Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Biochar Samples
2.3. Materials Characterization
2.4. Electrochemical Measurements
2.5. Catalytic Degradation Experiments
2.6. Analytical Methods
3. Results and Discussion
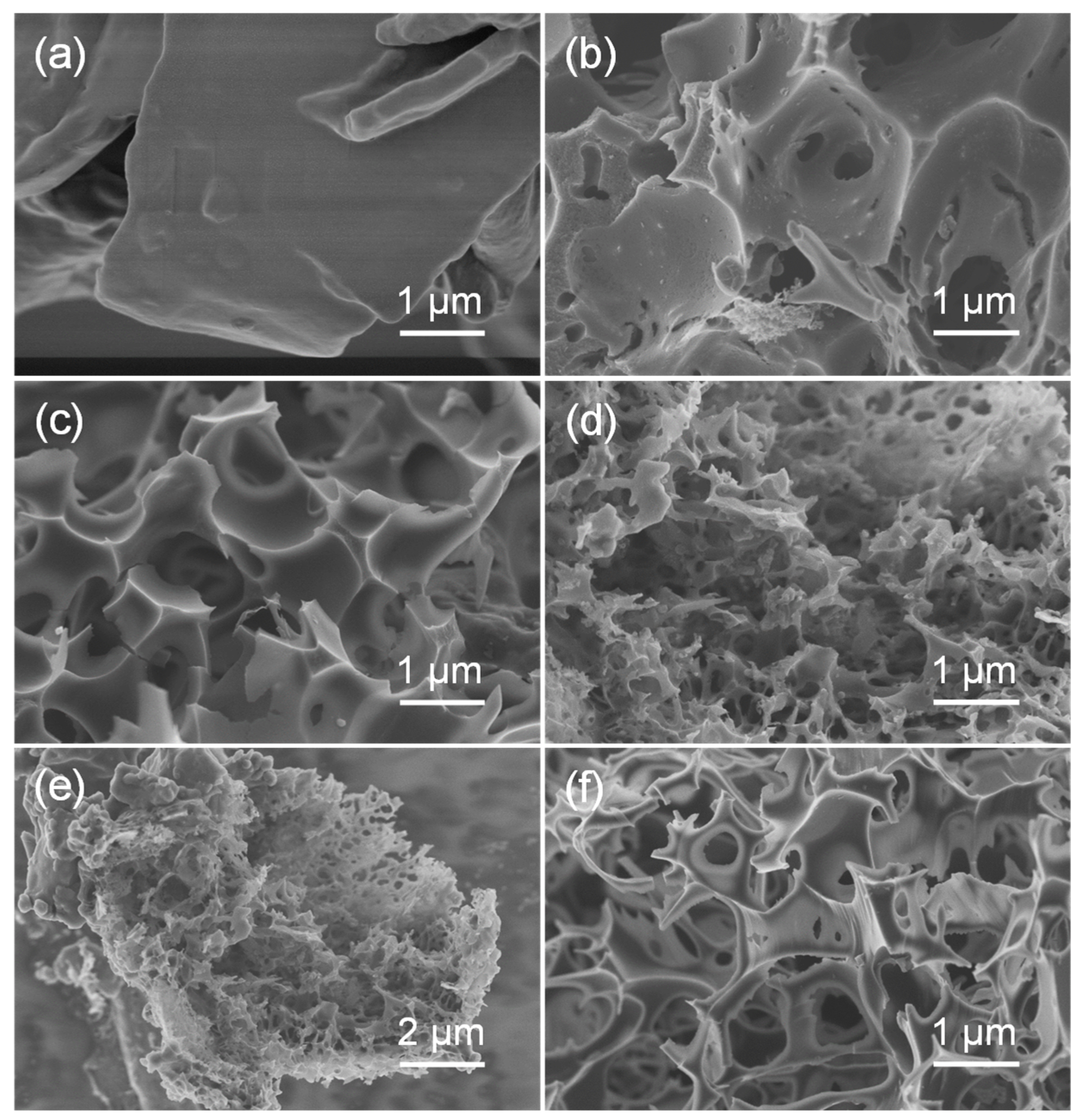
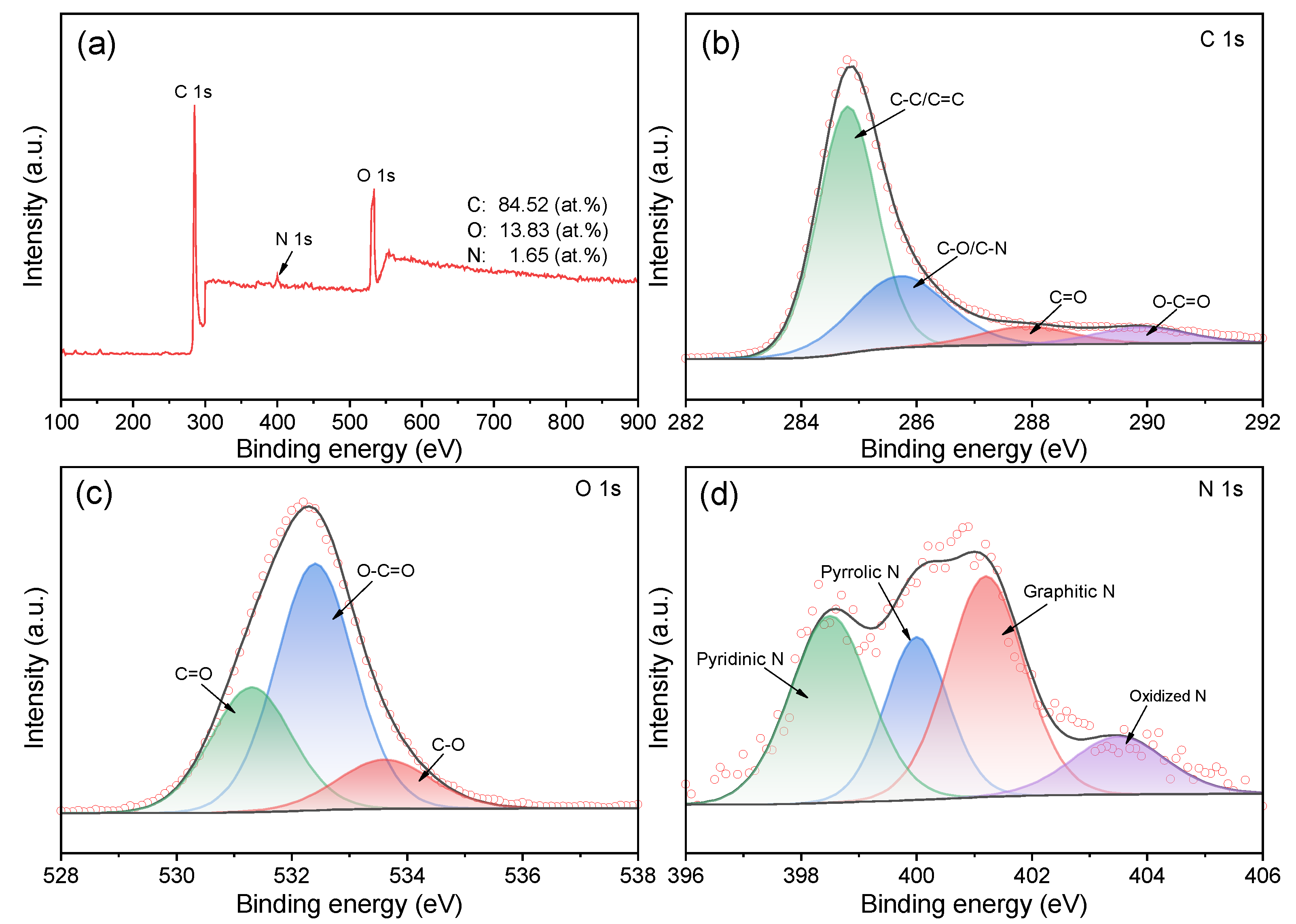
3.1. Morphological and Structural Characterization
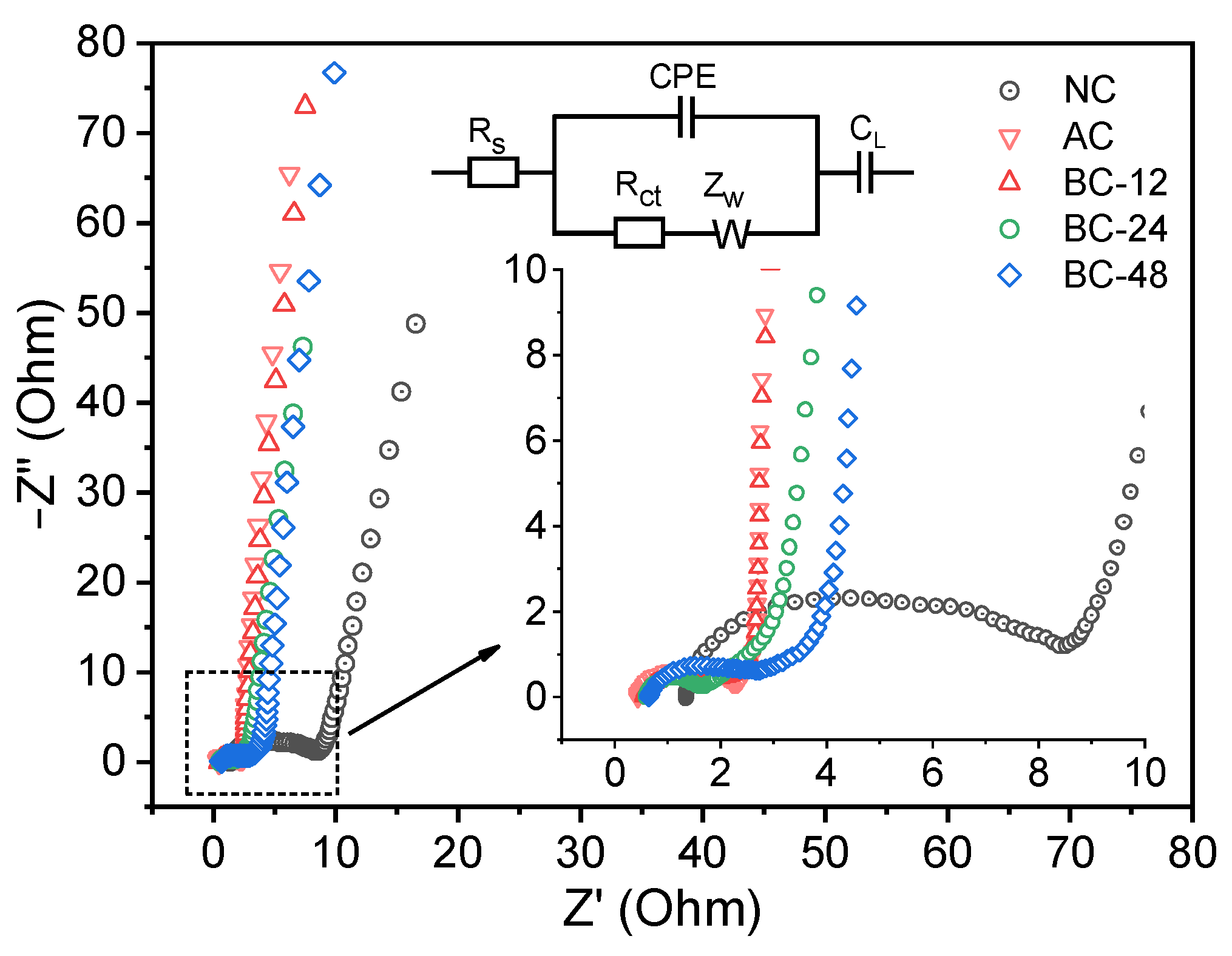
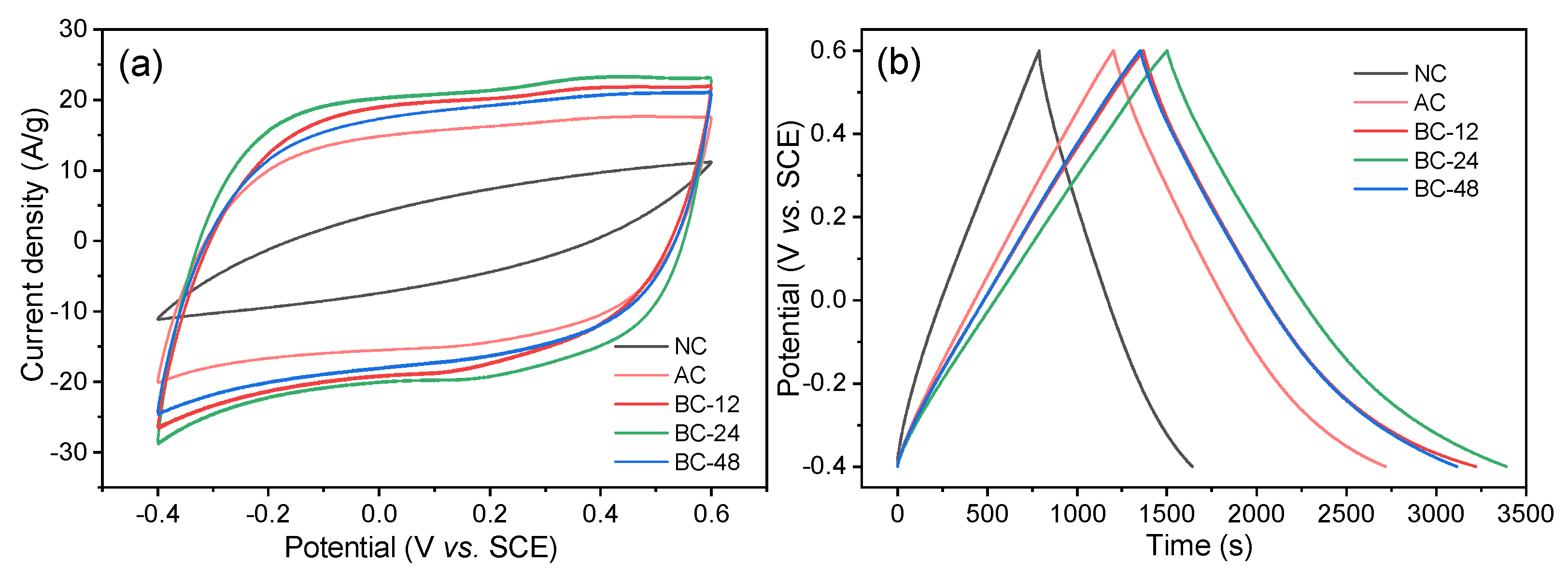
3.2. Electrochemical Measurements
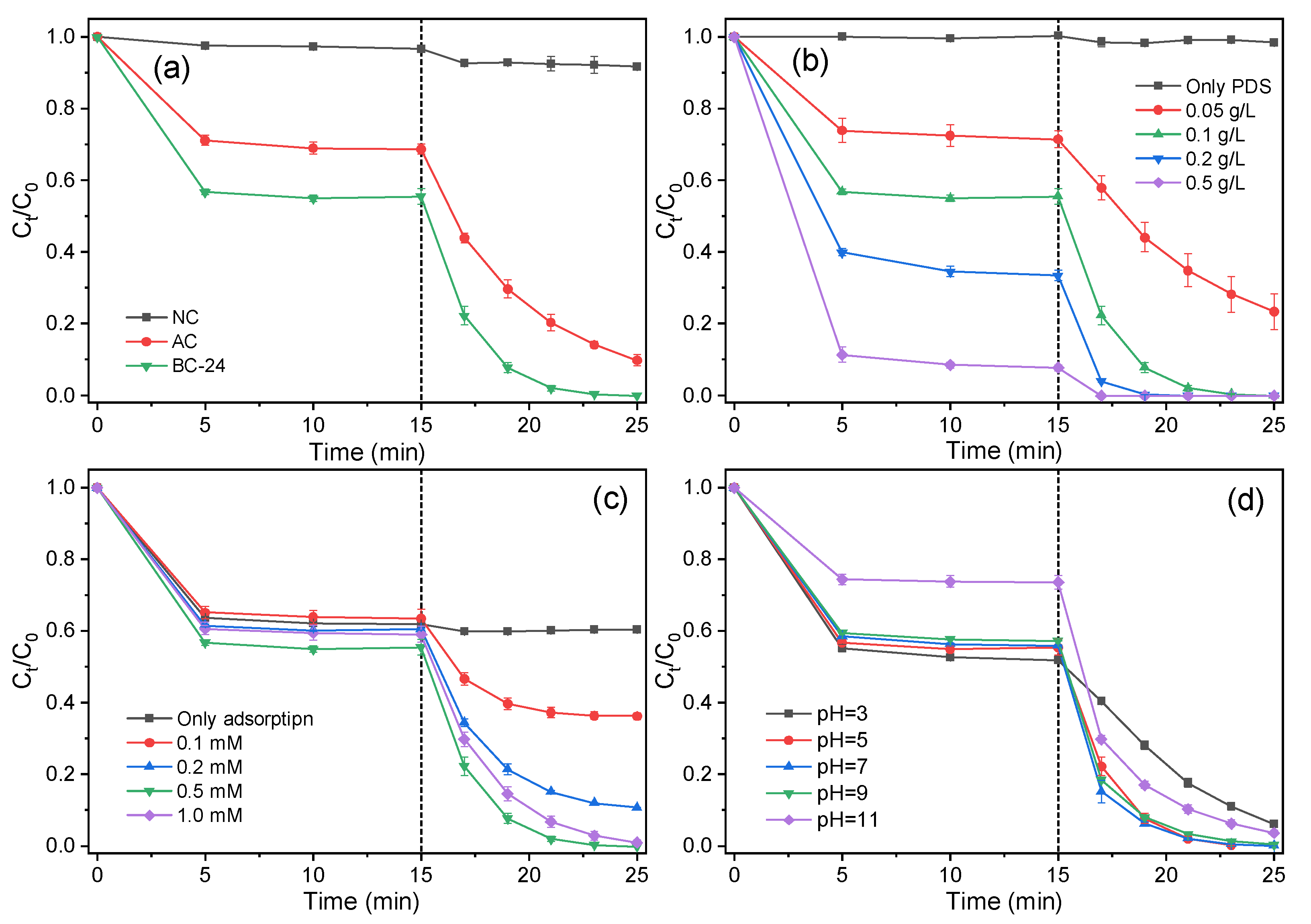
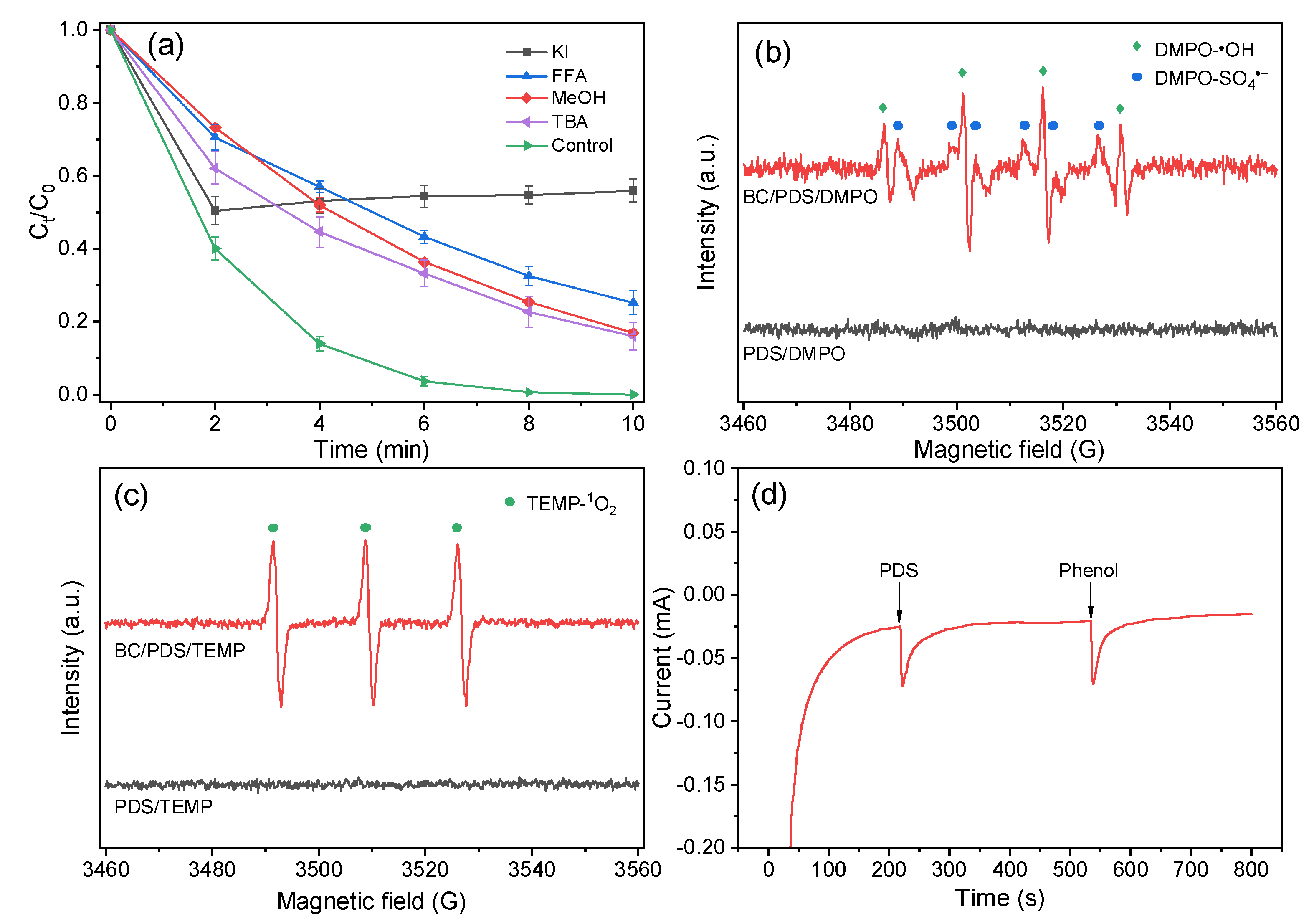
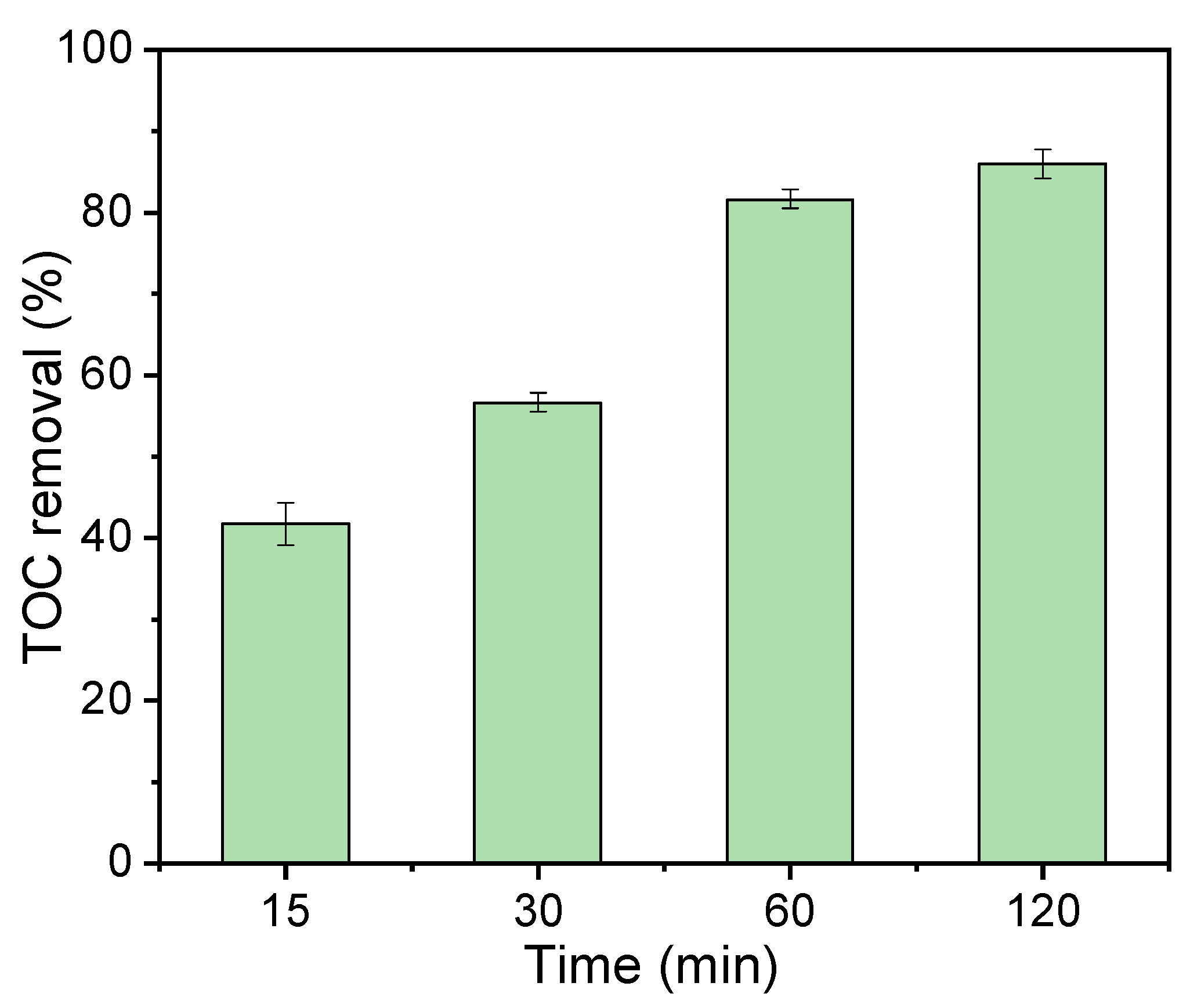
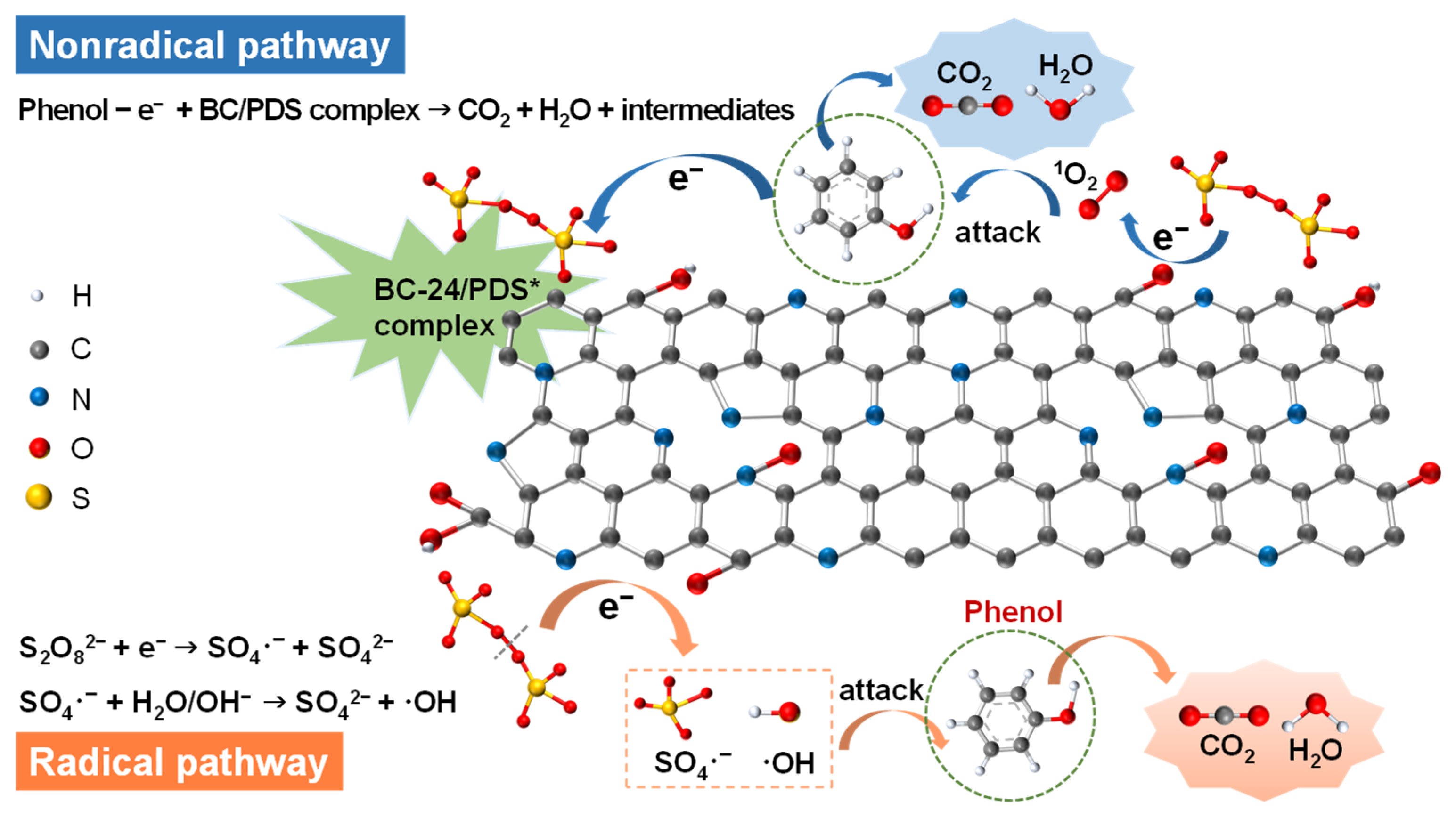
3.3. Catalytic Activity for Phenol Degradation through PDS Activation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.W.; Hou, C.-H. A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.F.; Han, X.; Han, B.X.; Yang, S.H. Emerging heterogeneous catalysts for biomass conversion: Studies of the reaction mechanism. Chem. Soc. Rev. 2021, 50, 11270–11292. [Google Scholar] [CrossRef]
- Bui, T.A.N.; Huynh, T.V.; Tran, H.L.; Doong, R.-A. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials 2022, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Surendran, S.; Ji, S.; Kim, D.; Chae, Y.; Kim, J.; Je, M.; Han, M.-K.; Choe, W.-S.; Choi, C.H.; et al. A sulfur self-doped multifunctional biochar catalyst for overall water splitting and a supercapacitor from Camellia japonica flowers. Carbon Energy 2022. [Google Scholar] [CrossRef]
- Lima, R.M.A.P.; dos Reis, G.S.; Thyrel, M.; Alcaraz-Espinoza, J.J.; Larsson, S.H.; de Oliveira, H.P. Facile Synthesis of Sustainable Biomass-Derived Porous Biochars as Promising Electrode Materials for High-Performance Supercapacitor Applications. Nanomaterials 2022, 12, 866. [Google Scholar] [CrossRef]
