Carbon Tube-Based Cathode for Li-CO2 Batteries: A Review
Abstract
:1. Introduction
2. Structure and Reaction Mechanism of a Lithium–Carbon Dioxide Battery
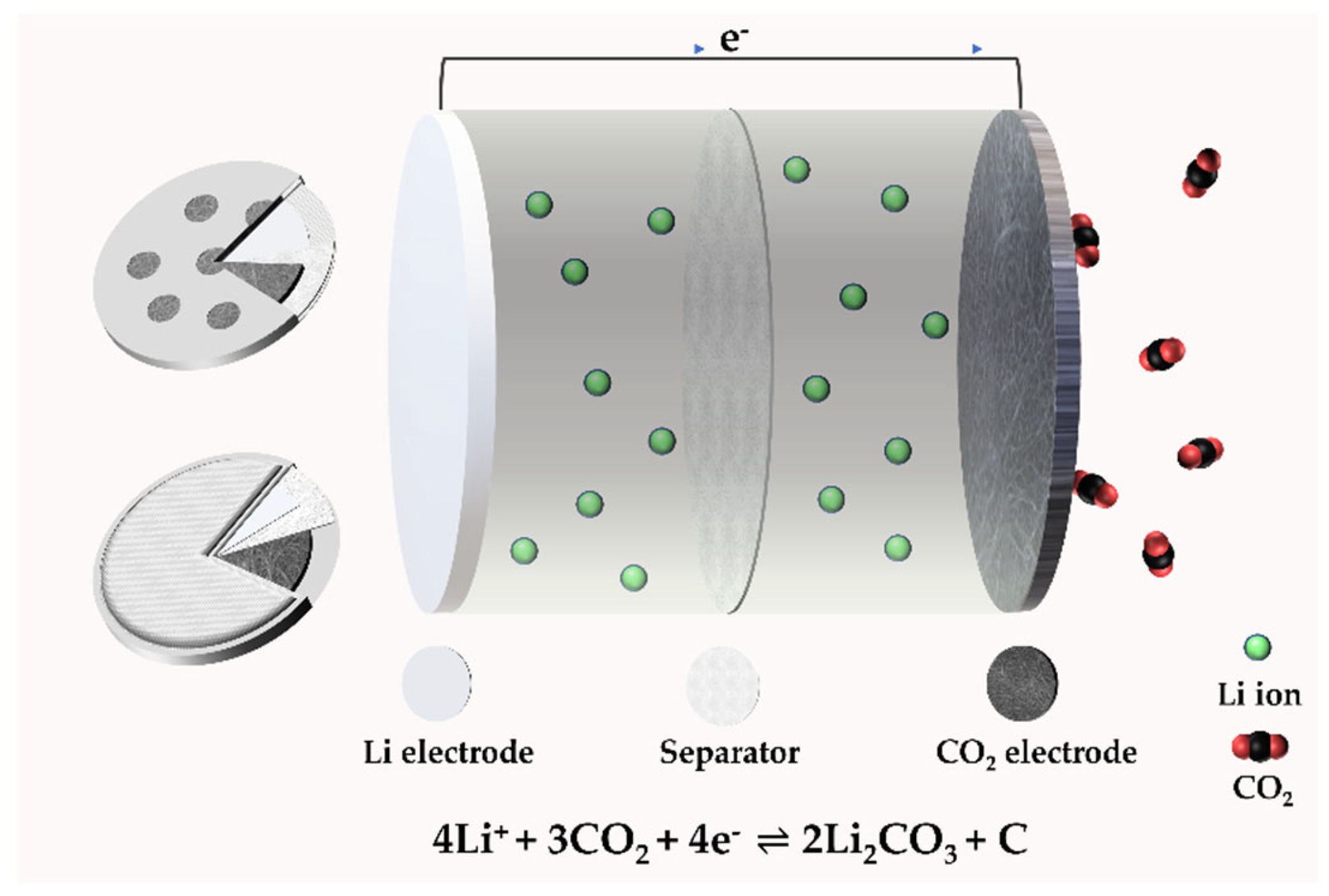
2.1. The Structure of a Li–CO2 Battery
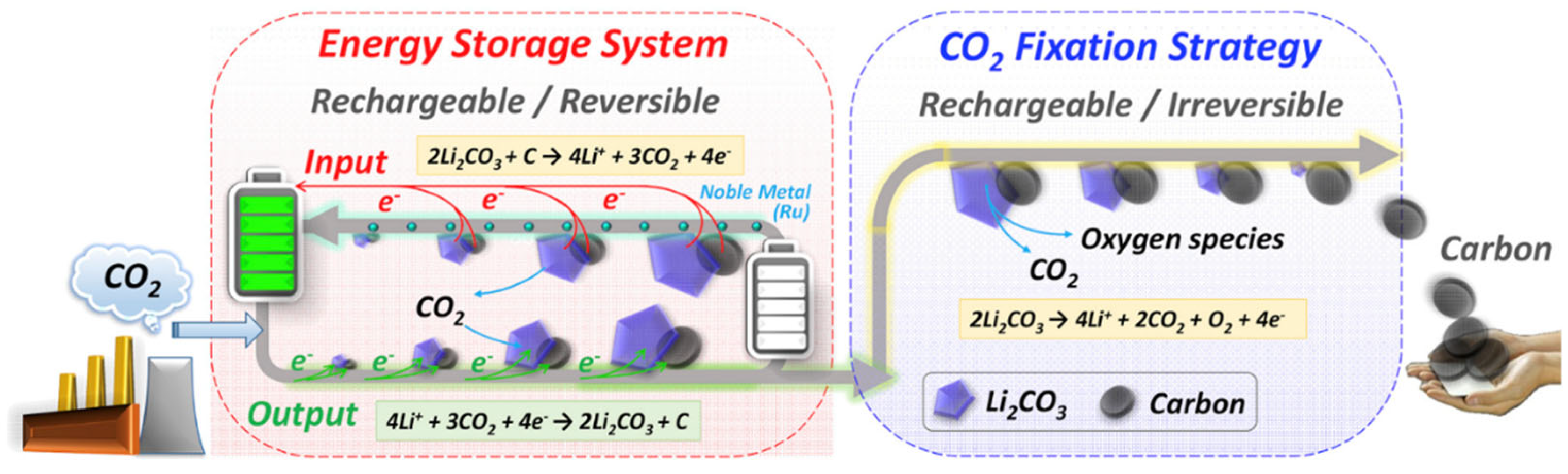
2.2. The Mechanism of a Li–CO2 Battery
2.3. The Application of DEMS in Electrode Interface Reaction
3. Carbon Tube-Based Cathode for Li–CO2 Battery
3.1. Carbon Tube–Noble Metal-Based Composites
3.2. Carbon Tube–Molybdenum-Based Composites
3.3. Carbon Tube–Other Metal-Based Composites
3.4. Heteroatom-Doped Carbon Tube-Based Composites
4. Prospect
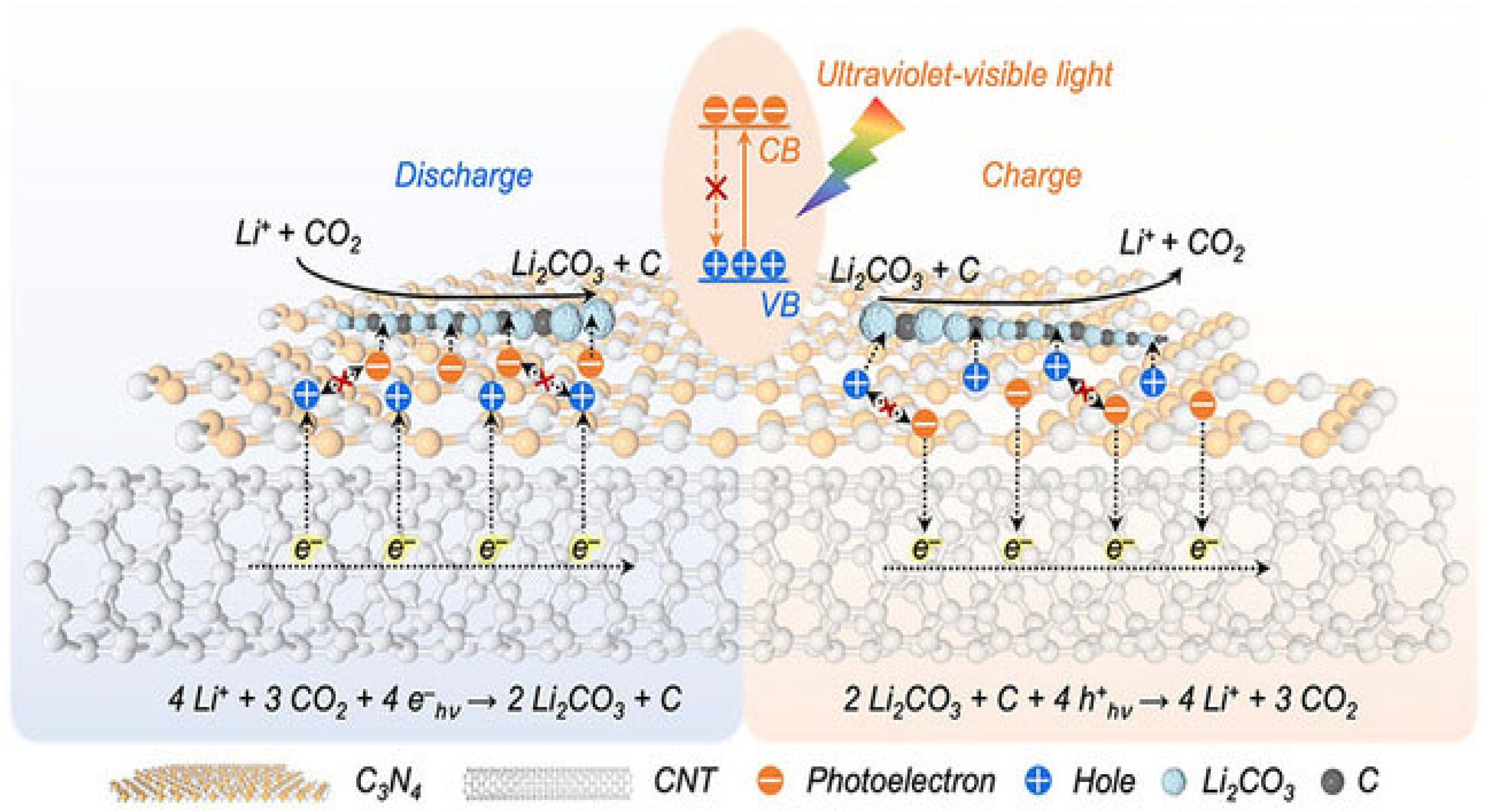
4.1. Light Field Assistance
4.2. Solid State
- They avoid electrolyte volatilization, which is not flammable;
- They can inhibit the growth of lithium dendrites with higher safety;
- They are not prone to inducing side reactions and have a better stability;
- They can effectively prevent water vapor in the air and reduce the corrosion of lithium anodes.
4.3. Other Metal–CO2 Batteries
5. Conclusions
- Mechanism problems in actual material systems. Through mass spectrometry or other in situ characterization techniques, the dynamic process of the battery system is usually explored by using simulated batteries, which may not effectively correspond to the actual battery system, especially in terms of the battery performance under different specific electrode material systems. For several steps of intermediate product formation, lithium carbonate or Li2C2O4 needs to be combined with the specific actual material system, and even with the different phase structures and tri-configuration systems of the same material, which is complex work.
