Self-Assembled Peptide Nanostructures for ECM Biomimicry
Abstract
:1. Introduction
2. Self-Assembling Short Peptides with Bioactive Motifs for Hydrogel Biomaterials
| Bioactive Sequence | Gelator | Function | Model | Ref. |
|---|---|---|---|---|
| RGD(S) and mimic | KFE-RGD KFE-RDG KFE-8 | Cell adhesion | hMSC | [58] |
| RADA16 | Cell adhesion | 3T3 cells | [59] | |
| G-Y sequence | Cell adhesion | L929 cells | [60] | |
| RGDSGAITIGC | Cell proliferation | 3T3 cells | [61] | |
| E3-PA E3G3Ada-PA | Cell adhesion | 3T3 cells | [62] | |
| Fmoc-FF Fmoc-RGD | Cell adhesion and Differentiation | 3A6 cells Mice | [63] | |
| Fmoc-FF Fmoc-RGD | Cell delivery | Osteoblast Fibroblast Mice | [64] | |
| Fmoc-F5-Phe Fmoc-K(Fmoc)-RGD | Antimicrobial Cell adhesion | 3T3 cells | [65] | |
| Silk fibroin Nap-FFRGD | Cell adhesion Angiogenesis | HUVEC Mice | [66] | |
| Silk fibroin Nap-FFRGD | Cell adhesion Osteogenesis | mBMSC Mice | [67] | |
| Collagen-like peptide | Neuronal cell maturation | Neuronal-glial cells | [68] | |
| Fmoc-FFβAR(K)βA-OH Fmoc-FFβAR(K)βA-NH2 | Cell adhesion | MSC-P5, N2a, A549 cells | [69] | |
| Fmoc-FFGGRGD | Inhibition of β1-integrin, FAK and Akt expression | Tenon’s capsule fibroblasts | [70] | |
| Fmoc-FRGDF Agarose | Laminin and fibronectin mimic | - | [71] | |
| C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA | Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] | |
| Fmoc-FRGDF Fmoc-PHSRN | Cell adhesion | HMFC | [73] | |
| E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG | Osteogenesis | MC3T3-E1 cells | [74] | |
| LDV | fFL and fFLDV | Cell adhesion | L929 cells | [75] |
| PHSRN | Fmoc-FRGDF Fmoc-PHSRN | Cell adhesion | HMFC | [73] |
| IKVAV | RADA4GGSIKVAV | Neuronal stem-cell delivery Anti-inflammatory | hMgSC Mice | [76] |
| Fmoc-DIKVAV | Neuronal cell differentiation | Mice | [77] | |
| Fmoc-DDIKVAV | Neuronal cell differentiation | hPSC Mice | [78] | |
| IKVAV-PA | Neuronal cell differentiation | hESC Mice Human temporal bone | [79] | |
| IKVAV-PA | Neuronal cell differentiation | BMSC | [80] | |
| IKVAV-PA YRSRKYSSWYVALKR | Spinal cord injury repair (laminin and FGF2 mimicry) | Mice | [81] | |
| Fmoc-DIKVAV Agarose | Laminin and fibronectin mimic | - | [71] | |
| Fmoc-IKVAV Fmoc-YIGSR | Neuronal cell growth | C6 cells SHSY5Y cells | [82] | |
| YIGSR | KLD-IKVAV KLD-YIGSR | Vasculogenesis | HUVEC, hMS cells | [83] |
| Nap-GFF(p)YIGSR | Anticancer Self-assembly directly on cells | HeLa cells | [84] | |
| Fmoc-IKVAVFmoc-YIGSR | Neuronal cell growth | C6 cells SHSY5Y cells | [82] | |
| YSV | FKFEYYSV | Anticancer | A549 cancer cells | [85] |
| Taxol-EYSV | Anticancer | HeLa, A2780 cells Mice | [86] | |
| Nap-GffyGYSV | Anticancer | BEL-7402, HeLa, MCF-7 cells Mice | [87] | |
| Nap-Gff(p)YSV | Anticancer Self-assembly directly on cells | HeLa, A549 cells | [88] | |
| Nap-GFF(p)YSV | Anticancer Self-assembly directly on cells | HeLa cells | [84] | |
| HAV | Fmoc/Nap-HAVDI | Cell adhesion | C6, L929 cells | [89] |
| HAV-PA E-PA | Chondrogenesis | rMSC | [90] | |
| KLD-12 | Chondrogenesis | hMSC | [91] | |
| SVVYGLR | RADA16 | Angiogenesis | HCN-A94-2 cells Zebrafish | [92] |
| C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA | Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] | |
| DGEA | C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA | Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] |
| KTT | C16KTTβAH | Collagen production | MCF-7, MDA-MB-231, HDFa cells | [93] |
| βAH | C16KTTβAH | Anticancer | MCF-7, MDA-MB-231, HDFa cells | [93] |
| ALKRQGRTLYGF | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG | Osteogenesis | MC3T3-E1 cells | [74] |
| DGRDSVAYG | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG | Osteogenesis | MC3T3-E1 cells | [74] |
