Fabrication of an Efficient N, S Co-Doped WO3 Operated in Wide-Range of Visible-Light for Photoelectrochemical Water Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of S-N-WO3
2.3. Fabrication of Electrodes
2.4. Measurement
3. Results
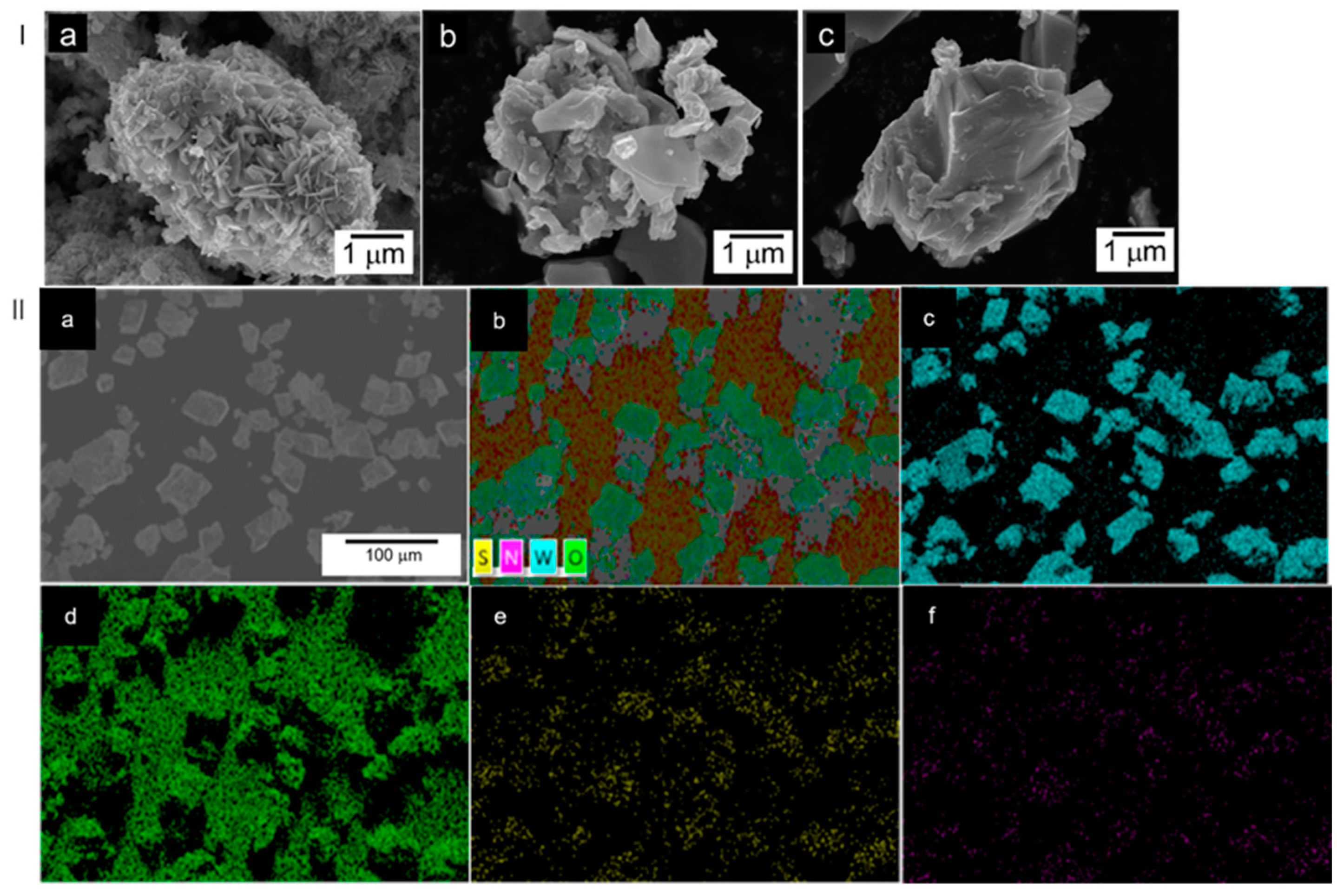
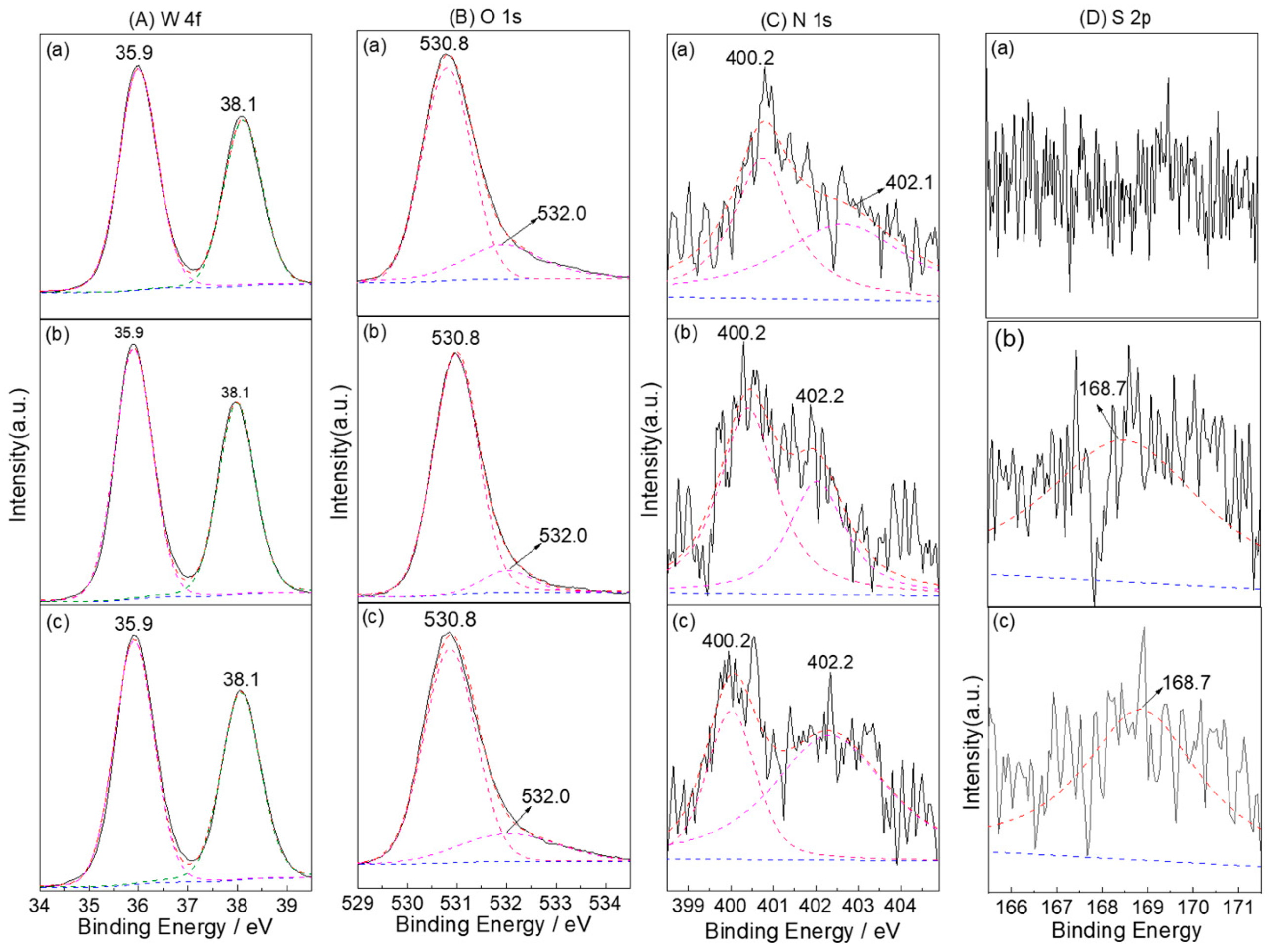
3.1. Characterization Structure of S-N-WO3
3.2. The Optical Properties of S-N-WO3
3.3. Photoelectrocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Tian, W.; Chen, C.; Xu, W.; Li, L. Tungsten Trioxide Nanostructures for Photoelectrochemical Water Splitting: Material Engineering and Charge Carrier Dynamic Manipulation. Adv. Func. Mater. 2019, 29, 1809036. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Non Legacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.; Chorkendorff, I. Towards Practical Implementation. Nat. Mater. 2012, 11, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Shi, C.W.; Zong-Bao, Y.U.; Hong-Da, W.U.; Xiao, W.; Geng, Z.X.; Ren, T.Q.; Han, Q.; Yang, Z.X. Preparation of WS2/C Composite Material and Its Electrocatalytic Hydrogen Evolution Performance. J. Fuel Chem. Technol. 2021, 49, 1362–1370. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Digdaya, I.A.; Adhyaksa, G.W.P.; Trześniewski, B.J.; Garnett, E.C.; Smith, W.A. Interfacial Engineering of Metal-Insulator-Semiconductor Junctions for Efficient and Stable Photoelectrochemical Water Oxidation. Nat. Commun. 2017, 8, 15968–15975. [Google Scholar] [CrossRef]
- Ding, Q.; Gou, L.; Wei, D.; Xu, D.; Fan, W.; Shi, W. Metal-Organic Framework Derived Co3O4/TiO2 Heterostructure Nanoarrays for Promote Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2021, 46, 24965–24976. [Google Scholar] [CrossRef]
