The Use of Modified Fe3O4 Particles to Recover Polyphenolic Compounds for the Valorisation of Olive Mill Wastewater from Slovenian Istria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Sample Collection
2.3. HPLC-DAD-MS/MS Analysis
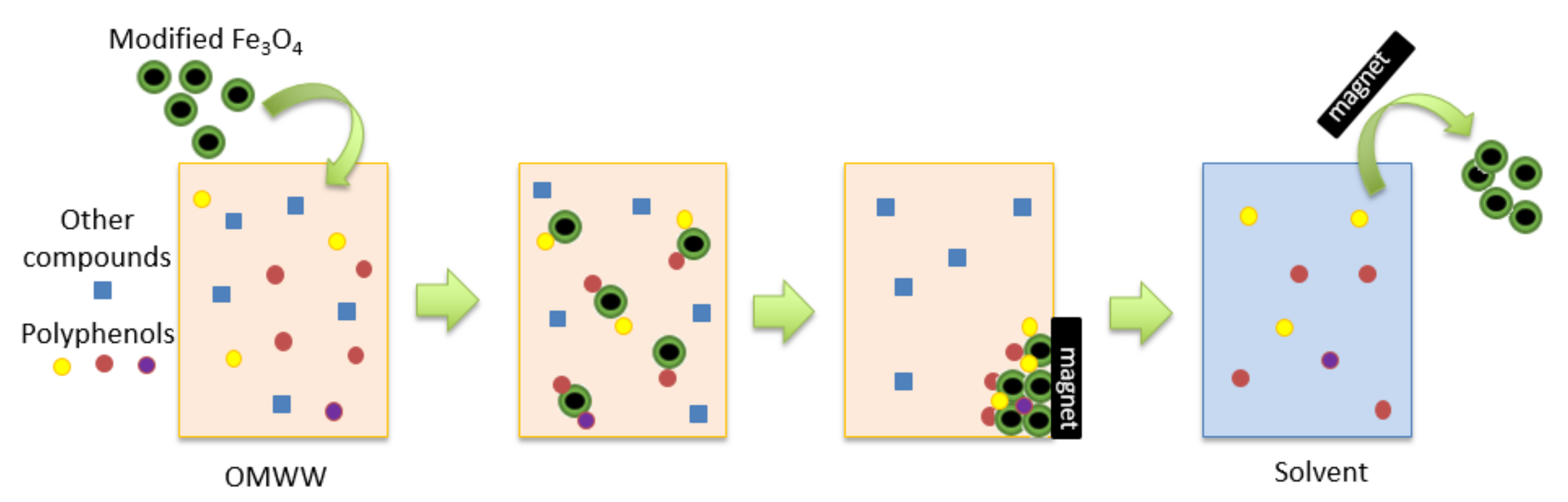
2.4. Modification of the Fe3O4 Particles and OMWW Treatment
2.5. Determination of the Total Phenol Content (TPC)
3. Results
3.1. Adsorption and Desorption of Polyphenols with the Unmodified Fe3O4
3.2. Adsorption and Desorption of Polyphenols with Fe3O4 Particles Modified with C18 Silica Gel
3.3. Adsorption and Desorption of Polyphenols with Fe3O4 Particles Modified with Citric Acid
3.4. Adsorption and Desorption of Polyphenols with Fe3O4 Particles Modified with SDS, Both with and without an Al2O3 Coating
4. Discussion
5. Conclusions
- In this study, it was found that the major advantage of (un)modified Fe3O4 particles is their easy multiple-cycle regeneration using low concentrations of low-cost chemicals.
- Their demonstrated adsorption capacity has the potential for successful commercialization in industrial applications.
- Differently modified Fe3O4 particles exhibit different extraction efficiencies for polyphenols with different chemical and physical properties.
