Experimental and Theoretical Investigation of the Thermophysical Properties of Cobalt Oxide (Co3O4) in Distilled Water (DW), Ethylene Glycol (EG), and DW–EG Mixture Nanofluids
Abstract
:1. Introduction
- Visible light is absorbed by transition metals and their oxides with band gaps smaller than 2.5 eV (such as Mn and Co).
- UV light can be transmitted through transition metals and their oxides with band gaps greater than 3.5 eV (e.g., Hf, Zr, Ce, Nd, Er, Dy).
2. Methodology
2.1. Preparation of Nanomaterial Samples
2.2. Characterization of Co3O4 Nanoparticles
2.3. Uncertainty Analysis
3. Theoretical and Experimental Density, Thermal Conductivity, and Viscosity of Nanofluids
3.1. Mathematical Model
3.2. Density, Thermal Conductivity, and Viscosity Measurement of Co3O4/DW, EG, and DW–EG Mixture Nanofluids
4. Results and Discussion
4.1. Experimental Density, Thermal Conductivity, and Viscosity of Co3O4/EG, DW, and EG–DW Mixture Nanofluids
4.1.1. The Experimental Density of Co3O4/EG, DW, and EG–DW Mixture Nanofluids
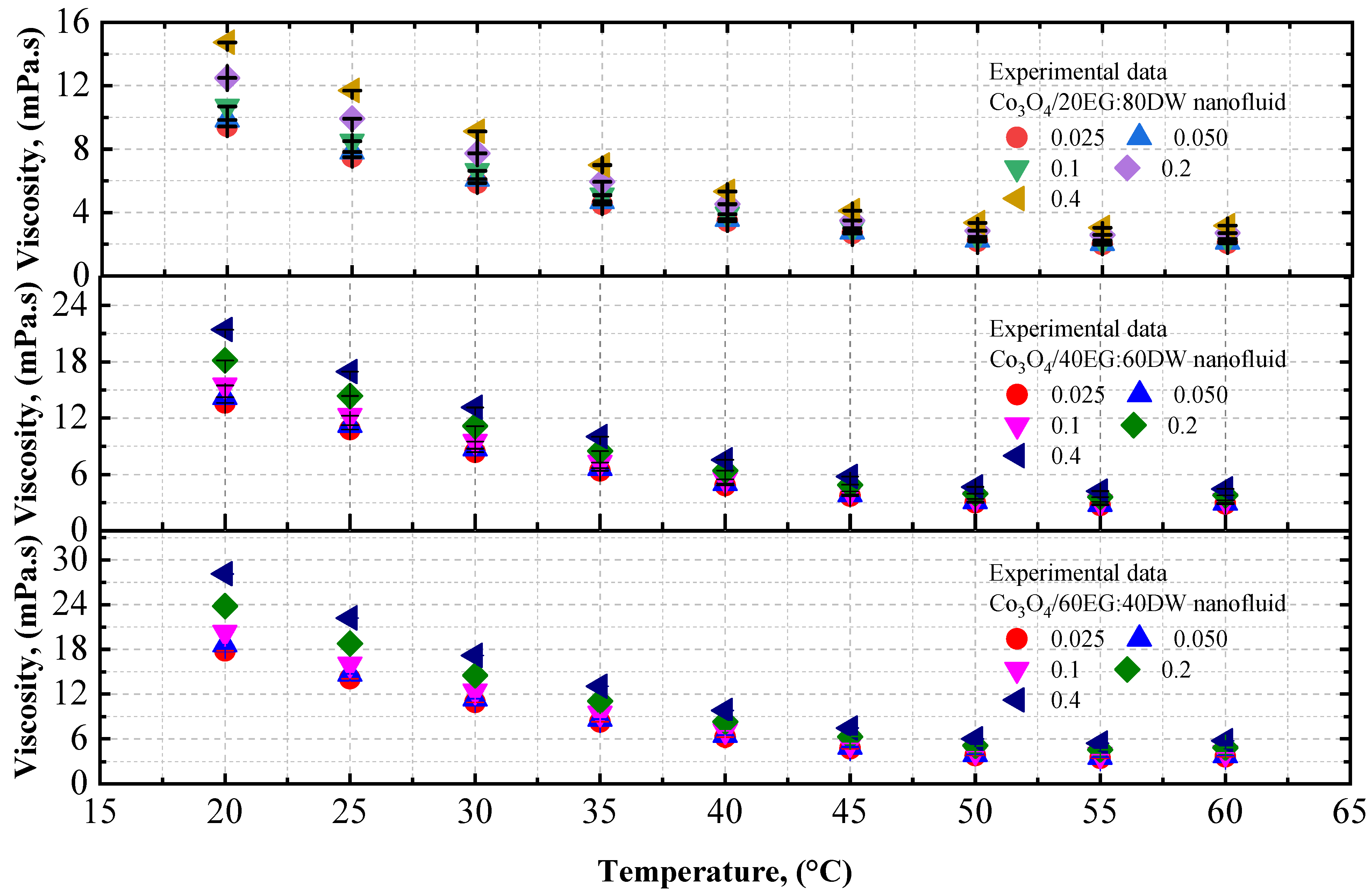
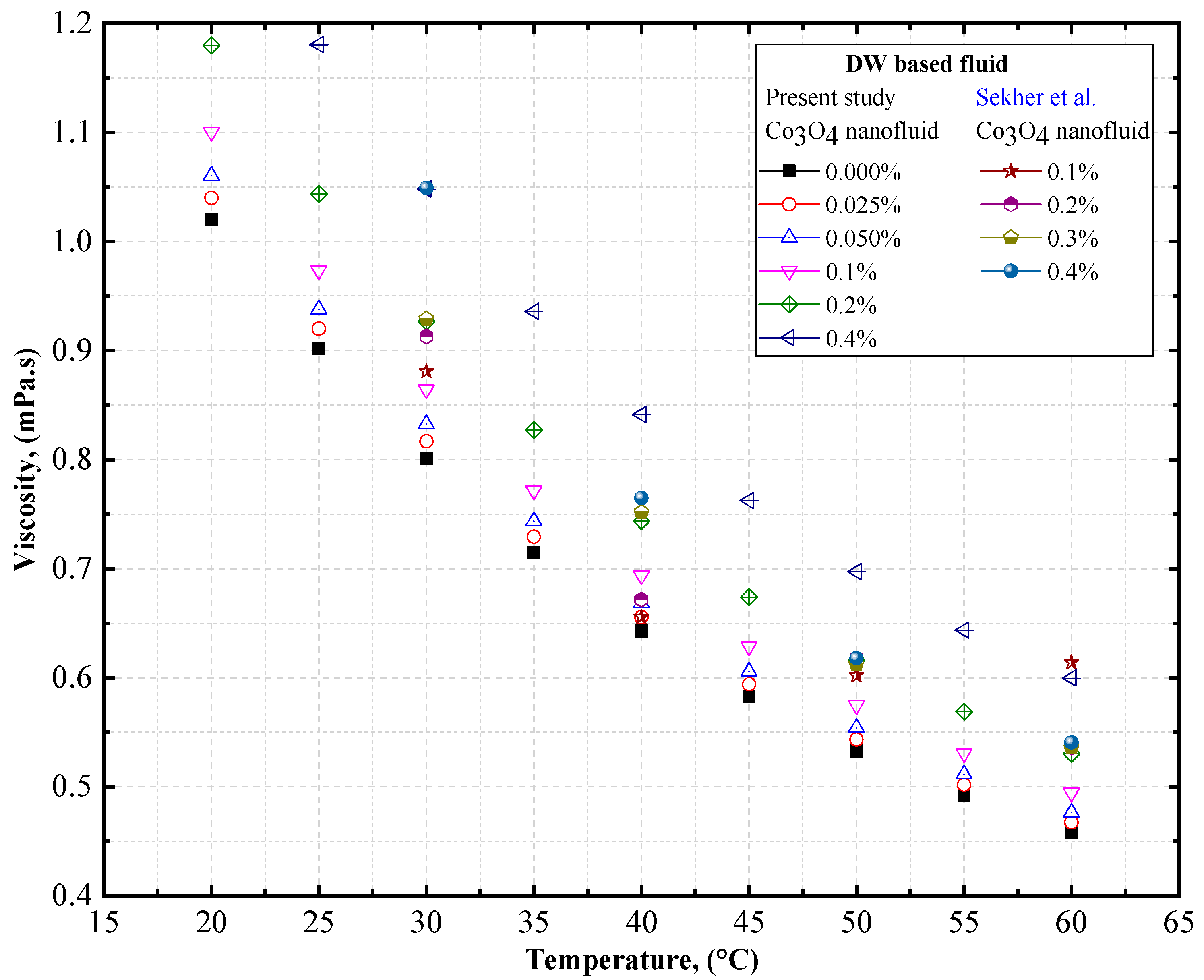
4.1.2. Experimental Viscosity of Co3O4/DW, Co3O4/EG, and Co3O4/EG–DW Mixture Nanofluids
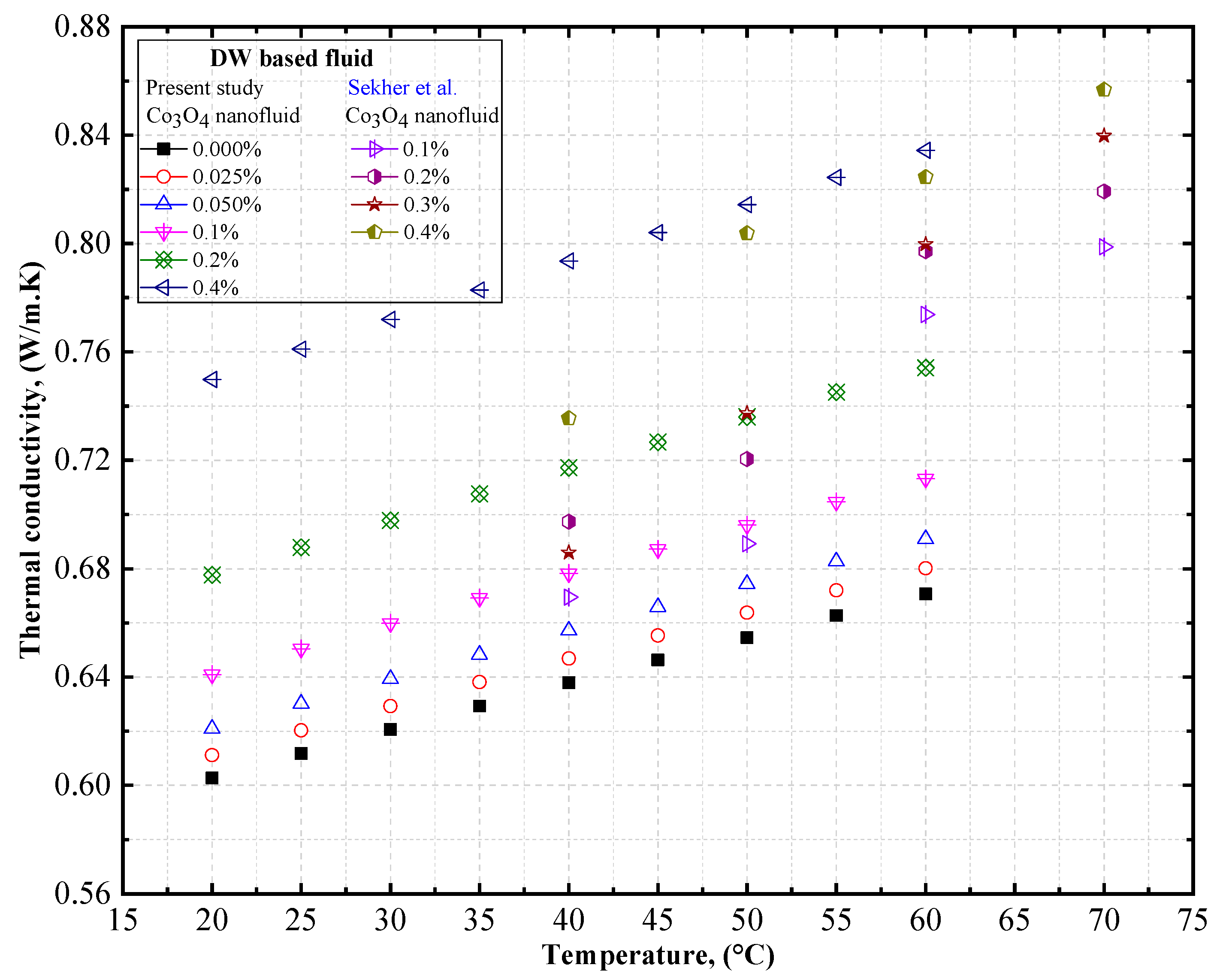
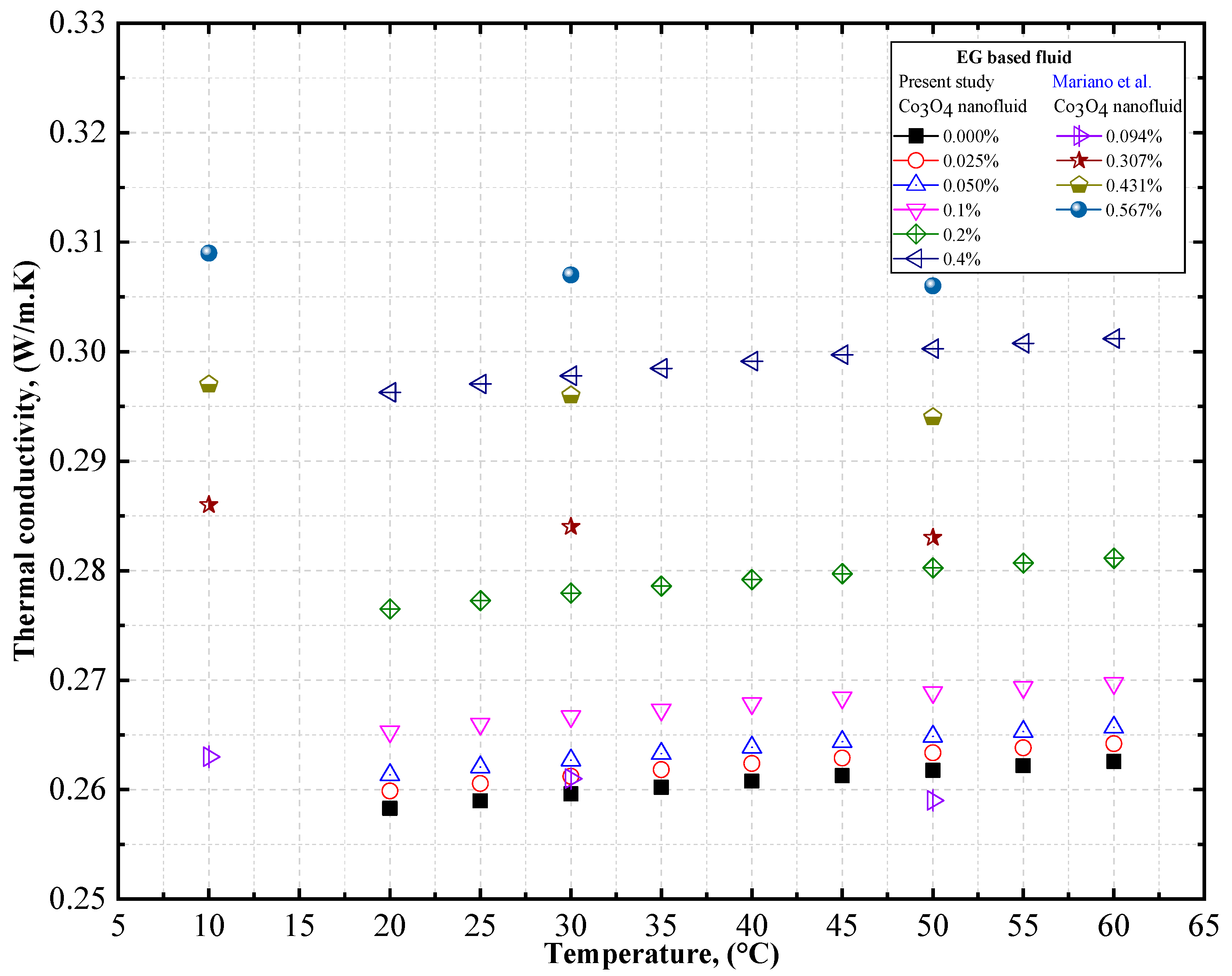
4.1.3. Experimental Thermal Conductivity of Co3O4/EG, Co3O4/DW, and Co3O4/EG–DW Mixture Nanofluids
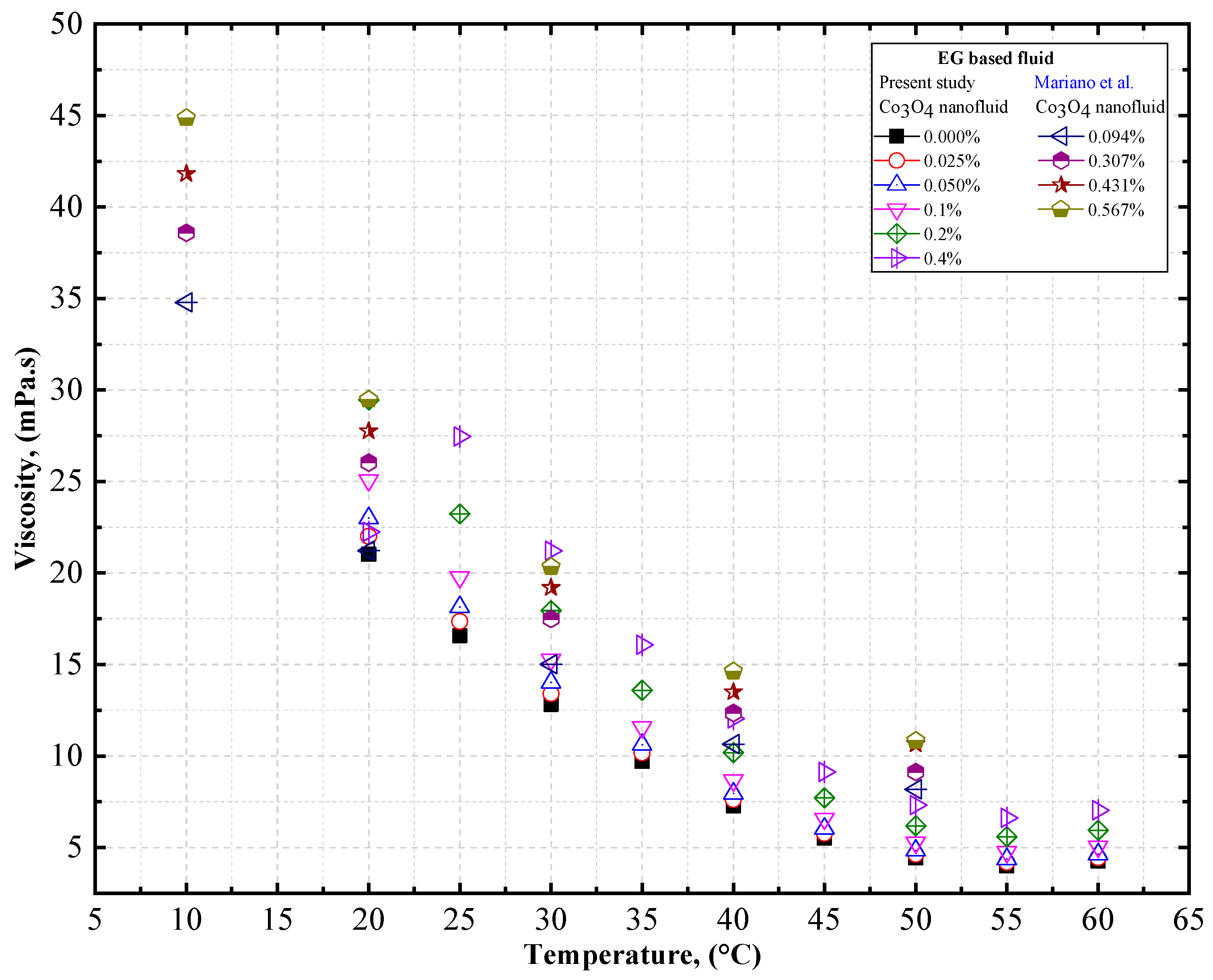
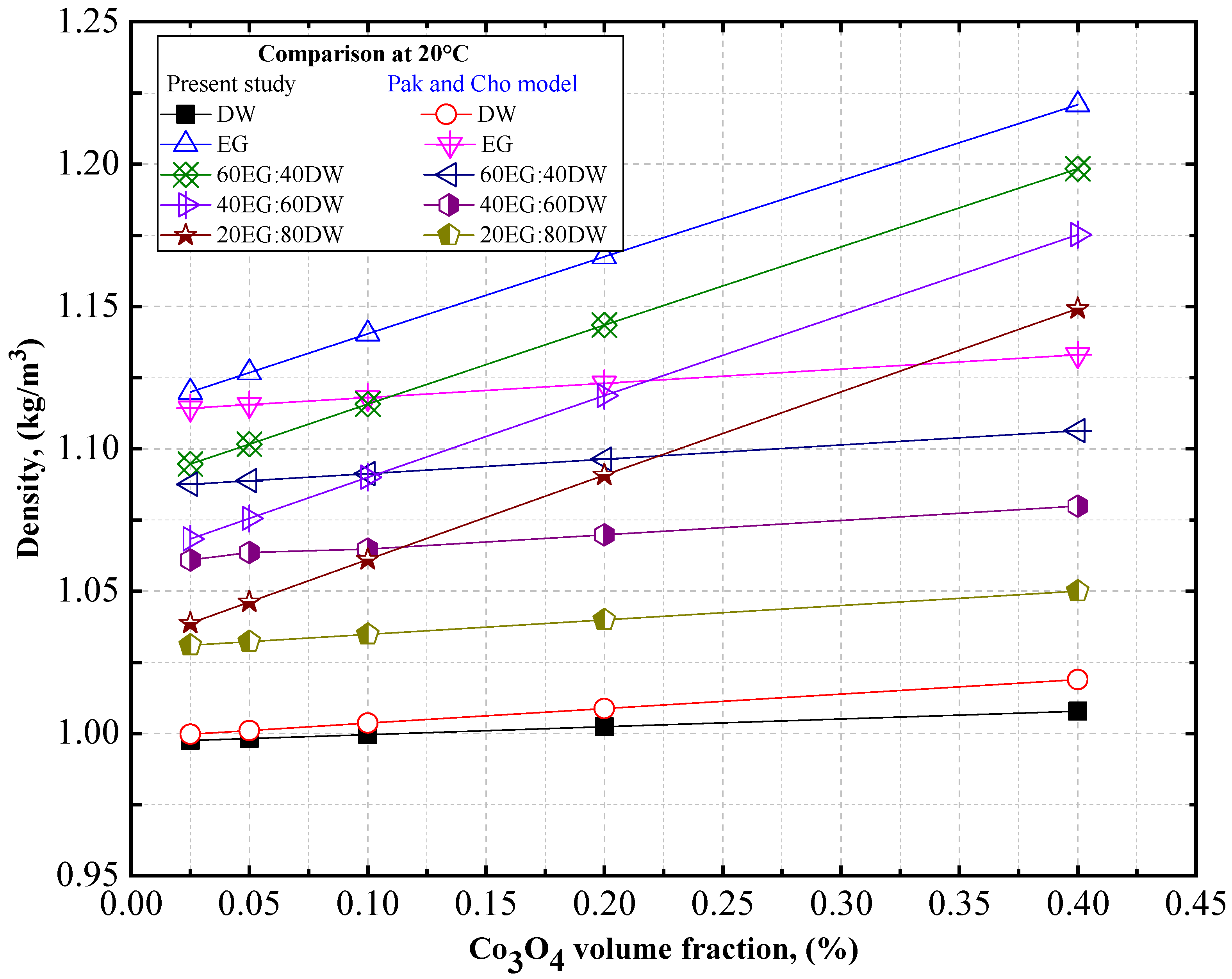
4.2. Experimental Density, Thermal Conductivity, and Viscosity Comparison of Co3O4/DW and Co3O4/EG Nanofluids
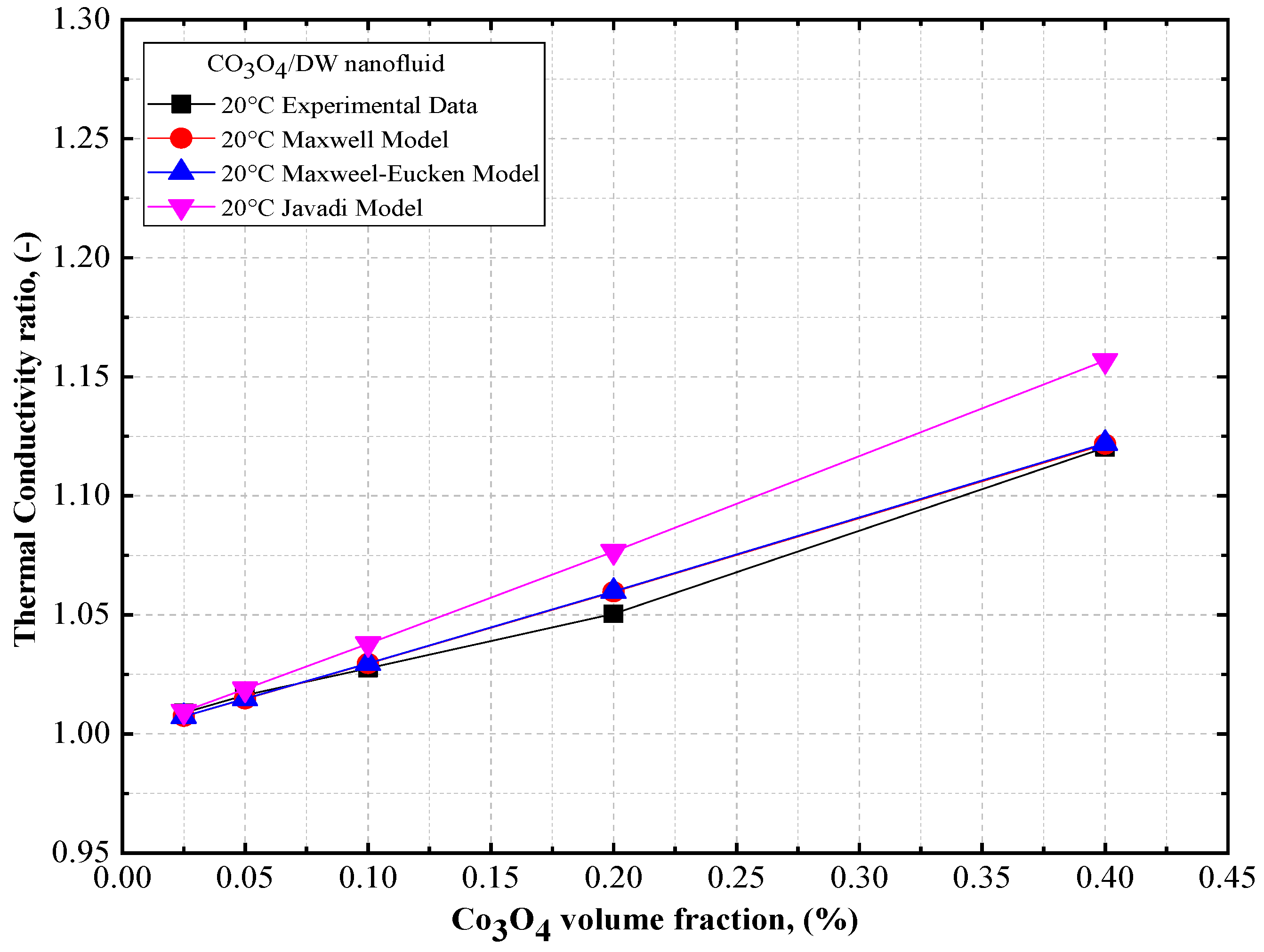
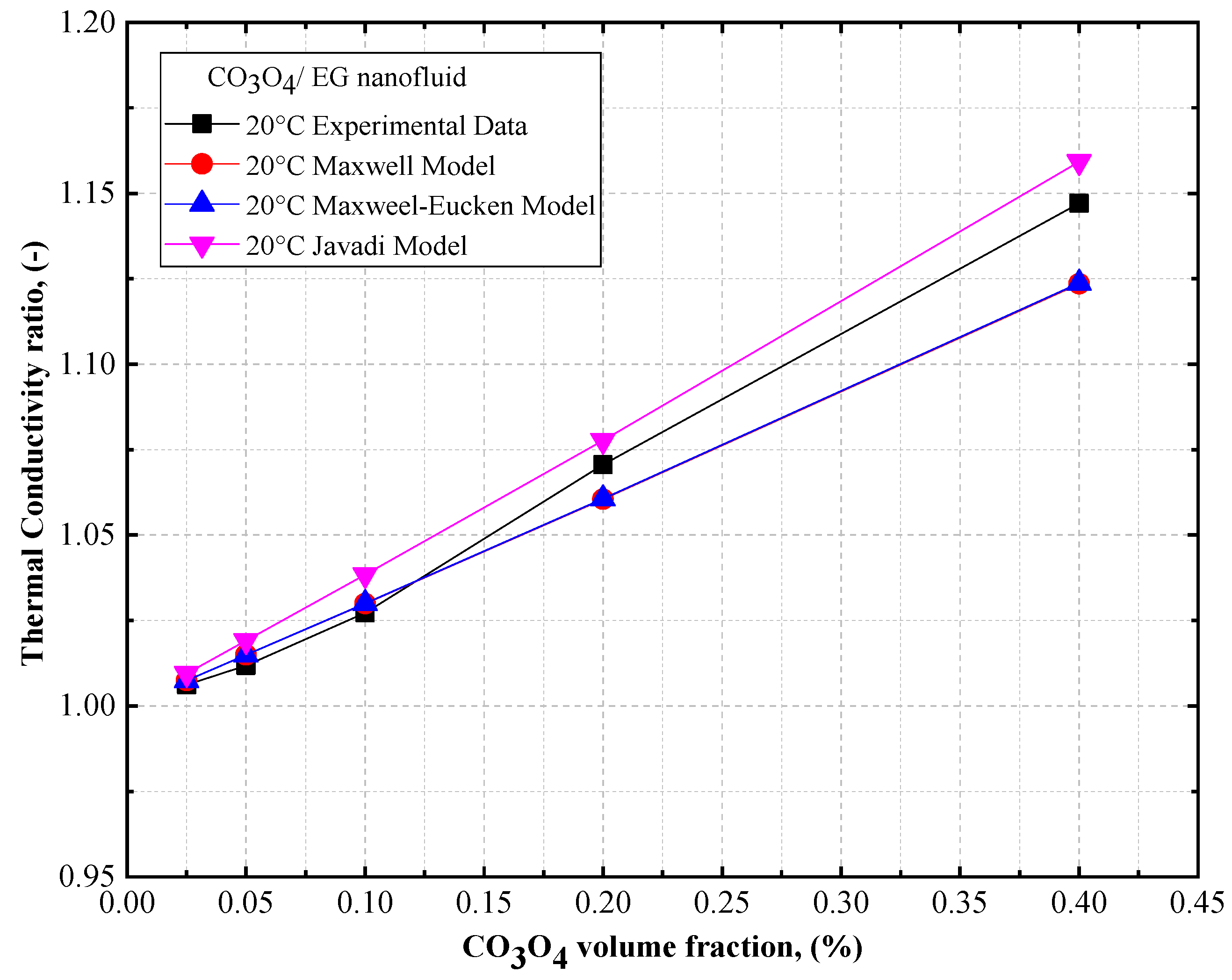
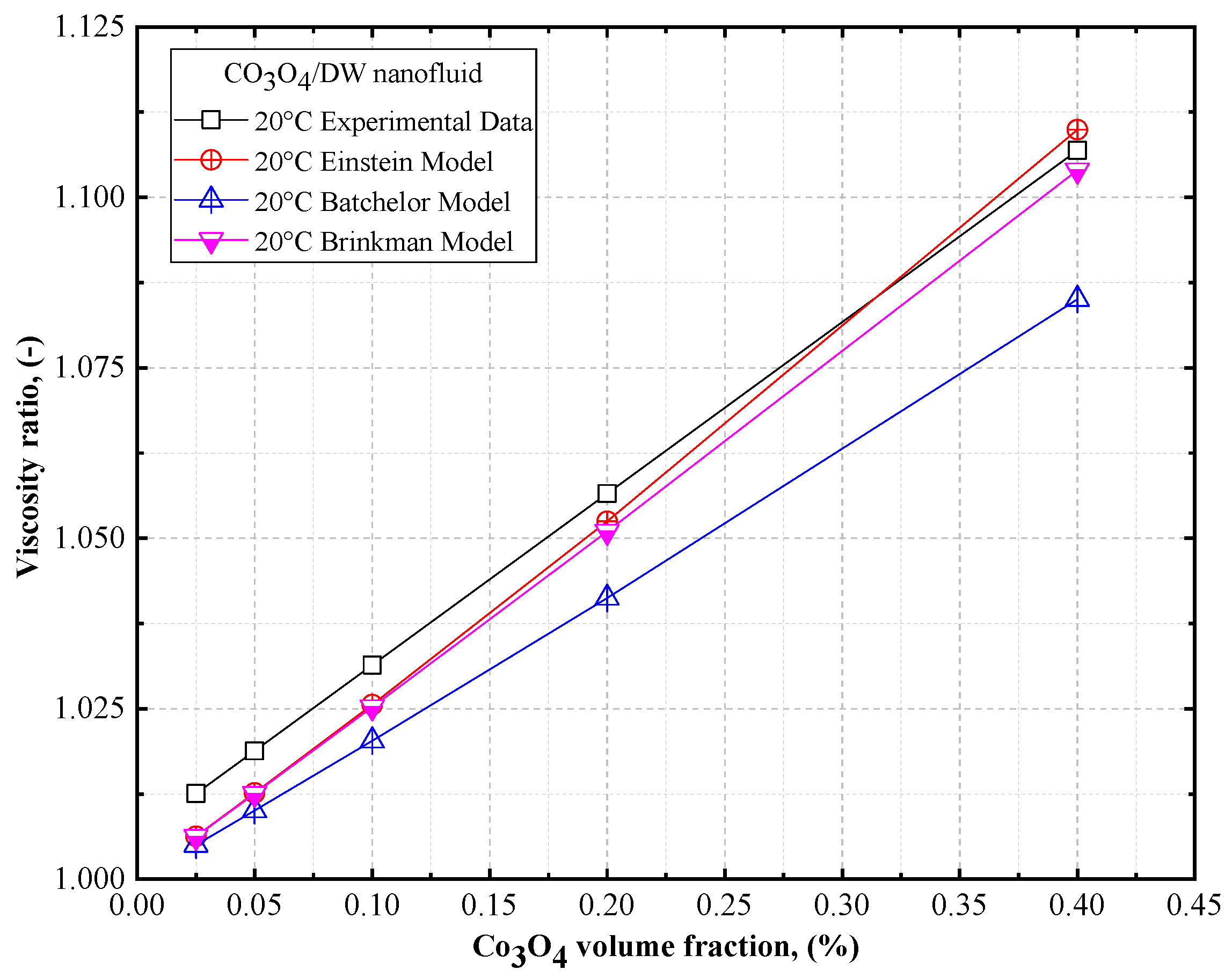
4.3. Comparison of the Theoretical Density, Thermal Conductivity, and Viscosity of Co3O4/DW, Co3O4/EG, Co3O4/60EG:40DW, Co3O4/40EG:60DW, and Co3O4/20EG:80DW Nanofluids
5. Conclusions
- The density of the Co3O4/DW, Co3O4/EG, and Co3O4/DW–EG mixture nanofluids decreased with increasing temperature, whereas it increased with increasing particle volume concentration. The lowest and highest density values were confirmed for the 0.025 vol.% Co3O4/DW nanofluid at 20 °C and the 0.4 vol.% Co3O4/EG60:DW40 at 60 °C, respectively.
- The density enhancement was about 0.73% and 11.48% for the temperatures 20 °C and 60 °C, respectively, compared to the base fluid (EG20:DW80).
- The viscosity of the Co3O4/DW, Co3O4/EG, and Co3O4/EG–DW mixture nanofluids also showed a similar variation trend to that of the density.
- The viscosity enhancement was about 0.70% and 1.61% at temperatures of 20 °C and 60 °C, respectively, compared to the base fluid (EG40:DW60).
- The thermal conductivity of the Co3O4/DW, Co3O4/EG, and Co3O4/DW–EG mixture nanofluids increased with temperature and particle volume concentration. The lowest and highest density values were confirmed for the 0.025 vol.% Co3O4/DW nanofluid and the 0.4 vol.% Co3O4/EG60:DW40 nanofluid, respectively.
- The thermal conductivity of the Co3O4/EG60:DW40 nanofluid (at a 0.4% volume concentrations) was enhanced by about 0.16% and 1.17% at temperatures of 20 °C and 60 °C, respectively, compared to the base fluid (EG60:DW40).