| PRGDSGYRGDS | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG | Osteogenesis | MC3T3-E1 cells | [74] |
2.1. RGD
2.2. LDV
2.3. PHSRN
2.4. IKVAV
2.5. YIGSR
2.6. YSV
2.7. HAV
2.8. SVVYGLR
2.9. DGEA
2.10. βAH and KTT
2.11. Other Bioactive Motifs Combined for Osteogenesis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, G.; Narayanan, G.; Garg, D.; Sachdev, A.; Matai, I. Biomaterials-based regenerative strategies for skin tissue wound healing. ACS Appl. Bio Mater. 2022, 5, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Abe, S.; Ueno, T. Engineering of protein crystals for use as solid biomaterials. Biomater. Sci. 2022, 10, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Kalouta, K.; Vetri, V.; Foderà, V. Protein materials as sustainable non- and minimally invasive strategies for biomedical applications. J. Control. Release 2022, 344, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liu, Q.; Zhang, Q.; Chew, T.G.; Ouyang, H. Modular protein engineering-based biomaterials for skeletal tissue engineering. Biomaterials 2022, 282, 121414. [Google Scholar] [CrossRef]
- Tinoco, A.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022, 40, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Kazimierska, K.; Kalinowska-Lis, U. Milk proteins-their biological activities and use in cosmetics and dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, L.; Dai, H.; Fu, Y.; Wang, H.; Zhang, Y. Advances in rational protein engineering toward functional architectures and their applications in food science. J. Agric. Food Chem. 2022, 70, 4522–4533. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-based biomaterials in periodontal regeneration: Current applications and future perspectives of plant-based collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef]
- Qin, D.; Wang, N.; You, X.G.; Zhang, A.D.; Chen, X.G.; Liu, Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: Ongoing research and perspectives. Biomater. Sci. 2022, 10, 318–353. [Google Scholar] [CrossRef]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3d cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Guerrini, A.; Ballestri, M.; Aluigi, A.; Zamboni, R.; Sotgiu, G.; Posati, T. Bioactive keratin and fibroin nanoparticles: An overview of their preparation strategies. Nanomaterials 2022, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.R.; Gong, J.S.; Su, C.; Liu, Y.L.; Qian, J.Y.; Xu, Z.H.; Shi, J.S. Preparation and applications of keratin biomaterials from natural keratin wastes. Appl. Microbiol. Biotechnol. 2022, 106, 2349–2366. [Google Scholar] [CrossRef]
- Ye, W.; Qin, M.; Qiu, R.; Li, J. Keratin-based wound dressings: From waste to wealth. Int. J. Biol. Macromol. 2022, 211, 183–197. [Google Scholar] [CrossRef]
- Lehmann, T.; Vaughn, A.E.; Seal, S.; Liechty, K.W.; Zgheib, C. Silk fibroin-based therapeutics for impaired wound healing. Pharmaceutics 2022, 14, 651. [Google Scholar] [CrossRef]
- Li, G.; Sun, S. Silk fibroin-based biomaterials for tissue engineering applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef]
- Ji, X.; Li, Q.; Song, H.; Fan, C. Protein-mimicking nanoparticles in biosystems. Adv. Mater. 2022, e202201562. [Google Scholar] [CrossRef]
- Caporale, A.; Adorinni, S.; Lamba, D.; Saviano, M. Peptide-protein interactions: From drug design to supramolecular biomaterials. Molecules 2021, 26, 1219. [Google Scholar] [CrossRef]
- Hamley, I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [Green Version]
- Cringoli, M.C.; Fornasiero, P.; Marchesan, S. Chapter 10 Minimalistic peptide self-assembly into supramolecular biomaterials. In Soft Matter for Biomedical Applications; The Royal Society of Chemistry: London, UK, 2021; pp. 236–263. [Google Scholar]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-assembling peptides: From design to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides. Adv. Mater. 2020, 32, e1805798. [Google Scholar] [CrossRef]