- A sequential extraction by differently modified particles offers the possibility of either a “complete extraction” of all polyphenols in the desired quantities, or a more targeted extraction of select molecules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Azaizeh, H.; Halahlih, F.; Najami, N.; Brunner, D.; Faulstich, M.; Tafesh, A. Antioxidant activity of phenolic fractions in ol-ive mill wastewater. Food Chem. 2012, 134, 2226–2234. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Vavouraki, A. Removal of polyphenols from olive mill wastewater by FPX 66 resin: Part II. Adsorption kinetics and equilibrium studies. Int. J. Waste Resour. 2020, 10, 1–7. [Google Scholar]
- Papaoikonomou, L.; Labanaris, K.; Kaderides, K.; Goula, A.M. Adsorption–desorption of phenolic compounds from olive mill wastewater using a novel low-cost biosorbent. Environ. Sci. Pollut. Res. 2021, 28, 24230–24244. [Google Scholar] [CrossRef] [PubMed]
- Annab, H.; Fiol, N.; Villaescusa, I.; Essamri, A. A proposal for the sustainable treatment and valorisation of olive mill wastes. J. Environ. Chem. Eng. 2019, 7, 102803. [Google Scholar] [CrossRef]
- Lissaneddine, A.; Mandi, L.; El Achaby, M.; Mousset, E.; Rene, E.R.; Ouazzani, N.; Pons, M.-N.; Aziz, F. Performance and dynamic modeling of a continuously operated pomace olive packed bed for olive mill wastewater treatment and phenol recovery. Chemosphere 2021, 280, 130797. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Pulido, J.M.; Martínez-Férez, A. About the recovery of the phenolic fraction from olive mill wastewater by micro and ultracentrifugation membranes. Chem. Eng. Trans. 2017, 60, 271–276. [Google Scholar]
- Garcia-Castello, E.M.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, M.; Rahimpour, A.; Nemati, S.; Alimohammady, M. Recovery of polyphenols from olive mill wastewater by nano-filtration. Cellul. Chem. Technol. 2016, 50, 961–966. [Google Scholar]
- Mudimu, O.A.; Peters, M.; Brauner, F.; Braun, G. Overview of membrane processes for the recovery of polyphenols from olive mill wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar]
- Boudissa, F.; Kadi, H. Transfer of phenolic compounds from olive mill wastewater to olive cake oil. J. Am. Oil Chem. Soc. 2013, 90, 717–723. [Google Scholar] [CrossRef]
- Dammak, I.; Neves, M.; Isoda, H.; Sayadi, S.; Nakajima, M. Recovery of polyphenols from olive mill wastewater using drowning-out crystallization based separation process. Innov. Food Sci. Emerg. Technol. 2016, 34, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Yahiaouia, N.; Kadia, H.; Moussaouia, R.; Sebaouia, O.; Fiallo, M. Treatment and valorization of olive mill wastewater by hydroxyapatite co-precipitation using experimental design. Desalin Water Treat 2020, 195, 232–239. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Rahmanian, N.; Mahdi Jafari, S.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2013, 91, 1–18. [Google Scholar] [CrossRef]
