Abstract
Herein, we describe pH and magnetism dual-responsive liquid paraffin-in-water Pickering emulsion stabilized by dynamic covalent Fe3O4 (DC-Fe3O4) nanoparticles. On one hand, the Pickerinfigureg emulsions are sensitive to pH variations, and efficient demulsification can be achieved by regulating the pH between 10 and 2 within 30 min. The dynamic imine bond in DC-Fe3O4 can be reversibly formed and decomposed, resulting in a pH-controlled amphiphilicity. The Pickering emulsion can be reversibly switched between stable and unstable states by pH at least three times. On the other hand, the magnetic Fe3O4 core of DC-Fe3O4 allowed rapid separation of the oil droplets from Pickering emulsions under an external magnetic field within 40 s, which was a good extraction system for purifying the aqueous solution contaminated by rhodamine B. The dual responsiveness enables Pickering emulsions to have better control of their stability and to be applied more broadly.
1. Introduction
Pickering emulsions, which were stabilized by solid particles, have gained much attention thanks to the low toxicity and long-term stability [1,2]. The outstanding stability of Pickering emulsions is required in the field of cosmetic formulations, food storage, and so on [3]. However, in some fields like heterogeneous catalysis [4,5], emulsion polymerization [6], and oil transportation and recovery [7,8,9], temporary stabilization is required. Chemical or physical methods are used in the industry to achieve demulsification, which are often energy-intensive or require extra complex additives, leading to increased economic and environmental costs [10,11]. Thus, stimuli-responsive Pickering emulsions are desired in the above applications because the stability of the emulsions can be easily controlled by external stimuli [12].
Recently, stimuli-responsive Pickering emulsions have received much attention [13,14,15,16,17]. So far, the stimuli of the Pickering emulsions include pH [18,19,20], magnetism [21,22,23], CO2 [24], temperature [25], light irradiation [26], redox state [27], specific ion concentration [28], and so on. Under outside stimuli, stable Pickering emulsions can be phase separated because of the inactivation of the stabilizer. There is merit in combining two stimuli to widen the controllable range or improve the degree of precision [16]. Pickering emulsions responding to dual triggers such as temperature–pH [29], CO2–temperature [16], CO2–magnetism [14,30], and redox–magnetism [9] have been investigated. For CO2-stimulated systems, a long time and high ventilation are needed [31]. Light-stimulated systems are difficult to achieve because of the cloudy appearance of the Pickering emulsions [32]. Temperature-stimulated systems are energy-demanding [31]. Among these stimuli, pH is readily implementable and magnetism is non-invasive and reversible [2,7,10,32,33]. Therefore, pH and magnetism dual-responsive Pickering emulsions have promising applications.
Magnetism-responsive Pickering emulsions have received considerable attention because of their application as extractors for dye molecules, such as Rhodamine B, methylene blue, and Nile blue [9]. Yang et al. [34] prepared a kind of magnetism-responsive Pickering emulsion using amphiphilic Fe3O4 particles as a stabilizer, which could be used as an extractor to remove methyl orange from the aqueous solution. Therefore, magnetism-responsive Pickering emulsion can be an attractive tool to remove dye molecules in water.
In the present study, dynamic covalent Fe3O4 (DC-Fe3O4) was prepared through pH sensitive dynamic imine bond (DIB) formation between amino-Fe3O4 (Fe3O4-NH2) and benzaldehyde. The amphiphilicity of DC-Fe3O4 nanoparticles could be modulated by pH because of the pH-responsiveness of DIB. The prepared amphiphilic DC-Fe3O4 could achieve effective emulsification of the oil phase at pH 10, while the following demulsification process could be completed by decreasing the pH from 10 to 2. Moreover, the droplets move in the direction of the magnet. The magnetism-responsive Pickering emulsion could be used as an extractor to adsorb Rhodamine B (RhB) from aqueous solution at least three times. This novel pH and magnetism dual-responsive Pickering emulsion has potential applications in oil recovery, emulsion polymerization, and dye extraction.
2. Experimental
2.1. Materials
Iron sulfate heptahydrate (FeSO4·7H2O, AR), ferric chloride (FeCl3, AR), sodium hydroxide (NaOH, AR), (3-aminopropyl) triethoxysilane (APTES, 98%), Nile red (≥95.0%), and Rhodamine B (RhB, AR) were obtained from Aladdin Reagents of China. Benzaldehyde (AR), liquid paraffin (AR), and hydrogen chloride (36.5 wt%) were supplied by Sinopharm Chemical Reagent Co. Ltd., Shanghai, China. All of the chemicals were used as received without any further purification.
2.2. Synthesis of DC-Fe3O4 Nanoparticles
Fe3O4 was synthesized using the co-precipitation method [35]. FeSO4 solution with a concentration of 0.5 M and FeCl3 solution with a concentration of 1 M were prepared using 0.2 M HCl (aq), respectively, as solvent. During preparation, 400 mL of 1.5 M NaOH (aq) was poured into the three flasks and heated to 82 °C, and then the mixture of FeSO4 solution (40 mL, 0.5 M) and FeCl3 solution (40 mL, 1 M) was added to the three flasks dropwise within 30 min under the atmosphere of N2 at 82 °C. As soon as the mixture turned to black, it was allowed to cool down to 25 °C under mechanical stirring. Then, the resultant black nanoparticles were washed with ethanol four times with the help of a magnet. The obtained product Fe3O4 nanoparticles were dried under a vacuum for 16 h.
The amino modified Fe3O4 (Fe3O4-NH2) nanoparticles were obtained by surface silanization of Fe3O4 nanoparticles (Figure 1a). In detail, 0.5 mL APTES and 0.95 g Fe3O4 were added into 25 mL ethanol at 25 °C. After stirring for 24 h, the obtained Fe3O4-NH2 was washed with ethanol four times with the help of a magnet and then dried under vacuum for 16 h.