- Webber, M.J.; Pashuck, E.T. (Macro)molecular self-assembly for hydrogel drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 275–295. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, W.; Qiu, F. Self-assembling peptides in current nanomedicine: Versatile nanomaterials for drug delivery. Curr. Med. Chem. 2020, 27, 4855–4881. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef]
- Yang, J.; An, H.W.; Wang, H. Self-assembled peptide drug delivery systems. ACS Appl. Bio Mater. 2021, 4, 24–46. [Google Scholar] [CrossRef]
- Pentlavalli, S.; Coulter, S.; Laverty, G. Peptide nanomaterials for drug delivery applications. Curr. Prot. Peptide Sci. 2020, 21, 401–412. [Google Scholar] [CrossRef]
- Nambiar, M.; Schneider, J.P. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J. Pept. Sci. 2022, 28, e3377. [Google Scholar] [CrossRef]
- Uzunalli, G.; Guler, M.O. Peptide gels for controlled release of proteins. Therap. Deliv. 2020, 11, 193–211. [Google Scholar] [CrossRef]
- Lyu, Y.; Azevedo, H.S. Supramolecular hydrogels for protein delivery in tissue engineering. Molecules 2021, 26, 873. [Google Scholar] [CrossRef]
- Cai, L.; Liu, S.; Guo, J.; Jia, Y.G. Polypeptide-based self-healing hydrogels: Design and biomedical applications. Acta Biomater. 2020, 113, 84–100. [Google Scholar] [CrossRef]
- Kurbasic, M.; Parisi, E.; Garcia, A.M.; Marchesan, S. Self-assembling, ultrashort peptide gels as antimicrobial biomaterials. Curr. Top. Med. Chem. 2020, 20, 1300–1309. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, C.; Xiong, F.; Ran, W.; Zhai, Y.; Zhu, H.H.; Wang, H.; Li, Y.; Zhang, P. Recent progress in the design and application of supramolecular peptide hydrogels in cancer therapy. Adv. Healthc. Mater. 2021, 10, e2001239. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-assembling peptide-based hydrogels for wound tissue repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Smith, A.M.; Ligorio, C.; Mykhaylyk, O.O.; Miller, A.F.; Saiani, A. Role of sheet-edge interactions in β-sheet self-assembling peptide hydrogels. Biomacromolecules 2020, 21, 2285–2297. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Patel, R.; Leach, J.; Mathomes, R.; Chhabria, V.; Patil-Sen, Y.; Hidalgo-Bastida, A.; Forbes, R.T.; Hayes, J.M.; Elsawy, M.A. Aromatic stacking facilitated self-assembly of ultrashort ionic complementary peptide sequence: β-sheet nanofibers with remarkable gelation and interfacial properties. Biomacromolecules 2020, 21, 2670–2680. [Google Scholar] [CrossRef]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, D. Designer peptide amphiphiles: Self-assembly to applications. Langmuir 2019, 35, 10704–10724. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Marchesan, S. Chapter 6 The use of D-amino acids for peptide self-assembled systems. In Peptide-Based Biomaterials; The Royal Society of Chemistry: London, UK, 2021; pp. 174–216. [Google Scholar]
- Vargiu, A.V.; Iglesias, D.; Styan, K.E.; Waddington, L.J.; Easton, C.D.; Marchesan, S. Design of a hydrophobic tripeptide that self-assembles into amphiphilic superstructures forming a hydrogel biomaterial. Chem. Commun. 2016, 52, 5912–5915. [Google Scholar] [CrossRef]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Scarel, M.; Marchesan, S. Diketopiperazine gels: New horizons from the self-assembly of cyclic dipeptides. Molecules 2021, 26, 3376. [Google Scholar] [CrossRef]
- Manchineella, S.; Govindaraju, T. Molecular self-assembly of cyclic dipeptide derivatives and their applications. ChemPlusChem 2017, 82, 88–106. [Google Scholar] [CrossRef]
- Kurbasic, M.; Semeraro, S.; Garcia, A.M.; Kralj, S.; Parisi, E.; Deganutti, C.; De Zorzi, R.; Marchesan, S. Microwave-assisted cyclization of unprotected dipeptides in water to 2,5-piperazinediones and self-assembly study of products and reagents. Synthesis 2019, 51, 2829–2838. [Google Scholar]
