Organic/Inorganic-Based Flexible Membrane for a Room-Temperature Electronic Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the ZnO/PVA/IL Membrane
2.3. Characterization
2.4. Sensor Fabrication and H2S Gas Sensing Tests
3. Results and Discussion
3.1. Structural and Morphological Characterization of ZnO NPs and ZnO/PVA/IL Membrane
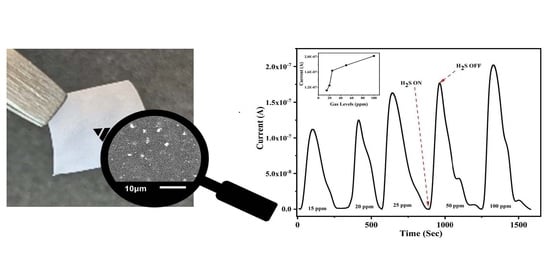
3.2. Gas Sensing Performance
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, R125. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Belley, R.; Bernard, N.; Côté, M.; Paquet, F.; Poitras, J. Hyperbaric oxygen therapy in the management of two cases of hydrogen sulfide toxicity from liquid manure. Can. J. Emerg. Med. 2005, 7, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, L.D.; Presnell, S.E. Death by sewer gas: Case report of a double fatality and review of the literature. Am. J. Forensic Med. Pathol. 2005, 26, 181–185. [Google Scholar] [CrossRef]
- Eun, S.; Reinhart, D.R.; Cooper, C.D.; Townsend, T.G.; Faour, A. Hydrogen sulfide flux measurements from construction and demolition debris (C&D) landfills. Waste Manag. 2007, 27, 220–227. [Google Scholar]
- Panza, D.; Belgiorno, V. Hydrogen sulphide removal from landfill gas. Process Saf. Environ. Prot. 2010, 88, 420–424. [Google Scholar] [CrossRef]
- Xu, Q.; Townsend, T. Factors affecting temporal H2S emission at construction and demolition (C&D) debris landfills. Chemosphere 2014, 96, 105–111. [Google Scholar]
- Cheng, Z.; Sun, Z.; Zhu, S.; Lou, Z.; Zhu, N.; Feng, L. The identification and health risk assessment of odor emissions from waste landfilling and composting. Sci. Total Environ. 2019, 649, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.; Baharuddin, A.A.; Wong, Y.H. Conducting polymers as chemiresistive gas sensing materials: A review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Amírola, J.; Rodríguez, A.; Castañer, L.; Santos, J.; Gutiérrez, J.; Horrillo, M. Micromachined silicon microcantilevers for gas sensing applications with capacitive read-out. Sens. Actuator B 2005, 111, 247–253. [Google Scholar] [CrossRef]
- Sedlak, P.; Sikula, J.; Majzner, J.; Vrnata, M.; Fitl, P.; Kopecky, D.; Vyslouzil, F.; Handel, P.H. Adsorption–desorption noise in QCM gas sensors. Sens. Actuators B 2012, 166, 264–268. [Google Scholar] [CrossRef]
- Manera, M.G.; Ferreiro-Vila, E.; García-Martín, J.M.; Cebollada, A.; García-Martín, A.; Giancane, G.; Valli, L.; Rella, R. Enhanced magneto-optical SPR platform for amine sensing based on Zn porphyrin dimers. Sens. Actuators B 2013, 182, 232–238. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chang, L.-F.; Hsueh, T.-J. Light-activated humidity and gas sensing by ZnO nanowires grown on LED at room temperature. Sens. Actuators B 2017, 249, 265–277. [Google Scholar] [CrossRef]
- Diep, V.M.; Armani, A.M. Flexible light-emitting nanocomposite based on ZnO nanotetrapods. Nano Lett. 2016, 16, 7389–7393. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Song, Z.; Kan, H.; Yu, H.; Liu, Q.; Zhang, G.; Liu, H. Sensitive H2S gas sensors employing colloidal zinc oxide quantum dots. Sens. Actuators B 2017, 249, 558–563. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Pan, Z.; Gu, M.; Punnoose, A. Synthesis of ZnO nanoparticles with controlled shapes, sizes, aggregations, and surface complex compounds for tuning or switching the photoluminescence. Cryst. Growth Des. 2015, 15, 3144–3149. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Gröttrup, J.; Mishra, A.K.; De Leeuw, N.H.; Carreira, J.F.; Rodrigues, J.; Ben Sedrine, N.; Correia, M.R.; Monteiro, T. Hybridization of zinc oxide tetrapods for selective gas sensing applications. ACS Appl. Mater. Interfaces 2017, 9, 4084–4099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çolak, H.; Karaköse, E. Synthesis and characterization of different dopant (Ge, Nd, W)-doped ZnO nanorods and their CO2 gas sensing applications. Sens. Actuators B 2019, 296, 126629. [Google Scholar] [CrossRef]
- Navaneethan, M.; Patil, V.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B 2018, 255, 672–683. [Google Scholar]