- Wang, N.; Wang, D.G.; Li, M.R.; Shi, J.Y.; Li, C. Photoelectrochemical Water Oxidation on Photoanodes Fabricated with Hexagonal Nanoflower and Nanoblock WO3. Nanocsale 2014, 6, 2061–2066. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the Photoelectrochemical Properties of Hematite (α-Fe2O3) Electrodes Using Hydrogen Peroxide as a Hole Scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of an Efficient BaTaO2N Photoanode Harvesting a Wide Range of Visible Light for Water Splitting. J. Am. Chem. Soc. 2013, 135, 10238–10241. [Google Scholar] [CrossRef]
- Li, D.; Takeuchi, R.; Chandra, D.; Saito, K.; Yui, T.; Yagi, M. Visible Light-Driven Water Oxidation on an In Situ N2-intercalated WO3 Nanorod Photoanode Synthesized by a Dual-Functional Structure-Directing Agent. ChemSusChem 2018, 11, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Saito, K.; Yui, T.; Yagi, M. Crystallization of Tungsten Trioxide Having Small Mesopores: Highly Efficient Photoanode for Visible-Light-Driven Water Oxidation. Angew. Chem. Int. Ed. 2013, 52, 12606–12609. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, K.; Cho, S.M.; Lee, T.-W.; Park, J.H. Synthesis of Transparent Mesoporous Tungsten Trioxide Films with Enhanced Photoelectro-Chemical Response: Application to Unassisted Solar Water Splitting. Energy Environ. Sci. 2011, 4, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Santato, C.; Ulmann, M.; Augustynski, J. Photoelectrochemical Properties of Nanostructured Tungsten Trioxide Films. J. Phys. Chem. B 2001, 105, 936–940. [Google Scholar] [CrossRef]
- Hisatomi, T.; Dotan, H.; Stefik, M.; Sivula, K.; Rothschild, A.; Grätzel, M.; Mathews, N. Enhancement in the Performance of Ultrathin Hematite Photoanode for Water Splitting by an Oxide Underlayer. Adv. Mater. 2012, 24, 2699–2702. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Cesar, I.; Grätzel, M. New Benchmark for Water Photooxidation by Nanostructured α-Fe2O3 Films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef] [PubMed]
