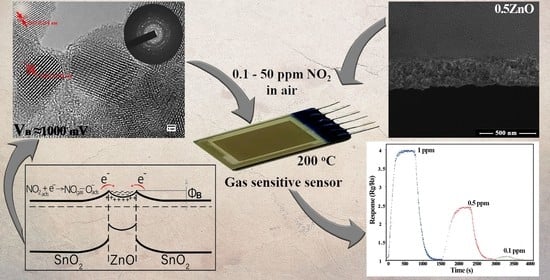
High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals for Synthesis of ZnO-SnO2 Thin Films
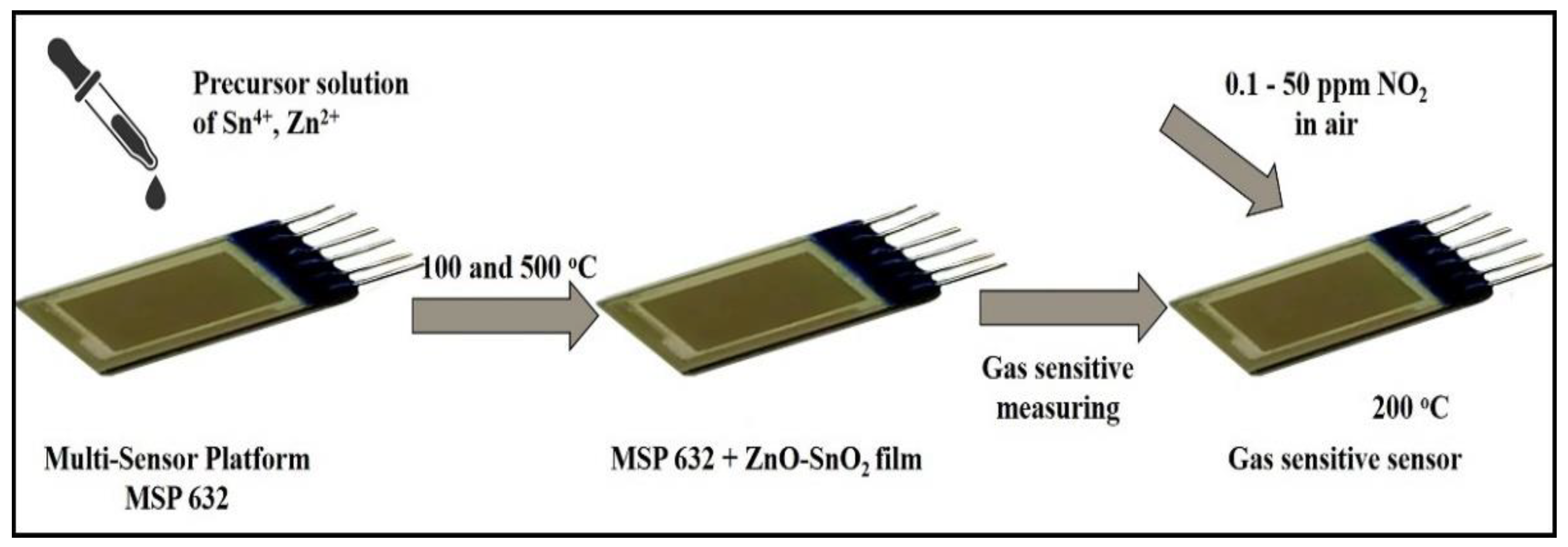
2.2. Synthesis of ZnO-SnO2 Thin Films
2.3. Characterization
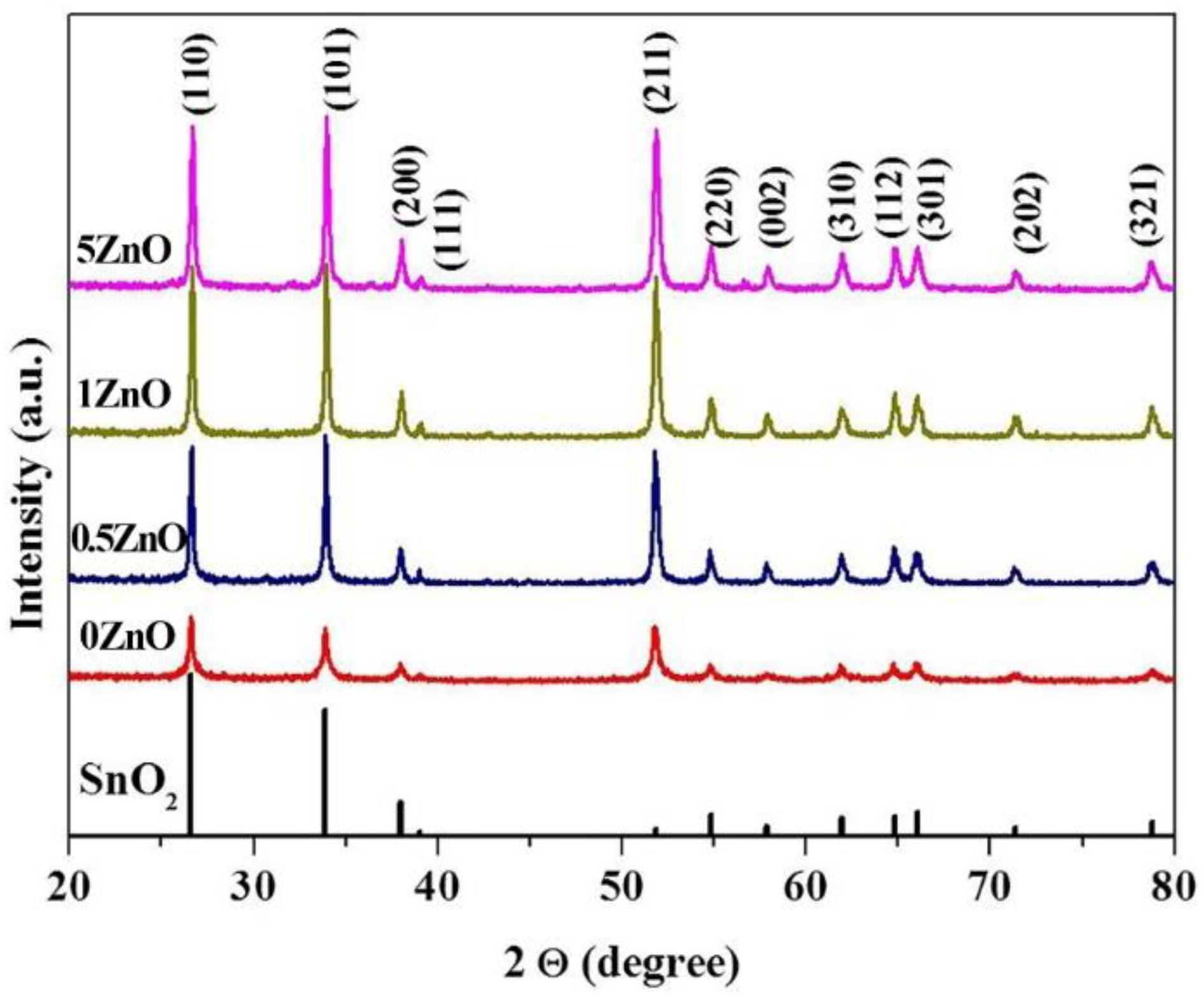
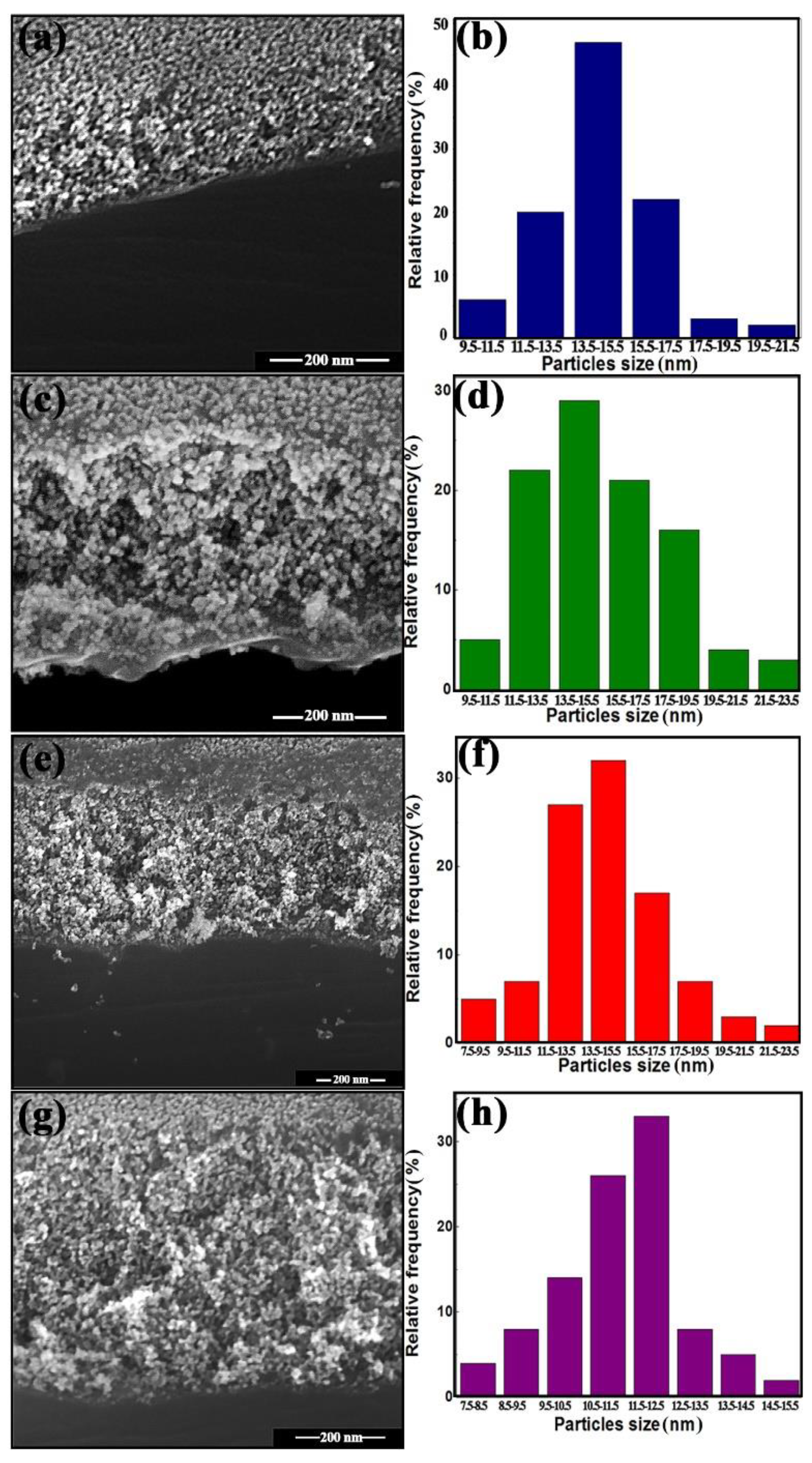
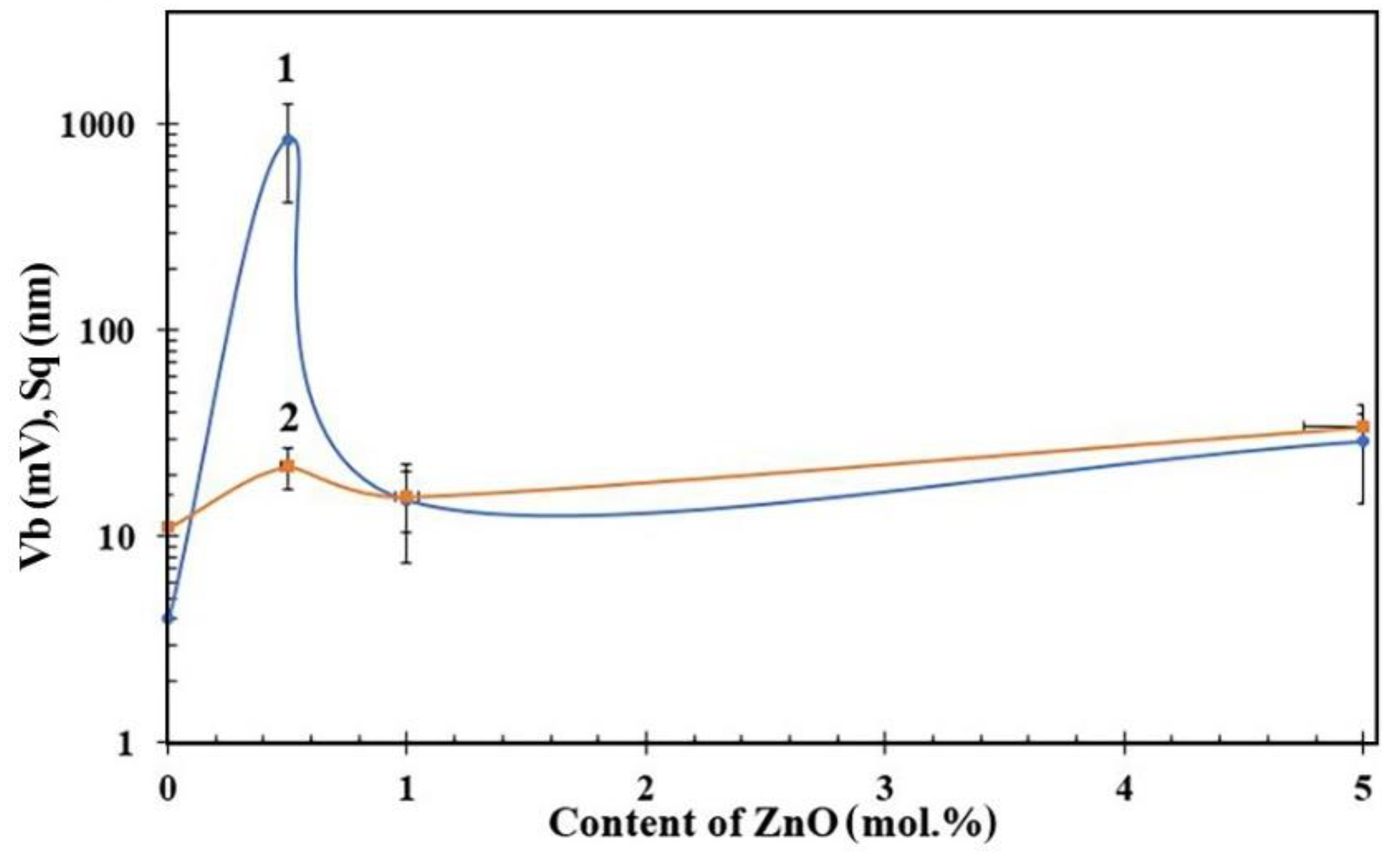
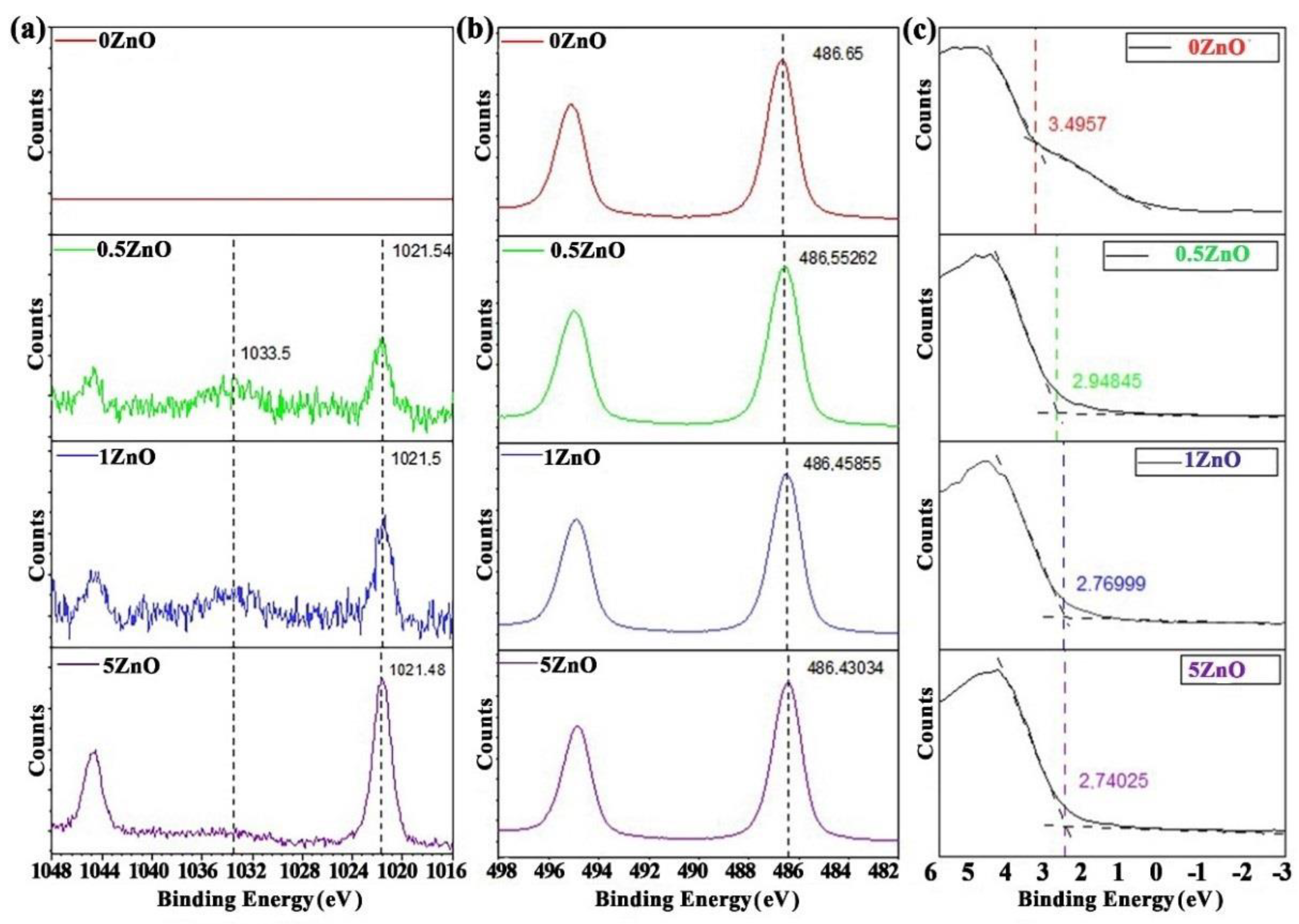
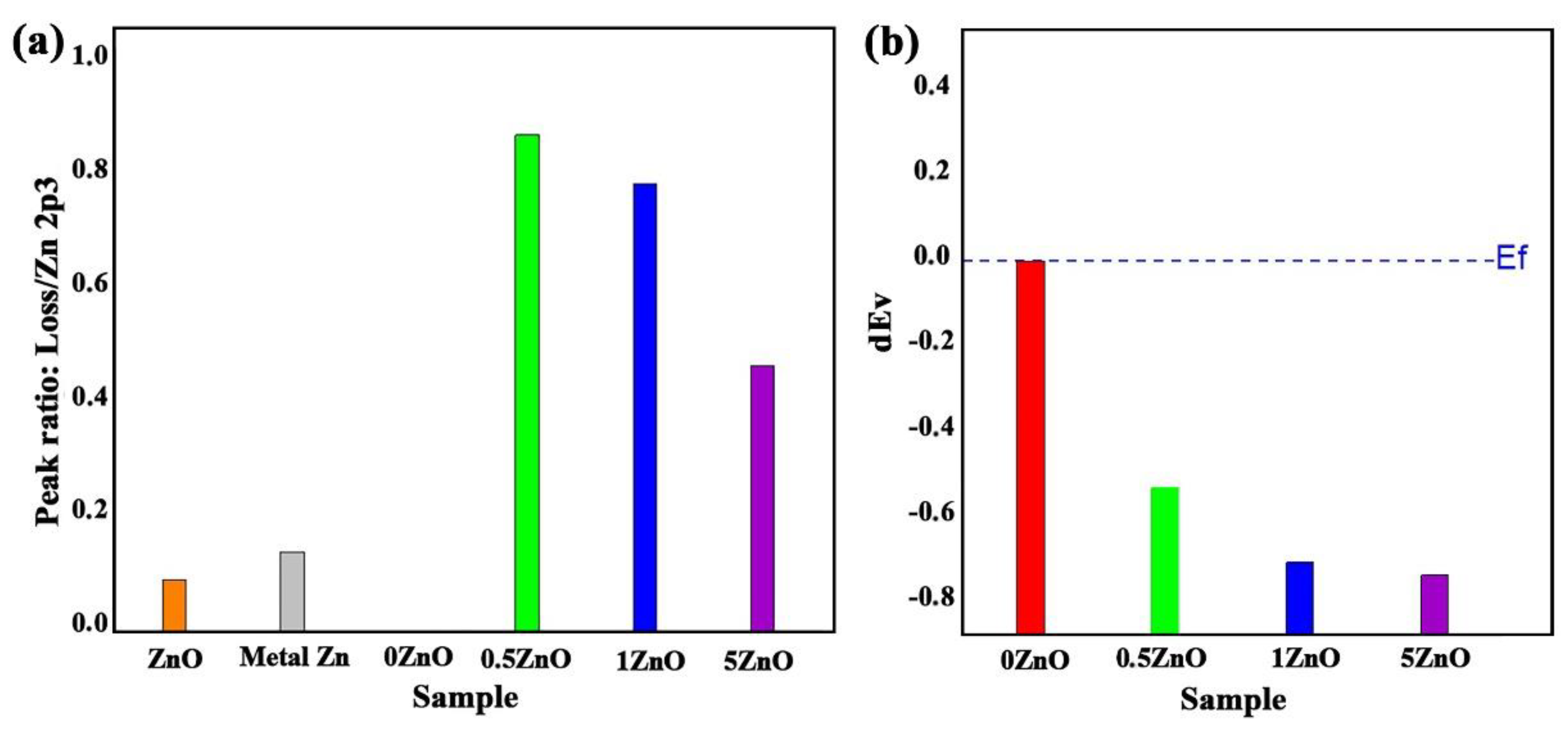
3. Results
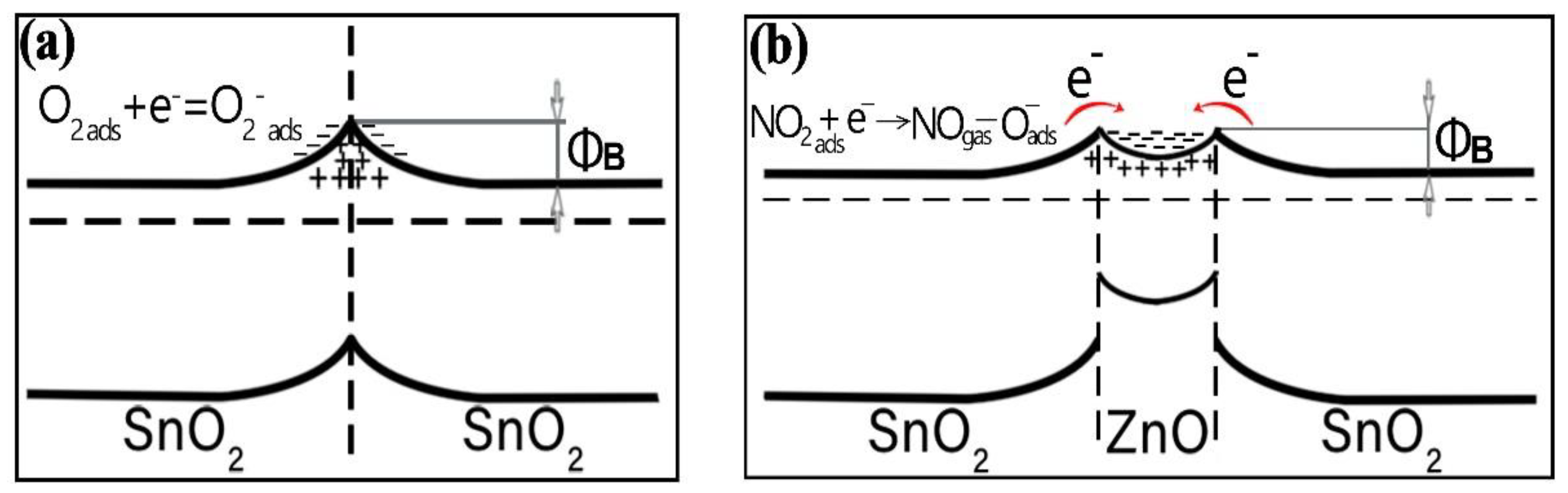
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 92, 607–627. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room temperature conductive type metal oxide semiconductor gas sensors for NO2 detection. Sens. Actuators A Phys. 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Mehraj, S.; Ansari, M.S. Annealed SnO2 thin films: Structural, electrical and their magnetic properties. Thin Solid Film. 2015, 589, 57–65. [Google Scholar] [CrossRef]
- Bayan, E.M.; Petrov, V.V.; Volkova, M.G.; Storozhenko, V.Y.; Chernyshev, A.V. SnO2-ZnO nanocomposite thin films: The influence of structure, composition and crystallinity on optical and electrophysical properties. J. Adv. Dielectr. 2021, 11, 2160008. [Google Scholar] [CrossRef]
- Kim, I.D.; Rothschild, A.; Lee, B.H.; Kim, D.Y.; Jo, S.M.; Tuller, H.L. Ultrasensitive Chemiresistors Based on Electrospun TiO2 Nanofibers. Nano Lett. 2006, 6, 2009–2013. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 3109–3144. [Google Scholar] [CrossRef]
- Velumani, M.; Meher, S.R.; Alex, Z.C. Composite metal oxide thin film based impedometric humidity sensors. Sens. Actuators B Chem. 2019, 301, 127084. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.; Hernández, D.M.; Makarov, V.; Febus, E.; Weiner, B.R.; Morell, G. Room temperature gas sensor based on tin dioxide-carbon nanotubes composite films. Sens. Actuators B Chem. 2014, 190, 227–233. [Google Scholar] [CrossRef]
