Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer
Abstract
1. Introduction
1.1. Imaging-Guided Interventional Approaches Offer Benefits
1.2. The Promise of Thermal Medicine and Challenges to Its Implementation
1.3. Magnetic Hyperthermia Offers Unique Solutions for Thermal Medicine
2. Current Challenges with MHT
2.1. MHT Requires Imaging of MNP Concentration and Distribution in Tissues
2.2. MRI of MNPs for MHT Has a Narrow Quantifiable Range
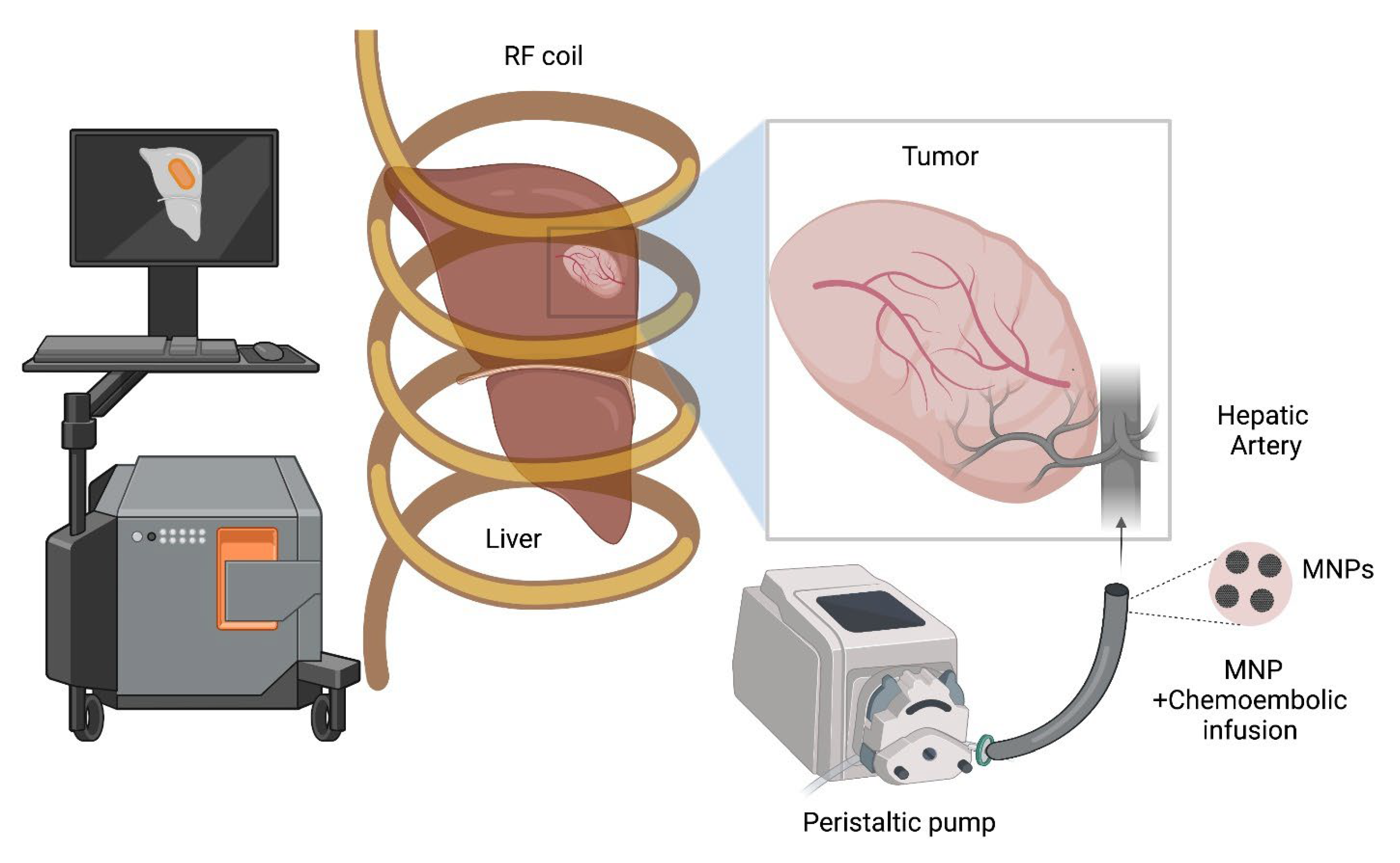
2.3. Imaging Guidance for TACE and ThermoTACE Indications in Unresectable HCC
2.4. MNPs + X-ray Contrast Fluids Are Feasible Dual-Contrast Agents for HCC Imaging
2.5. Magnetic Particle Imaging Offers MNP-Tracer Specific Imaging of Distribution and Concentration in Tissue
2.6. Liver Perfusion Imaging Can Provide Non-Invasive Diagnostic Imaging Modality
- Spatial and kinetic differences of perfusion/flow between small nodules and HCC can be accurately resolved.
- Arterial and venous flow can be accurately quantified.
- Image-tracer concentration can be accurately differentiated from the contrast.