- Carroll, G.M.; Zhong, D.K.; Gamelin, D.R. Mechanistic Insights into Solar Water Oxidation by Cobalt-Phosphate-Modified α-Fe2O3 Photoanodes. Energy Environ. Sci. 2015, 8, 577–584. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, Y.; Chen, T.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Gao, T.; Sun, X. Enhanced Photoelectrochemical Water Oxidation Performance of Fe2O3 Nanorods Array by S Doping. ACS Sustain. Chem. Eng. 2017, 5, 7502–7506. [Google Scholar] [CrossRef]
- Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Balijepalli, S.K.; Kaciulis, S.; Caneve, L.; Sarto, F.; Sarto, M.S. Synthesis and Characterization of ZnO Nanorods with a Narrow Size Distribution. RSC Adv. 2015, 5, 49861–49870. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Dakshinamurthy, A.C.; Pandey, B.; Roy, S.C.; Sudakar, C. Highly Efficient Photoelectrochemical ZnO and TiO2 Nanorod/Sb2S3 Heterostructured Photoanodes through One Step Thermolysis of Sb-MPA Complex. Sol. Energy 2021, 225, 333–343. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Graetzel, M.; Thimsen, E. Highly Active Oxide Photocathode for Photoelectrochemical Water Reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P. Highly Stable Copper Oxide Composite as an Effective Photocathode for Water Splitting via a Facile Electrochemical Synthesis Strategy. J. Mater. Chem. 2012, 22, 2456–2464. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, C.; Fu, J.; Wang, F.; Li, C.; Kunzelmann, V.F.; Jiang, C.-M.; Nakabayashi, M.; Shibata, N.; Sharp, I.D.; et al. Band Structure Engineering and Defect Control of ta3n5 for Efficient Photoelectrochemical Water Oxidation. Nat. Catal. 2020, 3, 932–940. [Google Scholar] [CrossRef]