- The maximum thermal conductivity of 10.8% prevailed at 20 °C at the volume fraction of 0.4%, and the maximum viscosity of 7.45% prevailed at 20 °C at the volume fraction of 0.4%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Jiménez-Pérez, J.L.; Cruz-Orea, A.; Sánchez-Ramírez, J.F.; Sánchez-Sinencio, F.; Martínez-Pérez, L.; Muñoz, G.A.L. Thermal Characterization of Nanofluids with Different Solvents. Int. J. Thermophys. 2009, 30, 1227–1233. [Google Scholar] [CrossRef]
- Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S. The effect of particle size on the thermal conductivity of alumina nanofluids. J. Nanoparticle Res. 2009, 11, 1129–1136. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.L.; Pineiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Paul, G.; Pal, T.; Manna, I. Thermo-physical property measurement of nano-gold dispersed water based nanofluids prepared by chemical precipitation technique. J. Colloid Interface Sci. 2010, 349, 434–437. [Google Scholar] [CrossRef]
- Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S. The thermal conductivity of alumina nanofluids in water, ethylene glycol, and ethylene glycol + water mixtures. J. Nanoparticle Res. 2010, 12, 1469–1477. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.D.; Kang, S.; Bang, I.C.; Kim, J.H. Investigation of viscosity and thermal conductivity of SiC nanofluids for heat transfer applications. Int. J. Heat Mass Transf. 2011, 54, 433–438. [Google Scholar] [CrossRef]
- Palabiyik, I.; Musina, Z.; Witharana, S.; Ding, Y. Dispersion stability and thermal conductivity of propyleneglycol based nanofluids. J. Nanopart. Res. 2011, 13, 5049–5055. [Google Scholar] [CrossRef]
- Ruan, B.; Jacobi, A.M. Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res. Lett. 2012, 7, 127. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P. Thermal properties of nanofluids. Adv. Colloid Interface Sci. 2012, 183–184, 30–45. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Thermophysical and electrokinetic properties of nanofluids—A critical review. Appl. Therm. Eng. 2008, 28, 2109–2125. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Christianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-Q.; Alvarado, J.L. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Namburu, P.; Kulkarni, D.; Dandekar, A.; Das, D. Experimental investigation of viscosity and specific heat of silicon dioxide nanofluids. Micro Nano Lett. 2007, 2, 67. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Tan, C. Rheological behaviour of nanofluids. New J. Phys. 2007, 9, 367. [Google Scholar] [CrossRef]
- Chevalier, O.; Tillement, F. Ayela, Rheological properties of nanofluids flowing through microchannels. Appl. Phys. Lett. 2007, 91, 233103. [Google Scholar] [CrossRef]
- Xie, H.; Chen, L.; Wu, Q. Measurements of the viscosity of suspensions (nanofluids) containing nanosized Al2O3 particles. High Temp.-High Press. 2008, 37, 127–135. [Google Scholar]