2.7. MHT Treatment Planning Models Require Experimental Validation
2.8. Perfusion Imaging Is Essential for MHT Treatment Planning to Capture Dynamic Tissue Responses
2.9. MHT Treatment Planning with Perfusion Modeling
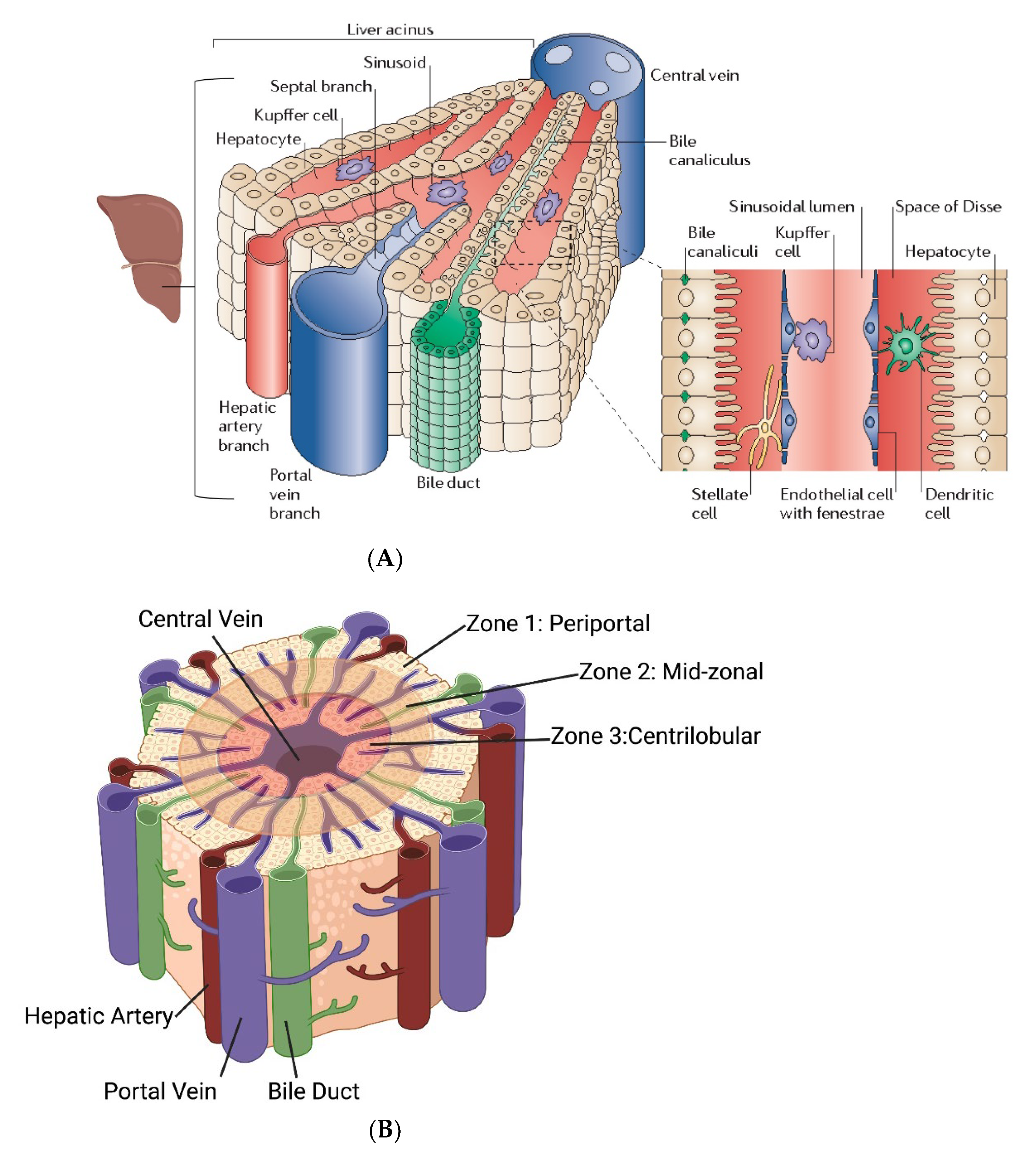
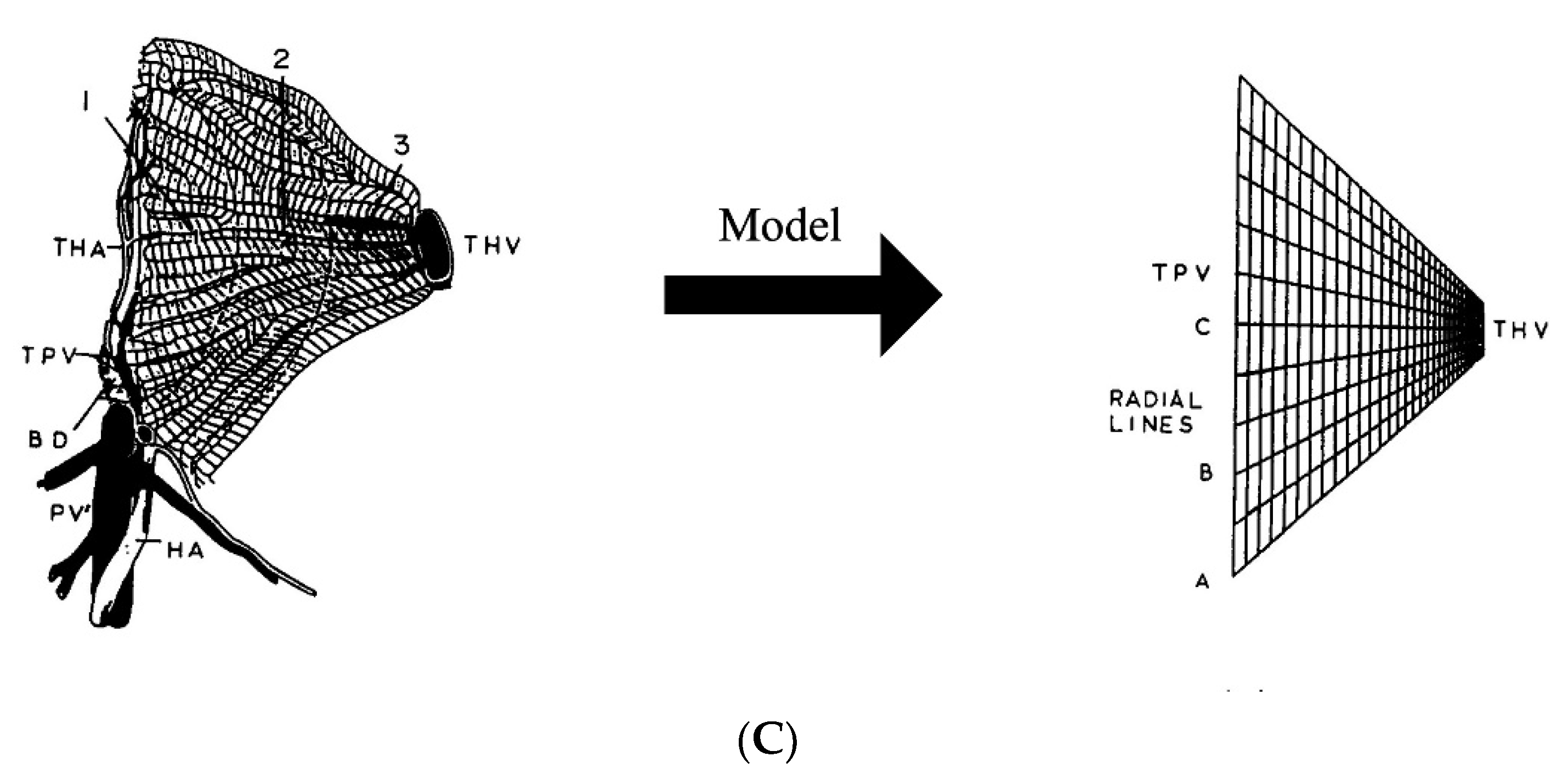
2.10. Need for Computational Modeling at the Liver Microcirculatory Level for MHT Treatment Planning
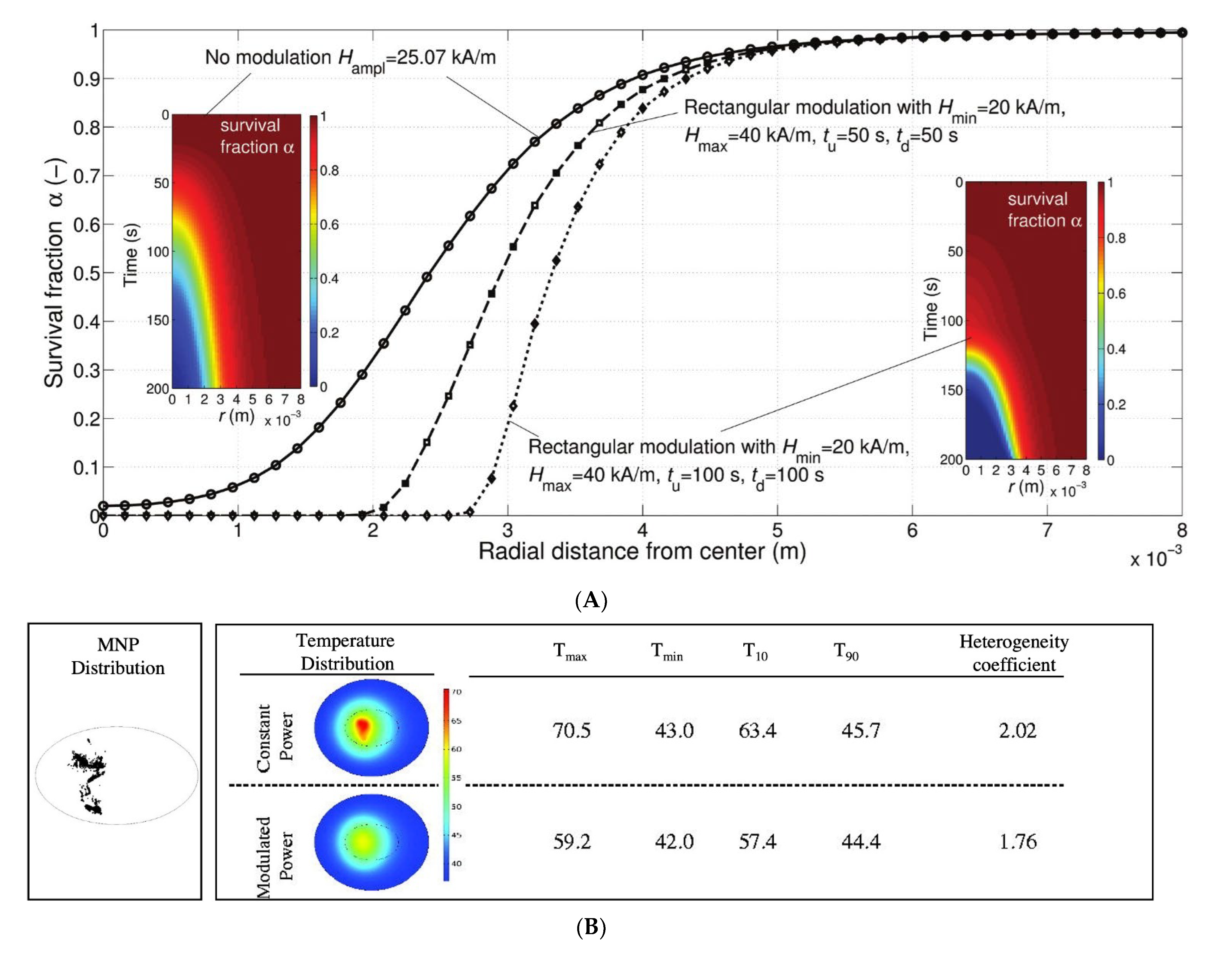
2.11. MHT Improved by Amplitude and Power Modulation
2.12. Theranostic Nanoparticle HT Requires Integrated Imaging
2.13. In Vivo Testing of MHT
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tapper, E.B.; Parikh, N.D. Mortality Due to Cirrhosis and Liver Cancer in the United States, 1999–2016: Observational Study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Foutz, J.; Gauntt, K.; Cafarella, M.; et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am. J. Transplant. 2022, 22, 204–309. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.; Benedict, S.; Diederich, C.; Gedroyc, W.; Klibanov, A.; Larner, J. MR-guided Focused Ultrasound Surgery, Present and Future. Med. Phys. 2013, 40, 080901. [Google Scholar] [CrossRef] [PubMed]
- Liapi, E.; Geschwind, J.-F.H. Transcatheter and Ablative Therapeutic Approaches for Solid Malignancies. J. Clin. Oncol. 2007, 25, 978–986. [Google Scholar] [CrossRef]
- Liapi, E.; Geschwind, J.-F.H. Transcatheter Arterial Chemoembolization for Liver Cancer: Is It Time to Distinguish Conventional from Drug-Eluting Chemoembolization? Cardiovasc. Interv. Radiol. 2011, 34, 37–49. [Google Scholar] [CrossRef]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef]
- Kong, J.-Y.; Li, S.-M.; Fan, H.-Y.; Zhang, L.; Zhao, H.-J.; Li, S.-M. Transarterial Chemoembolization Extends Long-Term Survival in Patients with Unresectable Hepatocellular Carcinoma. Medicine 2018, 97, e11872. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Yang, F.; Song, W.; Han, D.; Wen, W.; Qin, W. Quicker, Deeper and Stronger Imaging: A Review of Tumor-Targeted, near-Infrared Fluorescent Dyes for Fluorescence Guided Surgery in the Preclinical and Clinical Stages. Eur. J. Pharm. Biopharm. 2020, 152, 123–143. [Google Scholar] [CrossRef]
- Harvell-Smith, S.; Tung, L.D.; Thanh, N.T.K. Magnetic Particle Imaging: Tracer Development and the Biomedical Applications of a Radiation-Free, Sensitive, and Quantitative Imaging Modality. Nanoscale 2021, 14, 3658–3697. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef]