- Key material selection and structural design issues. The selection of catalytic electrode materials needs to comprehensively consider factors such as the source, cost, preparation process, catalytic performance, and stability. The selection of the electrolyte is primarily concerned with the safety of the voltage window range, as well as the cost. The cost of existing liquid and solid electrolyte systems is much higher than that of lithium-ion batteries. In addition to the electrode and electrolyte, the type and packaging process of the separator and battery casing will affect the final performance of the battery. That means each component plays a vital role in the final performance and application of Li–CO2 batteries.
- Characterization method. Non-in situ characterization methods can only provide complementary reference information for the disassembled battery. Effectively combining in situ infrared, Raman, scanning, and transmission electron microscopy with the battery system is the key to the study. In addition, with the combination of experimental characterization and theoretical calculation, the experimental data should provide more guidance to the calculation model, rather than a simple simulation, such as the reaction path, product type, formation, and decomposition of energy, reactant, product adsorption energy, Gibbs free energy change, electron, ion migration rate of thermodynamics, and kinetics analysis.
- Practical application problems. As a high-energy-density energy storage device and carbon dioxide treatment device, the actual reaction process, performance, and effect of the battery at the amplification scale should be considered in the actual application process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The IMBIE team. Mass balance of the Antarctic ice sheet from 1992 to 2017. Nature 2018, 558, 219–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Matter, J.M.; Stute, M.; Snaebjornsdottir, S.O.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, W.J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.K.; Frimer, A.A.; et al. Lithium–oxygen batteries and related systems: Potential, status, and future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef]
- Jung, J.W.; Cho, S.H.; Nam, J.S.; Kim, I.D. Current and future cathode materials for non-aqueous Li-air (O2) battery technology—A focused review. Energy Storage Mater. 2020, 24, 512–528. [Google Scholar] [CrossRef]
- Wang, D.; Mu, X.W.; He, P.; Zhou, H.S. Materials for advanced Li–O2 batteries: Explorations, challenges and prospects. Mater. Today 2019, 26, 87–99. [Google Scholar] [CrossRef]
- Zou, X.H.; Lu, Q.; Liao, K.M.; Shao, Z.P. Towards practically accessible aprotic Li–air batteries: Progress and challenges related to oxygen-permeable membranes and cathodes. Energy Storage Mater. 2022, 45, 869–902. [Google Scholar] [CrossRef]
- Mao, Y.J.; Tang, C.; Tang, Z.C.; Xie, J.; Chen, Z.; Tu, J.; Cao, G.S.; Zhao, X.B. Long-life Li–CO2 cells with ultrafine IrO2-decorated few-layered δ-MnO2 enabling amorphous Li2CO3 growth. Energy Storage Mater. 2019, 18, 405–413. [Google Scholar] [CrossRef]
- Prehal, C.; Freunberger, S.A. Li–O2 cell-scale energy densities. Joule 2019, 3, 321–323. [Google Scholar] [CrossRef] [Green Version]
- Takechi, K.; Shiga, T.; Asaoka, T. A Li–O2/CO2 battery. Chem. Commun. 2011, 47, 3463–3465. [Google Scholar] [CrossRef]
- Jiao, Y.N.; Qin, J.; Sari, H.M.K.; Li, D.J.; Li, X.F.; Sun, X.L. Recent progress and prospects of Li–CO2 batteries: Mechanisms, catalysts and electrolytes. Energy Storage Mater. 2021, 34, 148–170. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, L.; Zhao, Y.; Li, S.Y.; Fu, X.M.; Wang, B.J.; Peng, H.S. Li–CO2 batteries efficiently working at ultra-low temperatures. Adv. Funct. Mater. 2020, 30, 2001619–2001628. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.W.; Liu, P.F.; Xie, Y.P.; Cao, K.Z.; Zhou, Z. Identification of cathode stability in Li–CO2 batteries with Cu nanoparticles highly dispersed on N-doped graphene. J. Mater. Chem. A 2018, 6, 3218–3223. [Google Scholar] [CrossRef]
- Zhou, J.W.; Li, X.L.; Yang, C.; Li, Y.C.; Guo, K.K.; Cheng, J.L.; Yuan, D.W.; Song, C.H.; Lu, J.; Wang, B. A quasi-solid-state flexible fiber-shaped Li–CO2 battery with low overpotential and high energy efficiency. Adv. Mater. 2019, 31, 1804439–1804448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bai, W.L.; Wang, K.X.; Chen, J.S. Electrocatalyst design for aprotic Li–CO2 batteries. Energy Environ. Sci. 2020, 13, 4717–4737. [Google Scholar] [CrossRef]
