Enhanced Luminance of CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO-Oleic Acid/ZnO Quantum Dots Double Electron Transport Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Quantum Dots
2.2. Synthesis of ZnO-Oleic Acid (OA) QDs
2.3. QLEDs and Electron (Hole)-Only Device Fabrication
2.4. Characterization
3. Results and Discussions
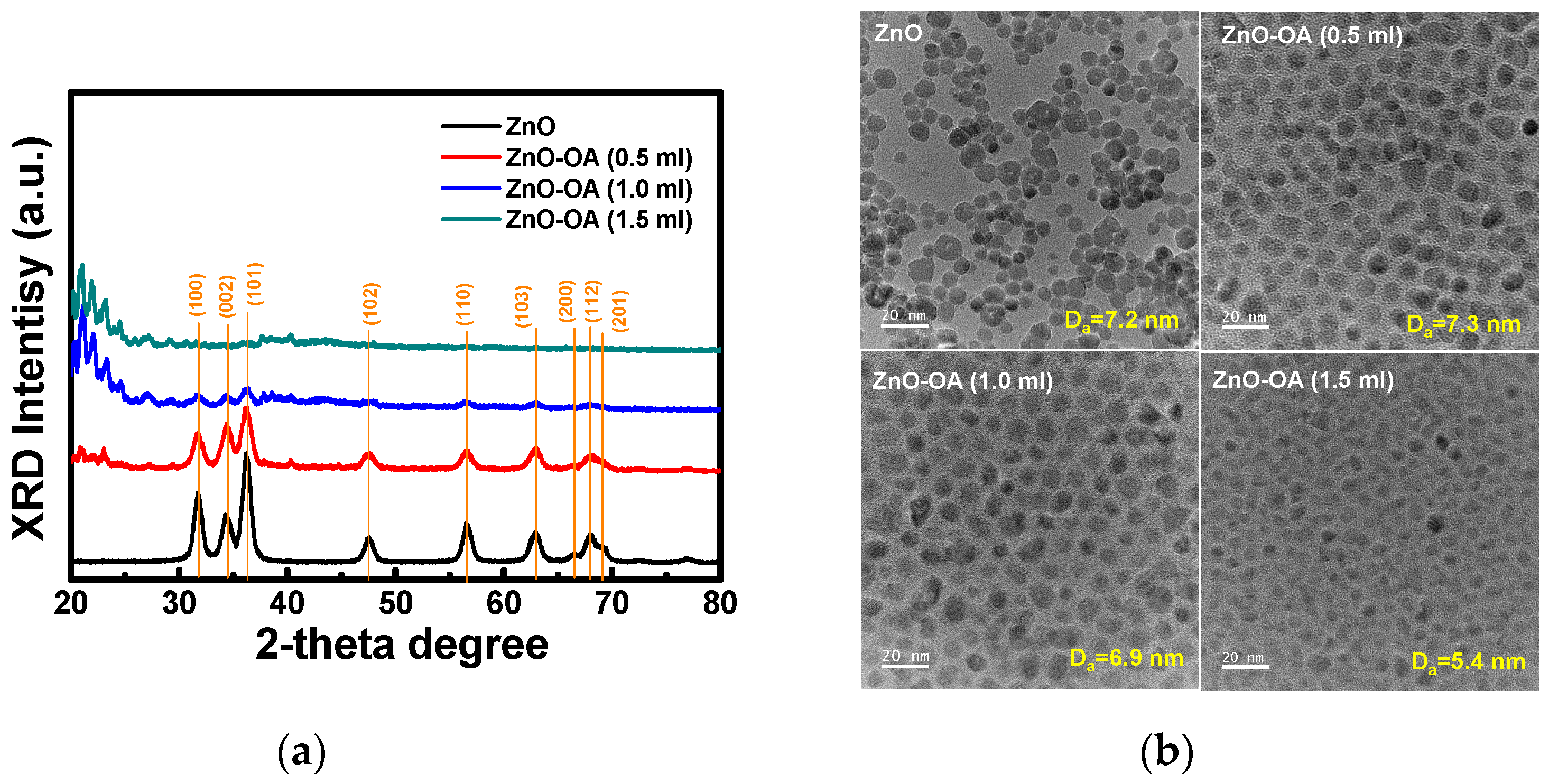
3.1. Synthesis of ZnO Quantum Dots
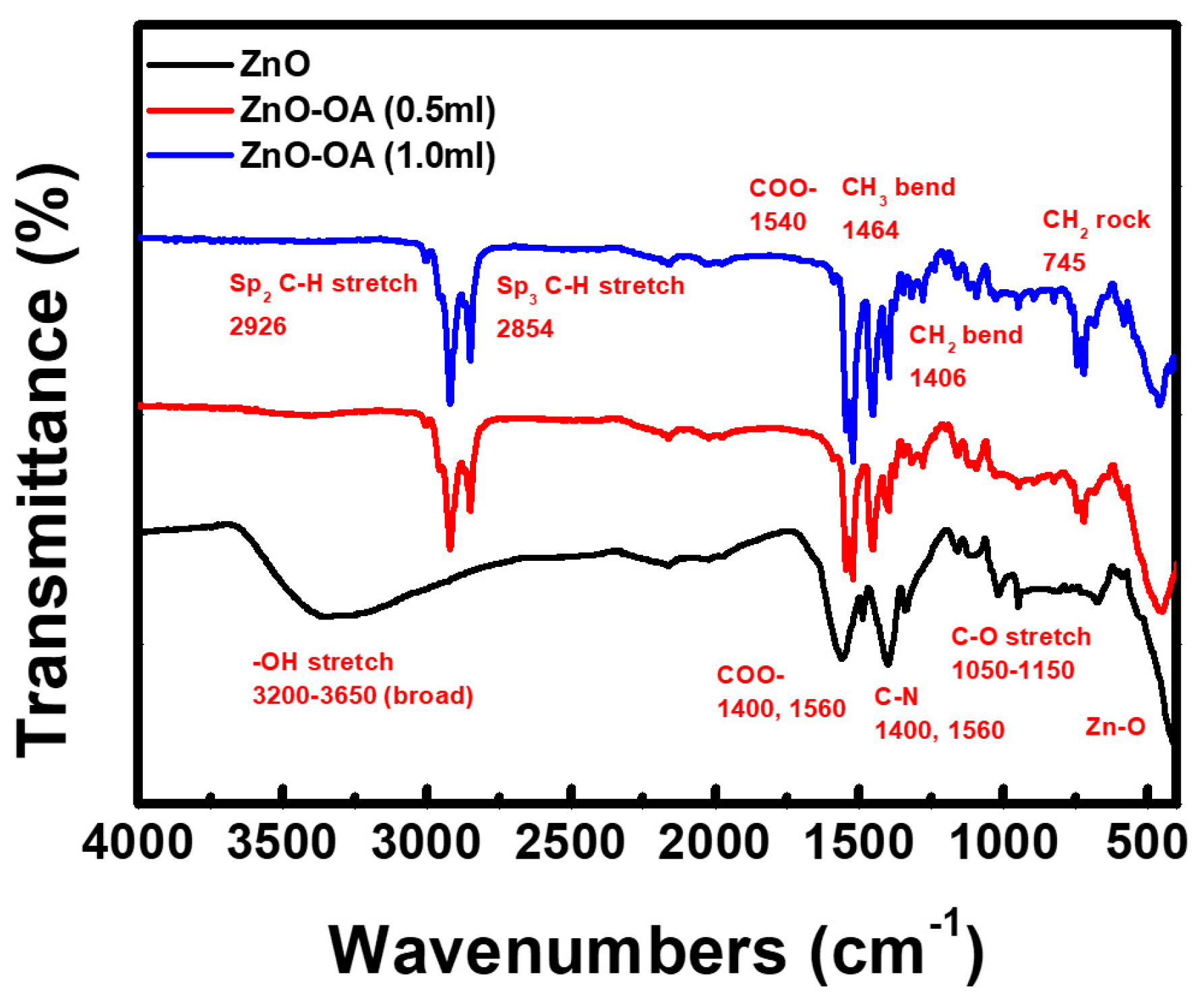
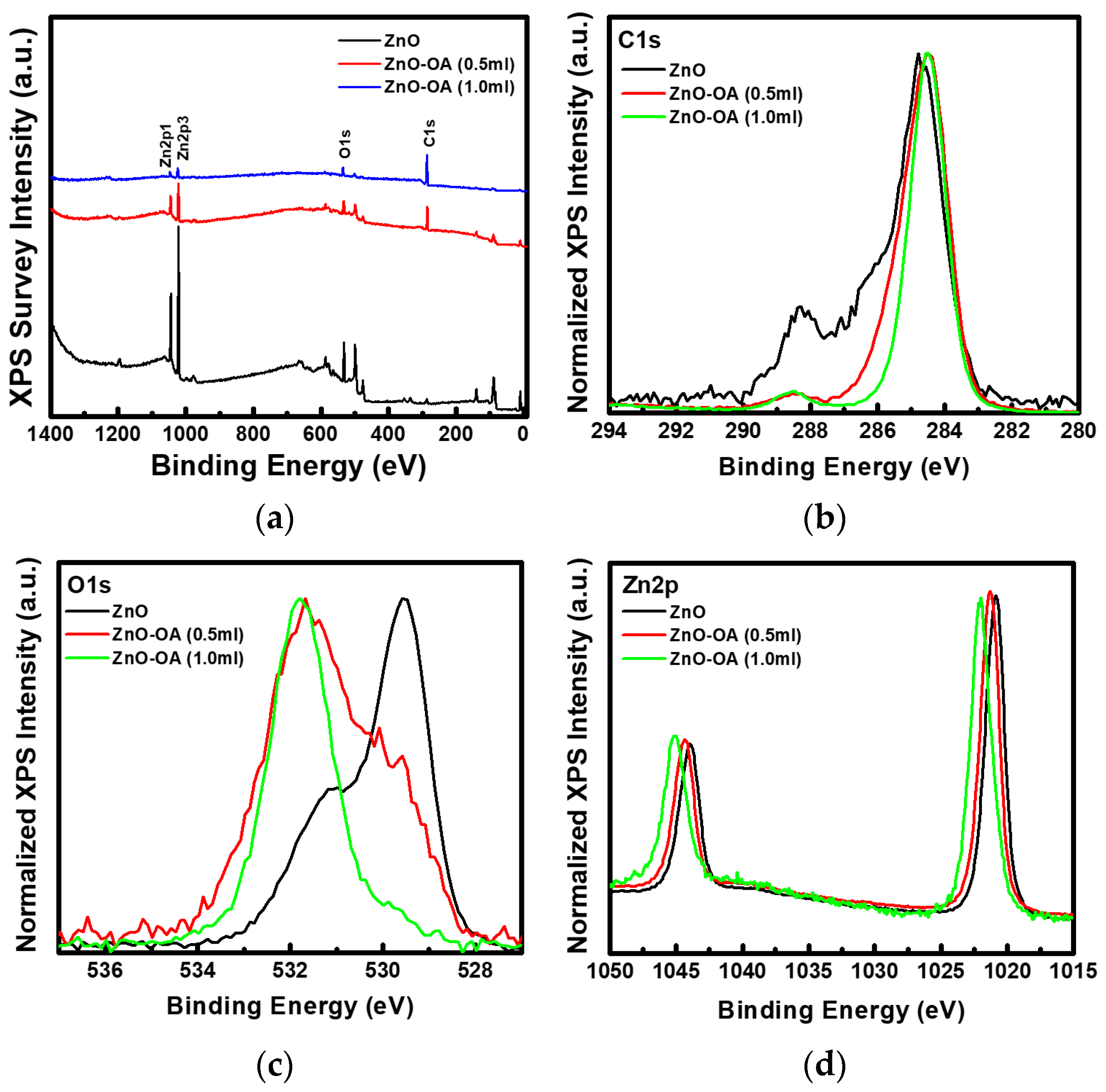
3.2. Surface Chemistry
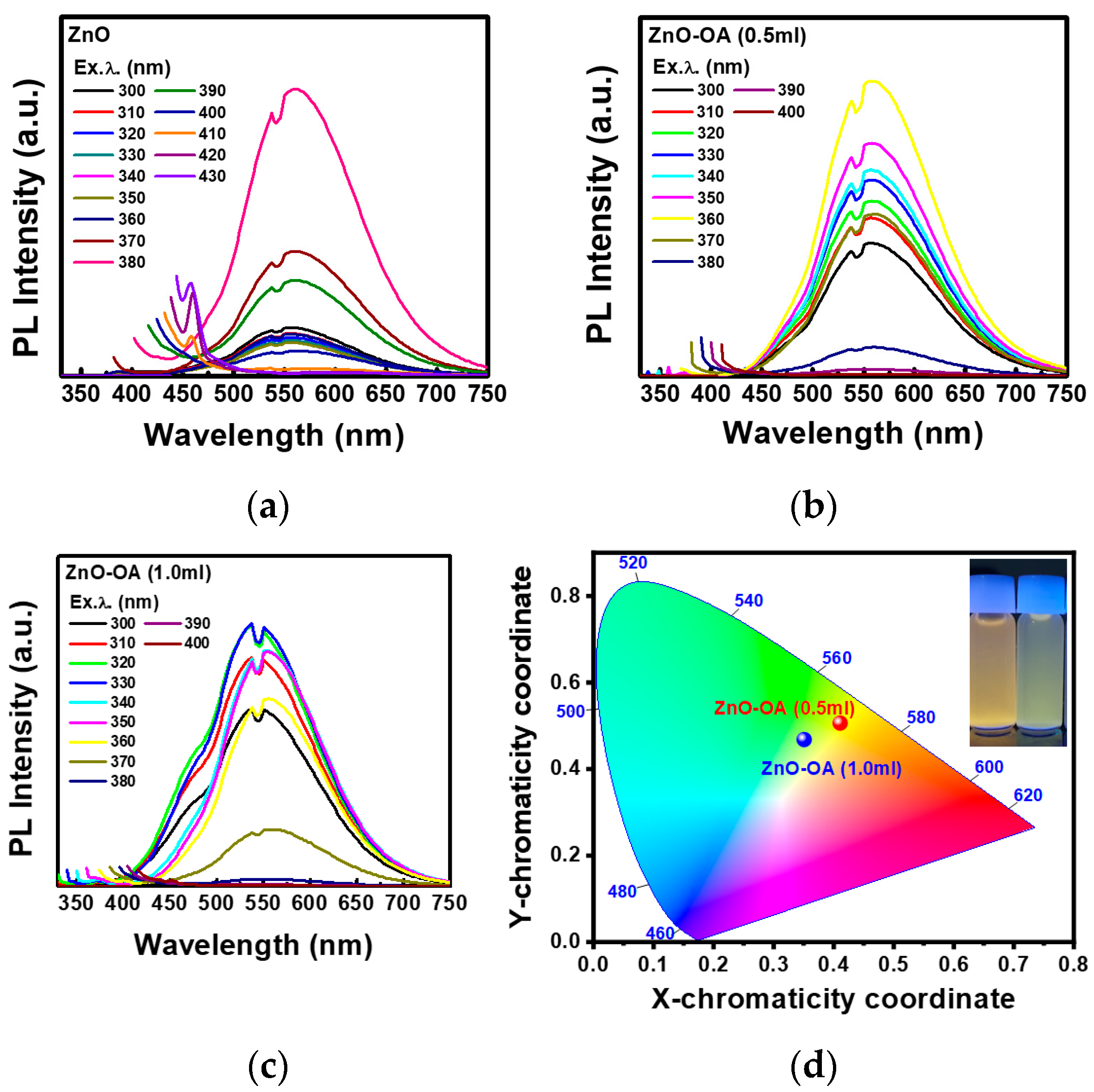
3.3. Optical Properties
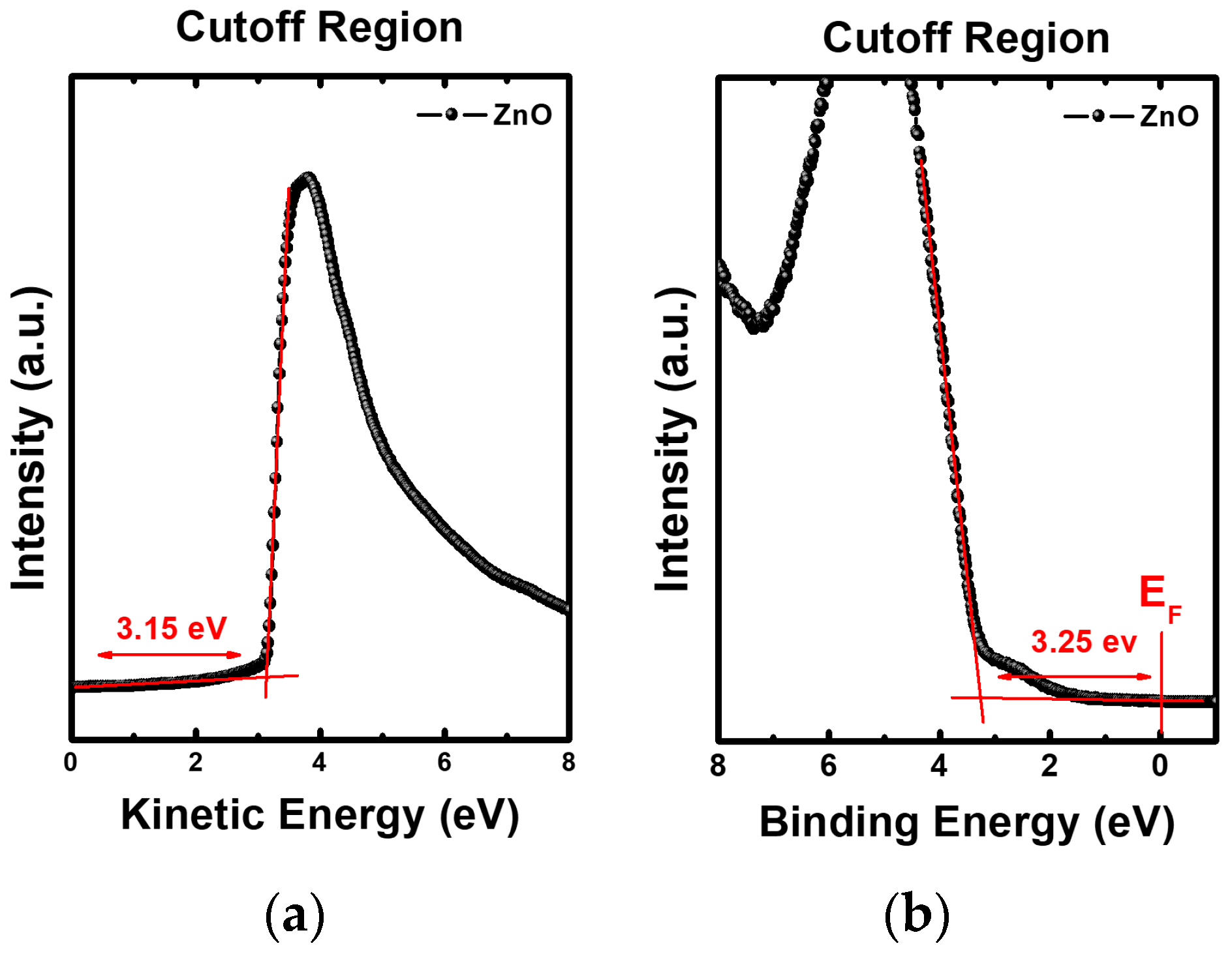
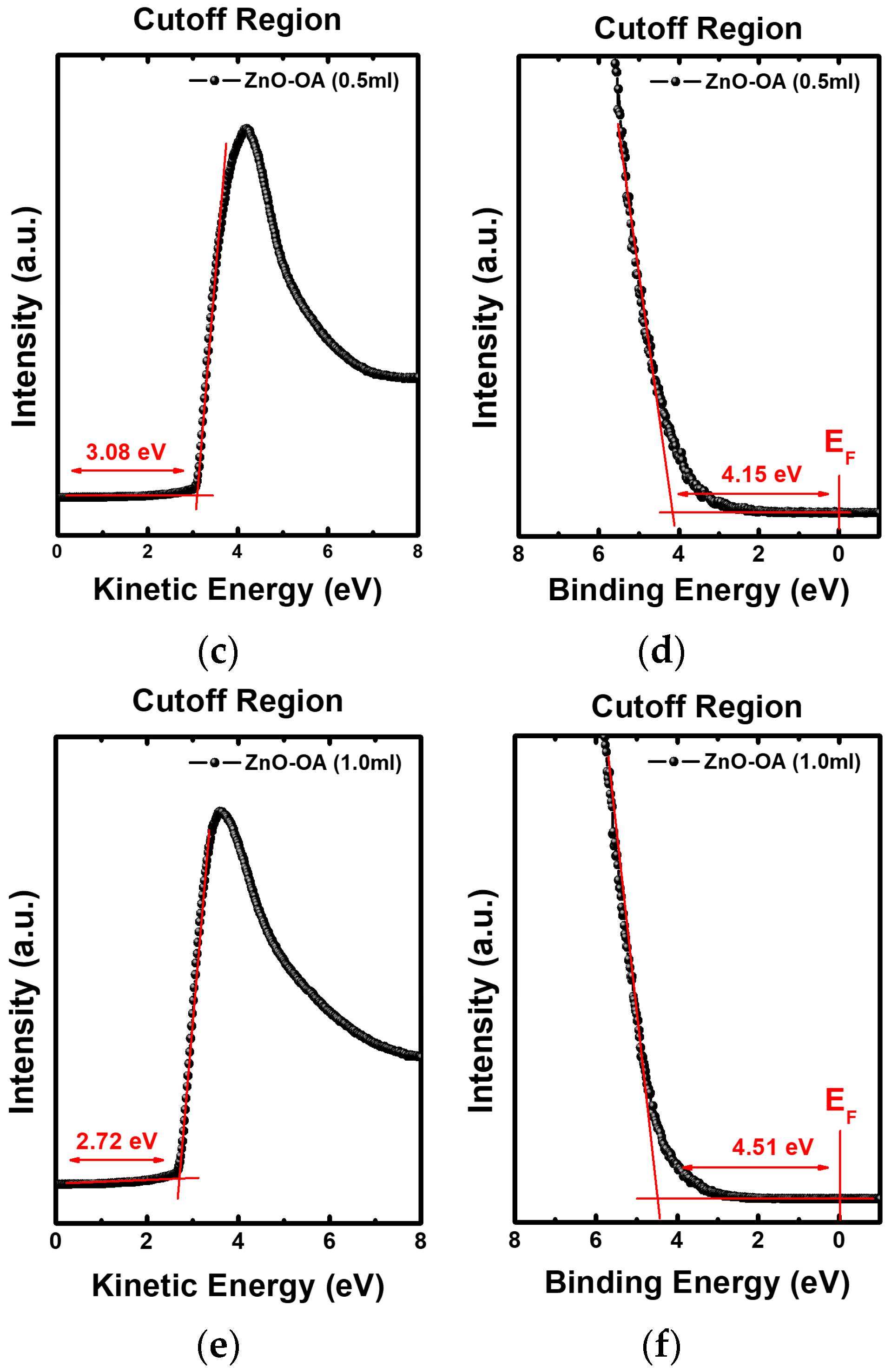
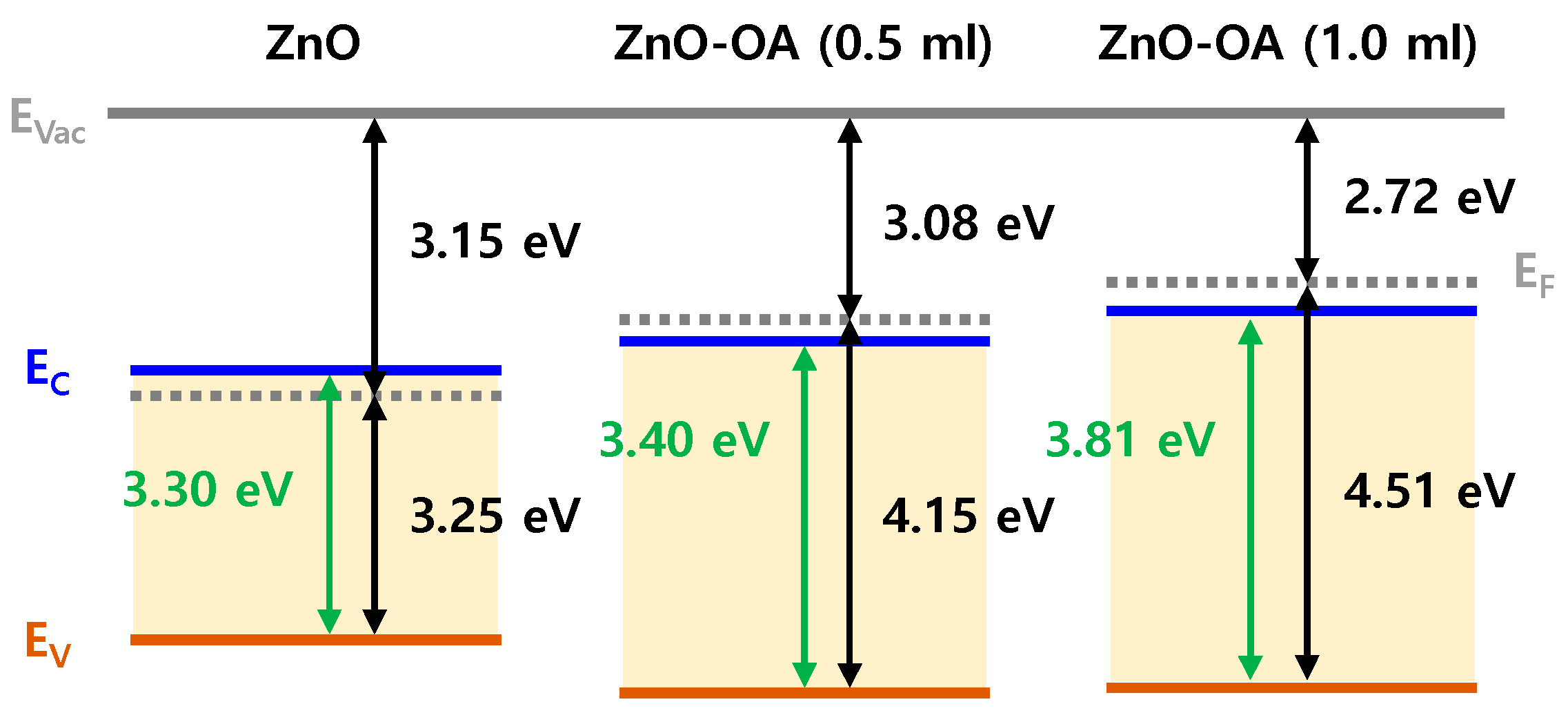
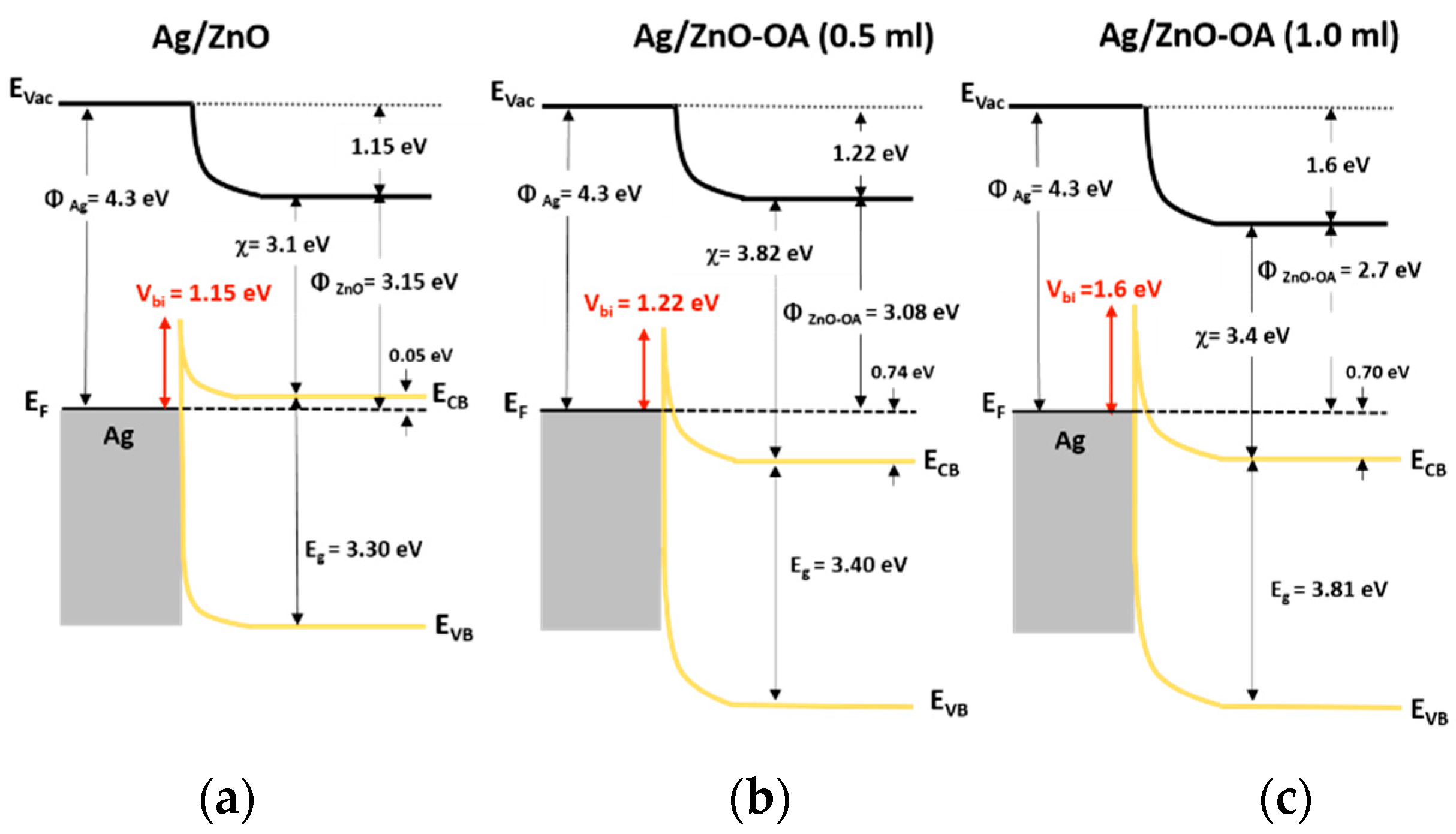
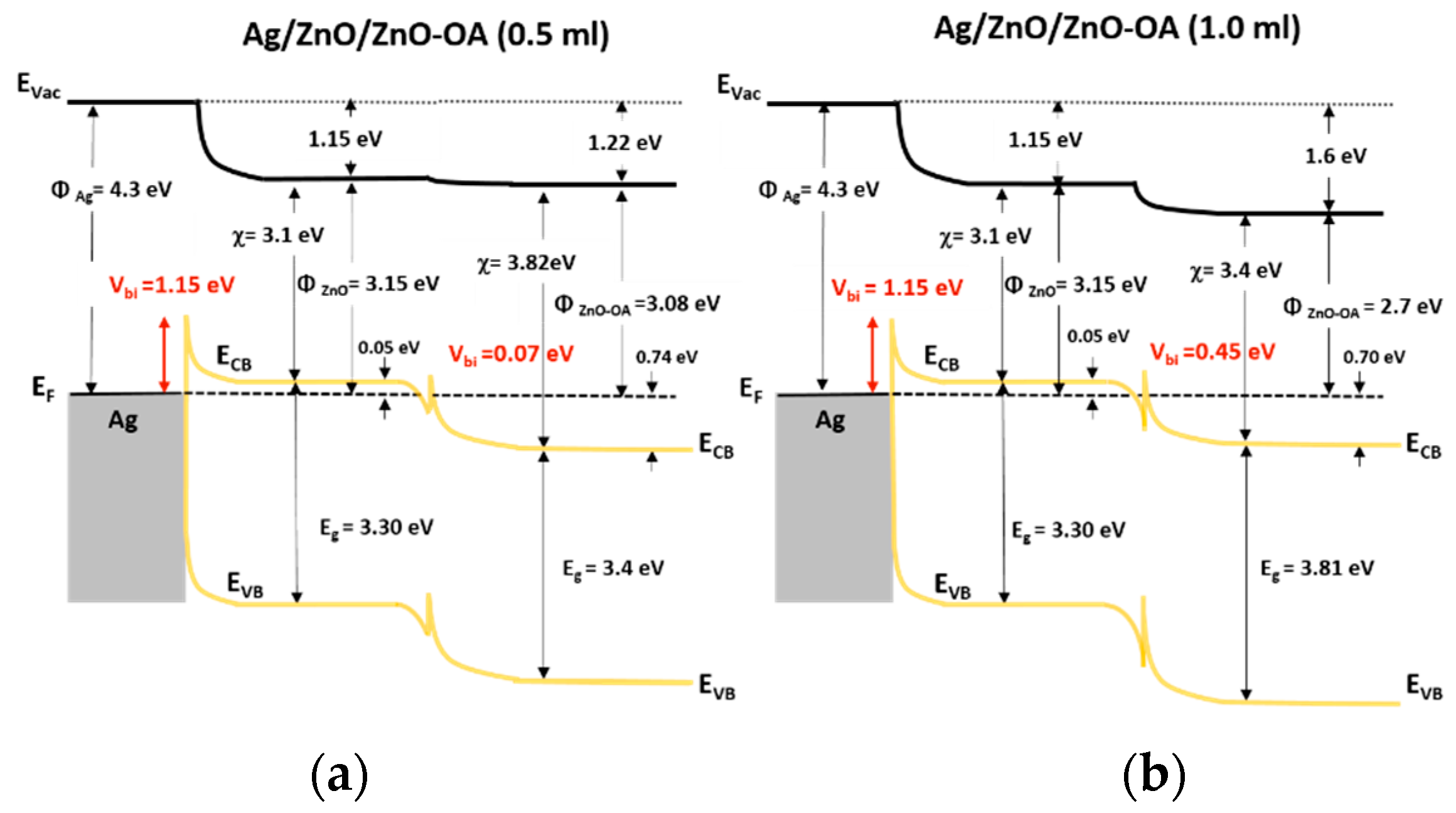
3.4. Electronic Energy Level Structure
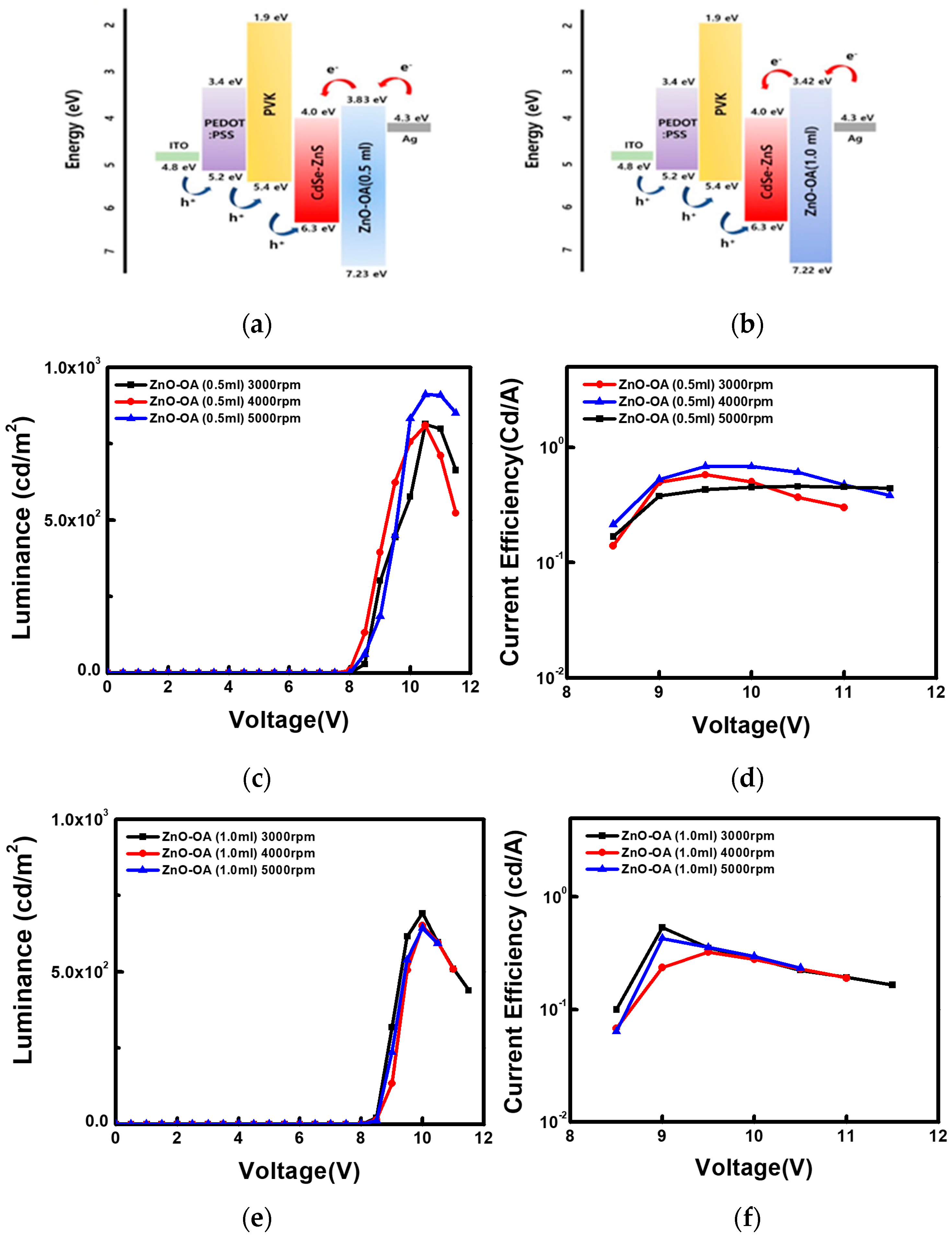
3.5. Improved EL of CdSe/ZnS QLEDs by Optimization of ETL
3.5.1. QLEDs with a Single ETL
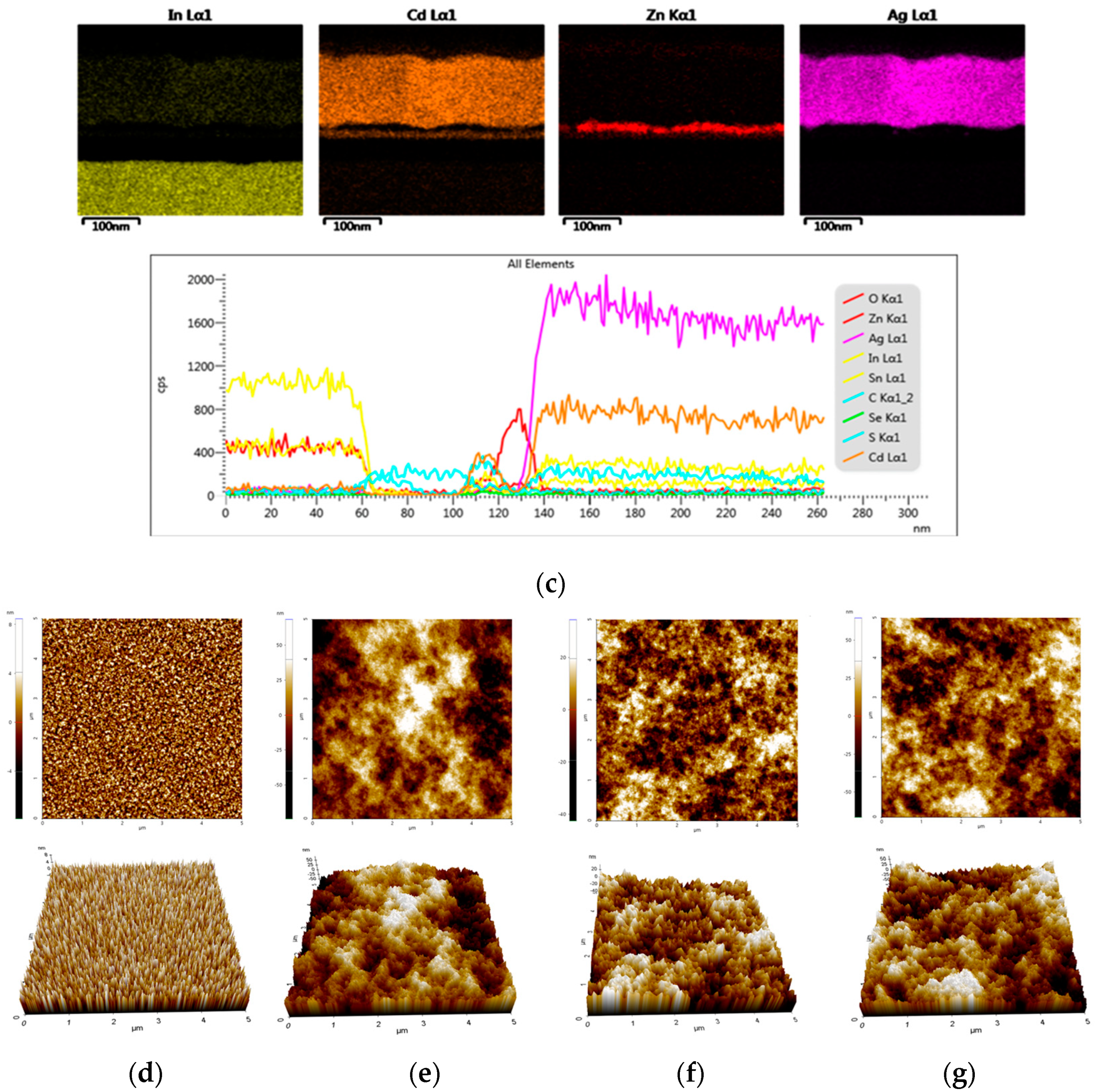
3.5.2. Interface Morphology
3.5.3. EL with a Double ETLs
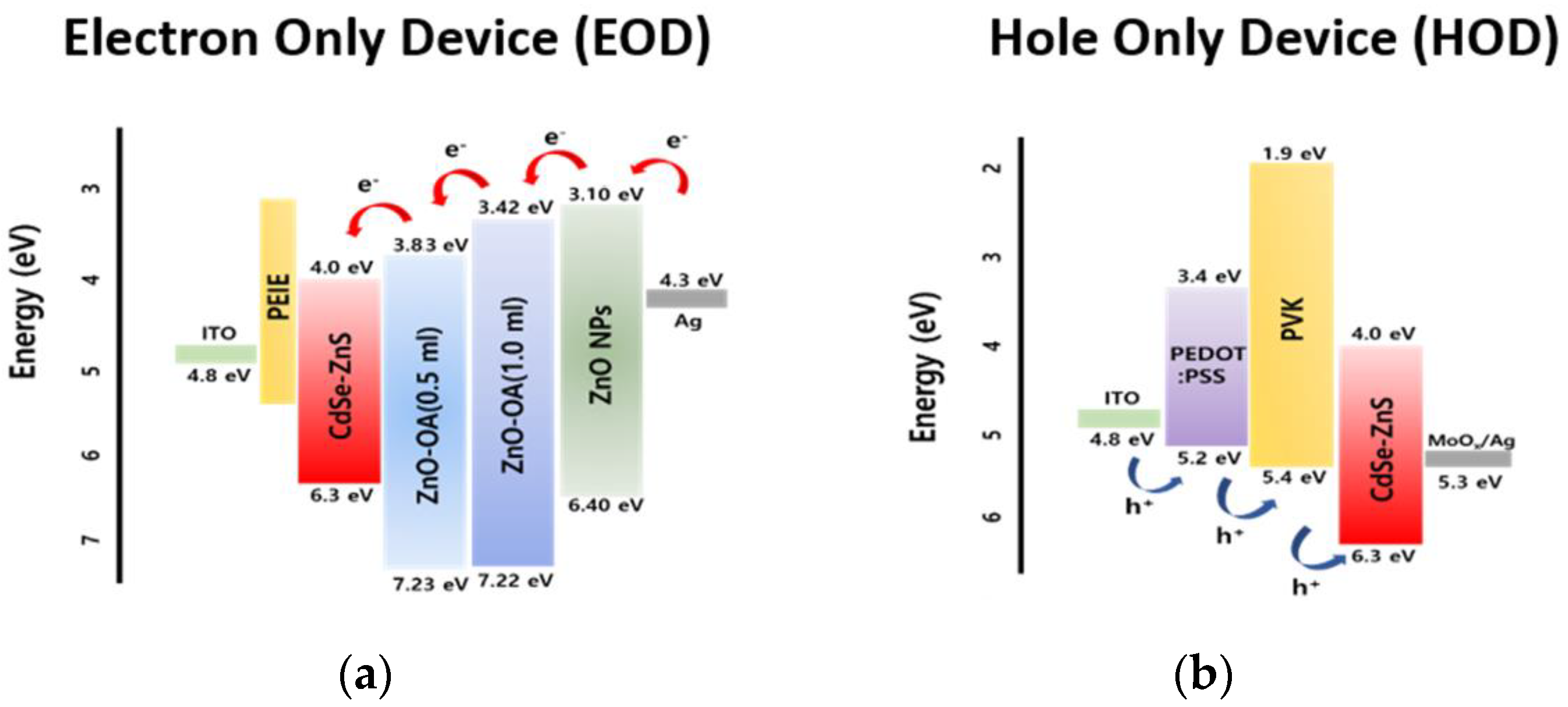
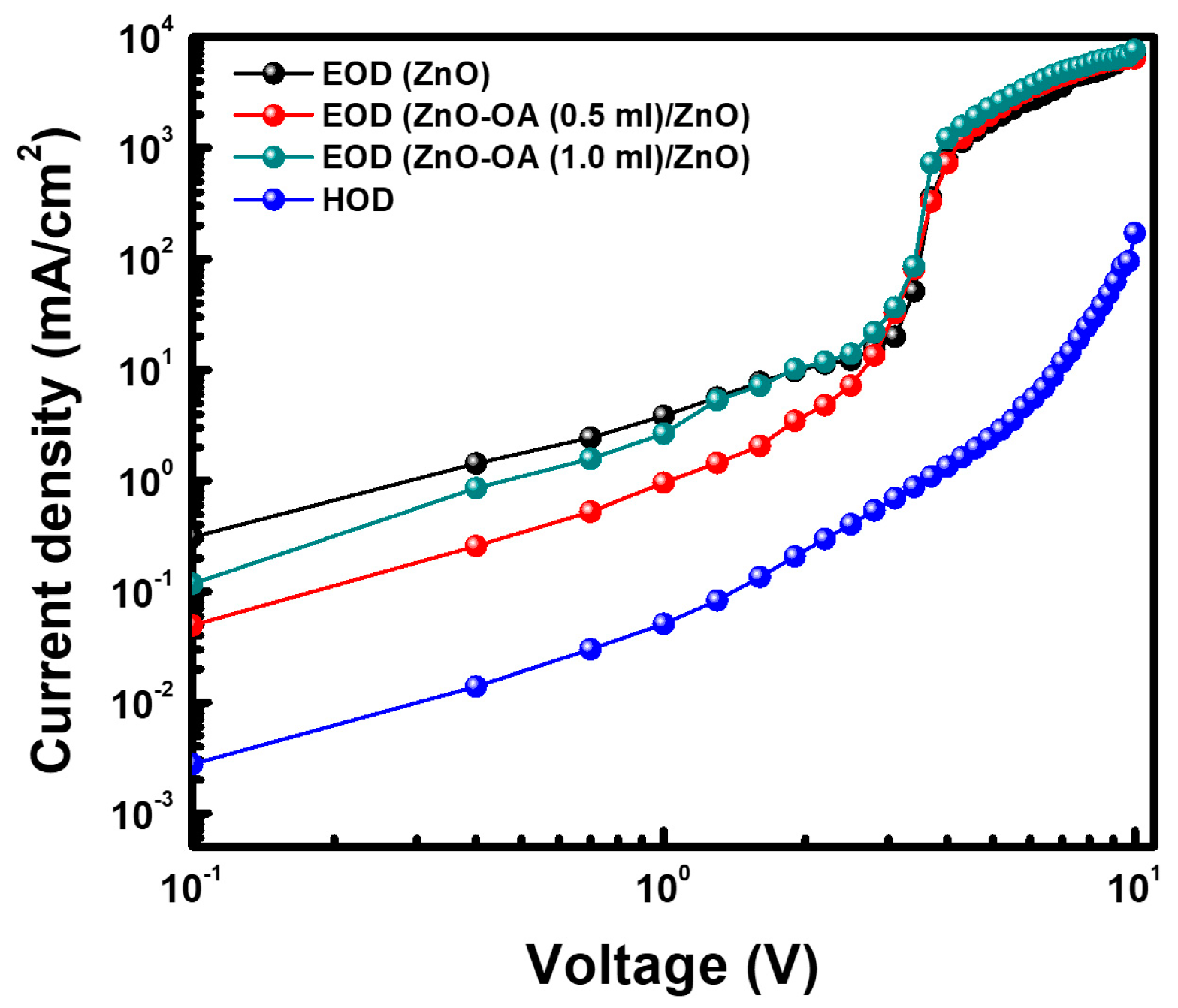