- Chen, Y.J.; Nie, L.; Xue, X.Y.; Wang, Y.G.; Wang, T.H. Linear ethanol sensing of SnO2 nanorods with extremely high sensitivity. Appl. Phys. Lett. 2006, 88, 083105. [Google Scholar] [CrossRef]
- Petrov, V.V.; Sysoev, V.V.; Starnikova, A.P.; Volkova, M.G.; Kalazhokov, Z.K.; Storozhenko, V.Y.; Khubezhov, S.A.; Bayan, E.M. Synthesis, Characterization and Gas Sensing Study of ZnO-SnO2 Nanocomposite Thin Films. Chemosensors 2021, 9, 124. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Li, P.; Wu, J.; Zong, X. Humidity-sensing performance of layer-by-layer self-assembled tungsten disulfide/tin dioxide nanocomposite. Sens. Actuators B Chem. 2018, 265, 529–538. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M.D. Novel Ag-SnO2-βC3N4 ternary nanocomposite based gas sensor for enhanced low-concentration NO2 sensing at room temperature. Sens. Actuators B Chem. 2020, 326, 128910. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Liu, X.; Zhang, L.; Zhang, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Ramos-Ramos, D.J.; Sotillo, B.; Urbieta, A.; Fernandez, P. Fabrication and Characterization of ZnO:CuO Electronic Composites for Their Application in Sensing Processes. IEEE Sens. J. 2021, 21, 2573–2580. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Zhang, L.; Chen, J.; Zho, Q.; Zhang, H.; Nie, L.; Don, Z.; Zhan, Z.; Wang, Z.; et al. Construction of a low-temperature, highly sensitive H2S sensor based on surfaces and interfaces reaction triggered by Au-doped hierarchical structured composites. Chem. Phys. Lett. 2021, 763, 138188. [Google Scholar] [CrossRef]