- Kiritsakis, A.K.; Kiritsakis, K.A.; Tsitsipas, C.K. A review of the evolution in the research of antioxidants in olives and olive oil during the last four decades. J. Food Bioact. 2020, 11, 31–56. [Google Scholar] [CrossRef]
- Takaç, S.; Karakaya, A. Recovery of phenolic antioxidants from olive mill wastewater. Recent Pat. Chem. Eng 2009, 2, 230–237. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Zhang, F.; Zhou, J.; Wu, J.; Alsaedi, A.; Li, J. Insight into the mechanism of adsorption of phenol and resorcinol on activated carbons with different oxidation degrees. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 22–30. [Google Scholar] [CrossRef]
- Singh, D.; Srivastava, B.; Va, V. Removal of Phenol Pollutants from Aqueous Solutions Using Various Adsorbents. J. Sci. Ind Res. 2002, 61, 208–218. [Google Scholar]
- Lin, S.-H.; Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Removal of phenol from water environment by surfactant-modified alumina through adsolubilization. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 63–68. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Trikas, E.D.; Papi, R.M.; Kyriakidis, D.A.; Zachariadis, G.A. Evaluation of ion exchange and sorbing materials for their adsorption/desorption performane towards anthocyanins, total phenolics, and sugars from a grape pomace extract. Separations 2017, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, D.; Beiser, N.; Regenbrecht, C.; Zirbes, M.; Waldvogel, S.R. Adsorption and separation of black liquor-derived phenol derivatives using anion exchange resins. Sep. Purif. Technol. 2017, 181, 8–17. [Google Scholar] [CrossRef]
- Pinto, P.R.; Mota, I.F.; Pereira, C.M.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Separation and recovery of polyphenols and carbohydrates from Eucalyptus bark extract by ultrafiltration/diafiltration and adsorption processes. Sep. Purif. Technol. 2017, 183, 96–105. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M.; Sayari, A. Amine-modified mesoporous silica for quantitative adsorption and release of hydroxytyrosol and other phenolic compounds from olive mill wastewater. J. Taiwan Inst. Chem. Eng. 2017, 70, 111–118. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, X.; Alula, Y.; Yao, S. Bionic multi-tentacled ionic liquid-modified silica gel for adsorption and separation of polyphenols from green tea (Camellia sinensis) leaves. Food Chem. 2017, 230, 637–648. [Google Scholar] [CrossRef]
- Mandel, K.; Hutter, F. The magnetic nanoparticle separation problem. Nano Today 2012, 7, 485–487. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesh, S.J.; Scott, T.B. Iron Nanoparticles for Water Treatment: Is the Future Free or Fixed? In Iron Oxides; Faivre, D., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 473–522. [Google Scholar]