- Li, L.; Xie, L.; Zheng, R.; Sun, R. Self-assembly dipeptide hydrogel: The structures and properties. Front. Chem. 2021, 9, 739791. [Google Scholar] [CrossRef]
- Bellotto, O.; Kralj, S.; Melchionna, M.; Pengo, P.; Kisovec, M.; Podobnik, M.; De Zorzi, R.; Marchesan, S. Self-assembly of unprotected dipeptides into hydrogels: Water-channels make the difference. ChemBioChem 2022, 23, e202100518. [Google Scholar] [CrossRef]
- Bellotto, O.; Pierri, G.; Rozhin, P.; Polentarutti, M.; Kralj, S.; D’Andrea, P.; Tedesco, C.; Marchesan, S. Dipeptide self-assembly into water-channels and gel biomaterial. Org. Biomol. Chem. 2022. [Google Scholar] [CrossRef]
- Scarel, E.; Bellotto, O.; Rozhin, P.; Kralj, S.; Tortora, M.; Vargiu, A.V.; De Zorzi, R.; Rossi, B.; Marchesan, S. Single-atom substitution enables supramolecular diversity from dipeptide building blocks. Soft Matter 2022, 18, 2129–2136. [Google Scholar] [CrossRef]
- Kralj, S.; Bellotto, O.; Parisi, E.; Garcia, A.M.; Iglesias, D.; Semeraro, S.; Deganutti, C.; D’Andrea, P.; Vargiu, A.V.; Geremia, S.; et al. Heterochirality and halogenation control Phe-Phe hierarchical assembly. ACS Nano 2020, 14, 16951–16961. [Google Scholar] [CrossRef]
- Bellotto, O.; Kralj, S.; De Zorzi, R.; Geremia, S.; Marchesan, S. Supramolecular hydrogels from unprotected dipeptides: A comparative study on stereoisomers and structural isomers. Soft Matter 2020, 16, 10151–10157. [Google Scholar] [CrossRef]
- Ung, P.; Winkler, D.A. Tripeptide motifs in biology: Targets for peptidomimetic design. J. Med. Chem. 2011, 54, 1111–1125. [Google Scholar] [CrossRef]
- Hellmund, K.S.; Koksch, B. Self-assembling peptides as extracellular matrix mimics to influence stem cell’s fate. Front. Chem. 2019, 7, 172. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Reinhardt, J.W.; Tram, N.K.; Debski, A.C.; Agarwal, G.; Reilly, M.A.; Gooch, K.J. Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel. Acta Biomater. 2018, 70, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Ishida, A.; Oshikawa, M.; Ajioka, I.; Muraoka, T. Sequence-dependent bioactivity and self-assembling properties of RGD-containing amphiphilic peptides as extracellular scaffolds. ACS Appl. Bio Mater. 2020, 3, 3605–3611. [Google Scholar] [CrossRef]
- Fujii, D.; Takase, K.; Takagi, A.; Kamino, K.; Hirano, Y. Design of RGDS peptide-immobilized self-assembling β-strand peptide from barnacle protein. Int. J. Mol. Sci. 2021, 22, 1240. [Google Scholar] [CrossRef]
- Deidda, G.; Jonnalagadda, S.V.R.; Spies, J.W.; Ranella, A.; Mossou, E.; Forsyth, V.T.; Mitchell, E.P.; Bowler, M.W.; Tamamis, P.; Mitraki, A. Self-assembled amyloid peptides with Arg-Gly-Asp (RGD) motifs as scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 1404–1416. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Padilla-Lopategui, S.; Azevedo, H.S.; Mata, A. Host–guest-mediated epitope presentation on self-assembled peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 4870–4880. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lin, S.-P.; Nelli, S.R.; Zhan, F.-K.; Cheng, H.; Lai, T.-S.; Yeh, M.-Y.; Lin, H.-C.; Hung, S.-C. Self-assembled peptide-based hydrogels as scaffolds for proliferation and multi-differentiation of mesenchymal stem cells. Macromol. Biosci. 2017, 17, 1600192. [Google Scholar] [CrossRef] [PubMed]