- Ha, N.H.; Thinh, D.D.; Huong, N.T.; Phuong, N.H.; Thach, P.D.; Hong, H.S. Fast response of carbon monoxide gas sensors using a highly porous network of ZnO nanoparticles decorated on 3D reduced graphene oxide. Appl. Surf. Sci. 2018, 434, 1048–1054. [Google Scholar] [CrossRef]
- Liangyuan, C.; Shouli, B.; Guojun, Z.; Dianqing, L.; Aifan, C.; Liu, C.C. Synthesis of ZnO-SnO2 nanocomposites by microemulsion and sensing properties for NO2. Sens. Actuators B Chem. 2008, 134, 360–366. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Bifunctional Sensing Mechanism of SnO2-ZnO Composite Nanofibers for Drastically Enhancing the Sensing Behavior in H2 Gas. ACS Appl. Mater. Interfaces 2015, 7, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Lia, T.; Zenga, W.; Wang, Z. Quasi-one-dimensional metal-oxide-based heterostructural gas-sensing materials: A review. Sens. Actuators B Chem. 2015, 221, 1570–1585. [Google Scholar] [CrossRef]
- Velumani, M.; Meher, S.R.; Alex, Z.C. Impedometric humidity sensing characteristics of SnO2 thin films and SnO2-ZnO composite thin films grown by magnetron sputtering. J. Mater. Sci. Mater. Electron. 2018, 29, 3999–4010. [Google Scholar] [CrossRef]
- Sin, N.D.M.; Ahmad, S.; Malek, M.F.; Mamat, M.H.; Rusop, M. Improvement sensitivity humidity sensor based on ZnO/SnO2 cubic structure. J. IOP Conf. Ser. Mater. Sci. Eng. 2013, 46, 012005. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Li, G.; Hao, F.; Han, C.; Liu, W.; Wei, D. Construction of ZnO-SnO2 n-n junction for dual-sensing of nitrogen dioxide and ethanol. Vacuum 2020, 18, 1109615. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Zhou, P.; Gao, F.; Xu, X.; Gao, S.; Wei, D.; Ao, Y.; Shen, Y. Enhanced NO2 sensing performance of ZnO nanowires functionalized with ultra-fine In2O3 nanoparticles. Sens. Actuator. B Chem. 2020, 308, 127729. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Kint, J.; Werbrouck, A.; Henderick, L.; Minjauw, M.; Dendooven, J.; Detavernier, C. Atomic layer deposition of ZnO-SnO2 composite thin film: The influence of structure, composition and crystallinity on lithium-ion battery performance. Electrochim. Acta 2019, 320, 134604. [Google Scholar] [CrossRef]