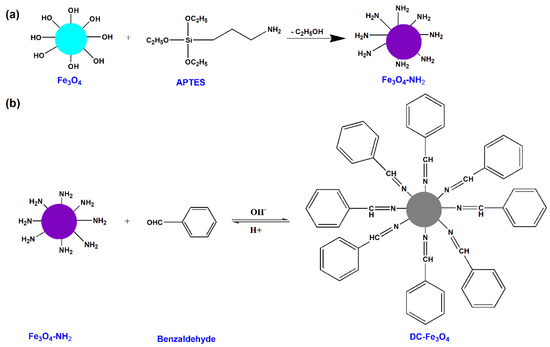
Figure 1.
Schematic illustration of the synthesis of Fe3O4-NH2 (a) and DC-Fe3O4 (b).
To prepare dynamic covalent Fe3O4 (DC-Fe3O4), Fe3O4-NH2 (1.0 g) and benzaldehyde were mixed in methanol (20 mL) under mechanical stirring for 1 h; the molar ratio of -NH2 and -CHO was 1. A schematic illustration of the synthesis of DC-Fe3O4 is presented in Figure 1b. The product was collected and then washed with ethanol four times with the help of a magnet. The product DC-Fe3O4 nanoparticles were dried under vacuum for 16 h.
2.3. Preparation of Pickering Emulsions
For Pickering emulsions’ preparation, water, DC-Fe3O4 nanoparticles, and liquid paraffin were placed into a glass bottle. The concentration of DC-Fe3O4 varied from 0.1 to 2.0 wt%. The water and liquid paraffin were in an equal volume ratio. Then, the mixture was homogenized using a JY88-IIN sonicator with a 6 mm probe at 50 W twelve times (5 s, 5 s off) to obtain Pickering emulsions. The Pickering emulsions were kept at room temperature for 1 month to observe the storage stability.
2.4. Characterization of DC-Fe3O4 Nanoparticles
DC-Fe3O4 nanoparticles were dispersed in water at a concentration of 0.05 wt%. Approximately 20 μL of the above DC-Fe3O4 dispersion was loaded onto a copper grid. The DC-Fe3O4 sample was allowed to dry and imaged using a transmission electron microscope (Hitachi HT7700).
The composition of the Fe3O4, Fe3O4-NH2, and DC-Fe3O4 was investigated by FTIR spectrometer (Bruker Optics, Germany). The morphology of DC-Fe3O4 was characterized using TEM (Hitachi HT7700). For solid/air/water three-phase contact angle measurement, samples of the Fe3O4, Fe3O4-NH2, and DC-Fe3O4 were compressed into films.
For three-phase (solid/air/water) contact angle measurements, samples of the Fe3O4, Fe3O4-NH2, and DC-Fe3O4 nanoparticles were compressed into films using a table press (Shimadzu Press). The contact angle was measured using the contact angle goniometer (DataPhysics Instruments GmbH, Filderstadt, Germany). The contact angle was recorded by placing a droplet of water with a volume of 3.0 μL onto the film in the air. When the water was in contact with the film, the image of the morphology of the water droplet on the film surface was recorded and later analyzed by the software Photoshop to obtain the contact angle.
2.5. Characterization of Pickering Emulsions
To conform the emulsion type, liquid paraffin was stained with Nile red. The Pickering emulsion droplets were detected with a confocal fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The micrographs of the Pickering emulsions were observed with an A1Pol optical microscope (ZEISS, Oberkochen, Germany).
2.6. pH Modulation of Pickering Emulsion
The pH modulation (between pH 10 and 2) of the Pickering emulsion was achieved by alternately adding 1 M HCl or 1 M NaOH, followed by stirring for 30 s and standing for 30 min.
2.7. Magnetic Modulation of Pickering Emulsion
The magnetism-responsive character of the Pickering emulsion was carried out by applying an NdFeB permanent magnet (Jiangsu Lingxi Magnetic Industry Co., Suzhou, China) with a size of 60 × 40 × 5 mm.
2.8. Extraction of RhB from the Aqueous Solution
The RhB-polluted aqueous solution (4 mg/L) was prepared by dissolving RhB in water. Pickering emulsion (1 mL) was added to RhB-polluted aqueous solution (5 mL) to extract RhB. After standing for 20 min, the purified water was separated over a magnet and poured out. Prior to and after extraction, the concentration of RhB was determined using a UV/Vis spectrophotometer at 553 nm. According to the following equation, the extraction efficiency (E) can be calculated:
where C0 (mg/L) and Ce (mg/L) are the concentrations of RhB aqueous solution before and after extraction, respectively. In addition, Pickering emulsion could be obtained again by washing with water, and the extraction process could be repeated at least three times.