- Mañas-Torres, M.C.; Gila-Vilchez, C.; Vazquez-Perez, F.J.; Kuzhir, P.; Momier, D.; Scimeca, J.-C.; Borderie, A.; Goracci, M.; Burel-Vandenbos, F.; Blanco-Elices, C.; et al. Injectable magnetic-responsive short-peptide supramolecular hydrogels: Ex vivo and in vivo evaluation. ACS Appl. Mater. Interfaces 2021, 13, 49692–49704. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Aviv, M.; Netti, F.; Cohen-Gerassi, D.; Adler-Abramovich, L. Molecular co-assembly of two building blocks harnesses both their attributes into a functional supramolecular hydrogel. Macromol. Biosci. 2022, 22, 2100439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yan, Y.; Qi, J.; Deng, L.; Shao, Z.-W.; Zhang, K.-Q.; Li, B.; Sun, Z.; Li, X. Cooperative assembly of a peptide gelator and silk fibroin afford an injectable hydrogel for tissue engineering. ACS Appl. Mater. Interfaces 2018, 10, 12474–12484. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Cheng, B.; Chen, K.; Cui, W.; Qi, J.; Li, X.; Deng, L. Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019, 8, 1801043. [Google Scholar] [CrossRef]
- Balion, Z.; Cėpla, V.; Svirskiene, N.; Svirskis, G.; Druceikaitė, K.; Inokaitis, H.; Rusteikaitė, J.; Masilionis, I.; Stankevičienė, G.; Jelinskas, T.; et al. Cerebellar cells self-assemble into functional organoids on synthetic, chemically crosslinked ECM-mimicking peptide hydrogels. Biomolecules 2020, 10, 754. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Yadav, S.; Kar, A.K.; Jha, D.; Gautam, H.K.; Patnaik, S.; Kumar, P.; Sharma, A.K. An injectable self-assembling hydrogel based on RGD peptidomimetic β-sheets as multifunctional biomaterials. Mater. Sci. Eng. C 2021, 133, 112633. [Google Scholar] [CrossRef]
- Chen, B.; Wu, P.; Liang, L.; Zhao, C.; Wang, Z.; He, L.; Zhang, R.; Xu, N. Inhibited effect of an RGD peptide hydrogel on the expression of β1-integrin, FAK, and Akt in Tenon’s capsule fibroblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1857–1865. [Google Scholar] [CrossRef]
- Firipis, K.; Boyd-Moss, M.; Long, B.; Dekiwadia, C.; Hoskin, W.; Pirogova, E.; Nisbet, D.R.; Kapsa, R.M.I.; Quigley, A.F.; Williams, R.J. Tuneable hybrid hydrogels via complementary self-assembly of a bioactive peptide with a robust polysaccharide. ACS Biomater. Sci. Eng. 2021, 7, 3340–3350. [Google Scholar] [CrossRef]
- Derkus, B.; Okesola, B.O.; Barrett, D.W.; D’Este, M.; Chowdhury, T.T.; Eglin, D.; Mata, A. Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater. 2020, 109, 82–94. [Google Scholar] [CrossRef]
- Aye, S.-S.S.; Li, R.; Boyd-Moss, M.; Long, B.; Pavuluri, S.; Bruggeman, K.; Wang, Y.; Barrow, C.R.; Nisbet, D.R.; Williams, R.J. Scaffolds formed via the non-equilibrium supramolecular assembly of the synergistic ECM peptides RGD and PHSRN demonstrate improved cell attachment in 3D. Polymers 2018, 10, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsumi, H.; Kawamura, M.; Mihara, H. Osteoblastic differentiation on hydrogels fabricated from Ca2+-responsive self-assembling peptides functionalized with bioactive peptides. Bioorg. Med. Chem. 2018, 26, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, M.C.; Romano, C.; Parisi, E.; Waddington, L.J.; Melchionna, M.; Semeraro, S.; De Zorzi, R.; Grönholm, M.; Marchesan, S. Bioadhesive supramolecular hydrogel from unprotected, short D,L-peptides with Phe-Phe and Leu-Asp-Val motifs. Chem. Commun. 2020, 56, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Sahab Negah, S.; Oliazadeh, P.; Jahanbazi Jahan-Abad, A.; Eshaghabadi, A.; Samini, F.; Ghasemi, S.; Asghari, A.; Gorji, A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019, 92, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Bruggeman, K.F.; Wang, Y.; Wang, T.Y.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. Using minimalist self-assembling peptides as hierarchical scaffolds to stabilise growth factors and promote stem cell integration in the injured brain. J. Tissue Eng. Regen. Med. 2018, 12, e1571–e1579. [Google Scholar] [CrossRef]