- Wang, Z.; Zhi, M.; Xu, M.; Guo, C.; Man, Z.; Zhang, Z.; Li, Q.; Lv, Y.; Zhao, W.; Yan, J.; et al. Ultrasensitive NO2 gas sensor based on Sb-doped SnO2 covered ZnO nano-heterojunction. J. Mater. Sci. 2021, 56, 7348–7356. [Google Scholar] [CrossRef]
- Lou, C.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Atomic layer deposition of ZnO on SnO2 nanospheres for enhanced formaldehyde detection. Sens. Actuators B Chem. 2020, 329, 129218. [Google Scholar] [CrossRef]
- Al-Jumaili, H.S.; Jasim, M.N. Preparation And Characterization Of ZnO:SnO2 Nanocomposite Thin Films On Porous Silicon as H2S Gas Sensor. J. Ovonic Res. 2019, 15, 81–87. [Google Scholar]
- Sharma, B.; Sharma, A.; Joshi, M.J. Myung, Sputtered SnO2/ZnO Heterostructures for Improved NO2 Gas Sensing Properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Shewale, P.S.; Yu, Y.S.; Kim, J.H.; Bobade, C.R.; Uplane, M.D. H2S gas sensitive Sn-doped ZnO thin films: Synthesis and characterization. J. Anal. Appl. Pyrolysis 2015, 112, 348–356. [Google Scholar] [CrossRef]
- Srinivasulu, T.; Saritha, K.; Reddy, K.T.R. Synthesis, and characterization of Fe-doped ZnO thin films deposited by chemical spray pyrolysis. Mod. Electron. Mater. 2017, 3, 76–85. [Google Scholar] [CrossRef]
- Petrov, V.V.; Bayan, E.M.; Khubezhov, S.A.; Varzarev, Y.N.; Volkova, M.G. Investigation of Rapid Gas-Sensitive Properties Degradation of ZnO-SnO2 Thin Films Grown on the Glass Substrate. Chemosensors 2020, 8, 40. [Google Scholar] [CrossRef]
