Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Biohybrids
2.1.1. Materials
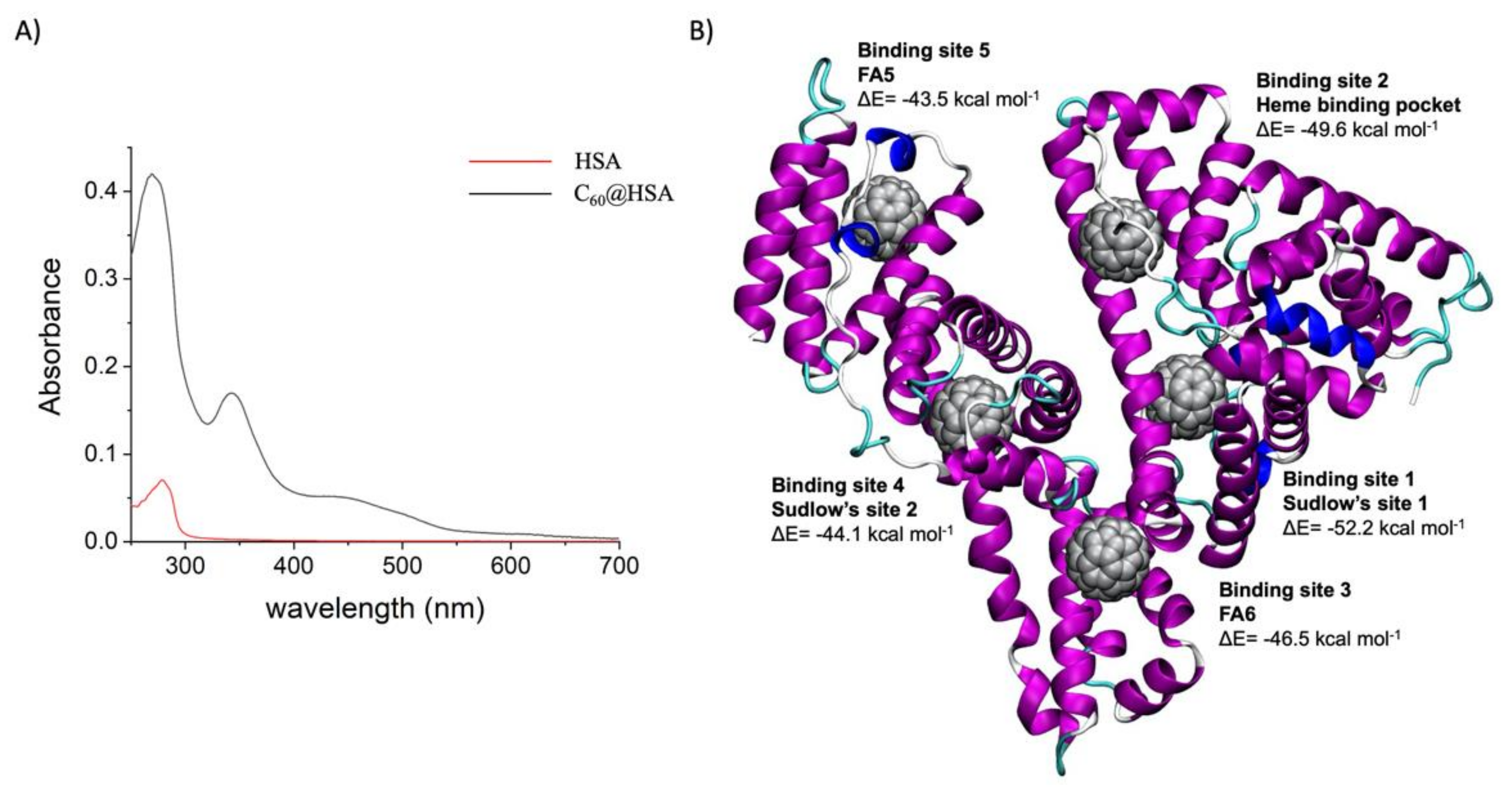
2.1.2. C60@HSA Synthesis and Purification
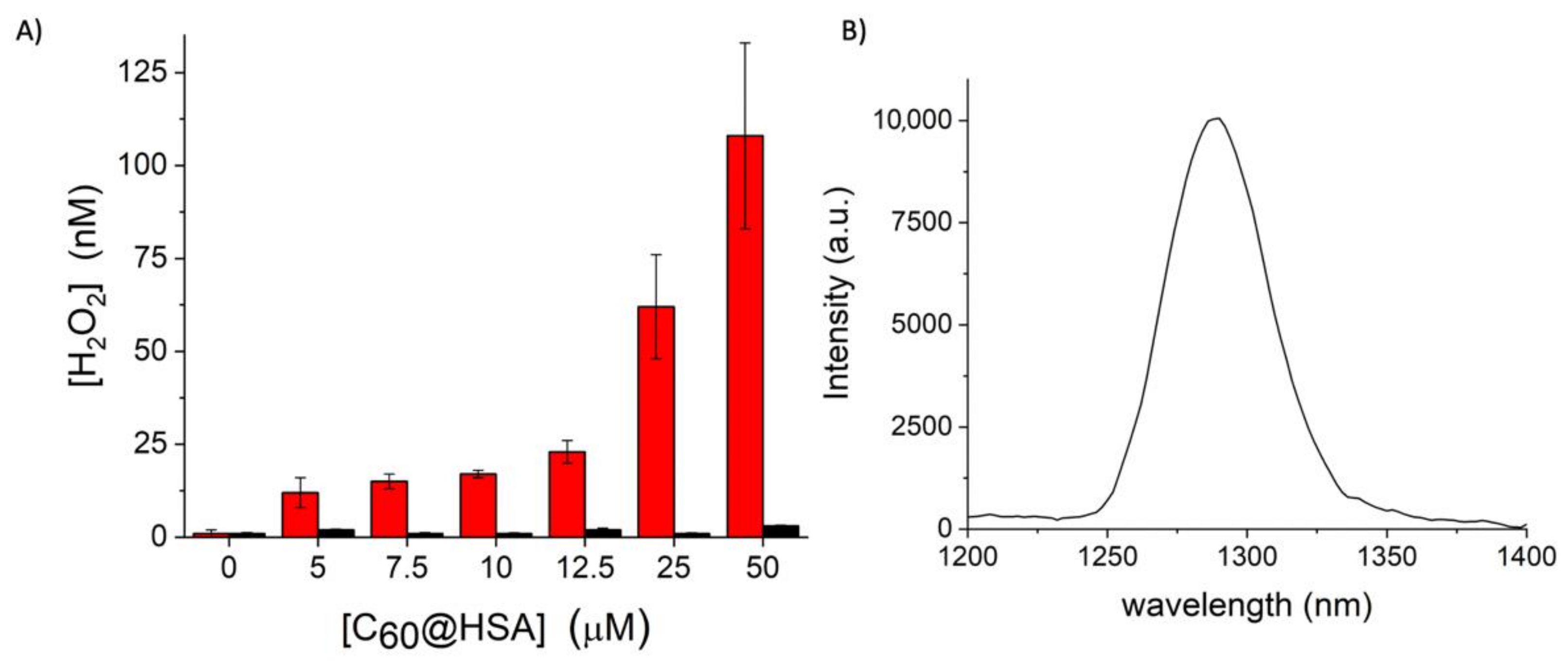
2.1.3. Amplex Red Assay for Peroxides Quantification
2.1.4. The 1O2 NIR Fluorometric Detection
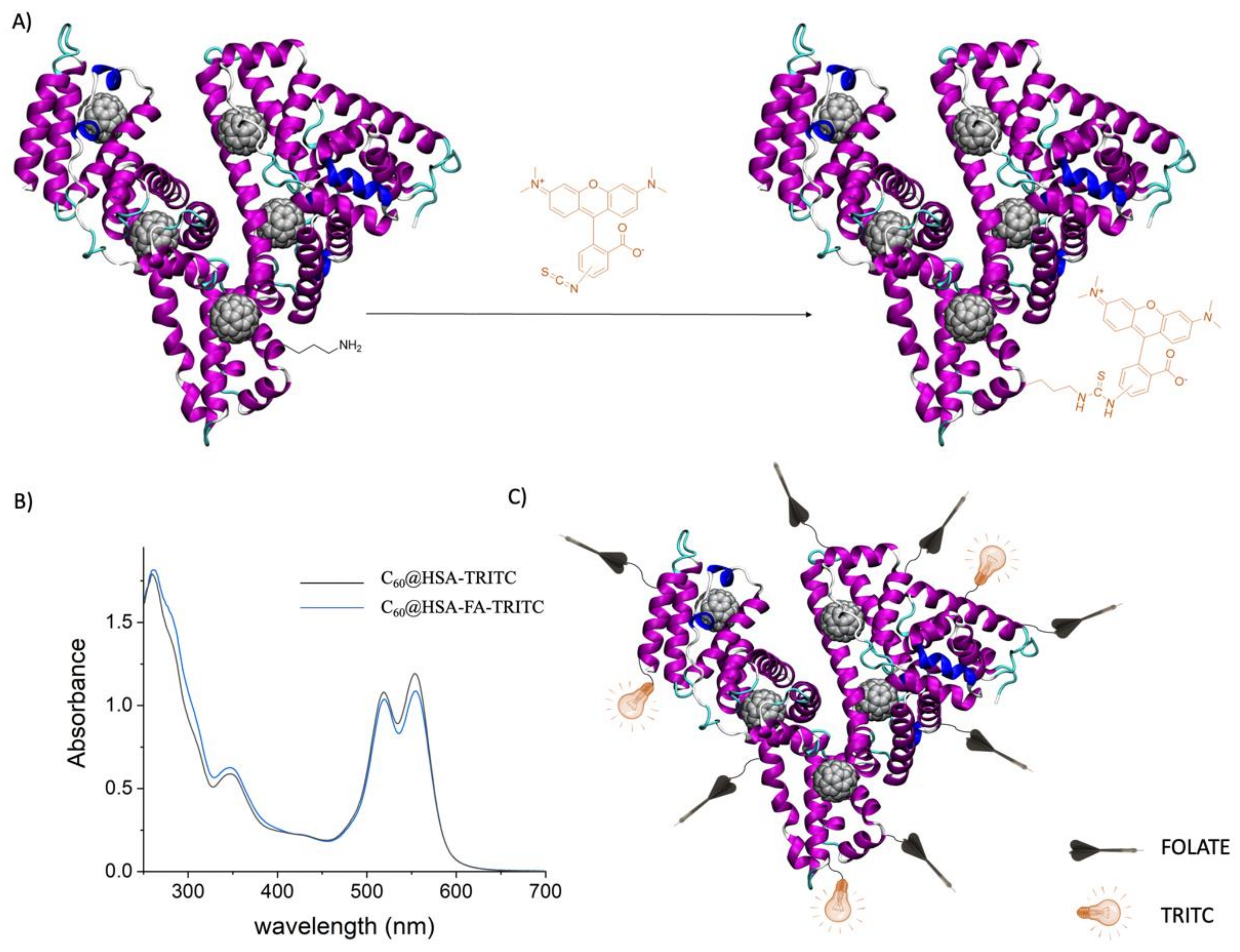
2.1.5. C60@HSA-FA Synthesis and Purification
2.1.6. C60@HSA-FA-TRITC Synthesis and Purification
2.1.7. Characterization of the HSA Bioconjugates
2.2. Computational Details
2.2.1. Docking
2.2.2. Minimization and MD Simulations
2.2.3. Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) Analysis
2.3. Cellular Experiments
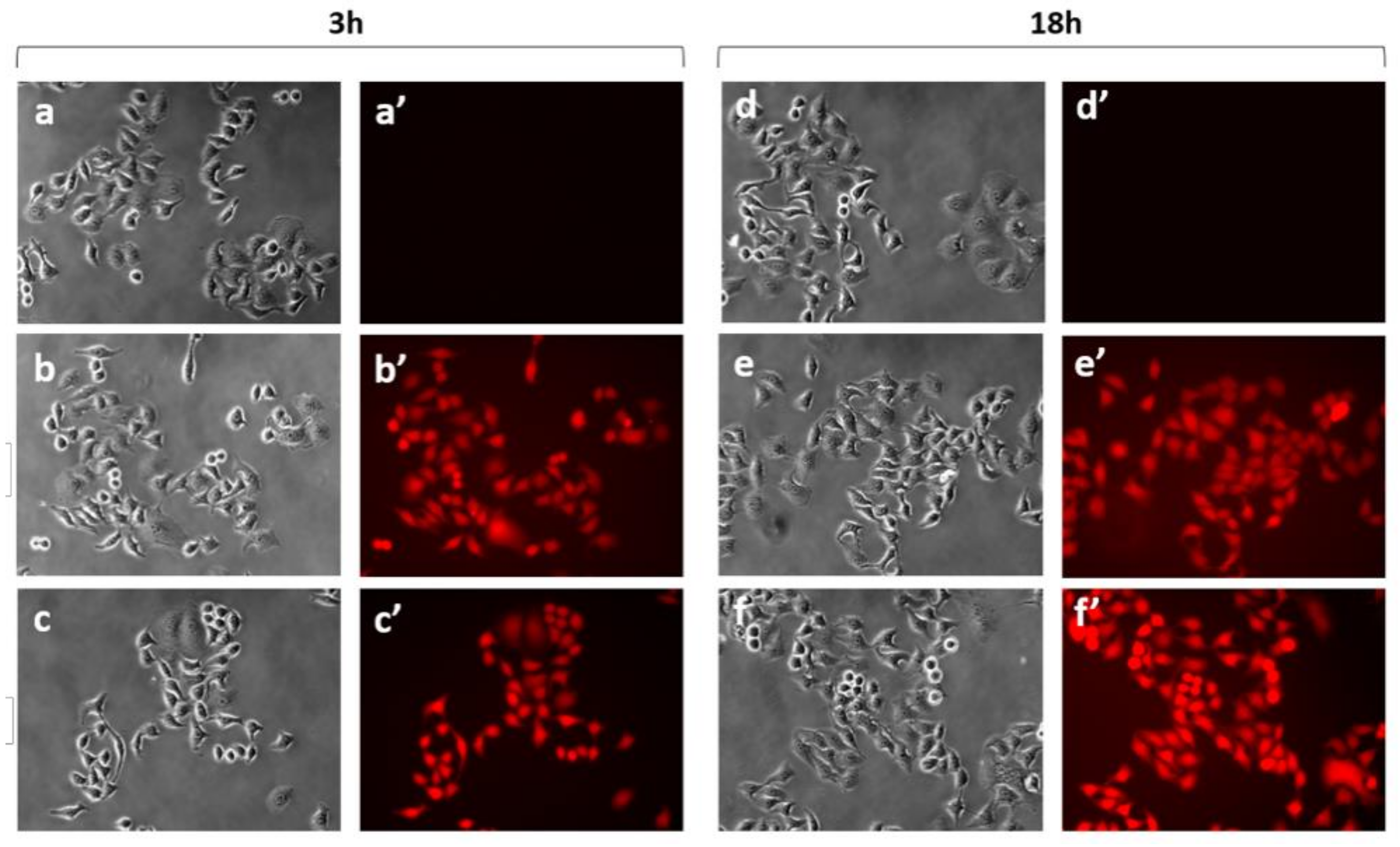
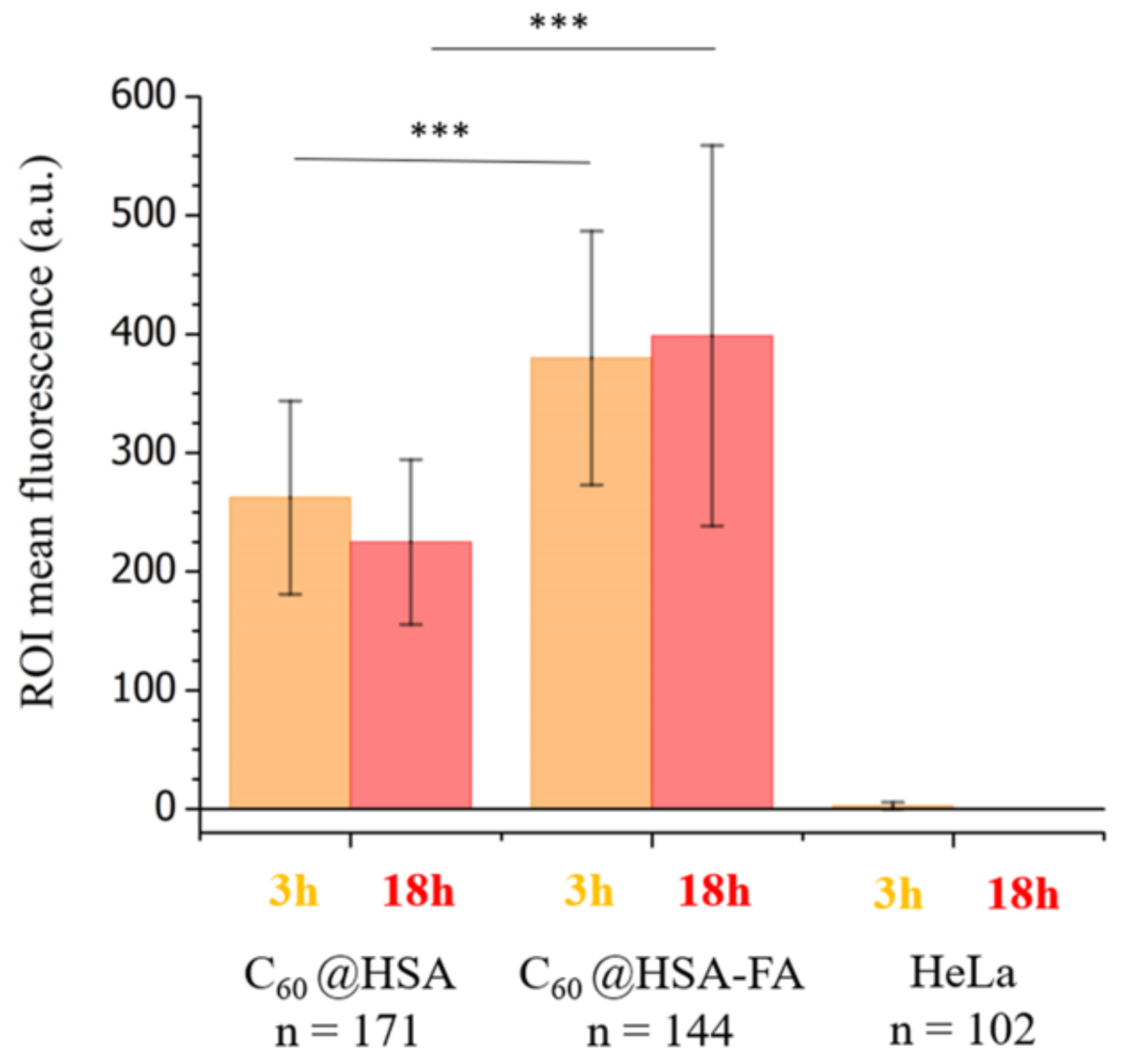
2.3.1. Internalization Studies of C60@HSA-TRITC and C60@HSA-FA-TRITC
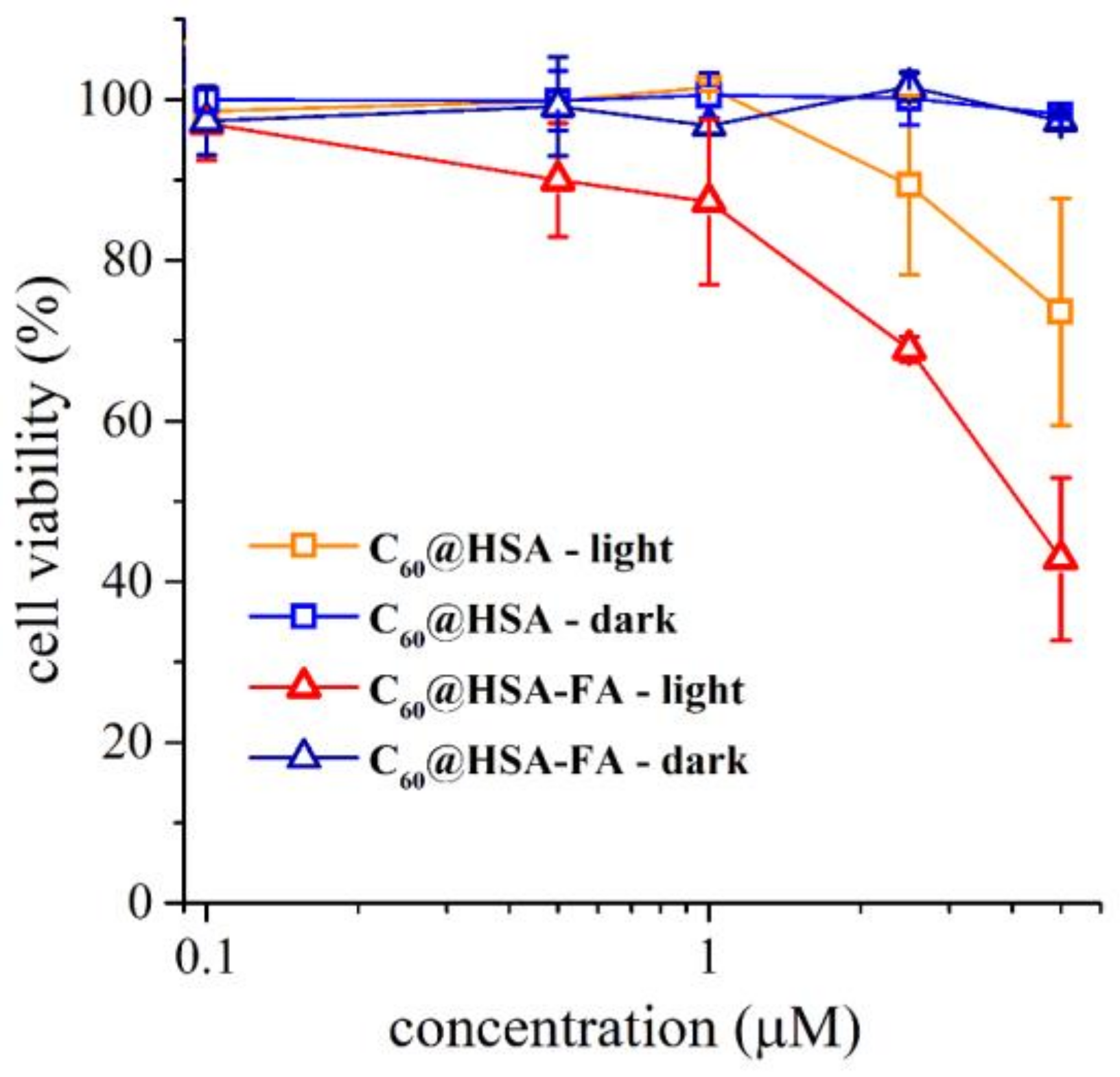
2.3.2. Cytotoxicity and Photodynamic Activity of C60@HSA and C60@HSA-FA
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Markovic, Z.; Todorovic-Markovic, B.; Kleut, D.; Nikolic, N.; Vranjes-Djuric, S.; Misirkic, M.; Vucicevic, L.; Janjetovic, K.; Isakovic, A.; Harhaji, L.; et al. The mechanism of cell-damaging reactive oxygen generation by colloidal fullerenes. Biomaterials 2007, 28, 5437–5448. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Antoku, D.; Sugikawa, K.; Ikeda, A. Photodynamic Activity of Fullerene Derivatives Solubilized in Water by Natural-Product-Based Solubilizing Agents. Chem.—A Eur. J. 2019, 25, 1854–1865. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Hotze, E.M.; Labille, J.; Alvarez, P.; Wiesner, M.R. Mechanisms of photochemistry and reactive oxygen production by fullerene suspensions in water. Environ. Sci. Technol. 2008, 42, 4175–4180. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.-Y.; Chignell, C.F.; Yin, J.-J.; Andley, U.; Roberts, J.E. Difference in Phototoxicity of Cyclodextrin Complexed Fullerene [(γ-CyD)2/C60] and Its Aggregated Derivatives toward Human Lens Epithelial Cells. Chem. Res. Toxicol. 2009, 22, 660–667. [Google Scholar] [CrossRef]
- Chae, S.R.; Badireddy, A.R.; Farner Budarz, J.; Lin, S.; Xiao, Y.; Therezien, M.; Wiesner, M.R. Heterogeneities in fullerene nanoparticle aggregates affecting reactivity, bioactivity, and transport. ACS Nano 2010, 4, 5011–5018. [Google Scholar] [CrossRef]
- Guldi, D.M.; Prato, M. Excited-state properties of C60 fullerene derivatives. Acc. Chem. Res. 2000, 33, 695–703. [Google Scholar] [CrossRef]