- Chen, H.; Ding, Y.; Lapkin, A.; Fan, X. Rheological behaviour of ethylene glycol-titanate nanotube nanofluids. J. Nanoparticle Res. 2009, 11, 1513–1520. [Google Scholar] [CrossRef]
- Heine, D.R.; Petersen, M.K.; Grest, G.S. Effect of particle shape and charge on bulk rheology of nanoparticle suspensions. J. Chem. Phys. 2010, 132, 184509. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Rheological non- Newtonian behaviour of ethylene glycol-based Fe2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 560. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Pérez-Rodríguez, M.J.; Gracia-Fernández, M.; Piñeiro, M.M. Study of viscoelastic properties of magnetic nanofluids: An insight into their internal structure. Soft Matt. 2013, 9, 11690–11698. [Google Scholar] [CrossRef]
- Zeroual, S.; Loulijat, H.; Achehal, E.; Estellé, P.; Hasnaoui, A.; Ouaskit, S. Viscosity of Ar-Cu nanofluids by molecular dynamics simulations: Effects of nanoparticle content, temperature and potential interaction. J. Mol. Liq. 2018, 268, 490–496. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Stubbs, L.P.; Ho, F.; Liu, R.; Ship, C.P.; Maguire, J.A.; Hosmane, N.S. Magnetic Nanocomposites: A New Perspective in Catalysis. ChemCatChem 2010, 2, 365–374. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, I.S. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today 2010, 5, 412–434. [Google Scholar] [CrossRef]
- Chen, G.J.; Wang, L.F. Design of magnetic nanoparticles-assisted drug delivery system. Curr. Pharm. Des. 2011, 17, 2331–2351. [Google Scholar] [CrossRef]
- Wan, T.-J.; Shen, S.-M.; Siao, S.-H.; Huang, C.-F.; Cheng, C.-Y. Using magnetic seeds to improve the aggregation and precipitation of nanoparticles from backside grinding wastewater. Water Res. 2011, 45, 6301–6307. [Google Scholar] [CrossRef]
- Kim, Y.C.; Han, S.; Hong, S. A feasibility study of magnetic separation of magnetic nanoparticle for forward osmo-sis. Water Sci. Technol. 2011, 64, 469–476. [Google Scholar] [CrossRef]
- Su, Q.; Yuan, W.; Yao, L.; Wu, Y.; Zhang, J.; Du, G. Microwave-assisted synthesis of Co3O4–graphene sheet-on-sheet nanocomposites and electrochemical performances for lithium ion batteries. Mater. Res. Bull. 2015, 72, 43–49. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Gómez-Villarejo, R.; Aguilar, T.; Hamze, S.; Estellé, P.; Navas, J. Experimental analysis of water-based nanofluids using boron nitride nanotubes with improved thermal properties. J. Mol. Liq. 2019, 277, 93–103. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hayashida, K.; Kozuka, Y.; Horiguchi, A.; Awaga, K.; Bandow, S.; Iijima, S. Preparation and magnetic properties of hollow nano-spheres of cobalt and cobalt oxide: Drastic cooling-field effects on remnant magnetization of antiferromagnet. Appl. Phys. Lett. 2004, 85, 5287–5289. [Google Scholar] [CrossRef]
- Makhlouf, S. Magnetic properties of Co3O4 nanoparticles. J. Magn. Magn. Mater. 2002, 246, 184–190. [Google Scholar] [CrossRef]