3. Results and Discussion
3.1. Characterization of Dynamic Covalent Fe3O4
The preparation of DC-Fe3O4 nanoparticles through DIB formation between amino-Fe3O4 (Fe3O4-NH2) and benzaldehyde is presented in Figure 1. FTIR was performed to prove the successful fabrication of DC-Fe3O4 nanoparticles. As shown in Figure 2, the bond at 580 cm−1 in the Fe3O4 FTIR spectra was the characteristic peak of the Fe-O functional groups. Just like the peak presented in Fe3O4, the Fe-O absorption peak (580 cm−1) also appears in the spectra of Fe3O4-NH2 and DC-Fe3O4, suggesting the presence of Fe3O4 in Fe3O4-NH2 and DC-Fe3O4. The FTIR spectrum of Fe3O4-NH2 shows sp3 C-H asymmetric and symmetric stretching vibration at 2974 and 2900 cm−1, respectively, demonstrating the presence of aminopropyl groups in Fe3O4-NH2. For DC-Fe3O4, the presence of aromatic stretching vibrations at 3134, 1624, and 633 cm−1, as well as the imine bond (C=N) stretching vibration at 1645 cm−1, confirmed the formation of DC-Fe3O4 between Fe3O4-NH2 and benzaldehyde via DIB. The morphology DC-Fe3O4 nanoparticles were characterized by TEM (Figure S1). The TEM image shows that the DC-Fe3O4 nanoparticles have a nearly spherical shape with a mean diameter of about 17 nm. The DC-Fe3O4 nanoparticles exhibit an instant magnetic response to the external magnetic field and can be separated completely from their liquid dispersions within 30 min. When the magnetic field is removed, the DC-Fe3O4 nanoparticles can be dispersed again by shaking.
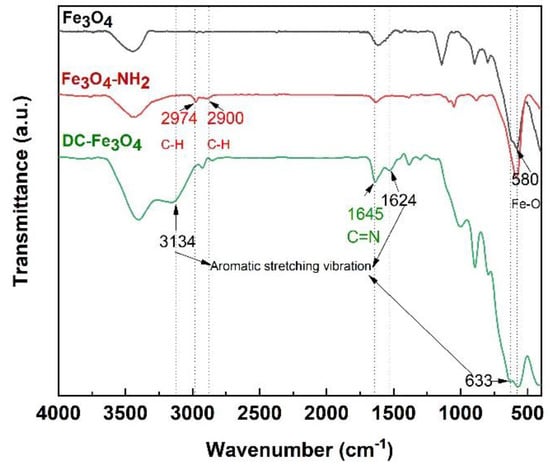
Figure 2.
FTIR spectra of Fe3O4, Fe3O4-NH2, and DC-Fe3O4 nanoparticles.
The stabilization of two immiscible phases was related to the wettability of particles at their interface, which was measured by contact angle [36,37,38]. Therefore, contact angle measurement was carried out for Fe3O4, Fe3O4-NH2, and DC-Fe3O4, respectively. Fe3O4 was found to have a contact angle of roughly 17° (Figure 3a), which was too hydrophilic for stabilizing Pickering emulsions; a similar result was reported by Sun et al. [9]. When Fe3O4 nanoparticles were modified by APTES, Fe3O4-NH2 nanoparticles also exhibit a hydrophilic character with a contact angle of about 25° (Figure 3b), which was also too hydrophilic for stabilizing Pickering emulsions. By modifying Fe3O4-NH2 nanoparticles with relatively hydrophobic benzaldehyde through DIB formation, the contact angle of modified Fe3O4 nanoparticles (DC-Fe3O4) increased to about 48° (Figure 3c). The improved amphiphilicity of DC-Fe3O4 is desirable to the formation of stable Pickering emulsions. It is well-known that the particle contact angle determines the type of Pickering emulsion [39,40]. If the contact angle of particles is less than 90°, they are located preferentially in the water phase, and the resulting curvature favors O/W Pickering emulsions. In contrast, if the contact angle exceeds 90°, the particles will reside primarily in the oil phase, which will lead to W/O Pickering emulsions [41]. Hence, amphiphilic DC-Fe3O4 nanoparticles with a contact angle of about 48° are expected to prepare O/W Pickering emulsions.
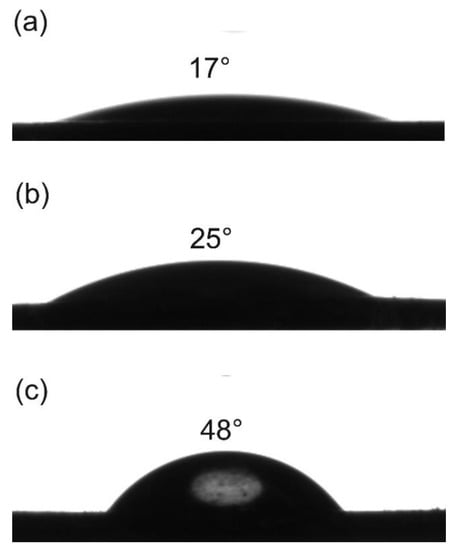
Figure 3.
Contact angles of water on Fe3O4 film (a), Fe3O4-NH2 film (b), and DC-Fe3O4 film (c).
The amphiphilicity of DC-Fe3O4 can be further proved by the dispersion behavior of DC-Fe3O4 on the liquid paraffin–water two phases. The DC-Fe3O4 nanoparticles straddle on the liquid paraffin–water interface instead of the aqueous phase even after shaking (Figure S2 and Figure 4a), indicating the amphipathic nature of the DC-Fe3O4 nanoparticles, consistent with the contact angle measurement.
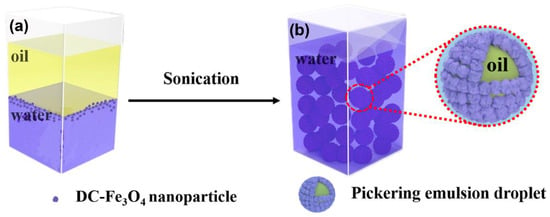
Figure 4.
Preparation process of Pickering emulsion stabilized by DC-Fe3O4 with a volume ratio of liquid paraffin and water of 1:1. (a) The mixture of liquid paraffin, water and DC-Fe3O4 nanoparticles before sonication, (b) the obtained Pickering emulsions after sonicating the mixture of liquid paraffin, water and DC-Fe3O4 nanoparticles in (a).