- Calvaresi, M.; Arnesano, F.; Bonacchi, S.; Bottoni, A.; Calò, V.; Conte, S.; Falini, G.; Fermani, S.; Losacco, M.; Montalti, M.; et al. C60@Lysozyme: Direct observation by nuclear magnetic resonance of a 1:1 fullerene protein adduct. ACS Nano 2014, 8, 1871–1877. [Google Scholar] [CrossRef]
- Di Giosia, M.; Bomans, P.H.H.; Bottoni, A.; Cantelli, A.; Falini, G.; Franchi, P.; Guarracino, G.; Friedrich, H.; Lucarini, M.; Paolucci, F.; et al. Proteins as supramolecular hosts for C60: A true solution of C60 in water. Nanoscale 2018, 10, 9908–9916. [Google Scholar] [CrossRef] [PubMed]
- Soldà, A.; Cantelli, A.; Di Giosia, M.; Montalti, M.; Zerbetto, F.; Rapino, S.; Calvaresi, M. C60@lysozyme: A new photosensitizing agent for photodynamic therapy. J. Mater. Chem. B 2017, 5, 6608–6615. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, M.; Nicolini, F.; Ferrazzano, L.; Soldà, A.; Valle, F.; Cantelli, A.; Marforio, T.D.; Bottoni, A.; Zerbetto, F.; Montalti, M.; et al. Stable and Biocompatible Monodispersion of C 60 in Water by Peptides. Bioconjug. Chem. 2019, 30, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Ko, D.-K.; Kim, Y.-T.; Kim, N.H.; Paul, J.; Zhang, S.-Q.; Murray, C.B.; Acharya, R.; DeGrado, W.F.; Kim, Y.H.; et al. Protein-directed self-assembly of a fullerene crystal. Nat. Commun. 2016, 7, 11429. [Google Scholar] [CrossRef]
- Vance, S.J.; Desai, V.; Smith, B.O.; Kennedy, M.W.; Cooper, A. Aqueous solubilization of C60 fullerene by natural protein surfactants, latherin and ranaspumin-2. Biophys. Chem. 2016, 214–215, 27–32. [Google Scholar]
- Di Giosia, M.; Valle, F.; Cantelli, A.; Bottoni, A.; Zerbetto, F.; Calvaresi, M. C60 bioconjugation with proteins: Towards a palette of carriers for all pH ranges. Materials 2018, 11, 691. [Google Scholar] [CrossRef]
- Liutkus, M.; López-Andarias, A.; Mejías, S.H.; López-Andarias, J.; Gil-Carton, D.; Feixas, F.; Osuna, S.; Matsuda, W.; Sakurai, T.; Seki, S.; et al. Protein-directed crystalline 2D fullerene assemblies. Nanoscale 2020, 12, 3614–3622. [Google Scholar] [CrossRef]