- Feng, X.; LaTempa, T.J.; Basham, J.I.; Mor, G.K.; Varghese, O.K.; Grimes, C.A. Ta3N5 Nanotube Arrays for Visible Light Water Photoelectrolysis. Nano Lett. 2010, 10, 948–952. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Template-Free Synthesis of Ta3N5 Nanorod Arrays for Efficient Photoelectrochemical Water Splitting. Chem. Commun. 2013, 49, 3019–3021. [Google Scholar] [CrossRef]
- Hodes, G.; Cahen, D.; Manassen, J. Tungsten Trioxide as a Photoanode for a Photoelectrochemical Cell (PEC). Nature 1976, 260, 312–313. [Google Scholar] [CrossRef]
- Radecka, M.; Sobas, P.; Wierzbicka, M.; Rekas, M. Photoelectrochemical Properties of Undoped and Ti-Doped WO3. Phys. B 2005, 364, 85–92. [Google Scholar] [CrossRef]
- Hameed, A.; Gondal, M.A.; Yamani, Z.H. Effect of Transition Metal Doping on Photocatalytic Activity of WO3 for Water Splitting under Laser Illumination: Role of 3d-Orbitals. Catal. Commun. 2004, 5, 715–719. [Google Scholar] [CrossRef]
- Bär, M.; Weinhardt, L.; Marsen, B.; Cole, B.; Gaillard, N.; Miller, E.; Heske, C. Mo Incorporation in WO3 Thin Film Photoanodes: Tailoring the Electronic Structure for Photoelectrochemical Hydrogen Production. Appl. Phys. Lett. 2010, 96, 032107–032109. [Google Scholar] [CrossRef]
- Liu, H.; Peng, T.; Ke, D.; Peng, Z.; Yan, C. Preparation and Photocatalytic Activity of Dysprosium Doped Tungsten Trioxide Nanoparticles. Mater. Chem. Phys. 2007, 104, 377–383. [Google Scholar] [CrossRef]
- Yang, B.; Luca, V. Enhanced Long-Wavelength Transient Photoresponsiveness of WO3 Induced by Tellurium Doping. Chem. Commun. 2008, 32, 4454–4456. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Duta, A.; Schoonman, J. Influence of Tantalum Dopant Ions (Ta5+) on the Efficiency of the Tungsten Trioxide Photoelectrode. Phys. Status Solidi A 2008, 205, 2038–2041. [Google Scholar] [CrossRef]
- Muthu Karuppasamy, K.; Subrahmanyam, A. Results on the Electrochromic and Photocatalytic Properties of Vanadium Doped Tungsten Oxide Thin Films Prepared by Reactive DC Magnetron Sputtering Technique. J. Phys. D Appl. Phys. 2008, 41, 035302. [Google Scholar] [CrossRef]