3.2. Preparation of Pickering Emulsions Stabilized by DC-Fe3O4 Nanoparticles
The liquid paraffin-in-water Pickering emulsion could not be stabilized by Fe3O4 or Fe3O4-NH2 alone for 30 min at the particle concentration of 1.0 wt% (a rather high concentration in Pickering emulsion preparation, Figure S3). The reason that Fe3O4 or Fe3O4-NH2 nanoparticles could not be used as the Pickering emulsifier might be attributed to the high hydrophilicity of Fe3O4 and Fe3O4-NH2, as proven by the result of the contact angle (Figure 3).
Based on the discussion of the contact angle, we presume that the amphiphilic DC-Fe3O4 nanoparticles with a contact angle of about 48° are anticipated to prepare O/W Pickering emulsions. The ability of DC-Fe3O4 nanoparticles to stabilize the Pickering emulsions was investigated; the preparation process of Pickering emulsions is presented in Figure 4. In the control experiment, we have proved that no emulsion was prepared without DC-Fe3O4 nanoparticles (Figure S4). With 0.1 wt% DC-Fe3O4 nanoparticles, no homogeneous Pickering emulsion could be prepared because of insufficient DC-Fe3O4 nanoparticles on the oil–water interface (Figure 5). Satisfactorily, when the DC-Fe3O4 nanoparticle concentrations were equal to or exceeding 0.25 wt%, stable Pickering emulsions were prepared (Figure 5), which might due to the effective adsorption of amphiphilic DC-Fe3O4 nanoparticles at the liquid paraffin–water interface (Figure 4b). The CLSM measurement shows that the labeled liquid paraffin is surrounded by the unlabeled water (Figure 6), indicating that O/W Pickering emulsion was formed.

Figure 5.
Photographs of the oil-in-water Pickering emulsions stabilized by DC-Fe3O4 nanoparticles at different concentrations. The photographs were taken at 24 h (a) and 1 month (b) after the sample preparation. The liquid paraffin and water were in an equal volume ratio. Concentrations of DC-Fe3O4 (wt%) in the Pickering emulsions are shown on the vessels.
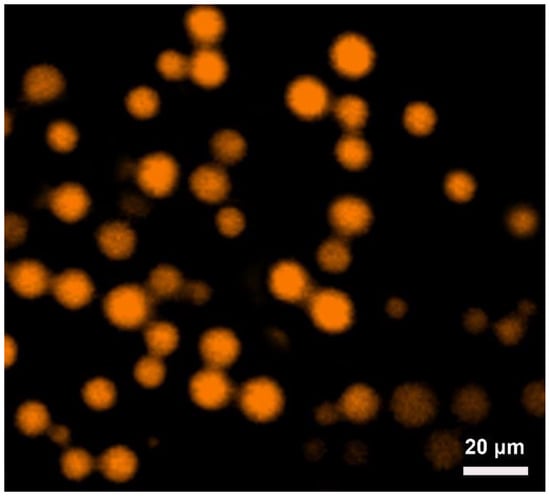
Figure 6.
CLSM image of 1.0 wt% DC-Fe3O4 nanoparticles stabilized liquid paraffin in water Pickering emulsion. The liquid paraffin (stained by Nile Red) and water were in an equal volume ratio.
The morphology of Pickering emulsions was observed by optical microscopy (Figure 7a–e). As shown in Figure 7a–e, small and spherical droplets were observed. According to the statistic, droplets became smaller with increasing DC-Fe3O4 nanoparticle concentrations: the mean droplet sizes of Pickering emulsions with a DC-Fe3O4 nanoparticle concentration of 0.25, 0.5, 1.0, 1.5, and 2.0 wt% were 107, 22, 18, 13, and 8 μm, respectively (Figure 7f). This was because more particles were able to stabilize larger interfaces at a constant oil–water ratio, which is a common feature for Pickering emulsions [10,17]. In this study, it was found that the size of Pickering emulsion droplets can be easily adjusted by choosing a suitable DC-Fe3O4 nanoparticle concentration.
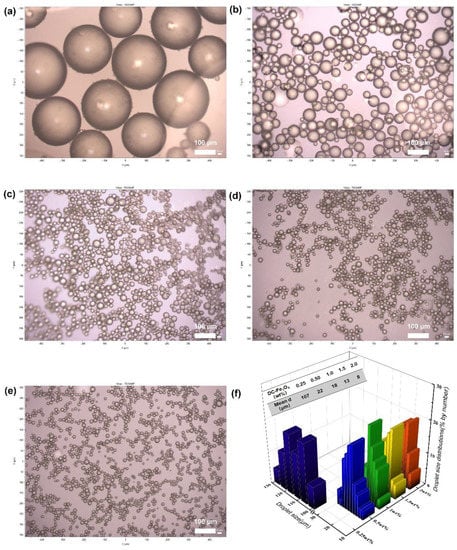
Figure 7.
Optical microscopes of the oil-in-water Pickering emulsions stabilized by DC-Fe3O4 nanoparticles at different concentrations with an equal volume of liquid paraffin and water. From (a–e), the DC-Fe3O4 concentration is 0.25, 0.5, 1.0, 1.5, and 2.0 wt%, respectively. The images were taken at 24 h preparation. (f) Droplet size distribution of Pickering emulsion stabilized DC-Fe3O4 nanoparticles at different concentrations. The liquid paraffin and water were in an equal volume ratio.
The Pickering emulsions showed almost no change in appearance after one month of storage, and no clear oil phase could be observed (Figure 5a,b), indicating the high stability of Pickering emulsions. Meanwhile, even after 1 month of storage, the droplet size of the Pickering emulsions remained nearly unchanged (Figure 8), further confirming their long-term stability. It was found that Pickering emulsions were stable for a long time because of the irreversible adsorption of DC-Fe3O4 nanoparticles at the O/W interface.