3.5.4. EOD and HOD
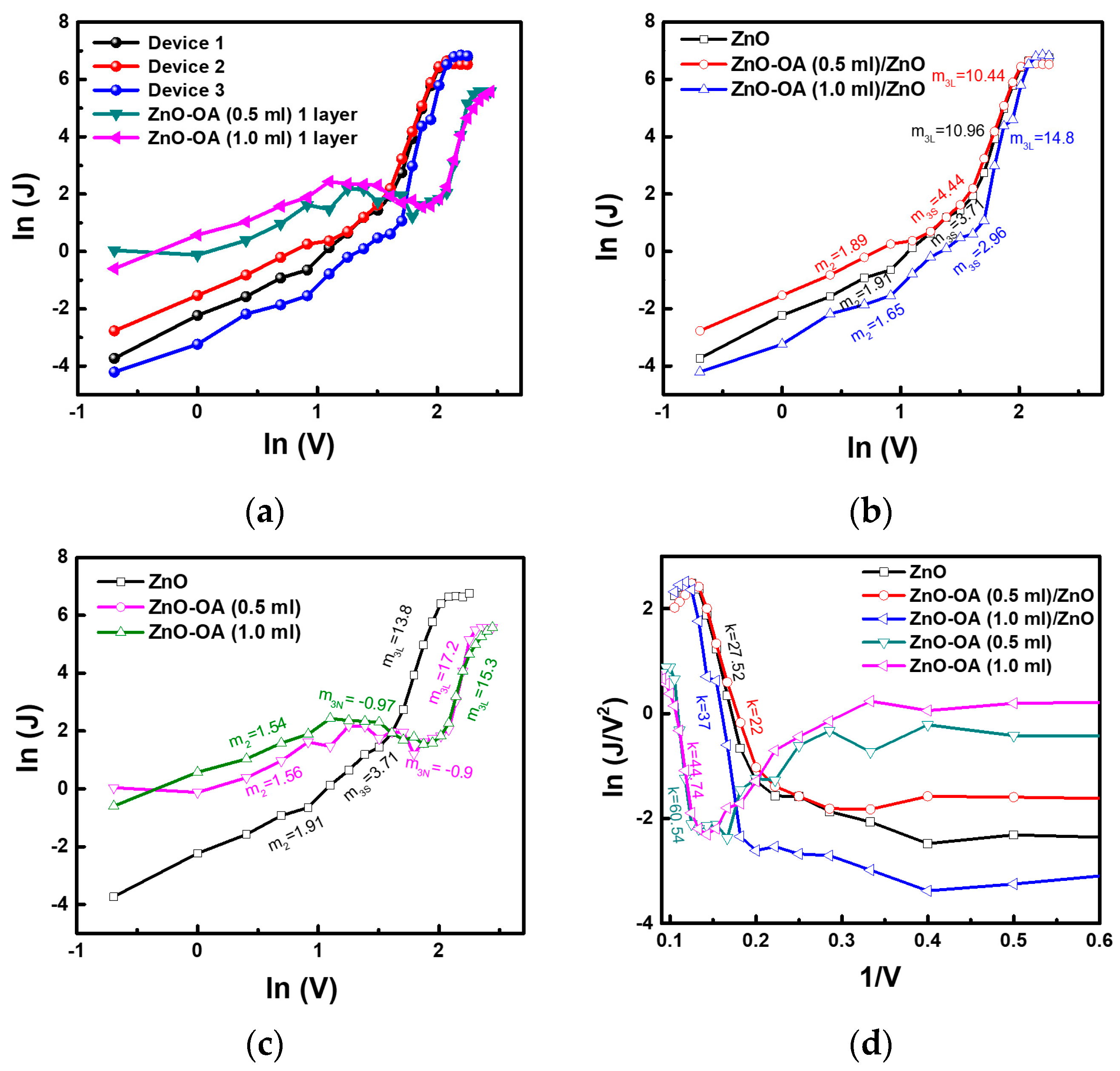
3.5.5. Conduction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size Tunable ZnO Nanoparticles To Enhance Electron Injection in Solution Processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient quantum-dot light-emitting diodes based on solution-processed multilayer structures. Nat. Photonics 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Moyen, E.; Kim, J.H.; Kim, J.; Jang, J. ZnO Nanoparticles for Quantum-Dot-Based Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 5203–5211. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Xue, J.; Xing, X.; Yu, C.; Huang, T.; Chu, J.; Wang, K.-L.; Dong, C.; Wei, Z.; et al. Core-Shell ZnO@SnO2 Nanoparticles for Efficient Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 17610–17616. [Google Scholar] [CrossRef]
- Janotti, A.; Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501–126529. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Ren, F.; Ming, X.; Long, Y.; Volinsky, A.A. Cu-Doped ZnO Electronic Structure and Optical Properties Studied by First-Principles Calculations and Experiments. Materials 2019, 12, 196. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Zheng, Y.; Choudhury, K.; Bera, D.; So, F.; Xue, J.; Holloway, P.H. Electroluminescence from light-emitting polymer/ZnO nanoparticle heterojunctions at sub-bandgap voltages. Nano Today 2010, 5, 384–389. [Google Scholar] [CrossRef]
- Kim, H.H.; Kumi, D.O.; Kim, K.; Park, D.; Yi, Y.; Cho, S.H.; Park, C.; Ntwaeaborwa, O.M.; Choi, W.K. Optimization of the electron transport in quantum dot light-emitting diodes by codoping ZnO with gallium (Ga) and magnesium (Mg). RSC Adv. 2019, 9, 32066–32071. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Han, C.Y.; Lee, K.H.; An, K.S.; Song, W.; Kim, J.; Oh, M.S.; Do, Y.R.; Yang, H. Performance Improvement of Quantum Dot-Light-Emitting Diodes Enabled by an Alloyed ZnMgO Nanoparticle Electron Transport Layer. Chem. Mater. 2015, 27, 197–204. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Li, C.; Xu, F.; Lei, W.; Sun, L.; Nathan, A.; Sun, X.W. All Solution-processed Stable White Quantum Dot Light-emitting Diodes with Hybrid ZnO@TiO2 as Blue Emitters. Sci. Rep. 2015, 4, 4085. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Park, S.; Yi, Y.; Son, D.I.; Park, C.; Hwang, D.K.; Choi, W.K. Inverted quantum dot light emitting diodes using polyethylenimine ethoxylated modified ZnO. Sci. Rep. 2015, 5, 8968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Lu, M.; Guo, J.; Yu, W.W.; Shi, Z.; Sun, G.; Zhang, Y. Double Electron Transport Layer and Optimized CsPbI3 Nanocrystal Emitter for Efficient Perovskite Light-Emitting Diodes. J. Phys. Chem. C 2020, 124, 28277–28284. [Google Scholar] [CrossRef]
- Kim, Y.C.; Baek, S.-D.; Myoung, J.-M. Enhanced brightness of methylammonium lead tribromide perovskite microcrystal-based green light-emitting diodes by adding hydrophilic polyvinylpyrrolidone with oleic acid-modified ZnO quantum dot electron transporting layer. J. Alloys Comp. 2019, 786, 11–17. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Du, X.-W.; Kulinich, S.A.; Qiu, J.-S.; Qin, W.-J.; Li, R.; Sun, J.; Liu, J. Stable Aqueous Dispersion of ZnO Quantum Dots with Strong Blue Emission via Simple Solution Route. J. Am. Chem. Soc. 2007, 129, 16029–16033. [Google Scholar] [CrossRef] [PubMed]