- Ding, J.; Zhao, Q.; Sun, L.; Ding, L.; Ren, N. Magnetic mixed hemimicelles solid-phase extraction of xanthohumol in beer coupled with high-performance liquid chromatography determination. J. Sep. Sci. 2011, 34, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Qu, F.; Li, N.B.; Luo, H.Q. Mixed hemimicelles solid-phase extraction of chlorophenols in environmental water samples with 1-hexadecyl-3-methylimidazolium bromide-coated Fe3O4 magnetic nanoparticles with high-performance liquid chromatographic analysis. Anal. Chim. Acta 2012, 715, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Yuan, D.; He, H.; Pham-Huy, C.; Dai, H.; Wang, C.; Zhang, C. Mixed hemimicelle solid-phase extraction based on magnetic carbon nanotubes and ionic liquids for the determination of flavonoids. Carbon 2014, 72, 274–286. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, C.; He, J.; Zeng, R.; Chen, R.; He, H. Platform construction and extraction mechanism study of magnetic mixed hemimicelles solid-phase extraction. Sci. Rep. 2016, 6, 38106. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-D.; Gao, D.; Xu, W.-J.; Li, F.; Yin, M.-N.; Fu, Q.-F.; Xia, Z.-N. Magnetic molecularly imprinted polymer for the selective extraction of hesperetin from the dried pericarp of Citrus reticulata Blanco. Talanta 2018, 184, 307–315. [Google Scholar] [CrossRef]
- Ma, W.; Dai, Y.; Row, K.H. Molecular imprinted polymers based on magnetic chitosan with different deep eutectic solvent monomers for the selective separation of catechins in black tea. Electrophoresis 2018, 39, 2039–2046. [Google Scholar] [CrossRef]
- Ying, L.-L.; Wang, D.-Y.; Yang, H.-P.; Deng, X.-Y.; Peng, C.; Zheng, C.; Xu, B.; Dong, L.-Y.; Wang, X.; Xu, L.; et al. Synthesis of boronate-decorated polyethyleneimine-grafted porous layer open tubular capillaries for enrichment of polyphenols in fruit juices. J. Chromatogr. A 2018, 1544, 23–32. [Google Scholar] [CrossRef]
- Rapa, M.; Vinci, G.; Ciano, S.; Cerra, S.; Fratoddi, I. Gold nanoparticles-based extraction of phenolic compounds from olive mill wastewater: A rapid and sustainable method. AIP Conf. Proc. 2020, 2257, 020010. [Google Scholar]
- Ottaviani, M.F.; Leonardis, I.; Cappiello, A.; Cangiotti, M.; Mazzeo, R.; Trufelli, H.; Palma, P. Structural modifications and adsorption capability of C18-silica/binary solvent interphases studied by EPR and RP-HPLC. J. Colloid Interface Sci. 2010, 352, 512–519. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef]
- Ryan, D.; Antolovich, M.; Prenzler, P.; Robards, K.; Lavee, S. Biotransformations of phenolic compounds in Olea europaea L. Sci. Hortic. 2002, 92, 147–176. [Google Scholar] [CrossRef]
- Peeters, K.; Miklavčič Višnjevec, A.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules 2021, 26, 6946. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with con-ventional methods. J. Agric. Food Chem. 2011, 59, 12725–12731. [Google Scholar] [CrossRef]