- Oshina, I.; Spigulis, J. Beer–Lambert Law for Optical Tissue Diagnostics: Current State of the Art and the Main Limitations. J. Biomed. Opt. 2021, 26, 100901. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of Hyperthermia in Driving the Future of Clinical Hyperthermia as Targeted Therapy: Translation into Clinical Application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef]
- Russell, A.H. Clinical Radiation Oncology. Edited by L. L. Gunderson and J. E. Tepper. Gynecol. Oncol. 2001, 81, 335. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; De Bruijne, M.; Canters, R.A.M.; Van Norden, Y.; Mens, J.W.; Van Rhoon, G.C.; Van Der Zee, J. Hyperthermia Dose-Effect Relationship in 420 Patients with Cervical Cancer Treated with Combined Radiotherapy and Hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of Thermal Dose as a Predictor of Local Control in Cervical Carcinoma Patients Treated with State-of-the-Art Radiation Therapy and Hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef]
- Dennis, C.L.; Krycka, K.L.; Borchers, J.A.; Desautels, R.D.; van Lierop, J.; Huls, N.F.; Jackson, A.J.; Gruettner, C.; Ivkov, R. Internal Magnetic Structure of Nanoparticles Dominates Time-Dependent Relaxation Processes in a Magnetic Field. Adv. Funct. Mater. 2015, 25, 4300–4311. [Google Scholar] [CrossRef]
- Krycka, K.L.; Jackson, A.J.; Borchers, J.A.; Shih, J.; Briber, R.; Ivkov, R.; Grüttner, C.; Dennis, C.L. Internal Magnetic Structure of Dextran Coated Magnetite Nanoparticles in Solution Using Small Angle Neutron Scattering with Polarization Analysis. J. Appl. Phys. 2011, 109, 07B513. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic Hyperthermia Therapy for the Treatment of Glioblastoma: A Review of the Therapy’s History, Efficacy and Application in Humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in Combined Treatment of Cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Lehmann, J.; Natarajan, A.; Denardo, G.L.; Ivkov, R.; Foreman, A.R.; Catapano, C.; Mirick, G.; Quang, T.; Gruettner, C.; Denardo, S.J. Short Communication: Nanoparticle Thermotherapy and External Beam Radiation Therapy for Human Prostate Cancer Cells. Cancer Biother. Radiopharm. 2008, 23, 265–271. [Google Scholar] [CrossRef]
- Kok, H.P.; Gellermann, J.; van den Berg, C.A.T.; Stauffer, P.R.; Hand, J.W.; Crezee, J. Thermal Modelling Using Discrete Vasculature for Thermal Therapy: A Review. Int. J. Hyperth. 2013, 29, 336–345. [Google Scholar] [CrossRef]