- Di Giosia, M.; Soldà, A.; Seeger, M.; Cantelli, A.; Arnesano, F.; Nardella, M.I.; Mangini, V.; Valle, F.; Montalti, M.; Zerbetto, F.; et al. A Bio-Conjugated Fullerene as a Subcellular-Targeted and Multifaceted Phototheranostic Agent. Adv. Funct. Mater. 2021, 31, 2101527. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Geremia, S. Atomic details of carbon-based nanomolecules interacting with proteins. Molecules 2020, 25, 3555. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, M.; Zerbetto, F.; Calvaresi, M. Incorporation of Molecular Nanoparticles Inside Proteins: The Trojan Horse Approach in Theranostics. Accounts Mater. Res. 2021, 2, 594–605. [Google Scholar] [CrossRef]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Z. Albumin Carriers for Cancer Theranostics: A Conventional Platform with New Promise. Adv. Mater. 2016, 28, 10557–10566. [Google Scholar] [CrossRef]
- Cantelli, A.; Malferrari, M.; Soldà, A.; Simonetti, G.; Forni, S.; Toscanella, E.; Mattioli, E.J.; Zerbetto, F.; Zanelli, A.; Di Giosia, M.; et al. Human Serum Albumin–Oligothiophene Bioconjugate: A Phototheranostic Platform for Localized Killing of Cancer Cells by Precise Light Activation. JACS Au 2021, 1, 925–935. [Google Scholar] [CrossRef]
- Tao, C.; Chuah, Y.J.; Xu, C.; Wang, D.A. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J. Mater. Chem. B 2019, 7, 357–367. [Google Scholar] [CrossRef]
- Teixeira, S.; Carvalho, M.A.; Castanheira, E.M.S. Functionalized Liposome and Albumin-Based Systems as Carriers for Poorly Water-Soluble Anticancer Drugs: An Updated Review. Biomedicines 2022, 10, 486. [Google Scholar] [CrossRef]
- Rozhkov, S.P.; Goryunov, A.S.; Sukhanova, G.A.; Borisova, A.G.; Rozhkova, N.N.; Andrievsky, G.V. Protein interaction with hydrated C60 fullerene in aqueous solutions. Biochem. Biophys. Res. Commun. 2003, 303, 562–566. [Google Scholar] [CrossRef]
- Belgorodsky, B.; Fadeev, L.; Ittah, V.; Benyamini, H.; Zelner, S.; Huppert, D.; Kotlyar, A.B.; Gozin, M. Formation and Characterization of Stable Human Serum Albumin−Tris-malonic Acid [C60]Fullerene Complex. Bioconjug. Chem. 2005, 16, 1058–1062. [Google Scholar] [CrossRef]
- Zhen, M.; Zheng, J.; Ye, L.; Li, S.; Jin, C.; Li, K.; Qiu, D.; Han, H.; Shu, C.; Yang, Y.; et al. Maximizing the relaxivity of Gd-complex by synergistic effect of HSA and carboxylfullerene. ACS Appl. Mater. Interfaces 2012, 4, 3724–3729. [Google Scholar] [CrossRef] [PubMed]