- Tang, X.F.; Liu, D.; Wang, Y.J.; Cui, L.F.; Ignaszak, A.; Yu, Y.; Zhang, J.J. Research advances in biomass-derived nanostructured carbons and their composite materials for electrochemical energy technologies. Prog. Mater Sci. 2021, 118, 100770. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Zheng, Y.; Zhao, Q.; Yu, H. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, M.; Tang, D.; Xu, Y.; Ran, H.; He, J.; Chen, K.; Sun, J. High H2O2 selectivity and enhanced Fe2+ regeneration toward an effective electro-Fenton process based on a self-doped porous biochar cathode. Appl. Catal. B Environ. 2022, 315, 121523. [Google Scholar] [CrossRef]
- He, M.; Zhao, P.; Duan, R.; Xu, S.; Cheng, G.; Li, M.; Ma, S. Insights on the electron transfer pathway of phenolic pollutant degradation by endogenous N-doped carbonaceous materials and peroxymonosulfate system. J. Hazard. Mater. 2022, 424, 127568. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Tang, D.Y.; Huang, L.; Chen, Y.F.; Ren, W.; Sun, J. High oxygen reduction reaction performance nitrogen-doped biochar cathode: A strategy for comprehensive utilizing nitrogen and carbon in water hyacinth. Bioresource Technol. 2018, 267, 524–531. [Google Scholar] [CrossRef]
- Liang, J.; Duan, X.; Xu, X.; Chen, K.; Wu, F.; Qiu, H.; Liu, C.; Wang, S.; Cao, X. Biomass-derived pyrolytic carbons accelerated Fe(III)/Fe(II) redox cycle for persulfate activation: Pyrolysis temperature-depended performance and mechanisms. Appl. Catal. B Environ. 2021, 297, 120446. [Google Scholar] [CrossRef]