- Crapanzano, R.; Villa, I.; Mostoni, S.; D’Arienzo, M.; Credico, B.D.; Fasoli, M.; Scotti, R.; Vedda, A. Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials 2020, 10, 1983. [Google Scholar] [CrossRef]
- Kim, H.H.; Park, S.; Lee, H.; Kang, J.K.; Choi, W.K. Blue-Light Emissive Type II ZnO@5-Amino-2Naphthalene Sulfonic Acid Core-Shell Quantum Dots. Adv. Photonics Res. 2022, 3, 2100315. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, H.; Kang, J.K.; Choi, W.K. Photoluminescence and Electron Paramagnetic Resonance Spectroscopy for Revealing Visible Emission of ZnO Quantum Dots. Ann. Phys. 2022, 534, 2100382. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Yang, G.; Zhang, S.; Yu, L.; Zhang, P. Tribological properties of oleic acid-modified zinc oxide nanoparticles as the lubricant additive in polyalpha olefin and diisooctyl sebacate base oils. RSC Adv. 2016, 6, 69836–69844. [Google Scholar] [CrossRef]
- Mariño, F.; López, E.R.; Arnosa, A.; Gómez, M.A.G.; Piñeiro, Y.; Rivas, J.; Lorenzo, C.A.; Fernández, J. ZnO nanoparticles coated with oleic acid as additives for a polyalphaolefin lubricant. J. Mol. Liq. 2022, 348, 118401–118412. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar]