- Thota, S.; Kumar, A.; Kumar, J. Optical, electrical and magnetic properties of Co3O4 nanocrystallites obtained by thermal de-composition of sol–gel derived oxalates. Mater. Sci. Eng. B 2009, 164, 30–37. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, J.; Liang, J.; Rao, G.; Li, J.B.; Zhang, J.; Du, Z. Synthesis and magnetic properties of antiferromagnetic Co3O4 nanoparticles. Phys. B Condens. Matter 2008, 403, 3141–3145. [Google Scholar] [CrossRef]
- Vickers, D.; Archer, L.; Floyd-Smith, T. Synthesis and characterization of cubic cobalt oxide nanocomposite fluids. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 348, 39–44. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ghasemi, E.; Fazlali, A.; Henneke, D.E. The effect of nanoparticle concentration on the rheological properties of paraffin-based Co3O4 ferrofluids. J. Nanoparticle Res. 2012, 14, 1–7. [Google Scholar] [CrossRef]
- Moita, A.; Moreira, A.; Pereira, J. Nanofluids for the next generation thermal management of electronics: A re-view. Symmetry 2021, 13, 1362. [Google Scholar] [CrossRef]
- Escher, W.; Brunschwiler, T.; Shalkevich, N.; Burgi, T.; Michel, B.; Poulikakos, D. On the Cooling of Electronics With Nanofluids. J. Heat Transf. 2011, 133, 051401. [Google Scholar] [CrossRef]
- Hatami, N.; Banari, A.K.; Malekzadeh, A.; Pouranfard, A. The effect of magnetic field on nanofluids heat transfer through a uniformly heated horizontal tube. Phys. Lett. A 2017, 381, 510–515. [Google Scholar] [CrossRef]
- Ahmad, S.; Saidur, R.; Mahbubul, I.; Al-Sulaiman, F. Optical properties of various nanofluids used in solar collector: A review. Renew. Sustain. Energy Rev. 2017, 73, 1014–1030. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Ferro, M.; Sousa, A.C. Experimental investigation of the thermal transport properties of graphene oxide/Co3O4 hybrid nanofluids. Int. Commun. Heat Mass Transf. 2017, 84, 1–10. [Google Scholar] [CrossRef]
- Sun, T.; Teja, A.S. Density, Viscosity, and Thermal Conductivity of Aqueous Ethylene, Diethylene, and Triethylene Glycol Mixtures between 290 K and 450 K. J. Chem. Eng. Data 2003, 48, 198–202. [Google Scholar] [CrossRef]
- Barai, D.P.; Bhanvase, B.A.; Żyła, G. Experimental Investigation of Thermal Conductivity of Water-Based Fe3O4 Nanofluid: An Effect of Ultrasonication Time. Nanomaterials 2022, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, A.M.; Baboli, Z.N.; Zamanizadeh, M.; Zareh, M. Experimental study on the thermal performance of mechanical cooling tower with rotational splash type packing. Energy Convers. Manag. 2014, 87, 530–538. [Google Scholar] [CrossRef]
- Askari, S.; Lotfi, R.; Seifkordi, A.; Rashidi, A.; Koolivand, H. A novel approach for energy and water conservation in wet cooling towers by using MWNTs and nanoporous graphene nanofluids. Energy Convers. Manag. 2016, 109, 10–18. [Google Scholar] [CrossRef]
- Vincely, D.A.; Natarajan, E. Experimental investigation of the solar FPC performance using graphene oxide nanofluid under forced circulation. Energy Convers. Manag. 2016, 117, 1–11. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Maxwell, J.C. Treatise on Electricity and Magnetism, 2nd ed.; Clarendon Press: Oxford, UK, 1881. [Google Scholar]