- Sun, X.Y.; Hou, Z.P.; He, P.; Zhou, H.S. Recent advances in rechargeable Li–CO2 batteries. Energy Fuels 2021, 35, 9165–9186. [Google Scholar] [CrossRef]
- Qiao, Y.; Yi, J.; Wu, S.C.; Liu, Y.; Yang, S.X.; He, P.; Zhou, H.S. Li–CO2 electrochemistry: A new strategy for CO2 fixation and energy storage. Joule 2017, 1, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.T.; Li, H.; Chen, J.Z.; Ye, H.J.; Yao, J.M.; Su, Y.W.; Guo, B.Y.; Peng, Z.Q.; Shen, T.D.; Tang, Y.F.; et al. In situ imaging electrocatalytic CO2 reduction and evolution reactions in all-solid-state Li–CO2 nanobatteries. Nanoscale 2020, 12, 23967–23974. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, W.T.; Shang, W.X.; Tan, P.; Dai, Y.W.; Cheng, C.; Ni, M. Investigation on the strategies for discharge capacity improvement of aprotic Li–CO2 batteries. Energy Fuels 2020, 34, 16870–16878. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Valery, A.; Luntz, A.C.; Gowda, S.R.; Wallraff, G.M.; Garcia, J.M.; Mori, T.; Krupp, L.E. Combining accurate O2 and Li2O2 assays to separate discharge and charge stability limitations in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 2013, 4, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.R.; Brunet, A.; Wallraff, G.M.; McCloskey, B.D. Implications of CO2 contamination in rechargeable nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 2013, 4, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Débart, A.; Holzapfel, M.; Novák, P.; Bruce, P.G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef]
- Peng, Z.Q.; Freunberger, S.A.; Chen, Y.H.; Bruce, P.G. A reversible and higher-rate Li–O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.J.; Zhang, X.; Zhang, Z.; Zhou, Z. Metal–CO2 batteries on the road: CO2 from contamination gas to energy source. Adv. Mater. 2017, 29, 1605891–1605899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Pang, L.; Su, Y.W.; Liu, T.F.; Wang, G.X.; Liu, C.T.; Wang, J.W.; Peng, Z.Q. Deciphering CO2 reduction reaction mechanism in aprotic Li–CO2 batteries using in situ vibrational spectroscopy coupled with theoretical calculations. ACS Energy Lett. 2022, 7, 624–631. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Du, F.H.; Xu, S.M.; Wang, Z.K.; Wang, K.X.; Chen, J.S. Hydroquinone resin induced carbon nanotubes on Ni foam as binder-free cathode for Li–O2 batteries. ACS Appl. Mater. Inter. 2016, 8, 3868–3873. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zhang, Z.; Chen, Y.N.; Xie, Z.J.; Wei, J.P.; Zhou, Z. Rechargeable Li–CO2 batteries with carbon nanotubes as air cathodes. Chem. Commun. 2015, 51, 14636–14639. [Google Scholar] [CrossRef]
- Yoon, K.R.; Kim, D.S.; Ryu, W.H.; Song, S.H.; Youn, D.Y.; Jung, J.W.; Jeon, S.; Park, Y.J.; Kim, I.D. Tailored combination of low dimensional catalysts for efficient oxygen reduction and evolution in Li–O2 batteries. ChemSusChem 2016, 9, 2080–2088. [Google Scholar] [CrossRef]
- Lin, J.F.; Ding, J.N.; Wang, H.Z.; Yang, X.Y.; Zheng, X.R.; Huang, Z.C.; Song, W.Q.; Ding, J.; Han, X.P.; Hu, W.B. Boosting energy efficiency and stability of Li–CO2 batteries via synergy between Ru atom clusters and single-atom Ru-N4 sites in the electrocatalyst cathode. Adv. Mater. 2022, 34, 2200559–2200569. [Google Scholar] [CrossRef]
- Savunthari, K.V.; Chen, C.H.; Chen, Y.R.; Tong, Z.Z.; Iputera, K.; Wang, F.M.; Hsu, C.C.; Wei, D.H.; Hu, S.F.; Liu, R.S. Effective Ru/CNT cathode for rechargeable solid-state Li–CO2 batteries. ACS Appl. Mater. Inter. 2021, 13, 44266–44273. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.L.; Gong, H.; Song, L.; Jiang, C.; Wang, T.; He, J.P. Nano-sized Au particle-modified carbon nanotubes as an effective and stable cathode for Li–CO2 batteries. Eur. J. Inorg. Chem. 2021, 2021, 590–596. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, S.M.; Liu, Y.; Dai, J.Q.; Xie, H.; Yao, Y.G.; Mu, X.W.; Chen, C.; Kline, D.J.; Hitz, E.M.; et al. Transient, in situ synthesis of ultrafine ruthenium nanoparticles for a high-rate Li–CO2 battery. Energy Environ. Sci. 2019, 12, 1100–1107. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.S.; Tan, J.Y.; Liu, Y.Q.; Jiao, M.L.; Liu, B.L.; Zhao, N.Q.; Zou, X.L.; Zhou, G.M.; Cheng, H.M. Designing electrophilic and nucleophilic dual centers in the ReS2 plane toward efficient bifunctional catalysts for Li–CO2 batteries. J. Am. Chem. Soc. 2022, 144, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yang, J.J.; Chen, C.U.; Wei, D.H.; Hu, S.F.; Liu, R.S. Improvement of lithium anode deterioration for ameliorating cyclabilities of non-aqueous Li–CO2 batteries. Nanoscale 2020, 12, 8385–8396. [Google Scholar] [CrossRef]