- Wei, W.; Zhao, J.; Shi, S.; Lin, H.; Mao, Z.; Zhang, F.; Qu, F. Boosting ppb-level triethylamine sensing of ZnO: Adjusting proportions of electron donor defects. J. Mater. Chem. C 2020, 8, 6734–6742. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.; Zhang, H. High temperature CO2 sensing and its cross-sensitivity towards H2 and CO gas using calcium doped ZnO thin film coated langasite SAW sensor. Sens. Actuators B 2019, 301, 126958. [Google Scholar] [CrossRef]
- Gupta, S.P.; Pawbake, A.S.; Sathe, B.R.; Late, D.J.; Walke, P.S. Superior humidity sensor and photodetector of mesoporous ZnO nanosheets at room temperature. Sens. Actuators B 2019, 293, 83–92. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.; Gaikwad, G.; Patil, D.; Naik, J. Synthesis of 1-D ZnO nanorods and polypyrrole/1-D ZnO nanocomposites for photocatalysis and gas sensor applications. Bull. Mater. Sci. 2016, 39, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Pan, Q.; Qin, J. Sensing characteristics of double layer film of ZnO. Sens. Actuators B 2000, 66, 161–163. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. A novel coral rock-like ZnO and its gas sensing. Mater. Lett. 2017, 209, 244–246. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B. The role of grain size on the thermal instability of nanostructured metal oxides used in gas sensor applications and approaches for grain-size stabilization. Prog. Cryst. Growth Charact. Mater. 2012, 58, 167–208. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Aziz, S.B.; Saeed, S.R. Structural and electrical properties of polyvinyl alcohol (PVA): Methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab. J. Chem. 2021, 14, 103388. [Google Scholar] [CrossRef]
- Ali, A.; Alzamly, A.; Greish, Y.E.; Bakiro, M.; Nguyen, H.L.; Mahmoud, S.T. A Highly Sensitive and Flexible Metal–Organic Framework Polymer-Based H2S Gas Sensor. ACS Omega 2021, 6, 17690–17697. [Google Scholar] [CrossRef]
- Allam, M.; Ayesh, A.I.; Mohsin, M.A.; Haik, Y. Physical properties of PVA doped with algal glycerol. J. Appl. Polym. Sci. 2013, 130, 4482–4489. [Google Scholar] [CrossRef]
- Abu-Hani, A.F.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Design, fabrication, and characterization of portable gas sensors based on spinel ferrite nanoparticles embedded in organic membranes. Sens. Actuators B 2017, 241, 1179–1187. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, A.; Kaushal, A.; Kaur, D.; Pandey, A.; Goyal, R. In situ high temperature XRD studies of ZnO nanopowder prepared via cost effective ultrasonic mist chemical vapour deposition. Bull. Mater. Sci. 2008, 31, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Ristić, M.; Musić, S.; Ivanda, M.; Popović, S. Sol–gel synthesis and characterization of nanocrystalline ZnO powders. J. Alloys Compd. 2005, 397, L1–L4. [Google Scholar] [CrossRef]
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar]
- Roy, A.S.; Gupta, S.; Sindhu, S.; Parveen, A.; Ramamurthy, P.C. Dielectric properties of novel PVA/ZnO hybrid nanocomposite films. Compos. Part B Eng. 2013, 47, 314–319. [Google Scholar] [CrossRef]
- Rawool, C.R.; Srivastava, A.K. A dual template imprinted polymer modified electrochemical sensor based on Cu metal organic framework/mesoporous carbon for highly sensitive and selective recognition of rifampicin and isoniazid. Sens. Actuators B 2019, 288, 493–506. [Google Scholar] [CrossRef]
- Ali, A.; AlTakroori, H.H.; Greish, Y.E.; Alzamly, A.; Siddig, L.A.; Qamhieh, N.; Mahmoud, S.T. Flexible Cu3 (HHTP) 2 MOF Membranes for Gas Sensing Application at Room Temperature. Nanomaterials 2022, 12, 913. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Kumari, B.S.; Rao, K.V.; Prabhu, Y. Germination and growth characteristics of mungbean seeds (Vigna radiata L.) affected by synthesized zinc oxide nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 2347–5161. [Google Scholar]