- Turian, R.M.; Sung, D.J.; Hsu, F.L. Thermal conductivity of granular coals, coal-water mixtures and mul-ti-solid/liquid suspensions. Fuel 1991, 70, 1157–1172. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Maxwell Model. J. Nanoparticle Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Einstein, A. Eine Neue Bestimmung der Moleküldimensionen. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 1905. [Google Scholar]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Enhancement of thermal conductivity and volumetric behavior of FexOy nanofluids. J. Appl. Phys. 2011, 110, 014309. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Pastoriza-Gallego, M.; Piñeiro, M.; Legido, J.; Lugo, L. Thermophysical properties of (diphenyl ether+biphenyl) mixtures for their use as heat transfer fluids. J. Chem. Thermodyn. 2012, 50, 80–88. [Google Scholar] [CrossRef]
- Sharma, K.V.; Sarma, P.K.; Azmi, W.H.; Mamat, R.; Kadirgama, K. Correlations to predict friction and forced convection heat transfer coefficients of water based nanofluids for turbulent flow in a tube. Int. J. Microscale Nanoscale Therm. Fluid Transp. Phenom. 2012, 3, 1–25. [Google Scholar]
- Sekhar, T.; Nandan, G.; Prakash, R.; Muthuraman, M. Investigations on Viscosity and Thermal Conductivity of Cobalt oxide- water Nano fluid. Mater. Today Proc. 2018, 5, 6176–6182. [Google Scholar] [CrossRef]
- Mariano, A.; Pastoriza-Gallego, M.J.; Lugo, L.; Mussari, L.; Piñeiro, M.M. Co3O4 ethylene glycol-based nanofluids: Thermal conductivity, viscosity and high pressure density. Int. J. Heat Mass Transf. 2015, 85, 54–60. [Google Scholar] [CrossRef]
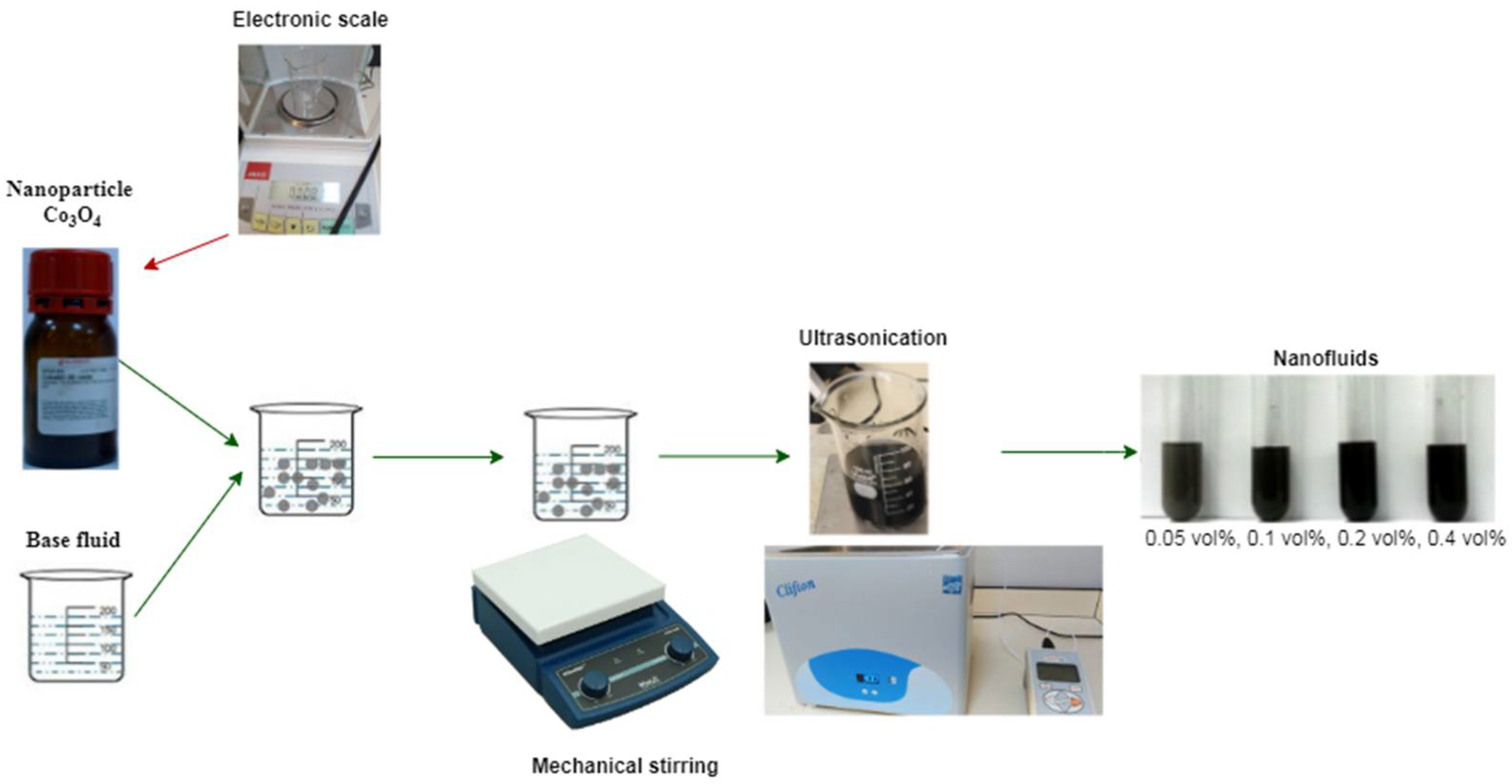
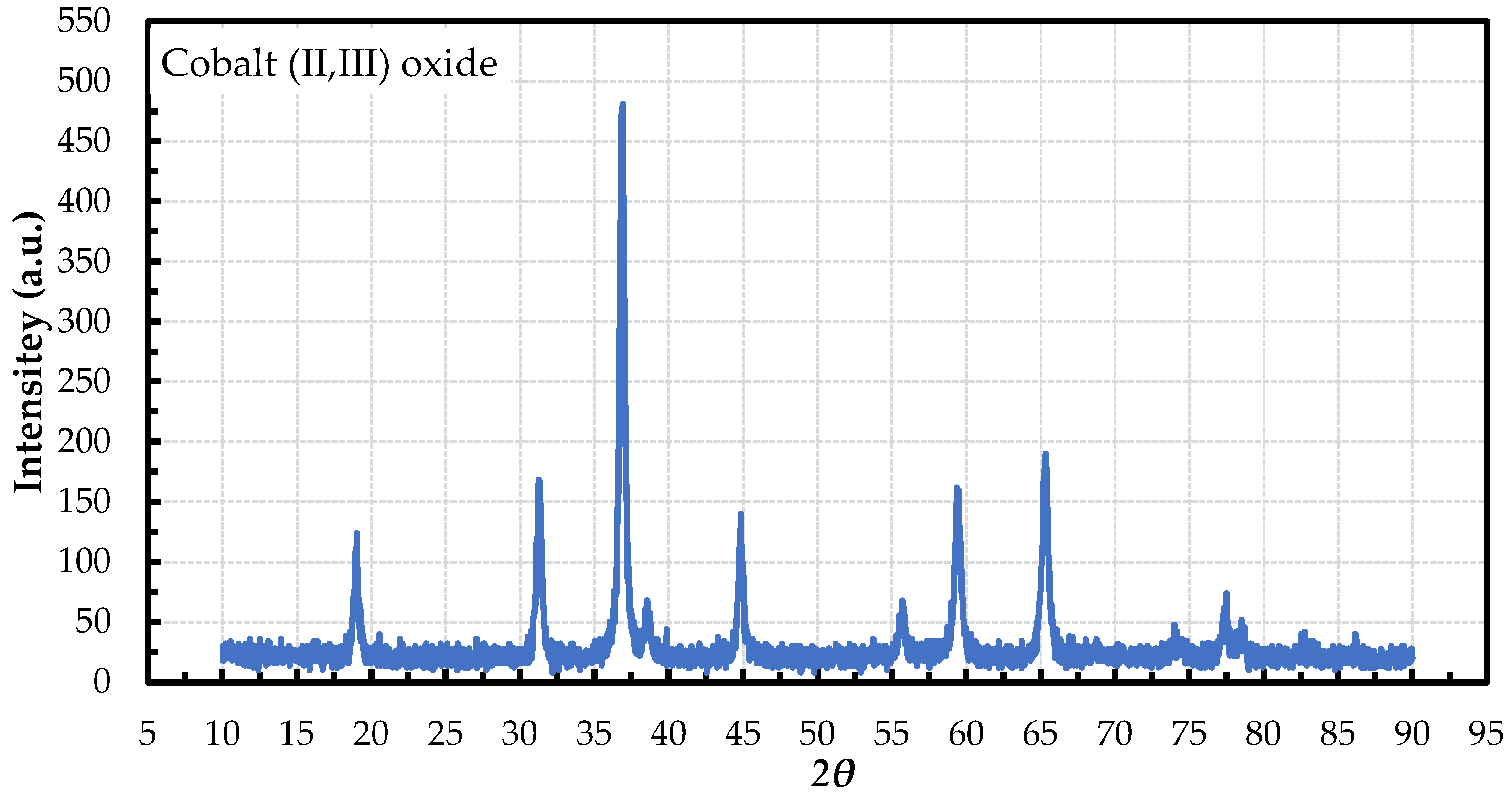

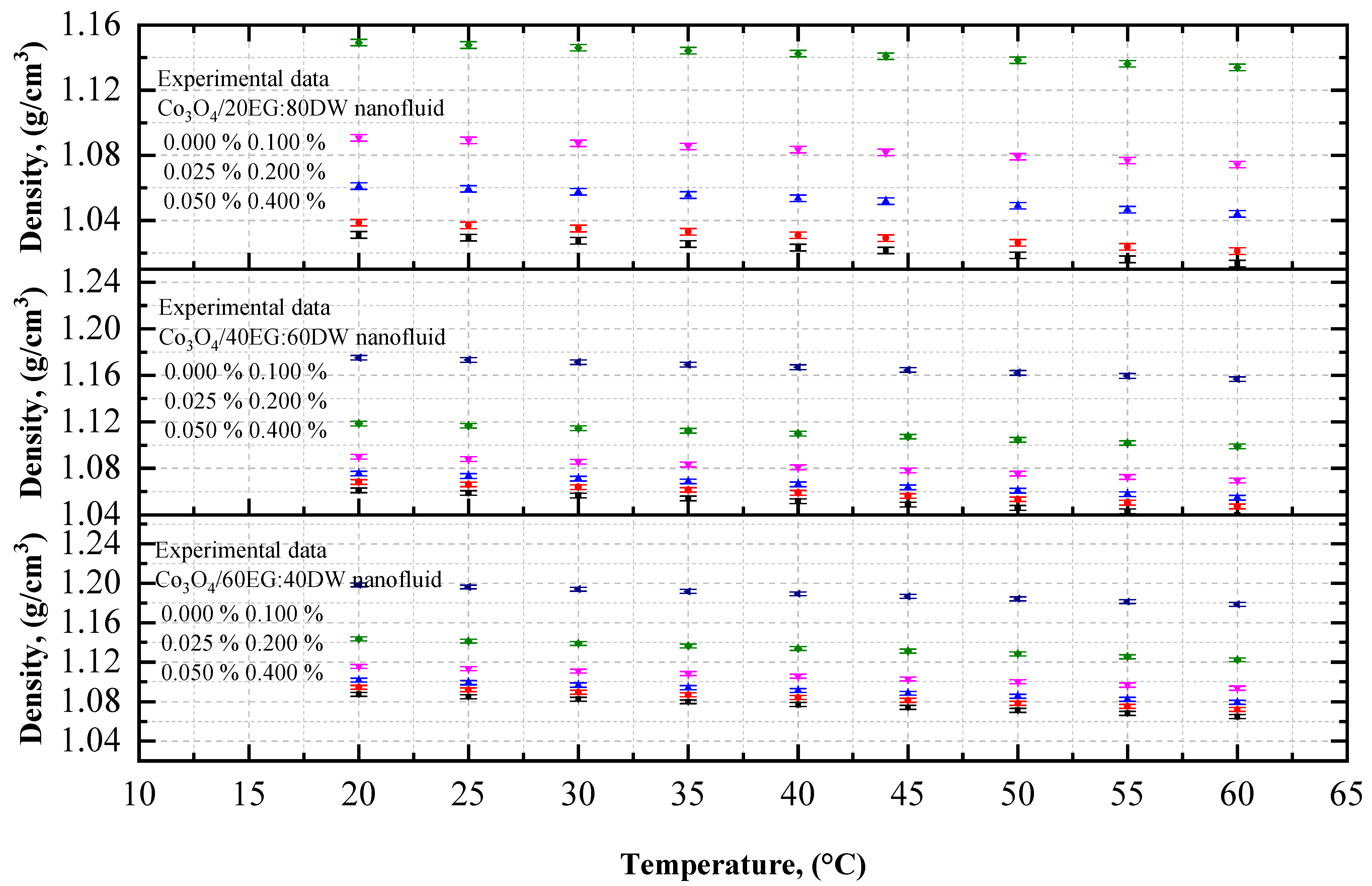
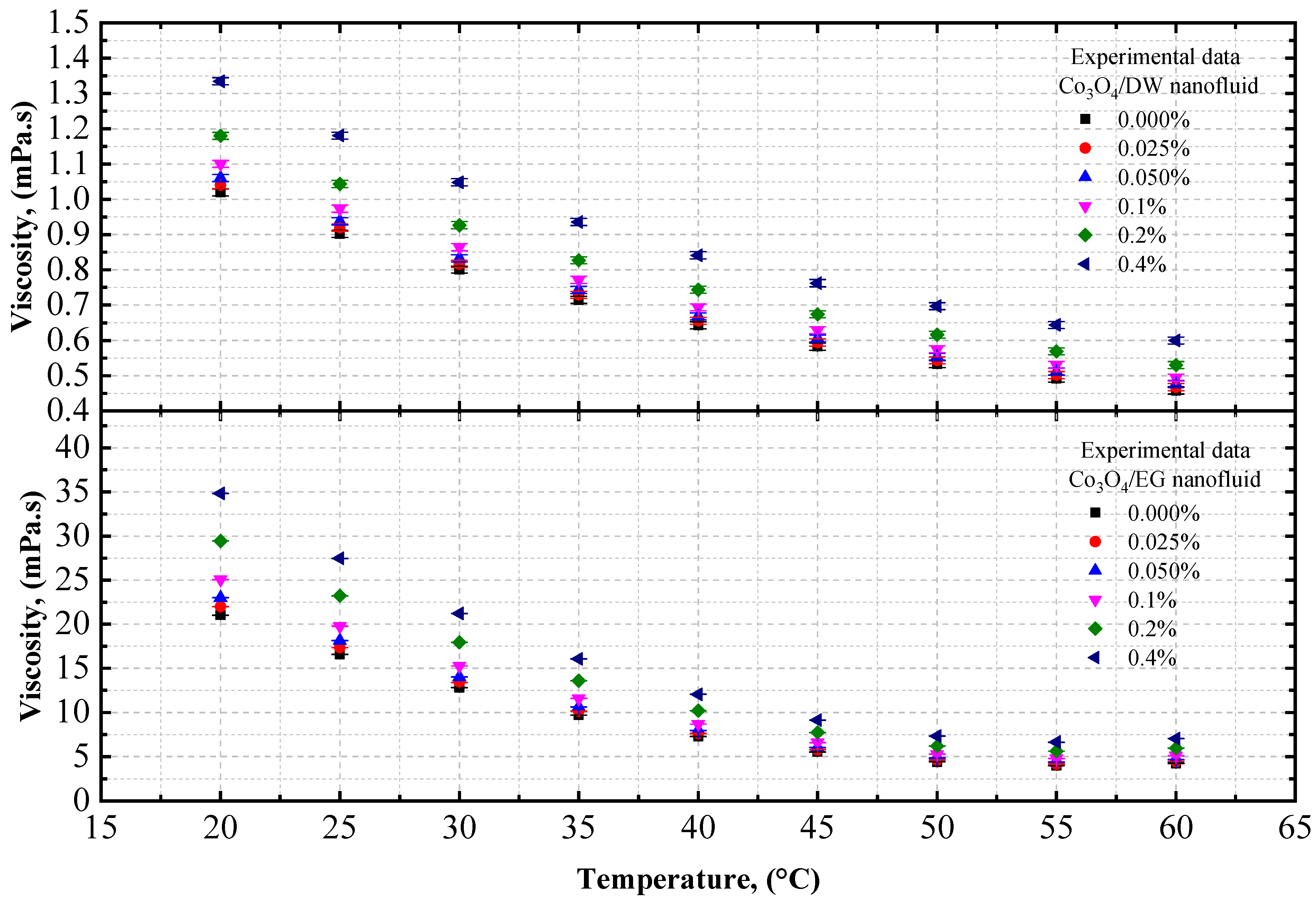



| Characteristics | Co3O4 | EG | DW | 60EG:40DW | 40EG:60DW | 20EG:80DW |
|---|---|---|---|---|---|---|
| Purity (%) | 99.5 | 99 | (-) | (-) | (-) | (-) |
| Black | color | (-) | (-) | (-) | (-) | (-) |
| Particle size measurement (nm) | ≤50 | (-) | (-) | (-) | (-) | (-) |
| Density (g/cm3) | 6.11 | 1.113 | 0.9985 | 1.08627 | 1.05968 | 1.02972 |
| Viscosity (mPa.s) | (-) | 21 | 0.89 | 5.38 | 2.96 | 1.65 |
| Thermal conductivity (W/mK) | 69 | 0.258 | 0.602 | 0.334 | 0.404 | 0.492 |
| ρ | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|
| 1 | 1127.68 | −0.65816 | −6.1765 × 10−4 | 0.30590 | 0.13781 | −1.8961 × 10−3 |
| 2 | 1132.35 | −0.67950 | −4.7565 × 10−4 | 0.90820 | −0.26348 | −3.3787 × 10−3 |
| 3 | 1139.48 | 0.71040 | −4.3663 × 10−4 | 1.1712 | −0.52694 | −3.8797 × 10−3 |
| , (%) | Mass of Co3O4 Required (g) for Several Base Fluids | ||||
|---|---|---|---|---|---|
| EG | DW | 60EG:40W | 40EG:60W | 20EG:80W | |
| 0.025 | 0.068604 | 0.076471 | 0.071548 | 0.073117 | 0.074756 |
| 0.05 | 0.136555 | 0.152215 | 0.142416 | 0.145539 | 0.148802 |
| 0.10 | 0.274209 | 0.305653 | 0.274209 | 0.274209 | 0.274209 |
| 0.20 | 0.547869 | 0.610694 | 0.571381 | 0.583911 | 0.597002 |
| 0.40 | 1.093542 | 1.218940 | 1.140472 | 1.165481 | 1.191612 |
| Parameters | Type | Uncertainty (%) |
|---|---|---|
| Density (ρ) | Pycnometer | ±0.2 |
| Velocity (μ) | A&D Vibro Viscometer (SV-10) | ±1 |