- Thoka, S.; Tsai, C.M.; Tong, Z.Z.; Jena, A.; Wang, F.M.; Hsu, C.C.; Chang, H.; Hu, S.F.; Liu, R.S. Comparative study of Li–CO2 and Na–CO2 batteries with Ru@CNT as a cathode catalyst. ACS Appl. Mater. Inter. 2021, 13, 480–490. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Chen, Z.X.; Xu, H.S.; Yu, W.; Liu, C.B.; Wang, X.W.; Zhang, K.; Xie, K.Y.; Loh, K.P. Covalent-organic-framework-based Li–CO2 batteries. Adv. Mater. 2019, 31, 1905879–1905887. [Google Scholar] [CrossRef]
- Bie, S.Y.; Du, M.L.; He, W.X.; Zhang, H.G.; Yu, Z.T.; Liu, J.G.; Liu, M.; Yan, W.W.; Zhou, L.; Zou, Z.G. Carbon nanotube@RuO2 as a high performance catalyst for Li–CO2 batteries. ACS Appl. Mater. Inter. 2019, 11, 5146–5151. [Google Scholar] [CrossRef]
- Jin, Y.C.; Chen, F.Y.; Wang, J.L.; Johnston, R.L. Tuning electronic and composition effects in ruthenium-copper alloy nanoparticles anchored on carbon nanofibers for rechargeable Li–CO2 batteries. Chem. Eng. J. 2019, 375, 121978–121987. [Google Scholar] [CrossRef]
- Kwak, W.J.; Lau, K.C.; Shin, C.D.; Amine, K.; Curtiss, L.A.; Sun, Y.K. A Mo2C/carbon nanotube composite cathode for lithium–oxygen batteries with high energy efficiency and long cycle life. ACS Nano 2015, 9, 4129–4137. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Xu, S.M.; Harris, M.M.; Ma, C.; Liu, Y.S.; Wei, X.; Xu, H.S.; Zhou, Y.X.; Cao, Y.C.; Wang, K.X.; et al. A composite of carbon-wrapped Mo2C nanoparticle and carbon nanotube formed directly on Ni foam as a high-performance binder-free cathode for Li–O2 batteries. Adv. Funct. Mater. 2016, 26, 8514–8520. [Google Scholar] [CrossRef]
- Qi, G.C.; Zhang, J.X.; Chen, L.; Wang, B.; Cheng, J.L. Binder-free MoN nanofibers catalysts for flexible 2-electron oxalate-based Li–CO2 batteries with high energy efficiency. Adv. Funct. Mater. 2022, 32, 2112501–2112511. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Wang, J.Z.; Liu, L.L.; Liu, Y.Q.; Chou, S.L.; Shi, D.Q.; Liu, H.K.; Wu, Y.P.; Zhang, W.M.; Chen, J. Mo2C/CNT: An efficient catalyst for rechargeable Li–CO2 batteries. Adv. Funct. Mater. 2017, 27, 1700564–1700571. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Guo, K.K.; Yuan, D.W.; Cheng, J.L.; Wang, B. Unraveling reaction mechanisms of Mo2C as cathode catalyst in a Li–CO2 battery. J. Am. Chem. Soc. 2020, 142, 6983–6990. [Google Scholar] [CrossRef]
- Feng, N.N.; Wang, B.L.; Yu, Z.; Gu, Y.M.; Xu, L.L.; Ma, J.; Wang, Y.G.; Xia, Y.Y. Mechanism-of-action elucidation of reversible Li–CO2 batteries using the water-in-salt electrolyte. ACS Appl. Mater. Inter. 2021, 13, 7396–7404. [Google Scholar] [CrossRef]
- Mao, D.Y.; Yi, S.L.; He, Z.R.; Zhu, Q.C. Non-woven fabrics derived binder-free gas diffusion catalyst cathode for long cycle Li–O2 batteries. J. Electroanal. Chem. 2022, 915, 116356–116361. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Xu, S.M.; Cai, Z.P.; Harris, M.M.; Wang, K.X.; Chen, J.S. Towards real Li-air batteries: A binder-free cathode with high electrochemical performance in CO2 and O2. Energy Storage Mater. 2017, 7, 209–215. [Google Scholar] [CrossRef]
- Chen, M.H.; Liu, Y.; Liang, X.Q.; Wang, F.; Li, Y.; Chen, Q.G. Integrated carbon nanotube/MoO3 core/shell arrays as freestanding air cathodes for flexible Li–CO2 batteries. Energy Technol. 2021, 9, 2100547. [Google Scholar] [CrossRef]
- Jin, Y.C.; Liu, Y.; Song, L.; Yu, J.H.; Li, K.R.; Zhang, M.D.; Wang, J.L. Interfacial engineering in hollow NiS2/FeS2-NSGA heterostructures with efficient catalytic activity for advanced Li–CO2 battery. Chem. Eng. J. 2022, 430, 133029–133038. [Google Scholar] [CrossRef]
- Chen, C.J.; Huang, C.S.; Huang, Y.C.; Wang, F.M.; Wang, X.C.; Wu, C.C.; Chang, W.S.; Dong, C.L.; Yin, L.C.; Liu, R.S. Catalytically active site identification of molybdenum disulfide as gas cathode in a nonaqueous Li-CO2 battery. ACS Appl. Mater. Inter. 2021, 13, 6156–6167. [Google Scholar] [CrossRef]