- Giełdoń, A.; Witt, M.M.; Gajewicz, A.; Puzyn, T. Rapid insight into C60 influence on biological functions of proteins. Struct. Chem. 2017, 28, 1775–1788. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Zhu, X. Computational studies of the binding mechanisms of fullerenes to human serum albumin. J. Mol. Model. 2015, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Leonis, G.; Avramopoulos, A.; Papavasileiou, K.D.; Reis, H.; Steinbrecher, T.; Papadopoulos, M.G. A Comprehensive Computational Study of the Interaction between Human Serum Albumin and Fullerenes. J. Phys. Chem. B 2015, 119, 14971–14985. [Google Scholar] [CrossRef] [PubMed]
- Krumkacheva, O.A.; Timofeev, I.O.; Politanskaya, L.V.; Polienko, Y.F.; Tretyakov, E.V.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Chubarov, A.S.; Bagryanskaya, E.G.; et al. Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR Spectroscopy. Angew. Chemie 2019, 131, 13405–13409. [Google Scholar] [CrossRef]
- Serda, M.; Szewczyk, G.; Krzysztyńska-Kuleta, O.; Korzuch, J.; Dulski, M.; Musioł, R.; Sarna, T. Developing [60]Fullerene Nanomaterials for Better Photodynamic Treatment of Non-Melanoma Skin Cancers. ACS Biomater. Sci. Eng. 2020, 6, 5930–5940. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, C.; Xie, L.; Wang, C.; Zhang, Y.; Xiang, J.; Li, L.; Tang, Y. Protein Conformation Changes Induced by a Novel Organophosphate-Containing Water-Soluble Derivative of a C60 Fullerene Nanoparticle. J. Phys. Chem. C 2007, 111, 14327–14333. [Google Scholar] [CrossRef]
- Belgorodsky, B.; Fadeev, L.; Kolsenik, J.; Gozin, M. Formation of a soluble stable complex between pristine C 60-fullerene and a native blood protein. ChemBioChem 2006, 7, 1783–1789. [Google Scholar] [CrossRef]
- Benyamini, H.; Shulman-Peleg, A.; Wolfson, H.J.; Belgorodsky, B.; Fadeev, L.; Gozin, M. Interaction of C60-Fullerene and Carboxyfullerene with Proteins: Docking and Binding Site Alignment. Bioconjugate Chem. 2006, 17, 378–386. [Google Scholar] [CrossRef]
- Zhang, M.F.; Xu, Z.Q.; Ge, Y.S.; Jiang, F.L.; Liu, Y. Binding of fullerol to human serum albumin: Spectroscopic and electrochemical approach. J. Photochem. Photobiol. B Biol. 2012, 108, 34–43. [Google Scholar] [CrossRef]
- Qu, X.; Komatsu, T.; Sato, T.; Glatter, O.; Horinouchi, H.; Kobayashi, K.; Tsuchida, E. Structure, Photophysical Property, and Cytotoxicity of Human Serum Albumin Complexed with Tris(dicarboxymethylene)[60]fullerene. Bioconjug. Chem. 2008, 19, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalik, A.; Hibah, A.; Zainy, B.M.; Makoto, A.; Daisuke, I.; Masaki, O.; Kaneto, U.; Fumitoshi, H. Preparation of soluble stable C60/human serum albumin nanoparticles via cyclodextrin complexation and their reactive oxygen production characteristics. Life Sci. 2013, 93, 277–282. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Mo, Y.; Cummings, P.T.; Heller, W.T. Human serum albumin interactions with C60 fullerene studied by spectroscopy, small-angle neutron scattering, and molecular dynamics simulations. J. Nanoparticle Res. 2013, 15, 1769. [Google Scholar] [CrossRef]
- Song, M.; Liu, S.; Yin, J.; Wang, H. Interaction of human serum album and C 60 aggregates in solution. Int. J. Mol. Sci. 2011, 12, 4964–4974. [Google Scholar] [CrossRef] [PubMed]
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Kang, Y.J.; Holley, C.K.; Abidian, M.R.; Madhankumar, A.B.; Connor, J.; Majd, S. Tumor Targeted Delivery of an Anti-Cancer Therapeutic: An In Vitro and In Vivo Evaluation. Adv. Healthc. Mater. 2021, 10, 2001207. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Ding, X.; Liu, J.; Luo, Z.; Liu, Y.; Ran, Q.; Cai, K. Multifunctional Fe2O3@PPy-PEG nanocomposite for combination cancer therapy with MR imaging. Nanotechnology 2015, 26, 425101. [Google Scholar] [CrossRef]
- Bartolini, L.; Malferrari, M.; Lugli, F.; Zerbetto, F.; Paolucci, F.; Pelicci, P.G.; Albonetti, C.; Rapino, S. Interaction of Single Cells with 2D Organic Monolayers: A Scanning Electrochemical Microscopy Study. ChemElectroChem 2018, 5, 2975–2981. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Calvaresi, M.; Zerbetto, F. Baiting proteins with C60. ACS Nano 2010, 4, 2283–2299. [Google Scholar] [CrossRef]
- Calvaresi, M.; Zerbetto, F. Fullerene sorting proteins. Nanoscale 2011, 3, 2873–2881. [Google Scholar] [CrossRef]
- Ahmed, L.; Rasulev, B.; Kar, S.; Krupa, P.; Mozolewska, M.A.; Leszczynski, J. Inhibitors or toxins? Large library target-specific screening of fullerene-based nanoparticles for drug design purpose. Nanoscale 2017, 9, 10263–10276. [Google Scholar] [CrossRef]
- Calvaresi, M.; Furini, S.; Domene, C.; Bottoni, A.; Zerbetto, F. Blocking the passage: C60 geometrically clogs K+ channels. ACS Nano 2015, 9, 4827–4834. [Google Scholar] [CrossRef][Green Version]
- Di Giosia, M.; Marforio, T.D.; Cantelli, A.; Valle, F.; Zerbetto, F.; Su, Q.; Wang, H.; Calvaresi, M. Inhibition of α-chymotrypsin by pristine single-wall carbon nanotubes: Clogging up the active site. J. Colloid Interface Sci. 2020, 571, 174–184. [Google Scholar] [CrossRef]