- Liew, S.L.; Zhang, Z.; Goh, T.W.G.; Subramanian, G.S.; Seng, H.L.D.; Hor, T.S.A.; Luo, H.K.; Chi, D.Z. Yb-doped WO3 Photocatalysts for Water Oxidation with Visible Light. Int. J. Hydrogen Energy 2014, 39, 4291–4298. [Google Scholar] [CrossRef]
- Chang, X.; Sun, S.; Zhou, Y.; Dong, L.; Yin, Y. Solvothermal Synthesis of Ce-Doped Tungsten Oxide Nanostructures as Visible-Light-Driven Photocatalysts. Nanotechnology 2011, 22, 265603. [Google Scholar] [CrossRef]
- Sun, Y.; Murphy, C.J.; Reyes-Gil, K.R.; Reyes-Garcia, E.A.; Thornton, J.M.; Morris, N.A.; Raftery, D. Photoelectrochemical and Structural Characterization of Carbon-Doped WO3 Films Prepared via Spray Pyrolysis. Int. J. Hydrogen Energy 2009, 34, 8476–8484. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, W.; Han, S.; Liu, C. Photoelectrochemical Properties and Photocatalytic Activity of Nitrogen-Doped Nanoporous WO3 Photoelectrodes under Visible Light. Appl. Surf. Sci. 2012, 258, 5038–5045. [Google Scholar] [CrossRef]
- Cole, B.; Marsen, B.; Miller, E.; Yan, Y.; To, B.; Jones, K.; Al-Jassim, M. Evaluation of Nitrogen Doping of Tungsten Oxide for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2008, 112, 5213–5220. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, X.; Chen, Q. Preparation and Water-Splitting Photocatalytic Behavior of S-Doped WO3. Appl. Surf. Sci. 2012, 263, 157–162. [Google Scholar] [CrossRef]
- Mi, Q.; Ping, Y.; Li, Y.; Cao, B.; Brunschwig, B.S.; Khalifah, P.G.; Galli, G.A.; Gray, H.B.; Lewis, N.S. Thermally Stable N2-Intercalated WO3 Photoanodes for Water Oxidation. J. Am. Chem. Soc. 2012, 134, 18318–18324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Chandra, D.; Takeuchi, R.; Togashi, T.; Kurihara, M.; Saito, K.; Yui, T.; Yagi, M. Dual-Functional Surfactant-Templated Strategy for Synthesis of an In Situ N2-Intercalated Mesoporous WO3 Photoanode for Efficient Visible-Light-Driven Water Oxidation. Chem. Eur. J. 2017, 23, 6596–6604. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Li, Y.; Gygi, F.; Galli, G. Tungsten Oxide Clathrates for Water Oxidation: A First Principles Study. Chem. Mater. 2012, 24, 4252–4260. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Z.; Ban, P.; Xu, X. C, N and S Codoped Rutile TiO2 Nanorods for Enhanced Visible-Light Photocatalytic Activity. Mater. Lett. 2017, 195, 143–146. [Google Scholar] [CrossRef]
- In, S.; Orlov, A.; Berg, R.; García, F.; Pedrosa-Jimenez, S.; Tikhov, M.S.; Wright, D.S.; Lambert, R.M. Effective Visible Light-Activated B-Doped and B,N-Codoped TiO2 Photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, V.; Olivo, A.; Pinna, F.; Signoretto, M.; Vindigni, F.; Cerrato, G.; Bianchi, C.L. C-N/TiO2 Photocatalysts: Effect of Co-doping on the Catalytic Performance under Visible Light. Appl. Catal. B Environ. 2014, 160–161, 152–160. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Chávez-Angulo, G.; Carrasco-Jaim, O.A.; Torres-Martínez, L.M.; Garza-Navarro, M.A. Enhanced Photoelectrochemical Water Splitting on Heterostructured α-Fe2O3-TiO2:X (X = Co, Cu, Bi) Photoanodes: Role of Metal Doping on Charge Carrier Dynamics Improvement. J. Photoch. Photobio A 2021, 410, 113077–113089. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Zhao, X. Preparation, Characterization and Photocatalytic Activity of In Situ N, S-Codoped TiO2 Powders. J. Mol. Catal. A Chem. 2006, 246, 176–184. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N−F−Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Xu, J.-H.; Li, J.; Dai, W.-L.; Cao, Y.; Li, H.; Fan, K. Simple Fabrication of Twist-Like Helix N, S-Codoped Titania Photocatalyst with Visible-Light Response. Appl. Catal. B Environ. 2008, 79, 72–80. [Google Scholar] [CrossRef]