- Somaa, F.A.; Wang, T.-Y.; Niclis, J.C.; Bruggeman, K.F.; Kauhausen, J.A.; Guo, H.; McDougall, S.; Williams, R.J.; Nisbet, D.R.; Thompson, L.H.; et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017, 20, 1964–1977. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, A.J.; Sayed, Z.A.; Stephanopoulos, N.; Berns, E.J.; Wadhwani, A.R.; Morrissey, Z.D.; Chadly, D.M.; Kobayashi, S.; Edelbrock, A.N.; Mashimo, T.; et al. Creating a stem cell niche in the inner ear using self-assembling peptide amphiphiles. PLoS ONE 2017, 12, e0190150. [Google Scholar] [CrossRef]
- Ruan, H.; Xiao, R.; Jiang, X.; Zhao, B.; Wu, K.; Shao, Z.; Zhang, Z.; Duan, H.; Song, Y. Biofunctionalized self-assembly of peptide amphiphile induces the differentiation of bone marrow mesenchymal stem cells into neural cells. Mol. Cell. Biochem. 2019, 450, 199–207. [Google Scholar] [CrossRef]
- Álvarez, Z.; Kolberg-Edelbrock, A.N.; Sasselli, I.R.; Ortega, J.A.; Qiu, R.; Syrgiannis, Z.; Mirau, P.A.; Chen, F.; Chin, S.M.; Weigand, S.; et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 2021, 374, 848–856. [Google Scholar] [CrossRef]
- Jain, R.; Roy, S. Controlling neuronal cell growth through composite laminin supramolecular hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 2832–2846. [Google Scholar] [CrossRef]
- Onak Pulat, G.; Gökmen, O.; Çevik, Z.B.Y.; Karaman, O. Role of functionalized self-assembled peptide hydrogels in in vitro vasculogenesis. Soft Matter 2021, 17, 6616–6626. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhang, Y.; Yang, Z.; Gao, J.; Shi, Y. Enzyme-instructed self-assembly (EISA) assists the self-assembly and hydrogelation of hydrophobic peptides. J. Mater. Chem. B 2022, 10, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, C.; Dai, G.; Feng, F.; Chi, Y.; Xu, K.; Zhong, W. Molecular self-assembly of a tyroservatide-derived octapeptide and hydroxycamptothecin for enhanced therapeutic efficacy. Nanoscale 2021, 13, 5094–5102. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gao, Y.; Guan, Y.; Wang, Z.; Yang, L.; Gao, J.; Fan, H.; Liu, J. Carrier-free supramolecular hydrogel composed of dual drugs for conquering drug resistance. ACS Appl. Mater. Interfaces 2019, 11, 33706–33715. [Google Scholar] [CrossRef]
- Ren, C.; Gao, Y.; Liu, J.; Zhang, Y.; Pu, G.; Yang, L.; Huang, F.; Yang, C.; Yang, Z.; Liu, J. Anticancer supramolecular hydrogel of D/L-peptide with enhanced stability and bioactivity. J. Biomed. Nanotechnol. 2018, 14, 1125–1134. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Chang, J.; Yang, C.; Liu, J.; Fan, S.; Ren, C. Enzyme-instructed self-assembly of a novel histone deacetylase inhibitor with enhanced selectivity and anticancer efficiency. Biomater. Sci. 2019, 7, 1477–1485. [Google Scholar] [CrossRef]
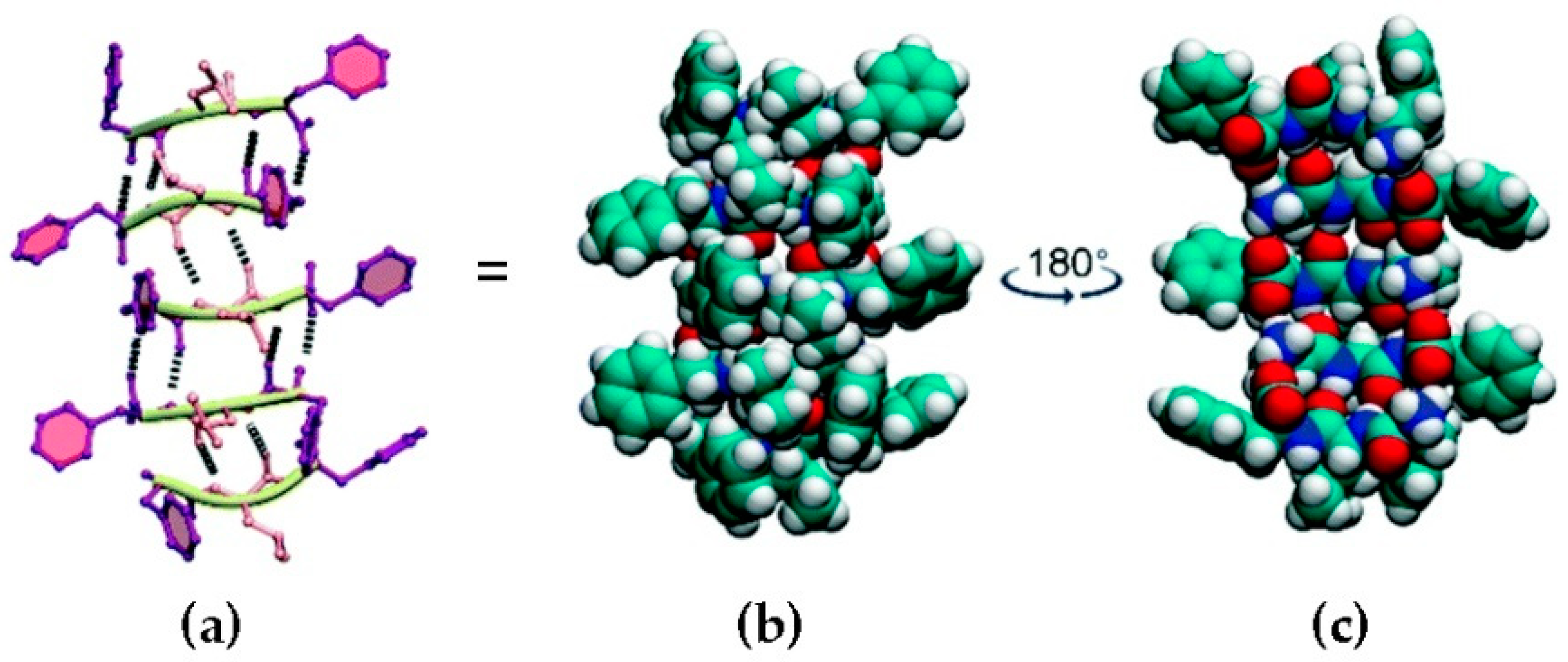
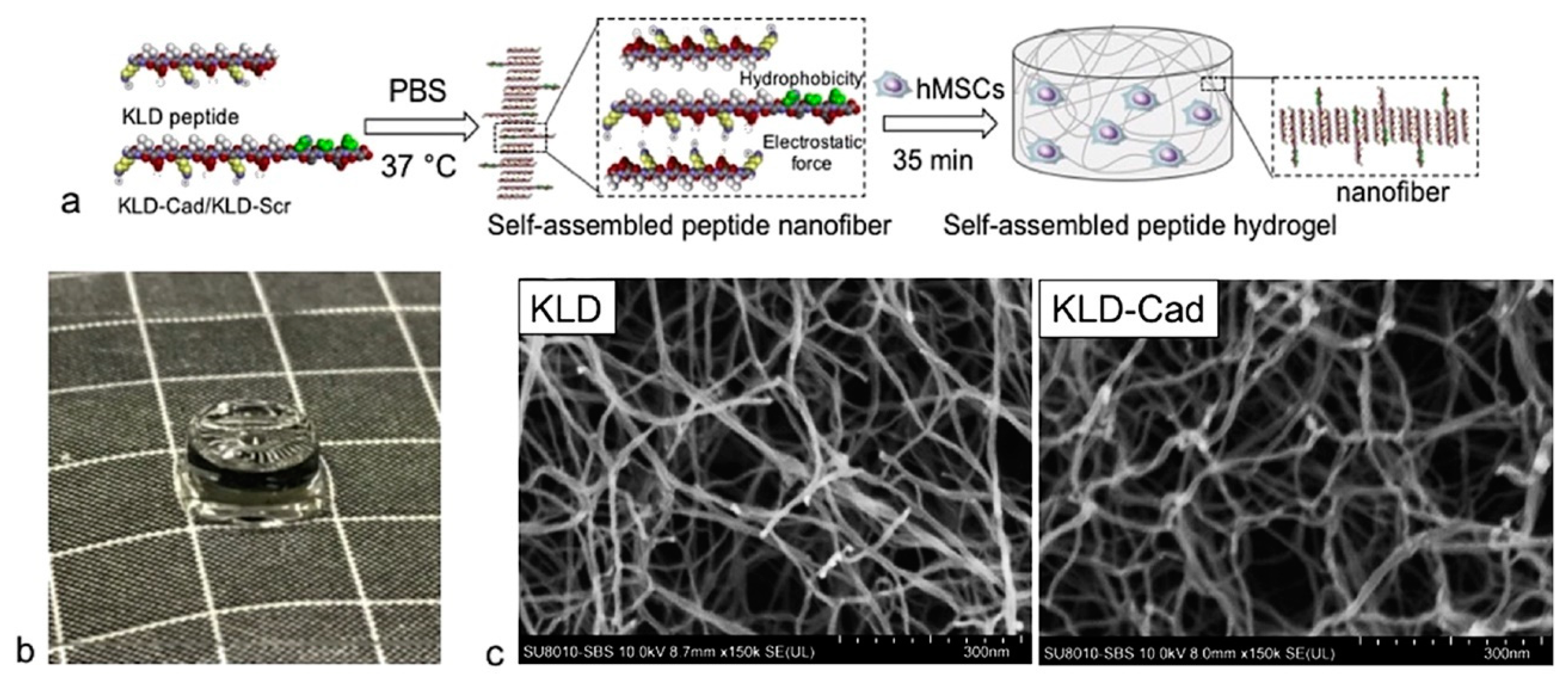
- Kaur, H.; Roy, S. Designing aromatic N-cadherin mimetic short-peptide-based bioactive scaffolds for controlling cellular behaviour. J. Mater. Chem. B 2021, 9, 5898–5913. [Google Scholar] [CrossRef]
- Eren Cimenci, C.; Kurtulus, G.U.; Caliskan, O.S.; Guler, M.O.; Tekinay, A.B. N-cadherin mimetic peptide nanofiber system induces chondrogenic differentiation of mesenchymal stem cells. Bioconjug. Chem. 2019, 30, 2417–2426. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef]
- Wang, T.-W.; Chang, K.-C.; Chen, L.-H.; Liao, S.-Y.; Yeh, C.-W.; Chuang, Y.-J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef]
- Castelletto, V.; Edwards-Gayle, C.J.C.; Greco, F.; Hamley, I.W.; Seitsonen, J.; Ruokolainen, J. Self-assembly, tunable hydrogel properties, and selective anti-cancer activity of a carnosine-derived lipidated peptide. ACS Appl. Mater. Interfaces 2019, 11, 33573–33580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Borchardt, T.R.; Wang, B. Orally active peptidomimetic RGD analogs that are glycoprotein IIb/IIa antagonists. Curr. Med. Chem. 2000, 7, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Gahane, A.Y.; Ranjan, P.; Singh, V.; Sharma, R.K.; Sinha, N.; Sharma, M.; Chaudhry, R.; Thakur, A.K. Fmoc-phenylalanine displays antibacterial activity against Gram-positive bacteria in gel and solution phases. Soft Matter 2018, 14, 2234–2244. [Google Scholar] [CrossRef]