| Thermal conductivity (k) | KD2-Pro | ±5 |
| Temperature (T) thermostat | Memmert SV 14–22 | ±0.1 |
| Characteristics | Value |
|---|---|
| KD2 sensor: | Model: KS-1 |
| Limit of measurement: | 0.02 to 2 (W/mK) |
| Accuracy: | 5% from 0.2–2 (W/mK) |
| Test Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | |
| Co3O4/DW | |||||||||
| 0.025 | 0.9985 | 0.9974 | 0.9964 | 0.9953 | 0.9943 | 0.9932 | 0.9922 | 0.9911 | 0.9901 |
| 0.05 | 0.9991 | 0.9981 | 0.9971 | 0.9960 | 0.9950 | 0.9939 | 0.9929 | 0.9918 | 0.9908 |
| 0.1 | 1.0005 | 0.9995 | 0.9984 | 0.9974 | 0.9963 | 0.9953 | 0.9942 | 0.9932 | 0.9921 |
| 0.2 | 1.0033 | 1.0022 | 1.0012 | 1.0001 | 0.9991 | 0.9980 | 0.9970 | 0.9959 | 0.9949 |
| 0.4 | 1.0087 | 1.0077 | 1.0066 | 1.0056 | 1.0045 | 1.0035 | 1.0024 | 1.0014 | 1.0004 |
| Co3O4/EG | |||||||||
| 0.025 | 1.1199 | 1.1164 | 1.1128 | 1.1092 | 1.1056 | 1.1019 | 1.0982 | 1.0945 | 1.0907 |
| 0.05 | 1.1268 | 1.1233 | 1.1197 | 1.1161 | 1.1125 | 1.1089 | 1.1052 | 1.1015 | 1.0978 |
| 0.1 | 1.1404 | 1.1369 | 1.1334 | 1.1299 | 1.1264 | 1.1228 | 1.1192 | 1.1155 | 1.1119 |
| 0.2 | 1.1675 | 1.1641 | 1.1607 | 1.1573 | 1.1539 | 1.1504 | 1.1469 | 1.1434 | 1.1398 |
| 0.4 | 1.2209 | 1.2177 | 1.2145 | 1.2113 | 1.2081 | 1.2049 | 1.2016 | 1.1983 | 1.1949 |
| Co3O4/20EG:80DW | |||||||||
| 0.025 | 1.0387 | 1.0369 | 1.0350 | 1.0330 | 1.0309 | 1.0291 | 1.0262 | 1.0237 | 1.0211 |
| 0.05 | 1.0462 | 1.0444 | 1.0426 | 1.0406 | 1.0385 | 1.0367 | 1.0339 | 1.0314 | 1.0288 |
| 0.1 | 1.0611 | 1.0594 | 1.0576 | 1.0556 | 1.0535 | 1.0518 | 1.049 | 1.0466 | 1.0440 |
| 0.2 | 1.0908 | 1.0891 | 1.0873 | 1.0855 | 1.0834 | 1.0818 | 1.0791 | 1.0767 | 1.0743 |
| 0.4 | 1.1492 | 1.1477 | 1.1460 | 1.1443 | 1.1424 | 1.1409 | 1.1384 | 1.1362 | 1.1339 |
| Co3O4/40EG:60DW | |||||||||
| 0.025 | 1.0683 | 1.0662 | 1.0640 | 1.0616 | 1.0590 | 1.0563 | 1.0535 | 1.0505 | 1.0473 |
| 0.05 | 1.0756 | 1.0735 | 1.0712 | 1.0689 | 1.0663 | 1.0637 | 1.0608 | 1.0579 | 1.0547 |
| 0.1 | 1.0900 | 1.0879 | 1.0857 | 1.0834 | 1.0809 | 1.0783 | 1.0755 | 1.0726 | 1.0695 |
| 0.2 | 1.1187 | 1.1167 | 1.1146 | 1.1123 | 1.1099 | 1.1073 | 1.1047 | 1.1018 | 1.0989 |
| 0.4 | 1.1752 | 1.1733 | 1.1714 | 1.1692 | 1.1670 | 1.1646 | 1.1621 | 1.1595 | 1.1568 |
| Co3O4/60EG:40DW | |||||||||
| 0.025 | 1.0946 | 1.0922 | 1.0897 | 1.0871 | 1.0844 | 1.0815 | 1.0785 | 1.0754 | 1.0722 |
| 0.05 | 1.1017 | 1.0993 | 1.0968 | 1.0942 | 1.0915 | 1.0886 | 1.0857 | 1.0826 | 1.0794 |
| 0.1 | 1.1157 | 1.1133 | 1.1109 | 1.1083 | 1.1057 | 1.1029 | 1.1000 | 1.0969 | 1.0938 |
| 0.2 | 1.1435 | 1.1412 | 1.1389 | 1.1364 | 1.1338 | 1.1311 | 1.1283 | 1.1254 | 1.1223 |
| 0.4 | 1.1984 | 1.1963 | 1.1941 | 1.1918 | 1.1893 | 1.1868 | 1.1842 | 1.1815 | 1.1786 |
| Test Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | |
| Co3O4/DW | |||||||||
| 0.025 | 1.04 | 0.92 | 0.82 | 0.73 | 0.66 | 0.59 | 0.54 | 0.5 | 0.47 |
| 0.05 | 1.06 | 0.94 | 0.83 | 0.74 | 0.67 | 0.61 | 0.55 | 0.51 | 0.48 |
| 0.1 | 1.10 | 0.97 | 0.86 | 0.77 | 0.69 | 0.63 | 0.57 | 0.53 | 0.49 |
| 0.2 | 1.18 | 1.04 | 0.93 | 0.83 | 0.74 | 0.67 | 0.62 | 0.57 | 0.53 |
| 0.4 | 1.34 | 1.18 | 1.05 | 0.94 | 0.84 | 0.76 | 0.7 | 0.64 | 0.60 |