- Volkova, M.G.; Storozhenko, V.Y.; Petrov, V.V.; Bayan, E.M. Characterization of nanocrystalline ZnO thin films prepared by new pyrolysis method. J. Phys. Conf. Ser. 2020, 1695, 012023. [Google Scholar] [CrossRef]
- El Khalidi, Z.; Daouli, A.; Jabraoui, H.; Hartiti, B.; Bouich, A.; Soucase, B.M.; Comini, E.; Arachchige, M.M.; Fadili, S.; Thevenin, P.; et al. Impact of Sn doping on the hydrogen detection characteristics of ZnO thin films: Insights from experimental and DFT combination. Appl. Surf. Sci. 2022, 574, 151585. [Google Scholar] [CrossRef]
- Park, J.A.; Moon, J.; Lee, S.J.; Kim, S.H.; Chu, H.Y.; Zyung, T. SnO2-ZnO hybrid nanofibers-based highly sensitive nitrogen dioxides sensor. Sens. Actuators B Chem. 2010, 145, 592–595. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Gao, S.; Fei, T.; Zhang, Y.; Zheng, X.; Zhang, T. An organometallic chemistry-assisted strategy for modification of zinc oxide nanoparticles by tin oxide nanoparticles: Formation of n-n heterojunction and boosting NO2 sensing properties. J. Colloid Interface Sci. 2020, 567, 328–338. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Kim, K.W.; Cho, P.S.; Kim, S.J.; Lee, J.H.; Kang, C.Y.; Kim, J.S.; Yoon, S.J. The selective detection of C2H5OH using SnO2-ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens. Actuators B Chem. 2007, 123, 318–324. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO-SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B Chem. 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Pakhare, K.S.; Sargar, B.M.; Potdar, S.S.; Sharma, A.K.; Patil, U.M. Facile Synthesis of Nano-Diced SnO2-ZnO Composite by Chemical Route for Gas Sensor Application. J. Electron. Mater. 2019, 48, 6269–6279. [Google Scholar] [CrossRef]
- Thanh Le, D.T.; Trung, D.D.; Chinh, N.D.; Thanh Binh, B.T.; Hong, H.S.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. Facile synthesis of SnO2–ZnO core–shell nanowires for enhanced ethanol-sensing performance. Curr. Appl. Phys. 2013, 13, 1637–1642. [Google Scholar] [CrossRef]