- Miklavčič Višnjevec, A.; Baker, P.; Charlton, A.; Preskett, D.; Peeters, K.; Tavzes, Č.; Kramberger, K.; Schwarzkopf, M. Developing an olive biorefinery in slovenia: Analysis of phenolic compounds found in olive mill pomace and wastewater. Molecules 2020, 26, 7. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Zhu, Y.; Wen, X.-E.; Zhuang, Y.-M. Preparation of Fe3O4-C18 nano-magnetic composite materials and their cleanup properties for organophosphorous pesticides. Anal. Bioanal. Chem. 2007, 387, 2227–2237. [Google Scholar] [CrossRef]
- Tekiye, E.-S.; Aghajani, Z.; Sharif, M.A. Synthesis and characterization of Fe3O4@Al2O3 nanoparticles and investigation its catalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 5360–5365. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.E.; Airinei, A. Citric-acid–coated magnetite nanoparticles for biological applications. Eur Phys. J. E 2006, 21, 117–121. [Google Scholar] [CrossRef]
- Augusto, P.A.; Castelo-Grande, T.; Vargas, D.; Pascual, A.; Hernández, L.; Estevez, A.M.; Barbosa, D. Upscale design, process development, and economic analysis of industrial plants for nanomagnetic particle production for environmental and biomedical use. Materials 2020, 13, 2477. [Google Scholar] [CrossRef]
- Pitroda, J.; Jethwa, B.; Dave, S.K. A critical review on carbon nanotubes. Int. J. Constr Res. Civ Eng 2016, 2, 36–42. [Google Scholar]
- Dehmania, Y.; lrashdi, A.A.A.; Lgaz, H.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.-M. Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): Mechanism exploration from both experimental and theoretical studies. Arab. J. Chem. 2020, 13, 5474–5486. [Google Scholar] [CrossRef]
- Yoon, S.U.; Mahanty, B.; Ha, H.M.; Kim, C.G. Phenol adsorption on surface-functionalized iron oxide nanoparticles: Modeling of the kinetics, isotherm, and mechanism. J. Nanopart. Res. 2016, 18, 170. [Google Scholar] [CrossRef]
- Sobiesiak, M. Chemical Structure of Phenols and Its Consequence for Sorption Processes Phenolic Compounds. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017; p. 456. [Google Scholar]
- Hosseini, S.; Gharachorloo, M.; Ghiassi-Tarzi, B.; Ghavami, M. Evaluation of the organic acids ability for extraction of anthocyanins and phenolic compounds from different sources and their degradation kinetics during cold storage. Pol. J. Food Nutr. Sci. 2016, 66, 261–269. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of anionic surfactant sodium dodecyl sulfate onto alpha alumina with small surface area. Colloid Polym. Sci. 2015, 293, 217–227. [Google Scholar] [CrossRef]
| Particle Type | Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| Fe3O4 | 247.7 | 16.53 |
| Fe3O4@C18 | 324.6 | 9.21 |
| Fe3O4@CA | 778.6 | −36.40 |
| Fe3O4@SDS | 325.0 | 1.86 |
| Phenolic Compounds | Polyphenol Content in First EtOH Fraction | Polyphenol Content in Fifteenth EtOH Fraction | Soluble Polyphenol Content in OMWW—Before Treatment | Soluble Polyphenol Concentration in OMWW—After Treatment |