- Garavand, N.T.; Mahdavi, S.M.; Irajizad, A.; Ahadi, K. Synthesis of Sodium Tungsten Oxide Nano-Thick Plates. Mater. Lett. 2012, 82, 214–216. [Google Scholar] [CrossRef]
- Shpak, A.P.; Korduban, A.M.; Medvedskij, M.M.; Kandyba, V.O. XPS Studies of Active Elements Surface of Gas Sensors Based on WO3−x Nanoparticles. J. Electron. Spectrosc. 2007, 156–158, 172–175. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Paraguay-Delgado, F.; Albiter, M.A.; Lara-Romero, J.; Martínez-Sánchez, R. Synthesis and Characterization of WO3 Nanostructures Prepared by an Aged-Hydrothermal Method. Mater. Charact. 2009, 60, 932–937. [Google Scholar] [CrossRef]
- Yang, X.; Wolcott, A.; Wang, G.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-Doped ZnO Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Frey, L.; Schmuki, P. Ion Implantation and Annealing for an Efficient N-Doping of TiO2 Nanotubes. Nano Lett. 2006, 6, 1080–1082. [Google Scholar] [CrossRef]
- Asahi, R.T.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yan, Z.; Xiao, T. Low-Temperature Solvothermal Synthesis of Visible-Light-Responsive S-Doped TiO2 Nanocrystal. Appl. Surf. Sci. 2012, 258, 4016–4022. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lin, Z.S.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Exploration on Anion Ordering, Optical Properties and Electronic Structure in K3WO3F3 Elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative Investigations of the Crystal Structure and Photoluminescence Property of Eulytite-Type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef]
- Ramana, C.V.; Carbajal-Franco, G.; Vemuri, R.S.; Troitskaia, I.B.; Gromilov, S.A.; Atuchin, V.V. Optical Properties and Thermal Stability of Germanium Oxide (GeO2) Nanocrystals with α-Quartz Structure. Mater. Sci. Eng. B 2010, 174, 279–284. [Google Scholar] [CrossRef]