- Mould, A.P.; Komoriya, A.; Yamada, K.M.; Humphries, M.J. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin α4β1. Inhibition of α4β1 function by RGD peptide homologues. J. Biol. Chem. 1991, 266, 3579–3585. [Google Scholar] [CrossRef]
- Komoriya, A.; Green, L.J.; Mervic, M.; Yamada, S.S.; Yamada, K.M.; Humphries, M.J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J. Biol. Chem. 1991, 266, 15075–15079. [Google Scholar] [CrossRef]
- Singh, J.; Adams, S.; Carter, M.B.; Cuervo, H.; Lee, W.-C.; Lobb, R.R.; Pepinsky, R.B.; Petter, R.; Scott, D. Rational design of potent and selective VLA-4 inhibitors and their utility in the treatment of asthma. Curr. Top. Med. Chem. 2004, 4, 1497–1507. [Google Scholar] [CrossRef]
- Kaneda, Y.; Yamamoto, Y.; Okada, N.; Tsutsumi, Y.; Nakagawa, S.; Kakiuch, M.; Maeda, M.; Kawasaki, K.; Mayumi, T. Antimetastatic effect of synthetic Glu-Ile-Leu-Asp-Val peptide derivatives containing D-amino acids. Anti-Cancer Drugs 1997, 8, 702–707. [Google Scholar] [CrossRef]
- Hattori, A.; Hozumi, K.; Ko, J.A.; Chikama, T.; Oomikawa, K.; Kato, J.; Ishida, K.; Hoshi, N.; Katagiri, F.; Kikkawa, Y.; et al. Sequence specificity of the PHSRN peptide from fibronectin on corneal epithelial migration. Biochem. Biophys. Res. Commun. 2009, 379, 346–350. [Google Scholar] [CrossRef]
- Aota, S.; Nomizu, M.; Yamada, K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994, 269, 24756–24761. [Google Scholar] [CrossRef]
- Aucoin, L.; Griffith, C.M.; Pleizier, G.; Deslandes, Y.; Sheardown, H. Interactions of corneal epithelial cells and surfaces modified with cell adhesion peptide combinations. J. Biomater. Sci. Polym. Ed. 2002, 13, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Shroff, K.; Pearce, T.R.; Kokkoli, E. Enhanced integrin mediated signaling and cell cycle progression on fibronectin mimetic peptide amphiphile monolayers. Langmuir 2012, 28, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Mardilovich, A.; Craig, J.A.; McCammon, M.Q.; Garg, A.; Kokkoli, E. Design of a novel fibronectin-mimetic peptide−amphiphile for functionalized biomaterials. Langmuir 2006, 22, 3259–3264. [Google Scholar] [CrossRef]
- Garcia, A.M.; Melchionna, M.; Bellotto, O.; Kralj, S.; Semeraro, S.; Parisi, E.; Iglesias, D.; D’Andrea, P.; De Zorzi, R.; Vargiu, A.V.; et al. Nanoscale Assembly of Functional Peptides with Divergent Programming Elements. ACS Nano 2021, 15, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Sephel, G.C.; Weeks, B.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Yamada, Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Cem. 1989, 264, 16174–16182. [Google Scholar] [CrossRef]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv. Sci. 2022, 9, e2103875. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Khooei, A.; Samini, F.; Gorji, A. Laminin-derived Ile-Lys-Val-Ala-Val: A promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res. 2018, 371, 223–236. [Google Scholar] [CrossRef]
- Graf, J.; Iwamoto, Y.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Robey, F.A.; Yamada, Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell 1987, 48, 989–996. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamada, Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J. Biol. Chem. 1987, 262, 17111–17117. [Google Scholar] [CrossRef]
- Pan, X.; Xu, J.; Jia, X. Research progress evaluating the function and mechanism of anti-tumor peptides. Cancer Manag. Res. 2020, 12, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, Y.; Shi, J.; Li, J.; Yuan, D.; Alberti, K.A.; Xu, Q.; Xu, B. Pericellular hydrogel/nanonets inhibit cancer cells. Angew. Chem. Int. Ed. 2014, 53, 8104–8107. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef] [Green Version]