| Co3O4/EG | |||||||||
| 0.025 | 22.00 | 17.35 | 13.40 | 10.20 | 7.61 | 5.77 | 4.62 | 4.18 | 4.44 |
| 0.05 | 23.00 | 18.14 | 14.01 | 10.60 | 7.96 | 6.03 | 4.83 | 4.37 | 4.64 |
| 0.1 | 25.07 | 19.77 | 15.27 | 11.60 | 8.67 | 6.57 | 5.27 | 4.76 | 5.06 |
| 0.2 | 29.45 | 23.23 | 17.94 | 13.60 | 10.2 | 7.72 | 6.19 | 5.60 | 5.94 |
| 0.4 | 34.82 | 27.46 | 21.21 | 16.10 | 12.00 | 9.12 | 7.32 | 6.62 | 7.03 |
| Co3O4/20EG:80DW | |||||||||
| 0.025 | 9.42 | 7.49 | 5.85 | 4.50 | 3.44 | 2.66 | 2.17 | 1.97 | 2.06 |
| 0.05 | 9.84 | 7.82 | 6.10 | 4.69 | 3.58 | 2.77 | 2.27 | 2.06 | 2.14 |
| 0.1 | 10.69 | 8.49 | 6.62 | 5.09 | 3.88 | 3.01 | 2.45 | 2.22 | 2.32 |
| 0.2 | 12.49 | 9.92 | 7.73 | 5.93 | 4.52 | 3.49 | 2.84 | 2.58 | 2.70 |
| 0.4 | 14.73 | 11.69 | 9.11 | 6.99 | 5.32 | 4.11 | 3.34 | 3.03 | 3.17 |
| Co3O4/40EG:60DW | |||||||||
| 0.025 | 13.62 | 10.78 | 8.368 | 6.38 | 4.83 | 3.70 | 2.99 | 2.71 | 2.85 |
| 0.05 | 14.23 | 11.26 | 8.741 | 6.67 | 5.04 | 3.86 | 3.12 | 2.83 | 2.98 |
| 0.1 | 15.48 | 12.25 | 9.509 | 7.25 | 5.48 | 4.19 | 3.39 | 3.07 | 3.23 |
| 0.2 | 18.14 | 14.35 | 11.13 | 8.49 | 6.41 | 4.90 | 3.96 | 3.59 | 3.78 |
| 0.4 | 21.42 | 16.95 | 13.15 | 10 | 7.56 | 5.78 | 4.67 | 4.23 | 4.46 |
| Co3O4/60EG:40DW | |||||||||
| 0.025 | 17.81 | 14.06 | 10.89 | 8.27 | 6.22 | 4.73 | 3.81 | 3.44 | 3.65 |
| 0.05 | 18.61 | 14.7 | 11.38 | 8.64 | 6.5 | 4.94 | 3.98 | 3.60 | 3.81 |
| 0.1 | 20.28 | 16.01 | 12.39 | 9.41 | 7.08 | 5.38 | 4.33 | 3.92 | 4.15 |
| 0.2 | 23.8 | 18.79 | 14.54 | 11.00 | 8.30 | 6.31 | 5.07 | 4.59 | 4.86 |
| 0.4 | 28.12 | 22.2 | 17.18 | 13.00 | 9.80 | 7.45 | 5.99 | 5.42 | 5.74 |
| Test Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | |
| Co3O4/DW | |||||||||
| 0.025 | 0.611 | 0.620 | 0.629 | 0.638 | 0.647 | 0.655 | 0.664 | 0.672 | 0.680 |
| 0.05 | 0.621 | 0.630 | 0.639 | 0.648 | 0.657 | 0.666 | 0.674 | 0.683 | 0.691 |
| 0.1 | 0.641 | 0.650 | 0.660 | 0.669 | 0.678 | 0.687 | 0.696 | 0.705 | 0.713 |
| 0.2 | 0.678 | 0.688 | 0.698 | 0.708 | 0.717 | 0.727 | 0.736 | 0.745 | 0.754 |
| 0.4 | 0.750 | 0.761 | 0.772 | 0.783 | 0.794 | 0.804 | 0.814 | 0.824 | 0.834 |
| Co3O4/EG | |||||||||
| 0.025 | 0.260 | 0.261 | 0.261 | 0.262 | 0.262 | 0.263 | 0.263 | 0.264 | 0.264 |
| 0.05 | 0.261 | 0.262 | 0.263 | 0.263 | 0.264 | 0.264 | 0.265 | 0.265 | 0.266 |
| 0.1 | 0.265 | 0.266 | 0.267 | 0.267 | 0.268 | 0.268 | 0.269 | 0.269 | 0.27 |
| 0.2 | 0.277 | 0.277 | 0.278 | 0.279 | 0.279 | 0.28 | 0.28 | 0.281 | 0.281 |
| 0.4 | 0.296 | 0.297 | 0.298 | 0.298 | 0.299 | 0.300 | 0.300 | 0.301 | 0.301 |
| Co3O4/20EG:80DW | |||||||||
| 0.025 | 0.471 | 0.476 | 0.482 | 0.488 | 0.493 | 0.498 | 0.504 | 0.509 | 0.514 |
| 0.05 | 0.477 | 0.483 | 0.489 | 0.494 | 0.500 | 0.505 | 0.511 | 0.516 | 0.521 |
| 0.1 | 0.491 | 0.497 | 0.503 | 0.508 | 0.514 | 0.520 | 0.525 | 0.531 | 0.536 |
| 0.2 | 0.517 | 0.524 | 0.530 | 0.536 | 0.542 | 0.548 | 0.554 | 0.559 | 0.565 |
| 0.4 | 0.568 | 0.575 | 0.582 | 0.589 | 0.596 | 0.602 | 0.609 | 0.615 | 0.621 |
| Co3O4/40EG:60DW | |||||||||
| 0.025 | 0.400 | 0.404 | 0.408 | 0.412 | 0.416 | 0.42 | 0.424 | 0.427 | 0.431 |