- Crezee, H.; Van Leeuwen, C.M.; Oei, A.L.; Stalpers, L.J.A.; Bel, A.; Franken, N.A.; Kok, H.P. Thermoradiotherapy Planning: Integration in Routine Clinical Practice. Int. J. Hyperth. 2015, 32, 41–49. [Google Scholar] [CrossRef]
- Kok, H.P.; Crezee, J. Adapt2Heat: Treatment Planning-Assisted Locoregional Hyperthermia by on-Line Visualization, Optimization and Re-Optimization of SAR and Temperature Distributions. Int. J. Hyperth. 2022, 39, 265–277. [Google Scholar] [CrossRef]
- Soetaert, F.; Dupré, L.; Ivkov, R.; Crevecoeur, G. Computational Evaluation of Amplitude Modulation for Enhanced Magnetic Nanoparticle Hyperthermia. Biomed. Eng. Biomed. Tech. 2015, 60, 491–504. [Google Scholar] [CrossRef]
- Kandala, S.K.; Liapi, E.; Whitcomb, L.L.; Attaluri, A.; Ivkov, R. Temperature-Controlled Power Modulation Compensates for Heterogeneous Nanoparticle Distributions: A Computational Optimization Analysis for Magnetic Hyperthermia. Int. J. Hyperth. 2018, 36, 115–129. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical Magnetic Hyperthermia Requires Integrated Magnetic Particle Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, P.; Croft, L.; Konkle, J.; Lu, K.; Saritas, E.; Zheng, B.; Conolly, S. A Novel SPIO Nanoparticle Imaging Technique. In Magnetic Particle Imaging; Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–265. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Kraitchman, D.L. Iron Oxide MR Contrast Agents for Molecular and Cellular Imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Pardoe, H.; Clark, P.R.; St. Pierre, T.G.; Moroz, P.; Jones, S.K. A Magnetic Resonance Imaging Based Method for Measurement of Tissue Iron Concentration in Liver Arterially Embolized with Ferrimagnetic Particles Designed for Magnetic Hyperthermia Treatment of Tumors. Magn. Reson. Imaging 2003, 21, 483–488. [Google Scholar] [CrossRef]
- Pardoe, H.; Chua-Anusorn, W.; St. Pierre, T.G.; Dobson, J. Detection Limits for Ferrimagnetic Particle Concentrations Using Magnetic Resonance Imaging Based Proton Transverse Relaxation Rate Measurements. Phys. Med. Biol. 2003, 48, N89–N95. [Google Scholar] [CrossRef]
- Ring, H.L.; Zhang, J.; Klein, N.D.; Eberly, L.E.; Haynes, C.L.; Garwood, M. Establishing the Overlap of IONP Quantification with Echo and Echoless MR Relaxation Mapping. Magn. Reson. Med 2018, 79, 1420–1428. [Google Scholar] [CrossRef]