- Ghosh, S.; Adak, D.; Bhattacharyya, R.; Mukherjee, N. ZnO/γ-Fe2O3 charge transfer interface toward highly selective H2S sensing at a low operating temperature of 30 °C. ACS Sens. 2017, 2, 1831–1838. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Shinde, A.B.; Kulkarni, S.; Maiti, N.; Koinkar, P.M.; Sonawane, K.M. Gas sensing performance of Al doped ZnO thin film for H2S detection. J. Alloys Compd. 2018, 748, 6–11. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, X.; Hao, X.; Dong, G. Facile fabrication of polyaniline nanocapsule modified zinc oxide hexagonal microdiscs for H2S gas sensing applications. Ind. Eng. Chem. Res. 2019, 58, 1906–1913. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, J.; Xu, L.; Liu, T.; Liu, Y.; Wang, X.; Suo, H.; Zhao, C. Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity. Crystals 2020, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Shewale, P.S.; Yun, K.-S. Synthesis and characterization of Cu-dped ZnO/RGO nanocomposites for room-temperature H2S gas sensor. J. Alloys Compd. 2020, 837, 155527. [Google Scholar] [CrossRef]
- Ding, P.; Xu, D.; Dong, N.; Chen, Y.; Xu, P.; Zheng, D.; Li, X. A high-sensitivity H2S gas sensor based on optimized ZnO-ZnS nano-heterojunction sensing material. Chin. Chem. Lett. 2020, 31, 2050–2054. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Xie, L.; Li, X.; Lin, D.; Zhu, Z. Low-temperature and highly sensitivity H2S gas sensor based on ZnO/CuO composite derived from bimetal metal-organic frameworks. Ceram. Int. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Narayana, A.; Bhat, S.A.; Fathima, A.; Lokesh, S.; Surya, S.G.; Yelamaggad, C. Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor-based carbon monoxide sensing. RSC Adv. 2020, 10, 13532–13542. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Bhatt, V.; Abhyankar, A.C.; Yun, J.-H.; Jeong, H.-J. Multifunctional dumbbell-shaped ZnO based temperature-dependent UV photodetection and selective H2 gas detection. Int. J. Hydrogen Energy 2020, 45, 15011–15025. [Google Scholar] [CrossRef]
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet. Sens. Actuators B 2019, 288, 1–11. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y. ZnO nanosheets abundant in oxygen vacancies derived from metal-organic frameworks for ppb-level gas sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tian, F.; Liang, H.; Wang, K.; Zhao, X.; Lu, Z.; Jiang, K.; Yang, L.; Lou, X. From the surface reaction control to gas-diffusion control: The synthesis of hierarchical porous SnO2 microspheres and their gas-sensing mechanism. J. Phys. Chem. C 2015, 119, 15963–15976. [Google Scholar] [CrossRef]








| Sensor/Material | Gas | Operating Temp °C | Detection Limit (ppm) | Response Value | Response Time/Recovery Time (Second) | Ref. |
|---|---|---|---|---|---|---|
| ZnO/PVA/IL | H2S | RT | 15 | 99% | 24/112 | This Work |
| Colloidal ZnO QDs | H2S | RT | 50 | 113.5 | 16/820 | [19] |
| ZnO/γ Fe2O3 Electrochemical | H2S | RT | 250 | 80% | 60/300 | [46] |
| Al-ZnO spray pyrolysis | H2S | 200 | 150 | 12.41% | 200/209 | [47] |
| Self-assembled polyaniline nanocapsules/ZnO hexagonal microdiscs | H2S | RT | 50 | 11.5% | 63/12 | [48] |
| Lettuce like ZnO 3D | H2S | 150 | 100 | 113 | 15/90 | [49] |
| Cu-doped ZnO RGO | H2S | RT | 100 | 0.87% | 14/32 | [50] |
| ZnO ZnS Heterostructure | H2S | 150 | 5 | 0.88 | N R | [51] |
| ZnO CuO composite | H2S | 40 | 10 | 393.35 | 173/179 | [52] |
| ZnO NPs | CO | RT | 25 | 6% | N R | [53] |
| Dumbbell-shaped ZnO 3D | H2 | 60 | 100 | 20% | 20/10 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlTakroori, H.H.D.; Ali, A.; Greish, Y.E.; Qamhieh, N.; Mahmoud, S.T. Organic/Inorganic-Based Flexible Membrane for a Room-Temperature Electronic Gas Sensor. Nanomaterials 2022, 12, 2037. https://doi.org/10.3390/nano12122037
AlTakroori HHD, Ali A, Greish YE, Qamhieh N, Mahmoud ST. Organic/Inorganic-Based Flexible Membrane for a Room-Temperature Electronic Gas Sensor. Nanomaterials. 2022; 12(12):2037. https://doi.org/10.3390/nano12122037
Chicago/Turabian StyleAlTakroori, Husam H. D., Ashraf Ali, Yaser E. Greish, Naser Qamhieh, and Saleh T. Mahmoud. 2022. "Organic/Inorganic-Based Flexible Membrane for a Room-Temperature Electronic Gas Sensor" Nanomaterials 12, no. 12: 2037. https://doi.org/10.3390/nano12122037
APA StyleAlTakroori, H. H. D., Ali, A., Greish, Y. E., Qamhieh, N., & Mahmoud, S. T. (2022). Organic/Inorganic-Based Flexible Membrane for a Room-Temperature Electronic Gas Sensor. Nanomaterials, 12(12), 2037. https://doi.org/10.3390/nano12122037