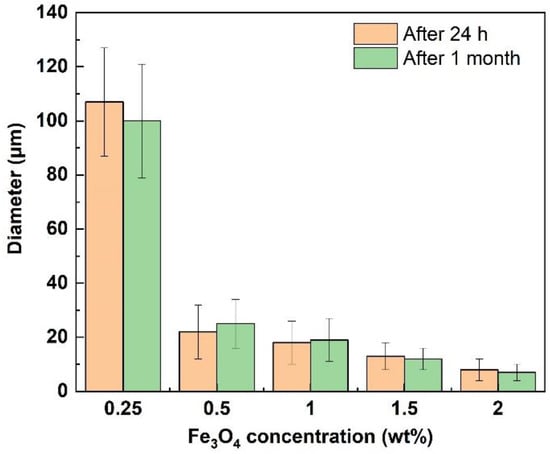
Figure 8.
The mean droplet size of Pickering emulsions after 24 h and after 1 month of storage at different stabilized DC-Fe3O4 concentrations. The liquid paraffin and water were in an equal volume ratio.
3.3. pH-Responsive Behavior of the Pickering Emulsions
On the basis of the above discussions, we can conclude that stable Pickering emulsions were obtained using the amphiphilic DC-Fe3O4 nanoparticles as a stabilizer. Considering the dynamic character of DIB to pH [42,43,44,45,46], the amphiphilicity of DC-Fe3O4 nanoparticles may be changed by transforming pH. To verify this, contact angle measurement for the DC-Fe3O4 film with acidic water (pH 2) was conducted. The contact angle of the DC-Fe3O4 nanoparticle film decreased from about 48° to about 25° after adding water droplets with a pH of 2 (Figure S5), indicating the dissociation of amphiphilicity DC-Fe3O4 into hydrophilic Fe3O4-NH2 and benzaldehyde in the acidic environment. That is to say, the amphiphilicity of DC-Fe3O4 nanoparticles could be changed by changing the pH.
In light of the change in amphiphilicity of DC-Fe3O4 nanoparticles when the pH is changed, we speculated that the pH could be used to adjust the stability of the obtained Pickering emulsions. To validate this hypothesis, liquid paraffin-in-water Pickering emulsion stabilized by 1.0 wt% DC-Fe3O4 nanoparticles was used as the representative sample. At pH 10, DC-Fe3O4 nanoparticles can be used as a stabilizer to prepare O/W Pickering emulsions because of the effective adsorption of amphiphilic DC-Fe3O4 at the liquid paraffin–water interface (Figure 9a and Scheme 1a). By decreasing the pH from 10 to 2, complete phase separation was achieved within 30 min (Figure 9b and Scheme 1b). At pH 2, the amphiphilic DC-Fe3O4 nanoparticles decomposed into hydrophilic Fe3O4-NH2 and inactive benzaldehyde, both of which were desorbed from the oil–water interface, causing demulsification (Figure 9b and Scheme 1b). The hydrophilic Fe3O4-NH2 nanoparticles participated in the aqueous phase (Scheme 1b). Additionally, benzaldehyde is surface-inactive and cannot stabilize emulsions effectively, as reported by our previous study [17]. We estimate that about 53% of the benzaldehyde migrates into the liquid paraffin phase based on the UV/Vis results (Scheme 1b). Moreover, after increasing the pH from 2 to 10, stable Pickering emulsion was reformed after re-sonication because of the re-formation of amphiphilic DC-Fe3O4 nanoparticles through DBI formation between hydrophilic Fe3O4-NH2 and surface inactive benzaldehyde (Figure 9a and Scheme 1a). Furthermore, pH-induced reversible emulsification and demulsification can be repeated up to three times without loss of efficacy (Figure 9a,b and Scheme 1a,b). Additionally, after three emulsification and demulsification cycles, the size of a newly formed Pickering emulsion shows a negligible increase compared with the original Pickering emulsion (13.1 μm vs. 13.5 μm, Figure S6). Emulsification and demulsification of the Pickering emulsion are determined by the pH responsiveness of the imine bond.
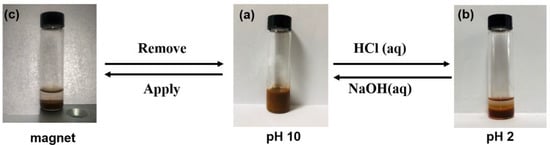
Figure 9.
The pH and magnetism dual-responsive oil-in-water Pickering emulsion prepared by 1.0 wt% DC-Fe3O4 nanoparticles. The Pickering emulsion sample at pH 10 (a), after reducing the pH to 2 (b), under an external magnetic field (c).
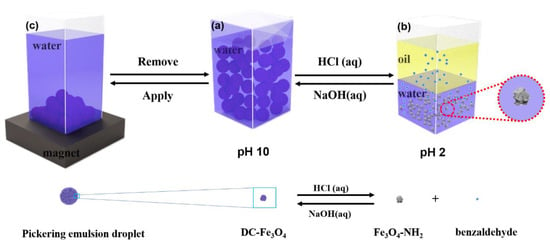
Scheme 1.
Illustration of pH and magnetism dual-responsive Pickering emulsion stabilized by DC-Fe3O4 nanoparticles. (a) Stable Pickering emulsion, (b) phase separated system after decreasing pH from 10 to 2 by adding 1 M HCl (1 M), and (c) Pickering emulsion droplets were collected by placing the magnet on the bottom of the bottle.