- St. Pierre, T.G.; Clark, P.R.; Chua-Anusorn, W.; Fleming, A.J.; Jeffrey, G.P.; Olynyk, J.K.; Pootrakul, P.; Robins, E.; Lindeman, R. Noninvasive Measurement and Imaging of Liver Iron Concentrations Using Proton Magnetic Resonance. Blood 2005, 105, 855–861. [Google Scholar] [CrossRef]
- Zhang, J.; Ring, H.L.; Hurley, K.R.; Shao, Q.; Carlson, C.S.; Idiyatullin, D.; Manuchehrabadi, N.; Hoopes, P.J.; Haynes, C.L.; Bischof, J.C.; et al. Quantification and Biodistribution of Iron Oxide Nanoparticles in the Primary Clearance Organs of Mice Using T1 Contrast for Heating. Magn. Reson. Med. 2017, 78, 702–712. [Google Scholar] [CrossRef]
- Stuber, M.; Gilson, W.D.; Schär, M.; Kedziorek, D.A.; Hofmann, L.V.; Shah, S.; Vonken, E.-J.; Bulte, J.W.; Kraitchman, D.L. Positive Contrast Visualization of Iron Oxide-labeled Stem Cells Using Inversion-recovery with ON-resonant Water Suppression (IRON). Magn. Reson. Med. 2007, 58, 1072–1077. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Wang, X.; Chen, W.; Jin, X.; Liu, X.; Ye, F.; Dai, Z.; Zheng, X.; Li, P.; et al. Hyperthermia Ablation Combined with Transarterial Chemoembolization versus Monotherapy for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Cancer Med. 2021, 10, 8432–8450. [Google Scholar] [CrossRef]
- Müller, L.; Stoehr, F.; Mähringer-Kunz, A.; Hahn, F.; Weinmann, A.; Kloeckner, R. Current Strategies to Identify Patients That Will Benefit from TACE Treatment and Future Directions a Practical Step-by-Step Guide. J. Hepatocell. Carcinoma 2021, 8, 403–419. [Google Scholar] [CrossRef]
- Hedayati, M.; Thomas, O.; Abubaker-Sharif, B.; Zhou, H.; Cornejo, C.; Zhang, Y.; Wabler, M.; Mihalic, J.; Gruettner, C.; Westphal, F.; et al. The Effect of Cell Cluster Size on Intracellular Nanoparticle-Mediated Hyperthermia: Is It Possible to Treat Microscopic Tumors? Nanomedicine 2013, 8, 29–41. [Google Scholar] [CrossRef]
- Guerbet. Lipiodol (Ethiodized Oil) [Package Insert]. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/009190s029s031lbl.pdf (accessed on 1 August 2022).
- Attaluri, A.; Seshadri, M.; Mirpour, S.; Wabler, M.; Marinho, T.; Furqan, M.; Zhou, H.; De Paoli, S.; Gruettner, C.; Gilson, W.; et al. Image-Guided Thermal Therapy with a Dual-Contrast Magnetic Nanoparticle Formulation: A Feasibility Study. Int. J. Hyperth. 2016, 32, 543–557. [Google Scholar] [CrossRef]
- Simulation Based Strategies for Clinical Translation of Magnetic Nanoparticle Hyperthermia. Available online: https://jscholarship.library.jhu.edu/handle/1774.2/60223 (accessed on 19 May 2021).