- Jiang, G.; Senthil, R.A.; Sun, Y.; Kumar, T.R.; Pan, J. Recent progress on porous carbon and its derivatives from plants as advanced electrode materials for supercapacitors. J. Power Sources 2022, 520, 230886. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Hu, F.; Gao, G.; Wu, G.; Yang, X. Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes. Adv. Mater. 2018, 30, 1705713. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wu, D.; Wang, T.; Zeng, P.; Jia, D. K2CO3-KCl acts as a molten salt flame retardant to prepare N and O doped honeycomb-like carbon in air for supercapacitors. J. Power Sources 2022, 532, 231072. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Han, X.; Li, Z.; Zhang, S.; Zong, M. A flexible Zinc-ion hybrid supercapacitor constructed by porous carbon with controllable structure. Appl. Surf. Sci. 2022, 579, 152247. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C.Z. 3D carbon based nanostructures for advanced supercapacitors. Energ. Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Luo, Y.; Lei, W.; Xiang, Q.; Ren, W.; Song, W.; Chen, K.; Sun, J. Hierarchical porous carbon materials derived from waste lentinus edodes by a hybrid hydrothermal and molten salt process for supercapacitor applications. Appl. Surf. Sci. 2018, 462, 862–871. [Google Scholar] [CrossRef]
- Lei, W.D.; Yang, B.K.; Sun, Y.J.; Xiao, L.W.; Tang, D.Y.; Chen, K.; Sun, J.; Ke, J.; Zhuang, Y.A. Self-sacrificial template synthesis of heteroatom doped porous biochar for enhanced electrochemical energy storage. J. Power Sources 2021, 488, 229455. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Shi, J.; Dou, Y.; Li, S.; You, R.; Zhang, S.; Miao, X.; Shi, S.; Ji, H.; et al. Super-hydrophilic microporous biochar from biowaste for supercapacitor application. Appl. Surf. Sci. 2021, 561, 150076. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, G.; Shao, P.; Ren, W.; Li, M.; Hu, Y.; Yang, L.; Shi, H.; Luo, X. A comparison of SMX degradation by persulfate activated with different nanocarbons: Kinetics, transformation pathways, and toxicity. Appl. Catal. B Environ. 