|---|---|---|---|---|
| Oleoside isomers | 8565 ± 690 | 9838 ± 120 | 728,146 ± 37,843 | 660,565 ± 15,705 |
| Hydroxytyrosol glucoside | 6713 ± 768 | 6338 ± 204 | 62,868 ± 8439 | 37,418 ± 5137 |
| Hydroxytyrosol | 9271 ± 744 | 8879 ± 664 | 379,040 ± 24,353 | 108,364 ± 10,466 |
| Trans p-coumaric acid 4-glucoside | 698 ± 75 | 537 ± 52 | 29,560 ± 92 | 34,050 ± 990 |
| Caffeic acid | 12,527 ± 307 | 12,549 ± 1414 | 285,428 ± 8107 | 134,697 ± 19,500 |
| Elenolic acid glucoside isomers | 530 ± 14 | 706 ± 129 | 25,333 ± 1073 | 26,746 ± 318 |
| β-OH-verbascoside isomers | 5658 ± 1140 | 5338 ± 122 | 136,176 ± 438 | 136,466 ± 6029 |
| Vanilin | <LOD | <LOD | <LOD | <LOD |
| Verbascoside isomers | 7533 ± 388 | 1350 ± 118 | <LOD | <LOD |
| Demethyloleuropein | 176 ± 68 | 103 ± 8 | 14,483 ± 38 | 2752 ± 1993 |
| Rutin | <LOD | <LOD | <LOD | <LOD |
| Luteolin-O-glucoside isomers | 404 ± 107 | 224 ± 87 | <LOD | <LOD |
| Luteolin rutinoside | 573 ± 97 | 404 ± 111 | <LOD | <LOD |
| Nuzhenide Isomers | 116 ± 40 | 123 ± 11 | 1361 ± 64 | 5331 ± 315 |
| Caffeoyl-6-secologanoside | 5840 ± 349 | 5569 ± 360 | 129,754 ± 7106 | 112,055 ± 14,199 |
| 3,4-DHPEA-EDA isomers | 174 ± 30 | 118 ± 29 | 4149 ± 743 | 2363 ± 1929 |
| Oleuropein/Oleuroside isomers | 428 ± 86 | 240 ± 49 | 23,635 ± 1135 | 12,346 ± 1231 |
| Oleuropein aglycone Isomers | 567 ± 113 | 241 ± 29 | 8297 ± 99 | 3815 ± 363 |
| p-HPEA-EDA | 155 ± 33 | 161 ± 7 | <LOD | 2758 ± 166 |
| Apigenin | 722 ± 38 | 435 ± 106 | <LOD | <LOD |
| Total (mg/mL) | 0.052 ± 0.010 | 0.044 ± 0.002 | 3.44 ± 0.09 | 1.85 ± 0.07 |
| Phenolic Compounds | Polyphenol Content in First EtOH Fraction | Polyphenol Content in Fifteenth EtOH Fraction | Soluble Polyphenol Content in OMWW—Before Treatment | Soluble Polyphenol Concentration in OMWW—After Treatment |
|---|---|---|---|---|
| Oleoside isomers | 12,483 ± 219 | 14,202 ± 52 | 429,192 ± 48,396 | 402,504 ± 878 |
| Hydroxytyrosol glucoside | 8214 ± 462 | 8610 ± 210 | 23,162 ± 6222 | 20,171 ± 1453 |
| Hydroxytyrosol | 6059 ± 86 | 3805 ± 500 | 284,414 ± 3023 | 35,406 ± 6058 |
| Trans p-coumaric acid 4-glucoside | 483 ± 61 | 566 ± 51 | 23,900 ± 5967 | <LOD |
| Caffeic acid | 6791 ± 1135 | 5307 ± 344 | 363,362 ± 42,318 | 54,073 ± 4322 |
| Elenolic acid glucoside isomers | 845 ± 94 | 800 ± 8 | 38,053 ± 2452 | 20,489 ± 231 |
| β-OH-verbascoside isomers | 10,514 ± 851 | 10,198 ± 663 | 16,641 ± 4222 | 138,969 ± 17,363 |
| Vanilin | 799 ± 133 | 791 ± 6 | <LOD | 27,458 ± 1018 |
| Verbascoside isomers | 10,743 ± 65 | 12,817 ± 476 | <LOD | <LOD |
| Demethyloleuropein | 429 ± 61 | 324 ± 68 | 19,732 ± 217 | 3256 ± 40 |
| Rutin | 2206 ± 255 | 1877 ± 81 | <LOD | <LOD |
| Luteolin-O-glucoside isomers | 1278 ± 83 | 769 ± 41 | <LOD | <LOD |
| Luteolin rutinoside | <LOD | <LOD | <LOD | <LOD |
| Nuzhenide Isomers | 158 ± 14 | 159 ± 5 | 6662 ± 429 | 5038 ± 116 |
| Caffeoyl-6-secologanoside | 10,291 ± 1405 | 9053 ± 802 | 117,566 ± 8118 | 119,630 ± 4237 |