- Hofmann, O.T.; Rinke, P. Band Bending Engineering at Organic/Inorganic Interfaces Using Organic Self-Assembled Monolayers. Adv. Electron. Mater. 2017, 3, 1600373–1600379. [Google Scholar] [CrossRef] [Green Version]
- Koitaya, T.; Mukai, K.; Yoshimoto, S.; Yoshinobu, J. Energy level alignment of cyclohexne on Rh(111) surfaces: The importance of. interfacial dipole and final-state screening. J. Chem. Phys. 2013, 138, 044702–044710. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-C. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef] [Green Version]
- Fowler, R.H.; Nordheim, L. Electron emission in intense electric fields. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1928, 119, 173–181. [Google Scholar]
- Sze, S.M. Physics of Semiconductor Devices, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1981; Chapter 2. [Google Scholar]
- Bhattacharjee, S.; Sarkar, P.K.; Roy, N.; Roy, A. Improvement of reliability of polymer nanocomposite based transparent memory device by oxygen vacancy rich ZnO nanorods. Microelectron. Eng. 2016, 164, 53–58. [Google Scholar] [CrossRef]
- Song, J.; Han, H.; Peng, B.; Hu, Y.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. Role of nanoparticle surface defects in the conduction mechanism of polymer–nanoparticle electrical bistable devices. RSC Adv. 2017, 7, 54128–54135. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Kim, H.H.; Lee, Y.J.; Kim, J.H.; Choi, H.-J.; Choi, W.K. Electron transport phenomena at the interface of Al electrode and heavily doped degenerate ZnO nanoparticles in quantum dot light emitting diode. Nanotechnology 2019, 30, 035207–035215. [Google Scholar] [CrossRef]





| Layer | Spin Coating (rpm) | Turn-On (V) | Maximum Luminance (cd/m2) | Current Efficiency (cd/A) |
|---|---|---|---|---|
| ZnO-OA (0.5 mL)/ZnO | 3000/4000 | 3.5 | 23,760 | 5.08 |
| ZnO-OA (0.5 mL)/ZnO | 4000/4000 | 3.0 | 24,430 | 7.64 |
| ZnO-OA (0.5 mL)/ZnO | 5000/4000 | 3.5 | 27,890 | 8.57 |
| ZnO-OA (1.0 mL)/ZnO | 3000/4000 | 4.0 | 25,940 | 5.84 |
| ZnO-OA (1.0 mL)/ZnO | 4000/4000 | 4.5 | 26,923 | 7.37 |
| ZnO-OA (1.0 mL)/ZnO | 5000/4000 | 5.0 | 26,980 | 7.48 |