2022, 310, 121345. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Ren, H.-Y.; Cao, G.-L.; Xie, G.-J.; Xing, D.-F.; Ren, N.-Q.; Liu, B.-F. Highly efficient activation of persulfate by encapsulated nano-Fe0 biochar for acetaminophen degradation: Rich electron environment and dominant effect of superoxide radical. Chem. Eng. J. 2022, 440, 135947. [Google Scholar] [CrossRef]
- Annamalai, S.; Shin, W.S. Efficient degradation of trimethoprim with ball-milled nitrogen-doped biochar catalyst via persulfate activation. Chem. Eng. J. 2022, 440, 135815. [Google Scholar] [CrossRef]
- Kumar, S.; Tewari, C.; Sahoo, N.G.; Philip, L. Mechanistic insights into carbo-catalyzed persulfate treatment for simultaneous degradation of cationic and anionic dye in multicomponent mixture using plastic waste-derived carbon. J. Hazard. Mater. 2022, 435, 128956. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Ma, T.; Liu, M.; Li, T.; Ren, H.; Zhou, R. Nitrogen-doped carbon nanotubes derived from carbonized polyaniline as a robust peroxydisulfate activator for the oxidation removal of organic pollutants: Singlet oxygen dominated mechanism and structure-activity relationship. Sep. Purif. Technol. 2022, 293, 121124. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Başer, B.; Yousaf, B.; Yetis, U.; Abbas, Q.; Kwon, E.E.; Wang, S.; Bolan, N.S.; Rinklebe, J. Formation of nitrogen functionalities in biochar materials and their role in the mitigation of hazardous emerging organic pollutants from wastewater. J. Hazard. Mater. 2021, 416, 126131. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xiong, L.; Nie, G.; Zhang, H.; Duan, X.; Wang, S. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, Q.; Wang, Z.; Hua, S.; Ding, D.; Cai, T.; Zhang, R. Pyrrolic N-rich biochar without exogenous nitrogen doping as a functional material for bisphenol A removal: Performance and mechanism. Appl. Catal. B Environ. 2021, 291, 120093. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Yin, H.Y.; Lu, B.H.; Xu, Y.; Tang, D.Y.; Mao, X.H.; Xiao, W.; Wang, D.H.; Alshawabkeh, A.N. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Sci. Technol. 2014, 48, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.; Chen, X.; Wu, W.; Lin, D.; Yang, K. Intrinsic defects enhanced biochar/peroxydisulfate oxidation capacity through electron-transfer regime. Chem. Eng. J. 2022, 438, 135606. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-d.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.-T.; Yoo, H.-Y.; Bae, H.; Kim, H.-I.; Lee, J. Exploring the Role of Persulfate in the Activation Process: Radical Precursor Versus Electron Acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ding, Y.; Huang, Y.; Li, N.; Hu, Y.; Zhao, C. Soybean root-derived N, O co-doped hierarchical porous carbon for supercapacitors. Appl. Surf. Sci. 2021, 555, 149726. [Google Scholar] [CrossRef]
- Teng, W.; Zhou, Q.; Wang, X.; Che, H.; Du, Y.; Hu, P.; Li, H.; Wang, J. Biotemplating preparation of N,O-codoped hierarchically porous carbon for high-performance supercapacitors. Appl. Surf. Sci. 2021, 566, 150613. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, K.; Chen, M.-s.; Zhang, M.; Shen, Z. One-pot synthesis of N-doped hierarchical porous carbon for high-performance aqueous capacitors in a wide pH range. J. Power Sources 2021, 491, 229587. [Google Scholar] [CrossRef]