| 3,4-DHPEA-EDA isomers | 15,507 ± 1088 | 12,357 ± 122 | <LOD | 29,595 ± 197 |
| Oleuropein/Oleuroside | 1131 ± 296 | 965 ± 13 | 26,995 ± 1438 | 17,485 ± 205 |
| Oleuropein aglycone Isomers | 2527 ± 775 | 854 ± 13 | 3378 ± 2605 | 19,622 ± 3150 |
| p-HPEA-EDA | 2360 ± 182 | 2332 ± 9 | 12,601 ± 85 | 17,819 ± 264 |
| Apigenin | 839 ± 160 | 293 ± 4 | <LOD | <LOD |
| Total (mg/mL) | 0.064 ± 0.005 | 0.058 ± 0.001 | 3.02 ± 0.12 | 1.63 ± 0.18 |
| Phenolic Compounds | Polyphenol Content in First EtOH Fraction | Polyphenol Content in Fifteenth EtOH Fraction | Soluble Polyphenol Content in OMWW—Before Treatment | Soluble Polyphenol Concentration in OMWW—After Treatment |
|---|---|---|---|---|
| Oleoside isomers | 154,399 ± 12,489 | 129,203 ± 4337 | 2308,995 ± 371,959 | 1506,070 ± 82,930 |
| Hydroxytyrosol glucoside | 15,281 ± 489 | 18,323 ± 2255 | 36,111 ± 36 | 173,218 ± 14,866 |
| Hydroxytyrosol | 11,419 ± 1154 | 28,553 ± 1403 | 124,526 ± 16,804 | 105,736 ± 26,879 |
| Trans p-coumaric acid 4-glucoside | 1138 ± 122 | <LOD | <LOD | 33,190 ± 5006 |
| Caffeic acid | 57,636 ± 2078 | 44,890 ± 5083 | 63,215 ± 14,291 | 438,410 ± 85,673 |
| Elenolic acid glucoside isomers | 11,889 ± 122 | 5144 ± 2582 | 152,095 ± 11,816 | 88,787 ± 21,987 |
| β-OH-verbascoside isomers | 13,574 ± 1434 | 14,020 ± 44 | 193,562 ± 77,384 | 520,700 ± 4087 |
| Vanilin | <LOD | <LOD | <LOD | <LOD |
| Verbascoside isomers | 1246 ± 44 | 2215 ± 205 | <LOD | <LOD |
| Demethyloleuropein | 223 ± 69 | 225 ± 16 | 1970 ± 583 | 3778 ± 40 |
| Rutin | <LOD | <LOD | <LOD | <LOD |
| Luteolin-O-glucoside isomers | 2800 ± 100 | 1814 ± 138 | 9843 ± 3992 | 18,080 ± 393 |
| Luteolin rutinoside | 1537 ± 420 | 1105 ± 90 | <LOD | <LOD |
| Nuzhenide Isomers | 128 ± 16 | 110 ± 35 | <LOD | 4661 ± 1752 |
| Caffeoyl-6-secologanoside | 12,005 ± 1320 | 9786 ± 234 | 118,057 ± 8281 | 266,715 ± 4646 |
| 3,4-DHPEA-EDA | 61 ± 26 | 210 ± 9 | <LOD | <LOD |
| Oleuropein/Oleuroside isomers | 1211 ± 279 | 456 ± 155 | 11,488 ± 2688 | 12,531 ± 3935 |
| Oleuropein aglycone Isomers | 341 ± 34 | <LOD | <LOD | <LOD |
| p-HPEA-EDA | 245 ± 1 | 126 ± 5 | 3781 ± 245 | 3317 ± 385 |
| Apigenin | 2426 ± 23 | 1391 ± 100 | <LOD | <LOD |
| Total (mg/mL) | 0.100 ± 0.009 | 0.095 ± 0.020 | 3.56 ± 0.18 | 2.84 ± 0.15 |
| Particle Type | Concentration SDS (g/mL) | pH | Total Phenol Concentration in EtOH (mg per mL OMWW in GAE) |
|---|---|---|---|
| Fe3O4@Al2O3 | 0.01 | 4.5 | 0.11 |
| Fe3O4@Al2O3 | 0.02 | 4.5 | 0.13 |
| Fe3O4@Al2O3 | 0.05 | 4.5 | 0.17 |
| Fe3O4@Al2O3 | 0.1 | 4.5 | 0.20 |
| Fe3O4@Al2O3 | 0.02 | 3.5 | 0.17 |
| Fe3O4@Al2O3 | 0.02 | 4.5 | 0.11 |
| Fe3O4@Al2O3 | 0.02 | 5.5 | 0.16 |
| Fe3O4@Al2O3 | 0.02 | 8 | 0.09 |
| Fe3O4 | 0.01 | 4.5 | 0.25 |
| Fe3O4 | 0.02 | 4.5 | 0.19 |
| Fe3O4 | 0.05 | 4.5 | 0.10 |
| Fe3O4 | 0.1 | 4.5 | 0.28 |
| Fe3O4 | 0.02 | 3.5 | 0.14 |
| Fe3O4 | 0.02 | 4.5 | 0.20 |
| Fe3O4 | 0.02 | 5.5 | 0.37 |
| Fe3O4 | 0.02 | 8 | 0.32 |