- He, B.; Li, G.Y.; Li, J.J.; Wang, J.; Tong, H.; Fan, Y.Q.; Wang, W.L.; Sun, S.H.; Dang, F. MoSe2@CNT core-shell nanostructures as grain promoters featuring a direct Li2O2 formation/decomposition catalytic capability in lithium-oxygen batteries. Adv. Energy Mater. 2021, 11, 2003263–2003274. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Gong, H.; Song, L.; Kong, Y.L.; Jiang, C.; Xue, H.R.; Li, P.; Huang, X.L.; He, J.P.; Wang, T. A highly efficient and free-standing copper single atoms anchored nitrogen-doped carbon nanofiber cathode toward reliable Li–CO2 batteries. Mater. Today Energy 2022, 25, 100967–100975. [Google Scholar] [CrossRef]
- Gong, H.; Yu, X.Y.; Xu, Y.Y.; Gao, B.; Xue, H.R.; Fan, X.L.; Guo, H.; Wang, T.; He, J.P. Long-life reversible Li–CO2 batteries with optimized Li2CO3 flakes as discharge products on palladium-copper nanoparticles. Inorg. Chem. Front. 2022, 9, 1533–1540. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhao, S.Y.; Wang, D.S.; Chen, B.; Zhang, Z.Y.; Sheng, J.Z.; Zhong, X.W.; Zou, X.L.; Jiang, S.P.; Zhou, G.M.; et al. Toward an understanding of the reversible Li–CO2 batteries over metal-N4-functionalized graphene electrocatalysts. ACS Nano 2022, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Jiang, C.; Gong, H.; Xue, H.R.; Gao, B.; Li, P.; Chang, K.; Huang, X.L.; Wang, T.; He, J.P. Single atom site conjugated copper polyphthalocyanine assisted carbon nanotubes as cathode for reversible Li–CO2 batteries. Nano Res. 2022, 15, 4100–4107. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhou, J.W.; Zhang, T.B.; Wang, T.S.; Li, X.L.; Jia, Y.F.; Cheng, J.L.; Guan, Q.; Liu, E.Z.; Peng, H.S.; et al. Highly surface-wrinkled and N-doped CNTs anchored on metal Wire: A novel fiber-shaped cathode toward high-performance flexible Li–CO2 batteries. Adv. Funct. Mater. 2019, 29, 1808117–1808129. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.Y.; Yoo, J.K.; Ryu, W.H. Capillary-driven formation of iron nanoparticles embedded in nanotubes for catalyzed lithium–carbon dioxide reaction. ACS Mater. Lett. 2021, 3, 815–825. [Google Scholar] [CrossRef]
- Thoka, S.; Chen, C.J.; Jena, A.; Wang, F.M.; Wang, X.C.; Chang, H.; Hu, S.F.; Liu, R.S. Spinel zinc cobalt oxide (ZnCo2O4) porous nanorods as a cathode material for highly durable Li–CO2 batteries. ACS Appl. Mater. Inter. 2020, 12, 17353–17363. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.Y.; Li, H.H.; Wang, X.G.; Chen, Y.N.; Xie, Z.J.; Zhou, Z. High performance Li–CO2 batteries with NiO-CNT cathodes. J. Mater. Chem. A 2018, 6, 2792–2796. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.J.; Yu, W.T.; Shang, W.X.; Ma, Y.Y.; Zhu, X.B.; Tan, P. Ultrafine Co-doped NiO nanoparticles decorated on carbon nanotubes improving the electrochemical performance and cycling stability of Li–CO2 batteries. ACS Appl. Energy Mater. 2021, 4, 11858–11866. [Google Scholar] [CrossRef]
- Lei, D.L.; Ma, S.Y.; Lu, Y.C.; Liu, Q.C.; Li, Z.J. High-performance Li–CO2 batteries with α-MnO2/CNT cathodes. J. Electron. Mater. 2019, 48, 4653–4659. [Google Scholar] [CrossRef]
- Liu, Q.N.; Hu, Z.; Li, L.; Li, W.J.; Zou, C.; Jin, H.L.; Wang, S.; Chou, S.L. Facile synthesis of birnessite δ-MnO2 and carbon nanotube composites as effective catalysts for Li–CO2 batteries. ACS Appl. Mater. Inter. 2021, 13, 16585–16593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Wang, T.S.; Yang, Y.J.; Zhang, X.; Lu, Z.J.; Wang, J.N.; Sun, C.; Diao, Y.Y.; Wang, X.; Yao, J.N. Breaking the stable triangle of carbonate via W-O bonds for Li–CO2 batteries with low polarization. ACS Energy Lett. 2021, 6, 3503–3510. [Google Scholar] [CrossRef]
- Gao, J.B.; Liu, Y.D.; Terayama, Y.; Katafuchi, K.; Hoshino, Y.; Inoue, G. Polyamine nanogel particles spray-coated on carbon paper for efficient CO2 capture in a milli-channel reactor. Chem. Eng. J. 2020, 401, 126059–126068. [Google Scholar] [CrossRef]
- Sun, Z.M.; Wang, D.; Lin, L.; Liu, Y.H.; Yuan, M.W.; Nan, C.Y.; Li, H.F.; Sun, G.B.; Yang, X.J. Ultrathin hexagonal boron nitride as a van der Waals’ force initiator activated graphene for engineering efficient non-metal electrocatalysts of Li–CO2 battery. Nano Res. 2022, 15, 1171–1177. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, J.W.; Zhang, J.X.; Li, M.; Bi, X.X.; Liu, T.C.; He, T.; Cheng, J.L.; Zhang, F.; Li, Y.P.; et al. Bamboo-like nitrogen-doped carbon nanotube forests as durable metal-free catalysts for self-powered flexible Li–CO2 batteries. Adv. Mater. 2019, 31, 1903852–1903860. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhang, J.X.; Qi, G.C.; Cheng, J.L.; Wang, B. Vertically aligned N-doped carbon nanotubes arrays as efficient binder-free catalysts for flexible Li–CO2 batteries. Energy Storage Mater. 2021, 35, 148–156. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, W.Y.; Zhang, B.; Fu, L.; Wan, M.T.; Li, G.C.; Cai, Z.; Tu, S.B.; Duan, X.R.; Seh, Z.W.; et al. Manipulating redox kinetics of sulfur species using Mott-Schottky electrocatalysts for advanced lithium-sulfur batteries. Nano Lett. 2021, 21, 6656–6663. [Google Scholar] [CrossRef]