- Bologna, F.; Mattioli, E.J.; Bottoni, A.; Zerbetto, F.; Calvaresi, M. Interactions between Endohedral Metallofullerenes and Proteins: The Gd@C 60 –Lysozyme Model. ACS Omega 2018, 3, 13782–13789. [Google Scholar] [CrossRef]
- Di Giosia, M.; Valle, F.; Cantelli, A.; Bottoni, A.; Zerbetto, F.; Fasoli, E.; Calvaresi, M. High-throughput virtual screening to rationally design protein—Carbon nanotube interactions. Identification and preparation of stable water dispersions of protein—Carbon nanotube hybrids and efficient design of new functional materials. Carbon N. Y. 2019, 147, 70–82. [Google Scholar] [CrossRef]
- Berto, M.; Di Giosia, M.; Giordani, M.; Sensi, M.; Valle, F.; Alessandrini, A.; Menozzi, C.; Cantelli, A.; Gazzadi, G.C.; Zerbetto, F.; et al. Green Fabrication of (6,5)Carbon Nanotube/Protein Transistor Endowed with Specific Recognition. Adv. Electron. Mater. 2021, 7, 2001114. [Google Scholar] [CrossRef]
- Marforio, T.D.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Fullerenes against COVID-19: Repurposing C60 and C70 to Clog the Active Site of SARS-CoV-2 Protease. Molecules 2022, 27, 1916. [Google Scholar] [CrossRef] [PubMed]
- Marforio, T.D.; Calza, A.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Dissecting the supramolecular dispersion of fullerenes by proteins/peptides: Amino acid ranking and driving forces for binding to c60. Int. J. Mol. Sci. 2021, 22, 11567. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2012, 20, 372–382. [Google Scholar] [CrossRef]
- Malferrari, M.; Ghelli, A.; Roggiani, F.; Valenti, G.; Paolucci, F.; Rugolo, M.; Rapino, S. Reactive Oxygen Species Produced by Mutated Mitochondrial Respiratory Chains of Entire Cells Monitored Using Modified Microelectrodes. ChemElectroChem 2018, 6, 627–633. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal bioconjugate for targeted Gram-negative antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852. [Google Scholar] [CrossRef]
- Bortot, B.; Apollonio, M.; Baj, G.; Andolfi, L.; Zupin, L.; Crovella, S.; di Giosia, M.; Cantelli, A.; Saporetti, R.; Ulfo, L.; et al. Advanced photodynamic therapy with an engineered M13 phage targeting EGFR: Mitochondrial localization and autophagy induction in ovarian cancer cell lines. Free Radic. Biol. Med. 2022, 179, 242–251. [Google Scholar] [CrossRef]