| Phenolic Compounds | Polyphenol Content in First EtOH Fraction | Polyphenol Content in Fifteenth EtOH Fraction | Soluble Polyphenol Content in OMWW—Before Treatment | Soluble Polyphenol Concentration in OMWW—After Treatment |
|---|---|---|---|---|
| Oleoside isomers | 54,046 ± 7914 | 73,216 ± 15,453 | 898,000 ± 1373 | 991,238 ± 71,747 |
| Hydroxytyrosol glucoside | 937 ± 319 | 11,109 ± 1372 | 75,035 ± 9793 | 69,315 ± 2171 |
| Hydroxytyrosol | 18,578 ± 3650 | 12,386 ± 1727 | 44,488 ± 16,232 | 98,379 ± 8494 |
| Trans p-coumaric acid 4-glucoside | 613 ± 109 | <LOD | 21,878 ± 3758 | 15,080 ± 1676 |
| Caffeic acid | 26,446 ± 1207 | 22,178 ± 132 | 86,608 ± 746 | 15,864 ± 6493 |
| Elenolic acid glucoside isomers | 11,965 ± 145 | 11,797 ± 330 | 72,928 ± 17,864 | 38,435 ± 8553 |
| β-OH-verbascoside isomers | 11,911 ± 884 | 14,883 ± 106 | 230,313 ± 9139 | 224,923 ± 19,315 |
| Vanilin | 2377 ± 141 | 7850 ± 3802 | <LOD | <LOD |
| Verbascoside isomers | 7818 ± 510 | 2310 ± 505 | <LOD | <LOD |
| Demethyloleuropein | 151 ± 21 | 179 ± 2 | 1853 ± 21 | 4127 ± 39 |
| Rutin | <LOD | <LOD | <LOD | <LOD |
| Luteolin-O-glucoside | 2124 ± 6 | 2101 ± 113 | <LOD | 56,390 ± 2592 |
| Luteolin rutinoside | 1100 ± 93 | <LOD | <LOD | <LOD |
| Nuzhenide Isomers | 82 ± 5 | 123 ± 23 | <LOD | <LOD |
| Caffeoyl-6-secologanoside | 7390 ± 280 | 8055 ± 923 | 139,568 ± 15,822 | 129,294 ± 3056 |
| 3,4-DHPEA-EDA isomers | 94 ± 15 | 144 ± 6 | <LOD | <LOD |
| Oleuropein/Oleuroside isomers | 308 ± 43 | 555 ± 174 | 25,820 ± 7122 | 27,072 ± 7686 |
| Oleuropein aglycone Isomers | 286 ± 5 | 215 ± 9 | <LOD | <LOD |
| p-HPEA-EDA | 139 ± 73 | 131 ± 6 | 4253 ± 43 | 2389 ± 434 |
| Apigenin | 1026 ± 49 | 1240 ± 59 | <LOD | 2371 ± 383 |
| Total (mg/mL) | 0.071 ± 0.009 | 0.083 ± 0.03 | 3.85 ± 0.14 | 2.57 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, K.; Miklavčič Višnjevec, A.; Tavzes, Č. The Use of Modified Fe3O4 Particles to Recover Polyphenolic Compounds for the Valorisation of Olive Mill Wastewater from Slovenian Istria. Nanomaterials 2022, 12, 2327. https://doi.org/10.3390/nano12142327
Peeters K, Miklavčič Višnjevec A, Tavzes Č. The Use of Modified Fe3O4 Particles to Recover Polyphenolic Compounds for the Valorisation of Olive Mill Wastewater from Slovenian Istria. Nanomaterials. 2022; 12(14):2327. https://doi.org/10.3390/nano12142327
Chicago/Turabian StylePeeters, Kelly, Ana Miklavčič Višnjevec, and Črtomir Tavzes. 2022. "The Use of Modified Fe3O4 Particles to Recover Polyphenolic Compounds for the Valorisation of Olive Mill Wastewater from Slovenian Istria" Nanomaterials 12, no. 14: 2327. https://doi.org/10.3390/nano12142327
APA StylePeeters, K., Miklavčič Višnjevec, A., & Tavzes, Č. (2022). The Use of Modified Fe3O4 Particles to Recover Polyphenolic Compounds for the Valorisation of Olive Mill Wastewater from Slovenian Istria. Nanomaterials, 12(14), 2327. https://doi.org/10.3390/nano12142327