| 0.05 | 0.405 | 0.409 | 0.413 | 0.417 | 0.421 | 0.425 | 0.429 | 0.432 | 0.436 |
| 0.1 | 0.416 | 0.420 | 0.424 | 0.428 | 0.432 | 0.436 | 0.440 | 0.443 | 0.447 |
| 0.2 | 0.437 | 0.441 | 0.446 | 0.450 | 0.454 | 0.459 | 0.463 | 0.467 | 0.470 |
| 0.4 | 0.478 | 0.483 | 0.487 | 0.492 | 0.497 | 0.501 | 0.506 | 0.510 | 0.514 |
| Co3O4/60EG:40DW | |||||||||
| 0.025 | 0.472 | 0.476 | 0.482 | 0.488 | 0.493 | 0.498 | 0.504 | 0.509 | 0.514 |
| 0.05 | 0.478 | 0.483 | 0.489 | 0.494 | 0.500 | 0.505 | 0.511 | 0.516 | 0.521 |
| 0.10 | 0.492 | 0.497 | 0.503 | 0.508 | 0.514 | 0.52 | 0.525 | 0.531 | 0.536 |
| 0.20 | 0.519 | 0.524 | 0.530 | 0.536 | 0.542 | 0.548 | 0.554 | 0.559 | 0.565 |
| 0.40 | 0.570 | 0.575 | 0.582 | 0.589 | 0.596 | 0.602 | 0.609 | 0.615 | 0.621 |
| Thermal Conductivity Ratio | ||||
|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | |
| 0.1 vol% | 1.056 | 1.059 | 1.160 | 1.170 |
| 0.2 vol% | 1.100 | 1.107 | 1.195 | 1.200 |
| 0.3 vol% | 1.082 | 1.133 | 1.199 | 1.230 |
| 0.4 vol% | 1.160 | 1.253 | 1.236 | 1.255 |
| Relative Viscosity | ||||
|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | |
| 0.1 vol% | 1.140 | 1.045 | 1.160 | 1.170 |
| 0.2 vol% | 1.100 | 1.020 | 1.130 | 1.340 |
| 0.3 vol% | 1.160 | 1.17 | 1.150 | 1.170 |
| 0.4 vol% | 1.310 | 1.190 | 1.160 | 1.180 |
| Thermal Conductivity (Wm−1k−1) | |||
|---|---|---|---|
| 283.15 K | 303.15 K | 323.15 K | |
| 0.0094 | 0.263 | 0.261 | 0.259 |
| 0.0307 | 0.286 | 0.284 | 0.283 |
| 0.0431 | 0.297 | 0.296 | 0.294 |
| 0.0567 | 0.309 | 0.307 | 0.306 |
| Viscosity (mPa.s) | |||||
|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | |
| 0.0094 | 34.79 | 22.24 | 15.01 | 10.64 | 8.17 |
| 0.0307 | 38.59 | 26.04 | 17.49 | 12.35 | 9.12 |
| 0.0431 | 41.82 | 27.75 | 19.20 | 13.49 | 10.64 |
| 0.0570 | 44.86 | 29.48 | 20.34 | 14.63 | 10.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsboul, M.; Ghazali, M.S.M.; Gomaa, M.R.; Albani, A. Experimental and Theoretical Investigation of the Thermophysical Properties of Cobalt Oxide (Co3O4) in Distilled Water (DW), Ethylene Glycol (EG), and DW–EG Mixture Nanofluids. Nanomaterials 2022, 12, 2779. https://doi.org/10.3390/nano12162779
Alsboul M, Ghazali MSM, Gomaa MR, Albani A. Experimental and Theoretical Investigation of the Thermophysical Properties of Cobalt Oxide (Co3O4) in Distilled Water (DW), Ethylene Glycol (EG), and DW–EG Mixture Nanofluids. Nanomaterials. 2022; 12(16):2779. https://doi.org/10.3390/nano12162779
Chicago/Turabian StyleAlsboul, Monther, Mohd Sabri Mohd Ghazali, Mohamed R. Gomaa, and Aliashim Albani. 2022. "Experimental and Theoretical Investigation of the Thermophysical Properties of Cobalt Oxide (Co3O4) in Distilled Water (DW), Ethylene Glycol (EG), and DW–EG Mixture Nanofluids" Nanomaterials 12, no. 16: 2779. https://doi.org/10.3390/nano12162779
APA StyleAlsboul, M., Ghazali, M. S. M., Gomaa, M. R., & Albani, A. (2022). Experimental and Theoretical Investigation of the Thermophysical Properties of Cobalt Oxide (Co3O4) in Distilled Water (DW), Ethylene Glycol (EG), and DW–EG Mixture Nanofluids. Nanomaterials, 12(16), 2779. https://doi.org/10.3390/nano12162779