- Ulfo, L.; Cantelli, A.; Petrosino, A.; Costantini, P.E.; Nigro, M.; Starinieri, F.; Turrini, E.; Zadran, S.K.; Zuccheri, G.; Saporetti, R.; et al. Orthogonal nanoarchitectonics of M13 phage for receptor targeted anticancer photodynamic therapy. Nanoscale 2022, 14, 632–641. [Google Scholar] [CrossRef]
- Butzbach, K.; Rasse-Suriani, F.A.O.; Gonzalez, M.M.; Cabrerizo, F.M.; Epe, B. Albumin–Folate Conjugates for Drug-targeting in Photodynamic Therapy. Photochem. Photobiol. 2016, 92, 611–619. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Brusa, P.; Cattel, L. Folate-mediated targeting of albumin conjugates of paclitaxel obtained through a heterogeneous phase system. Int. J. Pharm. 2009, 382, 117–123. [Google Scholar] [CrossRef]
- Dubey, R.D.; Alam, N.; Saneja, A.; Khare, V.; Kumar, A.; Vaidh, S.; Mahajan, G.; Sharma, P.R.; Singh, S.K.; Mondhe, D.M.; et al. Development and evaluation of folate functionalized albumin nanoparticles for targeted delivery of gemcitabine. Int. J. Pharm. 2015, 492, 80–91. [Google Scholar] [CrossRef]
- Akbarian, A.; Ebtekar, M.; Pakravan, N.; Hassan, Z.M. Folate receptor alpha targeted delivery of artemether to breast cancer cells with folate-decorated human serum albumin nanoparticles. Int. J. Biol. Macromol. 2020, 152, 90–101. [Google Scholar] [CrossRef]
- Kranz, D.M.; Patrick, T.A.; Brigle, K.E.; Spinella, M.J.; Roy, E.J. Conjugates of folate and anti-T-cell-receptor antibodies specifically target folate-receptor-positive tumor cells for lysis. Proc. Natl. Acad. Sci. USA 1995, 92, 9057–9061. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O.; Mitin, D.; Suvorov, D.; Tormyshev, V.; Fedin, M.; Bowman, M.K.; Bagryanskaya, E. Reversible dimerization of human serum albumin. Molecules 2021, 26, 108. [Google Scholar] [CrossRef] [PubMed]
- Babcock, J.J.; Brancaleon, L. Bovine serum albumin oligomers in the E- and B-forms at low protein concentration and ionic strength. Int. J. Biol. Macromol. 2013, 53, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Alargova, R.G.; Tsujii, K. Stable dispersions of fullerenes, C60 and C70, in water. Preparation and characterization. Langmuir 2001, 17, 6013–6017. [Google Scholar] [CrossRef]
- Bellotti, E.; Cascone, M.G.; Barbani, N.; Rossin, D.; Rastaldo, R.; Giachino, C.; Cristallini, C. Targeting cancer cells overexpressing folate receptors with new terpolymer-based nanocapsules: Toward a novel targeted dna delivery system for cancer therapy. Biomedicines 2021, 9, 1275. [Google Scholar] [CrossRef]