- Lai, T.Y.; Fang, T.H.; Hsiao, Y.J.; Chan, C.A. Characteristics of Au-doped SnO2-ZnO heteronanostructures for gas sensing applications. Vacuum 2019, 166, 155–161. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Yalovega, G.E.; Shmatko, V.A.; Funik, A.O.; Petrov, V.V. SiO2CuOx films for nitrogen dioxide detection: Correlation between technological conditions and properties. Sens. Actuators B Chem. 2016, 230, 167–175. [Google Scholar] [CrossRef]
- Shalimova, K.V. Physics Semiconductors, 2nd ed.; Revised and Expanded, Energy; Scientific Research: Moscow, Russia, 1976. [Google Scholar]
- Korovkin, M.; AnanIeva, L.; Nebera, T.; Antsiferova, A. Assessment of quartz materials crystallinity by X-ray diffraction. IOP Conf. Ser. Mater. Sci. Eng. 2016, 110, 012095. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.J.; Norman, M.B. An index of crystallinity for quartz. Am. J. Sci. 1976, 276, 1120–1130. [Google Scholar] [CrossRef]
- Vorobyeva, N.A.; Rumyantseva, M.N.; Forsh, P.A.; Gaskov, A.M. Conductivity of Nanocrystalline ZnO(Ga). Semiconductors 2013, 47, 650–654. [Google Scholar] [CrossRef]
- Clifford, P.K.; Tuma, D.T. Characteristics of Semiconductor Gas Sensors II. Transient Response to Temperature Change. Sens. Actuators B Chem. 1983, 3, 255–281. [Google Scholar] [CrossRef]
- Hunsicker, R.A.; Klier, K.; Gaffney, T.S.; Kirchner, J.G. Framework Zinc-Substituted Zeolites: Synthesis, and Core-Level and Valence-Band XPS. Chem. Mater. 2002, 14, 4807–4811. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti-recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Precise Determination of the Valence-Band Edge in X-Ray Photoemission Spectra: Application to Measurement of Semiconductor Interface Potentials. Phys. Rev. Lett. 1980, 44, 1620–1623. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Tang, Y.; Wu, P. Band Gap Engineering of SnO2 by Epitaxial Strain: Experimental and Theoretical Investigations. Phys. Chem. C 2014, 118, 6448–6453. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaics. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1993; p. 460. [Google Scholar]
- Bonasewicz, P.; Hirschwald, W.; Neumann, G. Influence of surface processes on electrical, photochemical, and thermodynamical properties of zinc oxide film. Electrochem. Soc. 1986, 133, 2270–2278. [Google Scholar] [CrossRef]
- Juna, J.H.; Yuna, J.; Choa, K.; Hwangb, I.-S.; Leeb, J.-H.; Kim, S. Necked ZnO nanoparticle-based NO2 sensors with high and fast response. Sens. Actuators B Chem. 2009, 140, 412–417. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ridha, N.J. Synthesis, and characterization of ZnO/SnO2 nanorods core-shell arrays for high performance gas sensors. Appl. Phys. A 2021, 127, 203. [Google Scholar] [CrossRef]
- Wei, M.; Li, C.F.; Deng, X.R.; Deng, H. Surface work function of transparent conductive ZnO films. Energy Procedia 2012, 16, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Mishra, Y.; Chakravadhanula, V.; Hrkac, V.; Jebril, S.; Agarwal, D.; Mohapatra, S.; Avasthi, D.; Kienle, L.; Adelung, R. Crystal growth behaviour in Au-ZnO nanocomposite under different annealing environments and photoswitchability. Appl. Phys. 2012, 112, 064308. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Basics of oxygen and SnO2 interaction; work function change and conductivity measurements. Sens. Actuators B Chem. 2006, 118, 78–83. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Roles of Shape and Size of Component Crystals in Semiconductor Gas Sensors: II. Response to NO2 and H2. Electrochem. Soc. 2008, 155, J93–J98. [Google Scholar] [CrossRef]