- Tay, Z.W.; Savliwala, S.; Hensley, D.W.; Fung, K.L.B.; Colson, C.; Fellows, B.D.; Zhou, X.; Huynh, Q.; Lu, Y.; Zheng, B.; et al. Superferromagnetic Nanoparticles Enable Order-of-Magnitude Resolution & Sensitivity Gain in Magnetic Particle Imaging. Small Methods 2021, 5, 2100796. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic Imaging Using the Nonlinear Response of Magnetic Particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Mason, E.E.; Cooley, C.Z.; Cauley, S.F.; Griswold, M.A.; Conolly, S.M.; Wald, L.L. Design Analysis of an MPI Human Functional Brain Scanner. Int. J. Magn. Part Imaging 2017, 3, 1703008. [Google Scholar] [CrossRef]
- Chiandussi, L.; Greco, F.; Sardi, G.; Vaccarino, A.; Ferraris, C.M.; Curti, B. Estimation of Hepatic Arterial and Portal Venous Blood Flow by Direct Catheterization of the Vena Porta through the Umbilical Cord in Man. Preliminary Results. Acta Hepato-Splenol. 1968, 15, 166–171. [Google Scholar]
- Wheater, P.R.; Burkitt, H.G.; Daniels, V.G. Wheater’s Functional Histology, 6th ed.; Elsevier Health Sciences: London, UK, 2006. [Google Scholar]
- Cunningham, R.P.; Porat-Shliom, N. Liver Zonation—Revisiting Old Questions with New Technologies. Front. Physiol. 2021, 12, 732929. [Google Scholar] [CrossRef]
- Chew, S.A.; Moscato, S.; George, S.; Azimi, B.; Danti, S. Liver Cancer: Current and Future Trends Using Biomaterials. Cancers 2019, 11, 2026. [Google Scholar] [CrossRef]
- Adams, D.H.; Eksteen, B. Aberrant Homing of Mucosal T Cells and Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Nat. Rev. Immunol. 2006, 6, 244–251. [Google Scholar] [CrossRef]
- Ruebner, B.H.; Montgomery, C.K.; French, S.W. Diagnostic Pathology of the Liver and Biliary Tract, 2nd ed.; Hemisphere Publishing Corp.: New York, NY, USA, 1991; p. 19. [Google Scholar]
- Ronot, M.; Leporq, B.; Van Beers, B.E.; Vilgrain, V. CT and MR Perfusion Techniques to Assess Diffuse Liver Disease. Abdom. Radiol. 2020, 45, 3496–3506. [Google Scholar] [CrossRef]
- Park, Y.N.; Kim, Y.-B.; Yang, K.M.; Park, C. Increased Expression of Vascular Endothelial Growth Factor and Angiogenesis in the Early Stage of Multistep Hepatocarcinogenesis. Arch. Pathol. Lab. Med. 2000, 124, 1061–1065. [Google Scholar] [CrossRef]
- Perkins, A.C.; Whalley, D.R.; Ballantyne, K.C.; Hardcastle, J.D. Reliability of the Hepatic Perfusion Index for the Detection of Liver Metastases. Nucl. Med. Commun. 1987, 8, 982–989. [Google Scholar] [CrossRef]
- Pandharipande, P.V.; Krinsky, G.A.; Rusinek, H.; Lee, V.S. Perfusion Imaging of the Liver: Current Challenges and Future Goals. Radiology 2005, 234, 661–673. [Google Scholar] [CrossRef]
- Annet, L.; Materne, R.; Danse, E.; Jamart, J.; Horsmans, Y.; Van Beers, B. Hepatic Flow Parameters Measured with MR Imaging and Doppler US: Correlations with Degree of Cirrhosis and Portal Hypertension. Radiology 2003, 229, 409–414. [Google Scholar] [CrossRef]
- Van Beers, B.E.; Leconte, I.; Materne, R.; Smith, A.M.; Jamart, J.; Horsmans, Y. Hepatic Perfusion Parameters in Chronic Liver Disease: Dynamic CT Measurements Correlated with Disease Severity. Am. J. Roentgenol. 2001, 176, 667–673. [Google Scholar] [CrossRef]
- Gülberg, V.; Haag, K.; Rössle, M.; Gerbes, A.L. Hepatic Arterial Buffer Response in Patients with Advanced Cirrhosis: Hepatic Arterial Buffer Response in Patients with Advanced Cirrhosis. Hepatology 2002, 35, 630–634. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1998, 85, 5–34. [Google Scholar] [CrossRef]
- Oberkampf, W.L.; Trucano, T.G.; Hirsch, C. Verification, Validation, and Predictive Capability in Computational Engineering and Physics. Appl. Mech. Rev. 2004, 57, 345–384. [Google Scholar] [CrossRef]
- Kandala, S.K.; Sharma, A.; Mirpour, S.; Liapi, E.; Ivkov, R.; Attaluri, A. Validation of a Coupled Electromagnetic and Thermal Model for Estimating Temperatures during Magnetic Nanoparticle Hyperthermia. Int. J. Hyperth. 2021, 38, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, D.; Cardan, R.; Stafford, R.J.; Yung, J.; Dodd, G.D.; Feng, Y. High-Fidelity Computer Models for Prospective Treatment Planning of Radiofrequency Ablation with In Vitro Experimental Correlation. J. Vasc. Interv. Radiol. 2010, 21, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yu, H.; Zhang, Y.; Ma, M.; Chen, Z.; Wang, C.; Teng, G.; Ma, J.; Sun, X.; Gu, N. Three-Dimensional Model for Determining Inhomogeneous Thermal Dosage in a Liver Tumor During Arterial Embolization Hyperthermia Incorporating Magnetic Nanoparticles. IEEE Trans. Magn. 2009, 45, 3085–3091. [Google Scholar] [CrossRef]
- Schutt, D.J.; Haemmerich, D. Effects of Variation in Perfusion Rates and of Perfusion Models in Computational Models of Radio Frequency Tumor Ablation. Med. Phys. 2008, 35, 3462–3470. [Google Scholar] [CrossRef]
- Materne, R.; Van Beers, B.E.; Smith, A.M.; Leconte, I.; Jamart, J.; Dehoux, J.-P.; Keyeux, A.; Horsmans, Y. Non-Invasive Quantification of Liver Perfusion with Dynamic Computed Tomography and a Dual-Input One-Compartmental Model. Clin. Sci. 2000, 99, 517–525. [Google Scholar] [CrossRef]