- Xiao, Y.; Du, F.; Hu, C.G.; Ding, Y.; Wang, Z.L.; Roy, A.; Dai, L.M. High-performance Li–CO2 batteries from free-standing, binder-free, bifunctional three-dimensional carbon catalysts. ACS Energy Lett. 2020, 5, 916–921. [Google Scholar] [CrossRef]
- Song, L.; Hu, C.G.; Xiao, Y.; He, J.P.; Lin, Y.; Connell, J.W.; Dai, L.M. An ultra-long life, high-performance, flexible Li–CO2 battery based on multifunctional carbon electrocatalysts. Nano Energy 2020, 71, 104595–104601. [Google Scholar] [CrossRef]
- Na, D.; Jeong, H.; Baek, J.; Yu, H.; Lee, S.M.; Lee, C.R.; Seo, H.K.; Kim, J.K.; Seo, I. Highly safe and stable Li–CO2 batteries using conducting ceramic solid electrolyte and MWCNT composite cathode. Electrochim. Acta 2022, 419, 140408–140415. [Google Scholar] [CrossRef]
- Xie, H.M.; Zhang, B.; Hu, C.G.; Xiao, N.; Liu, D. Boosting Li–CO2 battery performances by creating holey structure on CNT cathodes. Electrochim. Acta 2022, 417, 140310–140316. [Google Scholar] [CrossRef]
- Liu, L.L.; Guo, H.P.; Fu, L.J.; Chou, S.L.; Thiele, S.; Wu, Y.P.; Wang, J.Z. Critical advances in ambient air operation of nonaqueous rechargeable Li–Air batteries. Small 2021, 17, 1903854–1903885. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Zhang, K.; Zhao, Y.; Wang, C.; Wang, L.P.; Wang, L.; Liao, M.; Ye, L.; Zhang, Y.; Gao, Y.; et al. High-efficiency and stable Li–CO2 battery enabled by carbon nanotube/carbon nitride heterostructured photocathode. Angew Chem. Int. Ed. 2022, 61, e202114612. [Google Scholar]
- Zhang, K.; Li, J.X.; Zhai, W.J.; Li, C.F.; Zhu, Z.F.; Kang, X.Y.; Liao, M.; Ye, L.; Kong, T.Y.; Wang, C.; et al. Boosting cycling stability and rate capability of Li–CO2 batteries via synergistic photoelectric effect and plasmonic interaction. Angew Chem. Int. Ed. 2022, 61, e202201718. [Google Scholar]
- Ma, S.Y.; Lu, Y.C.; Yao, H.C.; Liu, Q.C.; Li, Z.J. Enhancing the process of CO2 reduction reaction by using CTAB to construct contact ion pair in Li–CO2 battery. Chin. Chem. Lett. 2022, 33, 2933–2936. [Google Scholar] [CrossRef]
- Wang, L.D.; Lu, Y.C.; Ma, S.Y.; Lian, Z.; Gu, X.L.; Li, J.; Li, Z.J.; Liu, Q.C. Optimizing CO2 reduction and evolution reaction mediated by o-phenylenediamine toward high performance Li–CO2 battery. Electrochim. Acta 2022, 419, 140424–140432. [Google Scholar] [CrossRef]
- Lai, J.N.; Xing, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R.J. Electrolytes for rechargeable lithium–air batteries. Angew Chem. Int. Ed. 2020, 59, 2974–2997. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Nie, J.H.; Li, F.; Wang, Z.L.; Sun, C.W. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Qiu, G.R.; Shi, Y.P.; Huang, B.L. A highly ionic conductive succinonitrile-based composite solid electrolyte for lithium metal batteries. Nano Res. 2022, 15, 5153–5160. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and properties of NaSICON-type LATP and LAGP solid electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, Z.Y.; Yang, B.C.; Liu, Y.; Wang, Y.G.; Xia, Y.Y. A rechargeable Li–CO2 battery with a gel polymer electrolyte. Angew Chem. Int. Ed. 2017, 56, 9126–9130. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Li, Z.F.; Chen, J. Flexible Li–CO2 batteries with liquid-free electrolyte. Angew Chem. Int. Ed. 2017, 56, 5785–5789. [Google Scholar] [CrossRef]
- Mu, X.W.; Pan, H.; He, P.; Zhou, H.S. Li–CO2 and Na–CO2 batteries: Toward greener and sustainable electrical energy storage. Adv. Mater. 2020, 32, 1903790–1903811. [Google Scholar]
- Tang, M.; Du, J.Y.; Ma, J.L.; Wang, X.D.; Zhang, X.; Shen, Q.Y.; Wang, F.P.; Wang, Y. Cobalt-decorated carbon nanofibers as a low overpotential cathode for nonaqueous Na–CO2 batteries. J. Alloys Compd. 2022, 911, 165054–165062. [Google Scholar] [CrossRef]
- Mao, Y.J.; Chen, X.; Cheng, H.; Lu, Y.H.; Xie, J.; Zhang, T.; Tu, J.; Xu, X.W.; Zhu, T.J.; Zhao, X.B. Forging ispired processing of sodium-fuorinated graphene composite as dendrite-free anode for long-life Na–CO2 cells. Energy Environ. Mater. 2022, 5, 572–581. [Google Scholar] [CrossRef]