- Pokutta, S.; Weis, W.I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell. Dev. Biol. 2007, 23, 237–261. [Google Scholar] [CrossRef] [Green Version]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Blaschuk, O.W.; Sullivan, R.; David, S.; Pouliot, Y. Identification of a cadherin cell adhesion recognition sequence. Dev. Biol. 1990, 139, 227–229. [Google Scholar] [CrossRef]
- Williams, E.-J.; Williams, G.; Gour, B.; Blaschuk, O.; Doherty, P. INP, a novel N-cadherin antagonist targeted to the amino acids that flank the HAV motif. Mol. Cell. Neurosci. 2000, 15, 456–464. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kobayashi, M.; Lee, P.P.; Lee, S.-W. Early osteogenic differentiation of mouse preosteoblasts induced by collagen-derived DGEA-peptide on nanofibrous phage tissue matrices. Biomacromolecules 2011, 12, 987–996. [Google Scholar] [CrossRef]
- Gariballa, S.E.; Sinclair, A.J. Carnosine: Physiological properties and therapeutic potential. Age Ageing 2000, 29, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Quinn, P.J.; Boldyrev, A.A.; Formazuyk, V.E. Carnosine: Its properties, functions and potential therapeutic applications. Mol. Asp. Med. 1992, 13, 379–444. [Google Scholar] [CrossRef]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Gaunitz, F.; Hipkiss, A.R. Carnosine and cancer: A perspective. Amino Acids 2012, 43, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.R.; Castelletto, V.; Connon, C.J.; Hamley, I.W. Collagen stimulating effect of peptide amphiphile C16–KTTKS on human fibroblasts. Mol. Pharm. 2013, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): A novel circulating stimulator of osteoblastic activity. EMBO J. 1992, 11, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Wang, X.; Gelain, F.; Zhang, S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE 2007, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, T.; Zhai, Y.; Ma, Y.; Xiao, Y. Designer functionalized self-assembling peptide scaffolds for adhesion, proliferation, and differentiation of MC3T3-E1. Soft Mater. 2014, 12, 79–87. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vukicevic, S.; Reddi, A.H. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev. Biol. 1991, 143, 303–308. [Google Scholar] [CrossRef]
- Lampel, A. Biology-inspired supramolecular peptide systems. Chem 2020, 6, 1222–1236. [Google Scholar] [CrossRef]
- Melchionna, M.; Styan, K.E.; Marchesan, S. The unexpected advantages of using D-amino acids for peptide self- assembly into nanostructured hydrogels for medicine. Curr. Top. Med. Chem. 2016, 16, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Clerici, F.; Erba, E.; Gelmi, M.L.; Pellegrino, S. Non-standard amino acids and peptides: From self-assembly to nanomaterials. Tetrahedron Lett. 2016, 57, 5540–5550. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Daniele, M.; Perez, V.; Gentili, A.; Gasperi, T.; Lupi, S.; Palocci, C. A physico-chemical approach to the study of genipin crosslinking of biofabricated peptide hydrogels. Process Biochem. 2018, 70, 110–116. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, D.; Marchesan, S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials 2022, 12, 2147. https://doi.org/10.3390/nano12132147
Marin D, Marchesan S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials. 2022; 12(13):2147. https://doi.org/10.3390/nano12132147
Chicago/Turabian StyleMarin, Davide, and Silvia Marchesan. 2022. "Self-Assembled Peptide Nanostructures for ECM Biomimicry" Nanomaterials 12, no. 13: 2147. https://doi.org/10.3390/nano12132147
APA StyleMarin, D., & Marchesan, S. (2022). Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials, 12(13), 2147. https://doi.org/10.3390/nano12132147