3.4. Magnetism-Responsive Behavior of the Pickering Emulsions
According to the reports, Pickering emulsion droplets stabilized by Fe3O4 nanoparticles can move in the direction of a magnet, or even coalesce when subjected to magnetic fields [9,23]. Upon exposure to a magnet, the DC-Fe3O4-coated Pickering emulsion droplets showed an instantaneous response with unidirectional movement toward the magnet, as demonstrated in Figure 9c and Scheme 1c, which was attributed to the superparamagnetic property of DC-Fe3O4 (magnetic saturation value, 43 emu/g). The time needed to remove all of the droplets was 40 s.
Magnetism-responsive Pickering emulsions have been widely used in extracting organic pollutants from aqueous solutions [9,34]. Here, we discuss the possibility of using the magnetism-responsive Pickering emulsion to extract a pollutant. The pink color could be seen in photos of the RhB solution (Figure S7a). Nonetheless, the pink color in RhB aqueous solution nearly disappeared after Pickering emulsion was added for 20 min, which suggested that RhB molecules were efficiently removed from the aqueous solution (Figure S7d). Based on the standard curve for RhB solution (Figure S8), the extraction efficiency for RhB adsorption was 97.5% (Figure S9). A similar adsorption experiment was conducted using only DC-Fe3O4 nanoparticle aqueous dispersion or liquid paraffin as an adsorbent in order to explore the adsorption mechanism. After adding DC-Fe3O4 nanoparticle aqueous dispersion or liquid paraffin into RhB aqueous solution for 1 h, the pink color did not disappear (Figure S7b,c). Compared with DC-Fe3O4 nanoparticle aqueous solution or liquid paraffin, Pickering emulsions have a higher specific surface area. Therefore, the high specific surface area of Pickering emulsions played an important role in the adsorption of RhB from the water phase into the oil–water interface. Besides, by replacing DC-Fe3O4 with conventional surfactant sodium lauryl polyoxyethylene ether sulfate (AES) without a phenyl ring, the pink color did not disappear either, indicating that the AES-stabilized emulsion cannot be used to adsorb the RhB from the aqueous solution (Figure S7e). The results demonstrate that the π–π stacking between benzene in RhB and phenyl ring in DC-Fe3O4 also played important roles for the adsorption of RhB into the oil–water interface. Pickering emulsions have good magnetic responsiveness and high stability, so emulsion droplets adsorbed by RhB can be regenerated upon washing and used to extract RhB again. Even after three times, the extraction efficiency was still above 78%. During extraction, the efficiency of the extraction decreased owing to the adsorption of residual RhB molecules at the droplet interface, which were difficult to remove. Pickering emulsions can potentially serve as extraction systems for dye molecules (such as RhB) because of their high efficiency through simple, yet rapid magnetic separation. Furthermore, the Pickering emulsions could be reused for extraction by washing. This simple magnetic separation method broadens the application of magnetism-responsive Pickering emulsions.
4. Conclusions
To summarize, this work has developed a dual-responsive oil-in-water Pickering emulsion prepared using dynamic covalent Fe3O4 (DC-Fe3O4) as a stabilizer. At pH 10, the hydrophobic functionalization with benzaldehyde through dynamic imine bond (DIB) allowed DC-Fe3O4 nanoparticles to reach the oil–water interface, forming stable Pickering emulsions. By reducing the pH from 10 to 2, DBI is decomposed so that amphiphilic DC-Fe3O4 breaks into highly hydrophilic Fe3O4-NH2 and surface-inactive benzaldehyde, resulting in a phase separation of the Pickering emulsion. Besides, Pickering emulsion stabilized by DC-Fe3O4 nanoparticles was also magnetically responsive. The magnetism-responsive Pickering emulsion could be used for the extraction of RhB-polluted aqueous solutions with a high extraction efficiency, and Pickering emulsion droplets can be used for extraction at least three times after washing, thanks to their magnetic responsiveness and the high stability of Pickering emulsion. The feasible and unique dual-responsiveness enrich the intelligent control of Pickering emulsions’ stability and broaden the applications of Pickering emulsion in field wastewater treatments.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano12152587/s1, Figure S1. TEM image of DC-Fe3O4, Figure S2. Photograph of DC-Fe3O4 nanoparticles partitioning at oil-water interface. The aqueous phase was stained with RbB, Figure S3. Photographs of liquid paraffin in water Pickering emulsions stabilized by 1.0 wt% Fe3O4 (a), 1.0 wt% Fe3O4-NH2 (b) at pH 10, taken 30 min after preparation, Figure S4. Photograph of the liquid paraffin and water after sonication for 2 min without DC-Fe3O4 nanoparticles, Figure S5. Image of contact measurement of acidic water droplet (pH = 2) on the DC-Fe3O4 film, Figure S6. Optical micrographs of initial Pickering emulsion and after 3 cycles are shown in (a) and (b), respectively. Pickering emulsion was prepared with 1.0 wt% DC-Fe3O4 at pH 10. The liquid paraffin and water were in an equal volume ratio, Figure S7. Photographs of 4 mg/L rhodamine B solution (a), the extraction of rhodamine B solution with DC-Fe3O4 nanoparticles (b), the extraction rhodamine B solution with liquid paraffin (c), extraction of rhodamine B solution with DC-Fe3O4 stabilized oil in water Pickering emulsion (d), and extraction of rhodamine B solution with AES stabilized oil in water emulsion (e), Figure S8. Standard curve of rhodamine B, Figure S9. The change of RhB concentration with standing time after adding 1 mL Pickering emulsion into 4 mL RhB aqueous solution. The initial concentration of RhB is 4 mg/L. The Pickering emulsion was stabilized by 1 wt% DC-Fe3O4 and the volume ratio of liquid paraffin and water is 1:1.