| ZnO single layer | 4000 | 4.5 | 25,570 | 6.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.Y.; Kim, H.H.; Noh, J.-H.; Lim, K.-Y.; Park, D.; Lee, I.-H.; Choi, W.K. Enhanced Luminance of CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO-Oleic Acid/ZnO Quantum Dots Double Electron Transport Layer. Nanomaterials 2022, 12, 2038. https://doi.org/10.3390/nano12122038
Lee DY, Kim HH, Noh J-H, Lim K-Y, Park D, Lee I-H, Choi WK. Enhanced Luminance of CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO-Oleic Acid/ZnO Quantum Dots Double Electron Transport Layer. Nanomaterials. 2022; 12(12):2038. https://doi.org/10.3390/nano12122038
Chicago/Turabian StyleLee, Da Young, Hong Hee Kim, Ji-Hyun Noh, Keun-Yong Lim, Donghee Park, In-Hwan Lee, and Won Kook Choi. 2022. "Enhanced Luminance of CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO-Oleic Acid/ZnO Quantum Dots Double Electron Transport Layer" Nanomaterials 12, no. 12: 2038. https://doi.org/10.3390/nano12122038
APA StyleLee, D. Y., Kim, H. H., Noh, J.-H., Lim, K.-Y., Park, D., Lee, I.-H., & Choi, W. K. (2022). Enhanced Luminance of CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO-Oleic Acid/ZnO Quantum Dots Double Electron Transport Layer. Nanomaterials, 12(12), 2038. https://doi.org/10.3390/nano12122038