- Thng, C.H.; Koh, T.S.; Collins, D.J.; Koh, D.M. Perfusion Magnetic Resonance Imaging of the Liver. World J. Gastroenterol. 2010, 16, 1598–1609. [Google Scholar] [CrossRef]
- Cuenod, C.A.; Leconte, I.; Siauve, N.; Frouin, F.; Dromain, C.; Clément, O.; Frija, G. Deconvolution Technique for Measuring Tissue Perfusion by Dynamic CT Application to Normal and Metastatic Liver. Acad. Radiol. 2002, 9, S205–S211. [Google Scholar] [CrossRef]
- Viglianti, B.L.; Lora-Michiels, M.; Poulson, J.M.; Lan, L.; Yu, D.; Sanders, L.; Craciunescu, O.; Vujaskovic, Z.; Thrall, D.E.; MacFall, J.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Predictor of Clinical Outcome in Canine Spontaneous Soft Tissue Sarcomas Treated with Thermoradiotherapy. Clin. Cancer Res. 2009, 15, 4993–5001. [Google Scholar] [CrossRef]
- Lüdemann, L.; Wust, P.; Gellermann, J. Perfusion Measurement Using DCE-MRI: Implications for Hyperthermia. Int. J. Hyperth. 2009, 24, 91–96. [Google Scholar] [CrossRef]
- Karçaaltıncaba, M.; Aktaş, A. Dual-Energy CT Revisited with Multidetector CT: Review of Principles and Clinical Applications. Diagn. Interv. Radiol. 2010, 17, 181–194. [Google Scholar] [CrossRef]
- Joe, E.; Kim, S.H.; Lee, K.B.; Jang, J.-J.; Lee, J.Y.; Lee, J.M.; Han, J.K.; Choi, B.I. Feasibility and Accuracy of Dual-Source Dual-Energy CT for Noninvasive Determination of Hepatic Iron Accumulation. Radiology 2012, 262, 126–135. [Google Scholar] [CrossRef]
- Lee, C.Y.C.; Rubinsky, B. A Multi-Dimensional Model of Momentum and Mass Transfer in the Liver. Int. J. Heat Mass Transf. 1989, 32, 2421–2434. [Google Scholar] [CrossRef]
- Sharma, A.; Özayral, S.; Caserto, J.S.; Cate, R.T.; Anders, N.M.; Barnett, J.D.; Kandala, S.K.; Henderson, E.; Stewart, J.; Liapi, E.; et al. Increased Uptake of Doxorubicin by Cells Undergoing Heat Stress Does Not Explain Its Synergistic Cytotoxicity with Hyperthermia. Int. J. Hyperth. 2019, 36, 712–720. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Thrall, D.E.; Palmer, G.; Schroeder, T.; Vujaskovic, Z.; Charles, H.C.; Macfall, J.; Wong, T. Utility of Functional Imaging in Prediction or Assessment of Treatment Response and Prognosis Following Thermotherapy. Int. J. Hyperth. 2010, 26, 283–293. [Google Scholar] [CrossRef]
- Karimian, N.; Matton, A.P.M.; Westerkamp, A.C.; Burlage, L.C.; Dries, S.O.D.; Leuvenink, H.; Lisman, T.; Uygun, K.; Markmann, J.F.; Porte, R.J. Ex Situ Normothermic Machine Perfusion of Donor Livers. J. Vis. Exp. 2015, 99, e52688. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of Available Methods for Quantifying the Heat Power Generation of Nanoparticles for Magnetic Hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple Models for Dynamic Hysteresis Loop Calculations of Magnetic Single-Domain Nanoparticles: Application to Magnetic Hyperthermia Optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Sharma, A.; Zhu, Y.; Thor, S.; Zhou, F.; Stadler, B.; Hubel, A. Magnetic Barcode Nanowires for Osteosarcoma Cell Control, Detection and Separation. IEEE Trans. Magn. 2012, 49, 453–456. [Google Scholar] [CrossRef]
- Madhukar, R.S.; Jin, P.J.; Maqableh, M.; Flatau, A.; Stadler, B. Magnetization Reversal Mechanisms in 35-Nm Diameter Fe1−XGaX/Cu Multilayered Nanowires. J. Appl. Phys. 2012, 111, 07A920. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, BME-31, 70–75. [Google Scholar] [CrossRef]
- Attaluri, A.; Ma, R.; Zhu, L. Using MicroCT Imaging Technique to Quantify Heat Generation Distribution Induced by Magnetic Nanoparticles for Cancer Treatments. J. Heat Transf. 2011, 133, 011003. [Google Scholar] [CrossRef]
- Attaluri, A.; Ma, R.; Qiu, Y.; Li, W.; Zhu, L. Nanoparticle Distribution and Temperature Elevations in Prostatic Tumours in Mice during Magnetic Nanoparticle Hyperthermia. Int. J. Hyperth. 2011, 27, 491–502. [Google Scholar] [CrossRef]
- Korganbayev, S.; Orrico, A.; Bianchi, L.; Paloschi, D.; Wolf, A.; Dostovalov, A.; Saccomandi, P. PID Controlling Approach Based on FBG Array Measurements for Laser Ablation of Pancreatic Tissues. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Korganbayev, S.; Orrico, A.; Bianchi, L.; De Landro, M.; Wolf, A.; Dostovalov, A.; Saccomandi, P. Closed-Loop Temperature Control Based on Fiber Bragg Grating Sensors for Laser Ablation of Hepatic Tissue. Sensors 2020, 20, 6496. [Google Scholar] [CrossRef]
- Ahmed, A.; Kim, E.; Jeon, S.; Kim, J.; Choi, H. Closed-Loop Temperature-Controlled Magnetic Hyperthermia Therapy with Magnetic Guidance of Superparamagnetic Iron-Oxide Nanoparticles. Adv. Ther. 2022, 5, 2100237. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef]
- Attaluri, A.; Kandala, S.K.; Zhou, H.; Wabler, M.; DeWeese, T.L.; Ivkov, R. Magnetic Nanoparticle Hyperthermia for Treating Locally Advanced Unresectable and Borderline Resectable Pancreatic Cancers: The Role of Tumor Size and Eddy-Current Heating. Int. J. Hyperth. 2021, 37, 108–119. [Google Scholar] [CrossRef]