- Karki, H.P.; Kim, H.; Jung, J.; Oh, J. Synthesis of Molybdenum Sulfide/Tellurium Hetero-Composite by a Simple One-Pot Hydrothermal Technique for High-Performance Supercapacitor Electrode Material. Nanomaterials 2021, 11, 2346. [Google Scholar] [CrossRef]
- Gou, G.; Huang, F.; Jiang, M.; Li, J.; Zhou, Z. Hierarchical porous carbon electrode materials for supercapacitor developed from wheat straw cellulosic foam. Renew. Energy 2020, 149, 208–216. [Google Scholar] [CrossRef]
- Jakubec, P.; Bartusek, S.; Dvořáček, J.J.; Šedajová, V.; Kupka, V.; Otyepka, M. Flax-Derived Carbon: A Highly Durable Electrode Material for Electrochemical Double-Layer Supercapacitors. Nanomaterials 2021, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: Performance and mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Ding, J.; Xu, W.; Liu, S.; Liu, Y.; Tan, X.; Li, X.; Li, Z.; Zhang, P.; Du, L.; Li, M. Activation of persulfate by nanoscale zero-valent iron loaded porous graphitized biochar for the removal of 17β-estradiol: Synthesis, performance and mechanism. J. Colloid Interface Sci. 2021, 588, 776–786. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Han, R.; Fang, Y.; Sun, P.; Xie, K.; Zhai, Z.; Liu, H.; Liu, H. N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7. Nanomaterials 2021, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, S.; Zhan, S. Superior photocatalytic disinfection effect of Ag-3D ordered mesoporous CeO2 under visible light. Appl. Catal. B Environ. 2018, 224, 27–37. [Google Scholar] [CrossRef]
- Dou, J.; Cheng, J.; Lu, Z.; Tian, Z.; Xu, J.; He, Y. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Q.; Yang, X.; Cai, Z.; Xiong, Y.; Huang, B. Preparation of phosphorus-doped porous carbon for high performance supercapacitors by one-step carbonization. RSC Adv. 2020, 10, 17768–17776. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Tran, T.T.V.; Van, C.N.; Vo, D.-V.N.; Kongparakul, S.; Zhang, H.; Guan, G.; Samart, C. Carbon sequestration through hydrothermal carbonization of expired fresh milk and its application in supercapacitor. Biomass Bioenerg. 2020, 143, 105836. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, Y.; Liu, Z.; Zhang, X.; Ma, W.; Wu, H.; Huang, X.; Zhang, Q. Novel synthesis route for the preparation of mesoporous nitrogen-doped carbons from forestry wastes for supercapacitors. Surf. Interfaces 2021, 24, 101132. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energ. Fuel. 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, H.-Q.; Lü, Q.-F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, Z.; Wang, Y.; Liu, Z.; Li, Z. Simple one-pot strategy for converting biowaste into valuable graphitized hierarchically porous biochar for high-efficiency capacitive storage. J. Energy Storage 2021, 44, 103259. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Wang, J.; Yu, J.; Feng, H.; Lu, Y.; Chen, Y.; Liu, Y.; Wang, J.; Xie, Q. Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil: Active sites and electron transfer mechanism. Colloids Surf. Physicochem. Eng. Aspects 2020, 599, 124904. [Google Scholar] [CrossRef]
- Fan, X.; Yu, Y.; Dong, S.; Liu, Y.; Song, C.; Li, Q.; Wang, X. Heteroatoms-doped biochar derived from deciduous resource as persulfate catalysts for efficient degradation of phenol. J. Water Process Eng. 2022, 48, 102866. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Zhao, B.; Lyu, H. Activation of peroxydisulfate by ball-milled α-FeOOH/biochar composite for phenol removal: Component contribution and internal mechanisms. Environ. Pollut. 