- Shimada, R.; Hino, S.; Yamana, K.; Kawasaki, R.; Konishi, T.; Ikeda, A. Improvement of Photodynamic Activity by a Stable System Consisting of a C60 Derivative and Photoantenna in Liposomes. ACS Med. Chem. Lett. 2022, 13, 641–647. [Google Scholar] [CrossRef]
- Kawasaki, R.; Antoku, D.; Ohdake, R.; Sugikawa, K.; Ikeda, A. Bacterial elimination via the photodynamic activity of a fullerene/light-harvesting antenna molecule assembled system integrated into liposome membranes. Nanoscale Adv. 2020, 2, 4395–4399. [Google Scholar] [CrossRef]
- Antoku, D.; Satake, S.; Mae, T.; Sugikawa, K.; Funabashi, H.; Kuroda, A.; Ikeda, A. Improvement of Photodynamic Activity of Lipid–Membrane-Incorporated Fullerene Derivative by Combination with a Photo-Antenna Molecule. Chem.—A Eur. J. 2018, 24, 7335–7339. [Google Scholar] [CrossRef]
- Liu, J.; Şen Karaman, D.; Zhang, J.; Rosenholm, J.M.; Guo, X.; Cai, K. NIR light-activated dual-modality cancer therapy mediated by photochemical internalization of porous nanocarriers with tethered lipid bilayers. J. Mater. Chem. B 2017, 5, 8289–8298. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Jung, S.; Chung, I.J.; Ahn, C.H. Targeted multimodal imaging modalities. Adv. Drug Deliv. Rev. 2014, 76, 60–78. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantelli, A.; Malferrari, M.; Mattioli, E.J.; Marconi, A.; Mirra, G.; Soldà, A.; Marforio, T.D.; Zerbetto, F.; Rapino, S.; Di Giosia, M.; et al. Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation. Nanomaterials 2022, 12, 3501. https://doi.org/10.3390/nano12193501
Cantelli A, Malferrari M, Mattioli EJ, Marconi A, Mirra G, Soldà A, Marforio TD, Zerbetto F, Rapino S, Di Giosia M, et al. Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation. Nanomaterials. 2022; 12(19):3501. https://doi.org/10.3390/nano12193501
Chicago/Turabian StyleCantelli, Andrea, Marco Malferrari, Edoardo Jun Mattioli, Alessia Marconi, Giulia Mirra, Alice Soldà, Tainah Dorina Marforio, Francesco Zerbetto, Stefania Rapino, Matteo Di Giosia, and et al. 2022. "Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation" Nanomaterials 12, no. 19: 3501. https://doi.org/10.3390/nano12193501
APA StyleCantelli, A., Malferrari, M., Mattioli, E. J., Marconi, A., Mirra, G., Soldà, A., Marforio, T. D., Zerbetto, F., Rapino, S., Di Giosia, M., & Calvaresi, M. (2022). Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation. Nanomaterials, 12(19), 3501. https://doi.org/10.3390/nano12193501