Author Contributions
Conceptualization, G.R. and Z.L.; methodology, D.L.; software, B.L.; validation, L.R.; formal analysis, W.D.; investigation, G.R.; resources, G.R. and W.D.; data curation, G.R.; writing-original draft preparation, G.R.; writing-review and editing, G.R.; visualization, G.R.; supervision, H.Y.; project administration, J.H.; funding acquisition, G.R. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (22002193), Key Scientific Research Project of Colleges and Universities in Henan Province (21A540005), Science and Technology Guidance Project plan of China National Textile and Apparel Association (2020059), and Strength Enhancement Plan of Advantageous Subjects of Zhongyuan University of Technology (SD202224).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahid, S.; Madhavan, N.; Mukherjee, M.; Basavaraj, M.G. Macroporous Ceramic Monolith from Nanoparticle–Polyelectrolyte-Stabilized Pickering Emulsions. J. Phys. Chem. B 2021, 125, 13575–13584. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lv, M.; Lu, G.; Cai, C.; Jiang, J.; Cui, Z. pH-Responsive Behavior of Pickering Emulsions Stabilized by a Selenium-Containing Surfactant and Alumina Nanoparticles. Langmuir 2021, 37, 10683–10691. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Bing, W.; Zhang, Z.; Li, Z.; Ren, J.; Qu, X. Light controlled reversible inversion of nanophosphor-stabilized pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 2014, 136, 7498–7504. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhu, A.; Zhao, Y.; Wang, H.; Binks, B.P.; Wang, J. Light-Responsive, Reversible Emulsification and Demulsification of Oil-in-Water Pickering Emulsions for Catalysis. Angew. Chem.-Int. Ed. 2021, 60, 3928–3933. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, W.; Wang, W.J.; Li, B.G.; Zhu, S. Highly CO2/N2-switchable zwitterionic surfactant for pickering emulsions at ambient temperature. Langmuir 2014, 30, 10248–10255. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Pu, J.; Zhao, Y.; Lin, B.; Bai, B.; Thomas, S.P. pH-Responsive crude oil-in-water Pickering emulsion stabilized by polyacrylamide nanogels. Fuel 2019, 258, 116159. [Google Scholar] [CrossRef]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef]
- Sun, N.; Li, Q.; Luo, D.; Sui, P.; Jiang, Q.; Liu, J.; Li, A.; Si, W.; Ma, Y. Dual-Responsive Pickering Emulsion Stabilized by Fe3O4 Nanoparticles Hydrophobized in Situ with an Electrochemical Active Molecule. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125588. [Google Scholar] [CrossRef]
- Wu, J.; Guan, X.; Wang, C.; Ngai, T.; Lin, W. pH-Responsive Pickering high internal phase emulsions stabilized by Waterborne polyurethane. J. Colloid Interface Sci. 2022, 610, 994–1004. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Wang, Q.; Sun, J.; Song, A. Pickering emulsions stabilized by surfactant particles with smart responses to pH and metal-ligands. J. Mol. Liq. 2021, 324, 114730. [Google Scholar] [CrossRef]
- Cui, F.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Development of pH-responsive emulsions stabilized by whey protein fibrils. Food Hydrocoll. 2022, 122, 107067. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual Responsive Pickering Emulsion Stabilized by Poly [2-(dimethylamino) ethyl methacrylate] Grafted Cellulose Nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, X.; Yang, W.; Wang, F.; Yu, H.; Song, A. Dual-responsive pickering emulsions triggered by CO2 and magnetism. J. Mol. Liq. 2021, 341, 116906. [Google Scholar] [CrossRef]
- Ren, G.; Li, B.; Ren, L.; Di, W.; Tian, L.; Zhang, P.; Shao, W.; He, J.; Sun, D. Dynamic Covalent Nanoparticles for Acid-Responsive Nonaqueous Pickering Emulsions. Langmuir 2021, 37, 6632–6640. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zheng, X.; Gu, H.; Di, W.; Wang, Z.; Guo, Y.; Xu, Z.; Sun, D. Temperature and CO2 Dual-Responsive Pickering Emulsions Using Jeffamine M2005-Modified Cellulose Nanocrystals. Langmuir 2019, 35, 13663–13670. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wang, M.; Wang, L.; Wang, Z.; Chen, Q.; Xu, Z.; Sun, D. Dynamic Covalent Silica Nanoparticles for pH-Switchable Pickering Emulsions. Langmuir 2018, 34, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.F.; Grigoriev, D.; Moehwald, H.; Tiersch, B.; Shchukin, D.G. Nanoparticle modification by weak polyelectrolytes for pH-sensitive pickering emulsions. Langmuir 2011, 27, 74–82. [Google Scholar] [CrossRef]
- Haase, M.F.; Grigoriev, D.; Moehwald, H.; Tiersch, B.; Shchukin, D.G. Encapsulation of amphoteric substances in a pH-sensitive pickering emulsion. J. Phys. Chem. C 2010, 114, 17304–17310. [Google Scholar] [CrossRef]
- Ma, R.; Zeng, M.; Huang, D.; Wang, J.; Cheng, Z.; Wang, Q. Amphiphilicity-adaptable graphene quantum dots to stabilize pH-responsive pickering emulsions at a very low concentration. J. Colloid Interface Sci. 2021, 601, 106–113. [Google Scholar] [CrossRef]
- Melle, S.; Lask, M.; Fuller, G.G. Pickering Emulsions with Controllable Stability. Langmuir 2005, 21, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Y.; Graff, R.W.; Lee, D.; Gao, H. Developing recyclable pH-responsive magnetic nanoparticles for oil–water separation. Polymer 2015, 72, 361–367. [Google Scholar] [CrossRef]