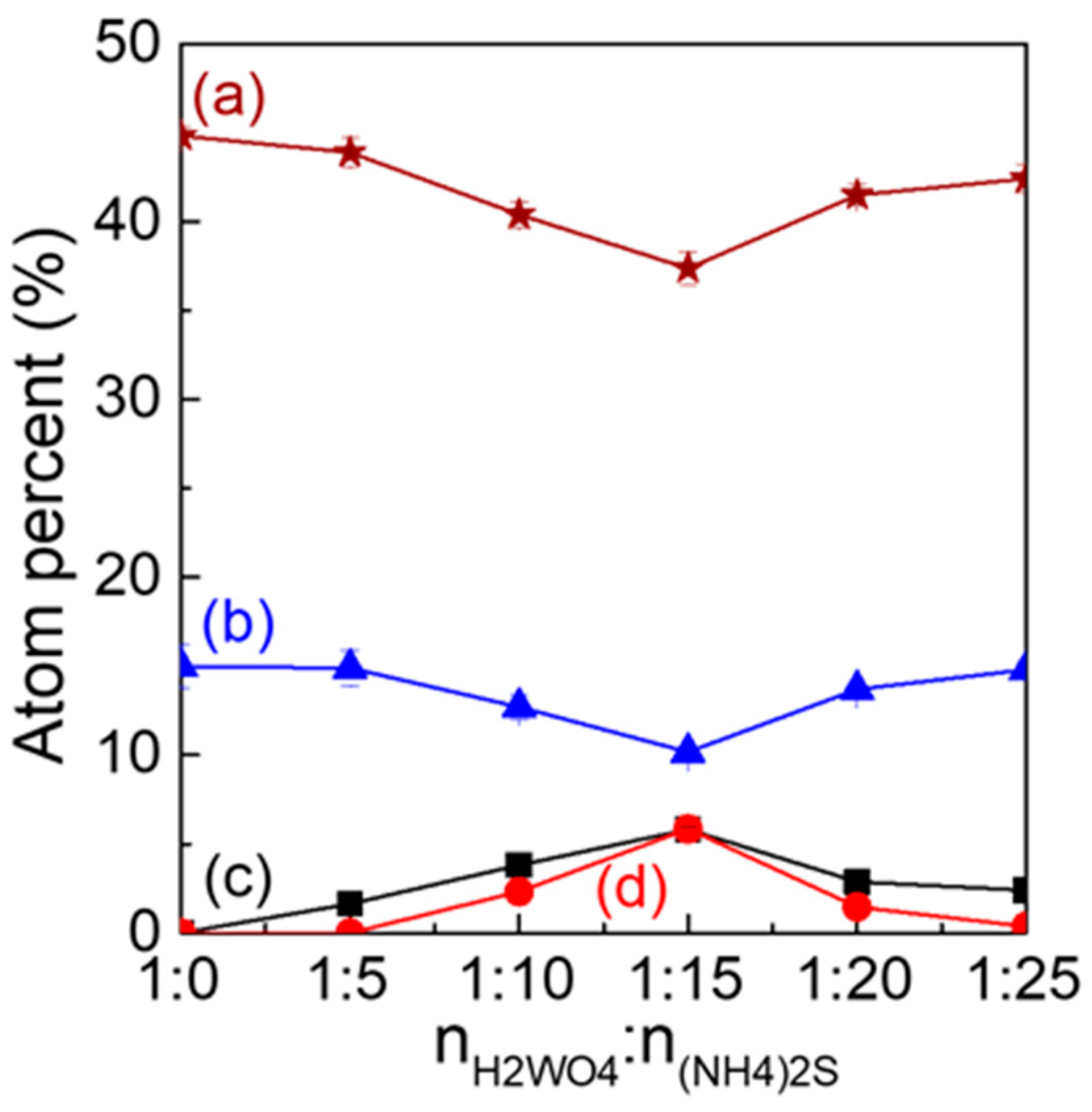
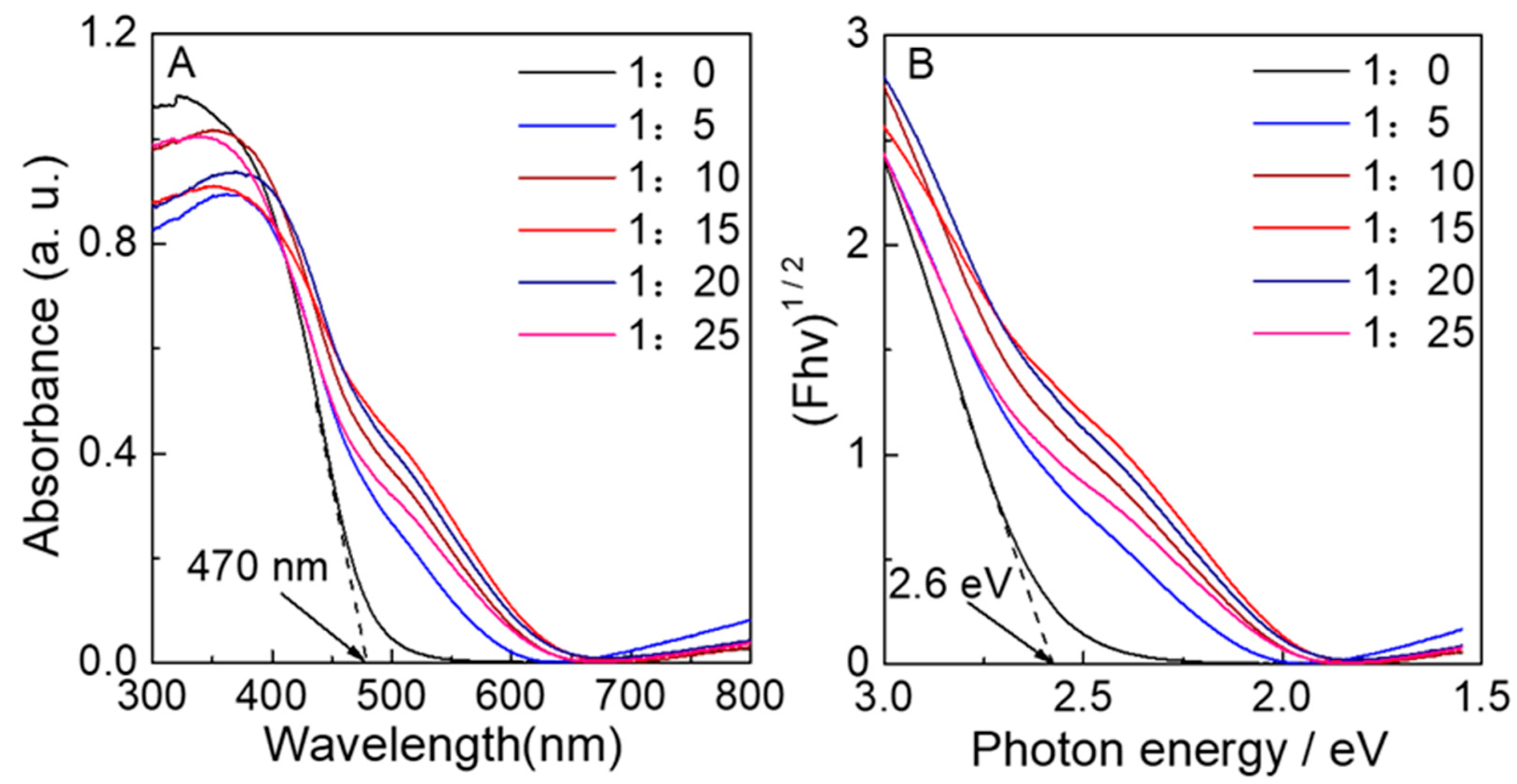
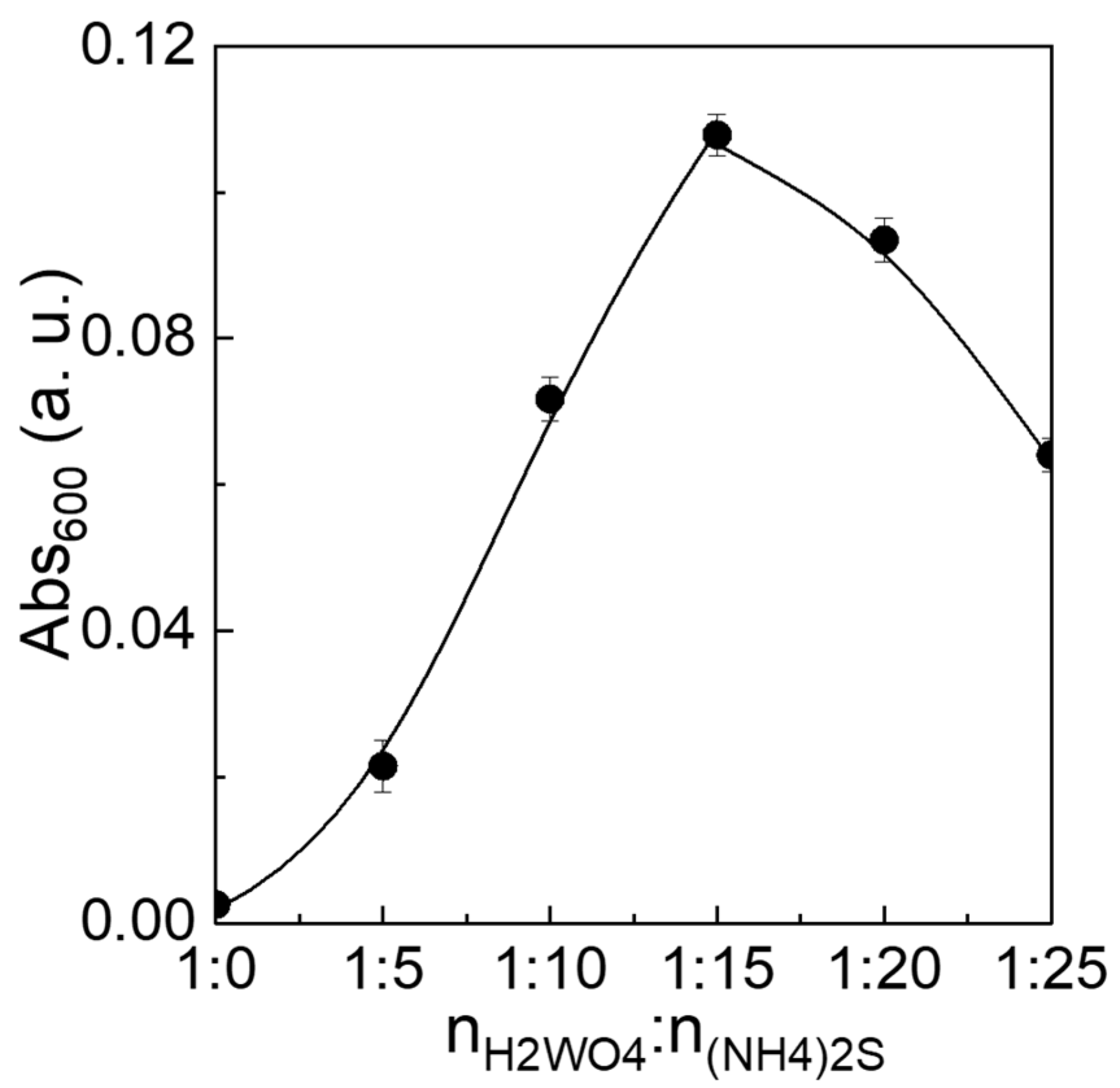
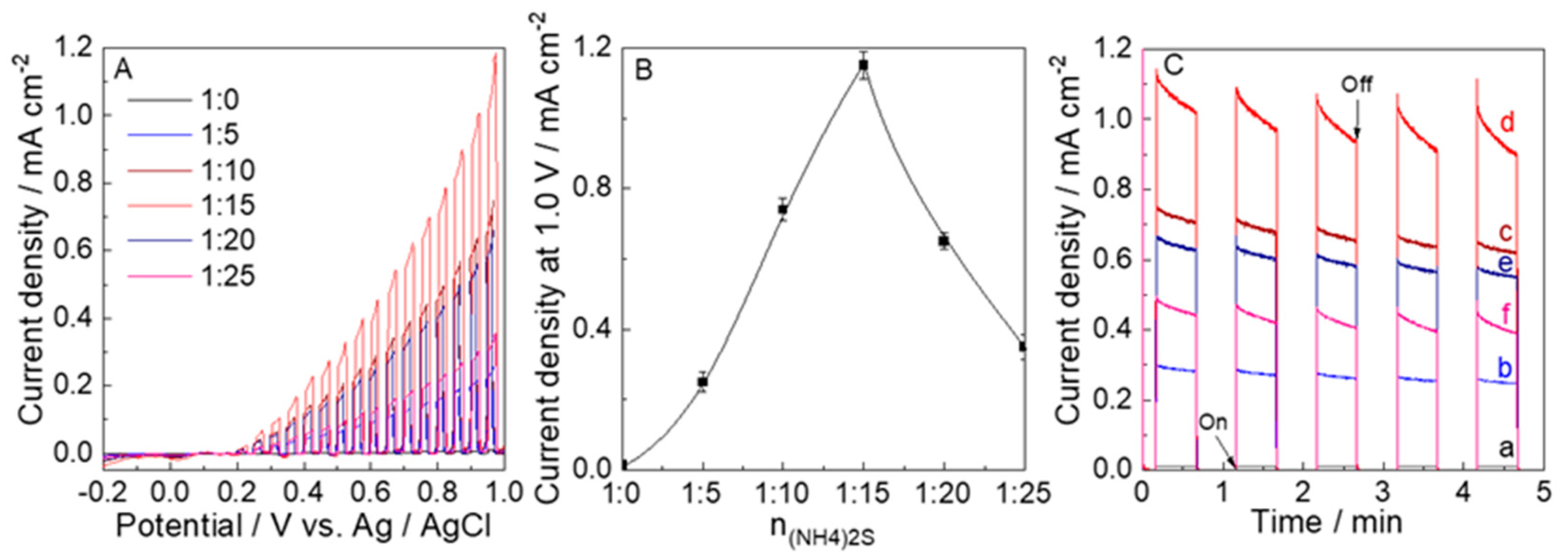
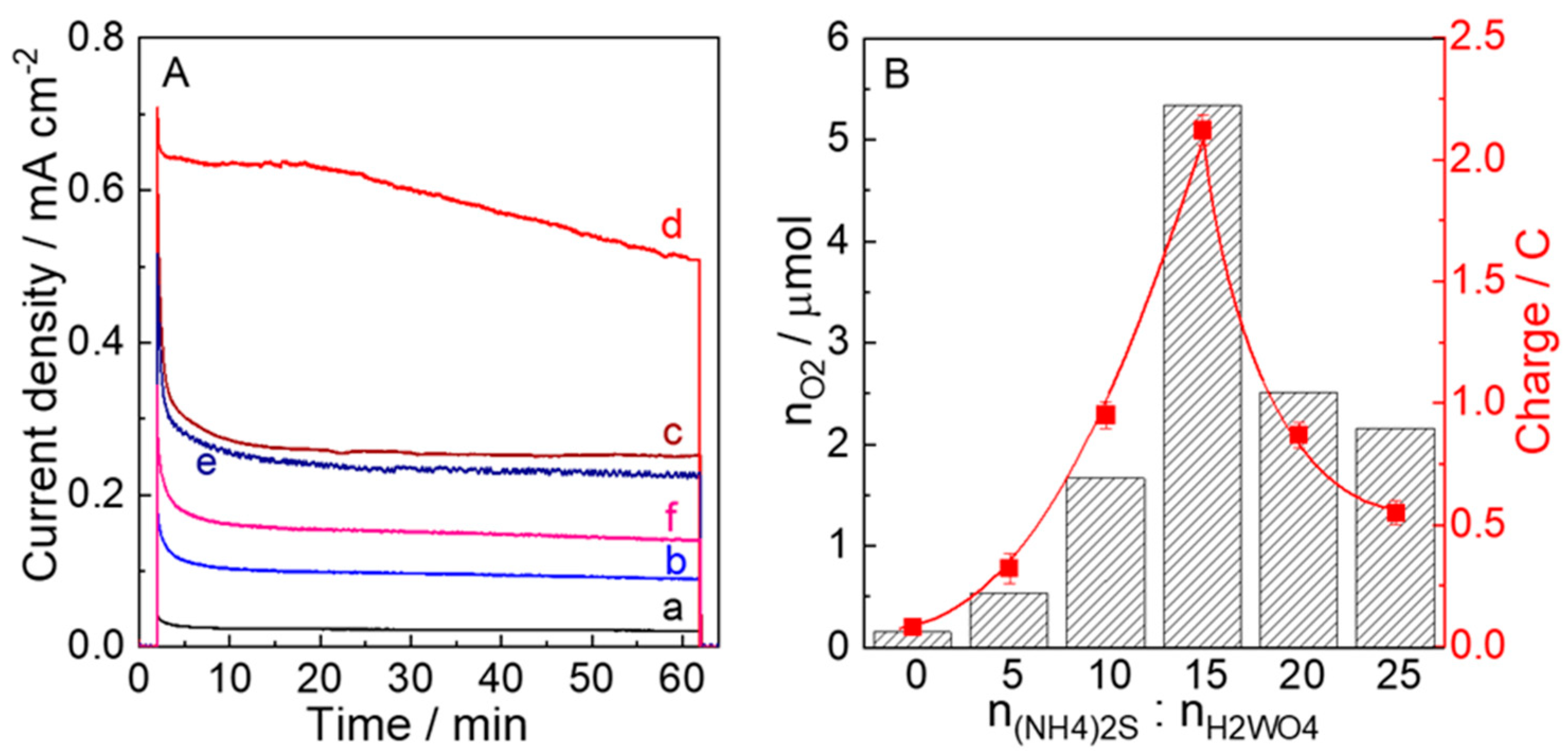

| Samples | nH2WO4:n(NH4)2S | Molar Ratio of nN:nW (a) | Molar Ratio of nS:nW (b) | Absorption Energies (c) (eV) |
|---|---|---|---|---|
| WO3−0 | 1:0 | 1:0 | 1:0 | 2.64, - |
| WO3−5 | 1:5 | 0.19:1 | 1:0 | 2.44, 2.10 |
| WO3−10 | 1:10 | 0.57:1 | 0.05:1 | 2.37, 2.02 |
| WO3−15 | 1:15 | 1.64:1 | 0.19:1 | 2.16, 1.95 |
| WO3−20 | 1:20 | 0.31:1 | 0.07:1 | 2.34, 1.97 |
| WO3−25 | 1:25 | 0.28:1 | 0.04:1 | 2.39, 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wu, F.; Gao, C.; Shen, H.; Han, F.; Han, F.; Chen, Z. Fabrication of an Efficient N, S Co-Doped WO3 Operated in Wide-Range of Visible-Light for Photoelectrochemical Water Oxidation. Nanomaterials 2022, 12, 2079. https://doi.org/10.3390/nano12122079
Li D, Wu F, Gao C, Shen H, Han F, Han F, Chen Z. Fabrication of an Efficient N, S Co-Doped WO3 Operated in Wide-Range of Visible-Light for Photoelectrochemical Water Oxidation. Nanomaterials. 2022; 12(12):2079. https://doi.org/10.3390/nano12122079
Chicago/Turabian StyleLi, Dong, Fachao Wu, Caiyun Gao, Hongfang Shen, Fei Han, Fenglan Han, and Zhanlin Chen. 2022. "Fabrication of an Efficient N, S Co-Doped WO3 Operated in Wide-Range of Visible-Light for Photoelectrochemical Water Oxidation" Nanomaterials 12, no. 12: 2079. https://doi.org/10.3390/nano12122079
APA StyleLi, D., Wu, F., Gao, C., Shen, H., Han, F., Han, F., & Chen, Z. (2022). Fabrication of an Efficient N, S Co-Doped WO3 Operated in Wide-Range of Visible-Light for Photoelectrochemical Water Oxidation. Nanomaterials, 12(12), 2079. https://doi.org/10.3390/nano12122079