2022, 293, 118596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Kinetics of PMS activation by graphene oxide and biochar. Chemosphere 2020, 239, 124812. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Yu, I.K.M.; Fan, J.; Clark, J.H.; Zhou, Y.; Cao, X.; Gao, B.; Ok, Y.S. A sustainable biochar catalyst synergized with copper heteroatoms and CO2 for singlet oxygenation and electron transfer routes. Green Chem. 2019, 21, 4800–4814. [Google Scholar] [CrossRef]



| Samples | SBET (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Proportion of Mesopore (%) | Average Pore Size (nm) |
|---|---|---|---|---|---|
| NC | 100.1 | 0.34 | 0.33 | 2.79 | 13.10 |
| AC | 971.4 | 0.96 | 0.59 | 38.78 | 2.42 |
| BC-12 | 1362.2 | 1.13 | 0.76 | 32.71 | 2.23 |
| BC-24 | 1501.9 | 1.14 | 0.80 | 30.08 | 2.12 |
| BC-48 | 1118.0 | 0.88 | 0.66 | 25.79 | 2.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, D.; Lu, L.; Luo, Z.; Yang, B.; Ke, J.; Lei, W.; Zhen, H.; Zhuang, Y.; Sun, J.; Chen, K.; et al. Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials 2022, 12, 2586. https://doi.org/10.3390/nano12152586
Tang D, Lu L, Luo Z, Yang B, Ke J, Lei W, Zhen H, Zhuang Y, Sun J, Chen K, et al. Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials. 2022; 12(15):2586. https://doi.org/10.3390/nano12152586
Chicago/Turabian StyleTang, Diyong, Li Lu, Zhipeng Luo, Baokun Yang, Jun Ke, Weidong Lei, Hongran Zhen, Yuan Zhuang, Jie Sun, Ke Chen, and et al. 2022. "Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation" Nanomaterials 12, no. 15: 2586. https://doi.org/10.3390/nano12152586
APA StyleTang, D., Lu, L., Luo, Z., Yang, B., Ke, J., Lei, W., Zhen, H., Zhuang, Y., Sun, J., Chen, K., & Sun, J. (2022). Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials, 12(15), 2586. https://doi.org/10.3390/nano12152586