- Brugger, B.; Richtering, W. Magnetic, Thermosensitive Microgels as Stimuli-Responsive Emulsifiers Allowing for Remote Control of Separability and Stability of Oil in Water-Emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Lv, M.; Meng, Q.; Si, W.; Hao, M.; Han, R.; Lai, Y.; Jiang, J.; Cui, Z. CO2-switchable oil-in-dispersion emulsions stabilized by tertiary amine surfactant and alumina particles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128541. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Ge, J.; Jiang, P.; Ding, L. pH- and thermo-responsive Pickering emulsion stabilized by silica nanoparticles and conventional nonionic copolymer surfactants. J. Colloid Interface Sci. 2022, 616, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, Y.; Cui, Z.; Binks, B.P. Pickering Emulsions Responsive to CO2/N2 and Light Dual Stimuli at Ambient Temperature. Langmuir 2016, 32, 8668–8675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, S.; Ren, X.; Liu, X.; Fang, Y. CO2 and Redox Dual Responsive Pickering Emulsion. Langmuir 2017, 33, 12973–12981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, J.; Liu, K.; Cui, Z.; Binks, B.P. Switchable Pickering Emulsions Stabilized by Silica Nanoparticles Hydrophobized in Situ with a Conventional Cationic Surfactant. Langmuir 2015, 31, 3301–3307. [Google Scholar] [CrossRef]
- Yi, C.; Liu, N.; Zheng, J.; Jiang, J.; Liu, X. Dual-responsive poly (styrene-alt-maleic acid)-graft-poly (N-isopropyl acrylamide) micelles as switchable emulsifiers. J. Colloid Interface Sci. 2012, 380, 90–98. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, X.; Cao, S.; Zhu, L.; Xi, L.; Wang, J. Pickering Interfacial Catalysts with CO2 and Magnetic Dual Response for Fast Recovering in Biphasic Reaction. ACS Appl. Mater. Interfaces 2019, 11, 16156–16163. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A.; Hejazi, S.H.; Gates, I. Dual Stimuli-Responsive Pickering Emulsions from Novel Magnetic Hydroxyapatite Nanoparticles and Their Characterization Using a Microfluidic Platform. Langmuir 2021, 37, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, J.; Cui, Z.; Binks, B.P. pH-Responsive Pickering Emulsions Stabilized by Silica Nanoparticles in Combination with a Conventional Zwitterionic Surfactant. Langmuir 2017, 33, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stöver, H.D.H. Doubly pH-Responsive Pickering Emulsion. Langmuir 2008, 24, 13237–13240. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Peng, J.; Zhang, Y.; Du, G.; Fang, Y. Smart magnetic ionic liquid-based Pickering emulsions stabilized by amphiphilic Fe3O4 nanoparticles: Highly efficient extraction systems for water purification. J. Colloid Interface Sci. 2017, 485, 213–222. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, X.; Binks, B.P.; Sun, K.; Bai, M.; Li, Y.; Liu, Y. Magnetic Pickering Emulsions Stabilized by Fe3O4 Nanoparticles. Langmuir 2011, 27, 3308–3316. [Google Scholar] [CrossRef]
- Shahid, S.; Gurram, S.R.; Basavaraj, M.G. Doubly pH Responsive Emulsions by Exploiting Aggregation of Oppositely Charged Nanoparticles and Polyelectrolytes. Langmuir 2018, 34, 5060–5071. [Google Scholar] [CrossRef]
- He, X.; Jia, K.; Yu, L.; Li, H.; Xin, J.; Zheng, X.; Ning, J.; Wu, H.; Huang, L.; Wen, W. Robust pH-switchable pickering emulsions stabilized solely by organic Rosin-based particles with adjustable wettability. J. Mol. Liq. 2022, 353, 118751. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, R.-X.; Guo, T.; Meng, T. Switchable Pickering Emulsions Stabilized by Awakened TiO2 Nanoparticle Emulsifiers Using UV/Dark Actuation. ACS Appl. Mater. Interfaces 2015, 7, 18240–18246. [Google Scholar] [CrossRef]
- Williams, M.; Warren, N.J.; Fielding, L.A.; Armes, S.P.; Verstraete, P.; Smets, J. Preparation of Double Emulsions using Hybrid Polymer/Silica Particles: New Pickering Emulsifiers with Adjustable Surface Wettability. ACS Appl. Mater. Interfaces 2014, 6, 20919–20927. [Google Scholar] [CrossRef]
- Morse, A.J.; Armes, S.P.; Thompson, K.L.; Dupin, D.; Fielding, L.A.; Mills, P.; Swart, R. Novel Pickering Emulsifiers Based on pH-Responsive Poly (2-(diethylamino) ethyl methacrylate) Latexes. Langmuir 2013, 29, 5466–5475. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Wang, T.; Fan, Y.; Kang, X.; Jia, R.; Kenzhebek, I.; Issakhov, M. Fabrication of a pH-responsive emulsifier for heavy oil recovery based on dynamic imine bond. J. Mol. Liq. 2021, 332, 115916. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Molecular assembly of Schiff base interactions: Construction and application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, G.; Wang, Z.; Zhang, X. Asymmetric and Symmetric Bolaform Supra-Amphiphiles: Formation of Imine Bond Influenced by Aggregation. Langmuir 2014, 30, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Minkenberg, C.B.; Li, F.; van Rijn, P.; Florusse, L.; Boekhoven, J.; Stuart, M.C.A.; Koper, G.J.M.; Eelkema, R.; van Esch, J.H. Responsive vesicles from dynamic covalent surfactants. Angew. Chem. Int. Ed. 2011, 50, 3421–3424. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Cui, W.; Yi, S. pH-responsive vesicles from supra-amphiphiles based on dynamic imine bond. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 28–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).