- Xu, C.F.; Wang, H.W.; Zhan, J.; Kang, Y.; Liang, F. Engineering NH3-induced 1D self-assembly architecture with conductive polymer for advanced hybrid Na–CO2 batteries via morphology modulation. J. Power Sources 2022, 520, 230909–230920. [Google Scholar] [CrossRef]
- Hu, X.F.; Li, Z.F.; Zhao, Y.R.; Sun, J.C.; Zhao, Q.; Wang, J.B.; Tao, Z.L.; Chen, J. Quasi-solid state rechargeable Na–CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 2017, 3, e1602396. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Cai, Y.C.; Zhang, Q.; Ni, Y.X.; Zhang, K.; Chen, J. Rechargeable K–CO2 batteries with a KSn anode and a carboxyl-containing carbon nanotube cathode catalyst. Angew Chem. Int. Ed. 2021, 60, 9540–9545. [Google Scholar] [CrossRef]







| Cathode | Discharge Capacity/ Current Density | Cycle Performance (Cutoff Specific Capacity/Current Density) | Discharge–Charge Voltage Platform | Year | Ref. |
|---|---|---|---|---|---|
| Mo2C/CNTs | 1150 μAh/20 μA | 40 (100 μAh/20 μA) | 2.65/3.35 V | 2017 | [43] |
| MoC/N-CNTs | 8227 mAh g−1/100 mA g−1 | 90 (1000 mAh g−1/1000 mA g−1) | 2.75/3.79 V | 2017 | [47] |
| NiO-CNTs | 9000 mAh g−1/100 mA g−1 | 42 (1000 mAh g−1/50 mA g−1) | 2.75/4.00 V | 2018 | [59] |
| COF-Ru@CNT | 27,348 mAh g−1/200 mA g−1 | 200 (1000 mAh g−1/1000 mA g−1) | 2.53/4.27 V | 2019 | [37] |
| CNTs@RuO2 | 2187 mAh g−1/50 mA g−1 | 55 (500 mAh g−1/50 mA g−1) | 2.48/3.90 V | 2019 | [38] |
| N-CNTs@Ti | 9292.3 mAh g−1/50 mA g−1 | 45 (1000 mAh g−1/250 mA g−1) | 2.60/4.18 V | 2019 | [56] |
| MnO2/CNTs | 7134 mAh g−1/50 mA g−1 | 50 (1000 mAh g−1/100 mA g−1) | 2.62/3.95 V | 2019 | [61] |
| N-CNTs | 23,328 mAh g−1/50 mA g−1 | 360 (1000 mAh g−1/1000 mA g−1) | 2.72/3.98 V | 2019 | [66] |
| Ru/CNTs | 2882 mAh g−1/100 mA g−1 | 268 (100 mAh g−1/100 mA g−1) | 2.56/4.01 V | 2020 | [35] |
| ZnCo2O4@CNTs | 4275 mAh g−1/100 mA g−1 | 230 (500 mAh g−1/100 mA g−1) | 2.52/4.22 V | 2020 | [58] |
| Co3O4@CNTs | 2473 mAh g−1/100 mA g−1 | 43 (500 mAh g−1/100 mA g−1) | 2.45/4.38 V | 2020 | [58] |
| 3D NCNTs/G | 17,534 mAh g−1/50 mA g−1 | 185 (1000 mAh g−1/100 mA g−1) | 2.77/3.90 V | 2020 | [69] |
| N,S-CNTs | 23,560 mAh g−1/200 mA g−1 | 538 (500 mAh g−1/200 mA g−1) | 2.63/4.52 V | 2020 | [70] |
| Ru/CNTs | 4541 mAh g−1/100 mA g−1 | 45 (500 mAh g−1/100 mA g−1) | 2.76/4.24 V | 2021 | [31] |
| AuNPs/CNTs | 6399 mAh g−1/100 mA g−1 | 46 (1000 mAh g−1/200 mA g−1) | 2.73/4.30 V | 2021 | [32] |
| Ru/CNTs | 23,102 mAh g−1/100 mA g−1 | 100 (500 mAh g−1/100 mA g−1) | 2.60/4.09 V | 2021 | [36] |
| Mo2C/CNTs | 0.5 mAh/0.05 mA | 20 (1000 mAh g−1/100 mA g−1) | 2.74/3.41 V | 2021 | [45] |
| MoO3@CNTs | 30.25 mAh cm−2/0.05 mA cm−2 | 300 (1 mAh cm−2/0.05 mA cm−2) | 2.68 /4.03 V | 2021 | [48] |
| MoS2/CNTs | 8551 mAh g−1/100 mA g−1 | 140 (500 mAh g−1/100 mA g−1) | 2.70/3.94 V | 2021 | [50] |
| Fe/CNTs | 3898 mAh g−1/100 mA g−1 | 30 (600 mAh g−1/100 mA g−1) | 2.62/4.24 V | 2021 | [57] |
| Co0.1Ni0.9Ox/CNT | 5871.4 mAh g−1/100 mA g−1 | 50 (500 mAh g−1/100 mA g−1) | 2.55/3.94 V | 2021 | [60] |
| CNT@MnO2 | - | 50 (1000 mAh g−1/200 mA g−1) | 2.64/4.19 V | 2021 | [62] |
| W2C-CNTs | 10,632 mAh g−1/100 mA g−1 | 75 (500 mAh g−1/200 mA g−1) | 2.81/3.20 V | 2021 | [63] |
| N-CNTs | 18,652 mAh g−1/100 mA g−1 | 120 (1000 mAh g−1/250 mA g−1) | 2.51/4.25 V | 2021 | [67] |
| CuPPc-CNTs | 18,652.7 mAh g−1/100 mA g−1 | 160 (1000 mAh g−1/200 mA g−1) | 2.87/4.32 V | 2022 | [55] |
| MWCNTs | 5255 mAh g−1/60 mA g−1 | 50 (600 mAh g−1/60 mA g−1) | 2.75/4.31 V | 2022 | [71] |
| Holey CNTs | 17,500 mAh g−1/500 mA g−1 | 150 (500 mAh g−1/100 mA g−1) | 2.75/4.31 V | 2022 | [72] |
| Metal-CO2 Battery | Earth’s Crust | Theoretical Potential | Theoretical Energy Density |
|---|---|---|---|
| Li | 0.0017 wt% | 2.80 V | 1876 Wh Kg−1 |
| Na | 2.3 wt% | 2.35 V | 1130 Wh Kg−1 |
| K | 1.5 wt% | 2.48 V | 921 Wh Kg−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, D.; He, Z.; Lu, W.; Zhu, Q. Carbon Tube-Based Cathode for Li-CO2 Batteries: A Review. Nanomaterials 2022, 12, 2063. https://doi.org/10.3390/nano12122063
Mao D, He Z, Lu W, Zhu Q. Carbon Tube-Based Cathode for Li-CO2 Batteries: A Review. Nanomaterials. 2022; 12(12):2063. https://doi.org/10.3390/nano12122063
Chicago/Turabian StyleMao, Deyu, Zirui He, Wanni Lu, and Qiancheng Zhu. 2022. "Carbon Tube-Based Cathode for Li-CO2 Batteries: A Review" Nanomaterials 12, no. 12: 2063. https://doi.org/10.3390/nano12122063
APA StyleMao, D., He, Z., Lu, W., & Zhu, Q. (2022). Carbon Tube-Based Cathode for Li-CO2 Batteries: A Review. Nanomaterials, 12(12), 2063. https://doi.org/10.3390/nano12122063