- Kuszyk, B.S.; Boitnott, J.K.; Choti, M.A.; Bluemke, D.A.; Sheth, S.; Magee, C.A.; Horton, K.M.; Eng, J.; Fishman, E.K. Local Tumor Recurrence Following Hepatic Cryoablation: Radiologic-Histopathologic Correlation in a Rabbit Model. Radiology 2000, 217, 477–486. [Google Scholar] [CrossRef]
- Tam, A.L.; Melancon, M.P.; Ensor, J.; Liu, Y.; Dixon, K.; McWatters, A.; Gupta, S. Rabbit Hepatic Arterial Anatomy Variations: Implications on Experimental Design. Acta Radiol. 2013, 55, 1226–1233. [Google Scholar] [CrossRef]
- Lee, K.-H.; Liapi, E.; Buijs, M.; Vossen, J.; Hong, K.; Georgiades, C.; Geschwind, J.-F.H. Considerations for Implantation Site of VX2 Carcinoma into Rabbit Liver. J. Vasc. Interv. Radiol. 2009, 20, 113–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.E.; Henderson, J.M.; Gorrell, M.D. Animal Models for Hepatocellular Carcinoma. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2019, 1865, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Schachtschneider, K.M.; Schwind, R.M.; Darfour-Oduro, K.A.; De, A.K.; Rund, L.A.; Singh, K.; Principe, D.R.; Guzman, G.; Ray, C.E.; Ozer, H.; et al. A Validated, Transitional and Translational Porcine Model of Hepatocellular Carcinoma. Oncotarget 2017, 8, 63620–63634. [Google Scholar] [CrossRef] [PubMed]
- Gaba, R.C.; Elkhadragy, L.; Boas, F.E.; Chaki, S.; Chen, H.H.; El-Kebir, M.; Garcia, K.D.; Giurini, E.F.; Guzman, G.; LoBianco, F.V.; et al. Development and Comprehensive Characterization of Porcine Hepatocellular Carcinoma for Translational Liver Cancer Investigation. Oncotarget 2020, 11, 2686–2701. [Google Scholar] [CrossRef]
- Tennant, B.C.; Toshkov, I.A.; Peek, S.F.; Jacob, J.R.; Menne, S.; Hornbuckle, W.E.; Schinazi, R.D.; Korba, B.E.; Cote, P.J.; Gerin, J.L. Hepatocellular Carcinoma in the Woodchuck Model of Hepatitis B Virus Infection. Gastroenterology 2004, 127, S283–S293. [Google Scholar] [CrossRef]
- Wilkins, L.R.; Stone, J.R.; Mata, J.; Hawrylack, A.; Kubicka, E.; Brautigan, D.L. The Use of the Woodchuck as an Animal Model for Evaluation of Transarterial Embolization. J. Vasc. Interv. Radiol. 2017, 28, 1467–1471. [Google Scholar] [CrossRef]
- Pritchard, W.F.; Woods, D.L.; Esparza-Trujillo, J.A.; Starost, M.F.; Mauda-Havakuk, M.; Mikhail, A.S.; Bakhutashvili, I.; Leonard, S.; Jones, E.C.; Krishnasamy, V.; et al. Transarterial Chemoembolization in a Woodchuck Model of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 812–819.e1. [Google Scholar] [CrossRef]
- Kim, A.Y.; Yacoub, J.H.; Field, D.H.; Park, B.U.; Kallakury, B.; Korolowicz, K.E.; Menne, S. Suitability of the Woodchuck HCC as a Preclinical Model for Evaluation of Intra-arterial Therapies. Anim. Model Exp. Med. 2020, 3, 98–102. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H.; Johnson, G.F. Canine Hepatocellular Carcinoma. Vet. Pathol. 1981, 18, 427–438. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug. Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Yu, J.I.; Park, H.C.; Jung, S.H.; Choi, C.; Shin, S.W.; Cho, S.K.; Sinn, D.H.; Paik, Y.-H.; Gwak, G.-Y.; Choi, M.S.; et al. Combination Treatment with Transarterial Chemoembolization, Radiotherapy, and Hyperthermia (CERT) for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: Final Results of a Prospective Phase II Trial. Oncotarget 2014, 5, 52651–52664. [Google Scholar] [CrossRef][Green Version]
- Dou, Y.; Hynynen, K.; Allen, C. To Heat or Not to Heat: Challenges with Clinical Translation of Thermosensitive Liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef]
- Tak, W.Y.; Lin, S.-M.; Wang, Y.; Zheng, J.; Vecchione, A.; Park, S.Y.; Chen, M.H.; Wong, S.; Xu, R.; Peng, C.-Y.; et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin. Cancer Res. 2018, 24, 73–83. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of ThermoDox with Standardized Radiofrequency Ablation (RFA) for Treatment of Hepatocellular Carcinoma (HCC) (OPTIMA). Available online: https://clinicaltrials.gov/ct2/show/NCT02112656 (accessed on 1 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Cressman, E.; Attaluri, A.; Kraitchman, D.L.; Ivkov, R. Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer. Nanomaterials 2022, 12, 2768. https://doi.org/10.3390/nano12162768
Sharma A, Cressman E, Attaluri A, Kraitchman DL, Ivkov R. Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer. Nanomaterials. 2022; 12(16):2768. https://doi.org/10.3390/nano12162768
Chicago/Turabian StyleSharma, Anirudh, Erik Cressman, Anilchandra Attaluri, Dara L. Kraitchman, and Robert Ivkov. 2022. "Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer" Nanomaterials 12, no. 16: 2768. https://doi.org/10.3390/nano12162768
APA StyleSharma, A., Cressman, E., Attaluri, A., Kraitchman, D. L., & Ivkov, R. (2022). Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer. Nanomaterials, 12(16), 2768. https://doi.org/10.3390/nano12162768






