanomaterials. 2022; 12(17):2897. https://doi.org/10.3390/nano12172897
Chicago/Turabian StyleChen, Bing, Yanhui Li, Qiuju Du, Xinxin Pi, Yuqi Wang, Yaohui Sun, Mingzhen Wang, Yang Zhang, Kewei Chen, and Jinke Zhu. 2022. "Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms" Nanomaterials 12, no. 17: 2897. https://doi.org/10.3390/nano12172897
APA StyleChen, B., Li, Y., Du, Q., Pi, X., Wang, Y., Sun, Y., Wang, M., Zhang, Y., Chen, K., & Zhu, J. (2022). Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms. Nanomaterials, 12(17), 2897. https://doi.org/10.3390/nano12172897