- Osburn, C.M.; Ormond, D.W. Dielectric breakdown properties in silicon dioxide films. Electrochem. Soc. 1972, 119, 591. [Google Scholar] [CrossRef]
- Harari, E. Dielectric Breakdown in electrically stressed thin Films of termal SiO2. Appl. Phys. 1978, 49, 2478. [Google Scholar] [CrossRef]
- Yasunaga, H.; Natori, A. Electromigration on semiconductor surfaces. Surf. Sci. Rep. 1992, 15, 205–280. [Google Scholar] [CrossRef]
- Korolev, A.N.; Sechenov, D.A.; Petrov, V.V. The nonlinear model of low temperature semiconductors impurity diffusion of direct electric field. Fiz. Khim. Obr. Mat. 1993, 5, 100. [Google Scholar]
- Petrov, V.V. Investigation of the gas molecules interaction features with the oxide gas-sensitive materials surface. Nano Microsyst. Technol. 2007, 1, 24–27. [Google Scholar]
- Kikoin, I.K. Tables of Physical Quantities; Guide; Atomizdat: Moscow, Russia, 1986. [Google Scholar]
- Grigoriev, I.E.; Meilikhov, E.Z. Physical Quantities; Guide; Energoatomizdat: Moscow, Russia, 1991. [Google Scholar]
- Nikolsky, B.P. Chemist’s Handbook (V.1), 2nd ed.; Reprint and Add, Chemistry; Goshimizdat: Moscow, Russia, 1976. [Google Scholar]
- Gerasimov, Y.I.; Dreving, V.P.; Eremin, E.N.; Kiselev, A.V.; Lebedev, V.P.; Panchenkov, G.M.; Shlygin, A.I. Physical Chemistry Course (V.1); Gerasimov, Y.I., Ed.; Chemistry: Moscow, Russia, 1964. [Google Scholar]
- Stromberg, A.G.; Semchenko, D.P. Physical Chemistry; Stromberg, A.G., Ed.; Higher School: Moscow, Russia, 1973. [Google Scholar]
- Henney, N. Solid State Chemistry; Mir: Moscow, Russia, 1971. [Google Scholar]
- Gerasimov, V.V.; Gerasimova, V.V.; Samoilov, A.G. Activation energy of the reaction in heterogeneous catalysis. Rep. USSR Acad. Sci. 1992, 332, 744–748. [Google Scholar]
- Francioso, L.; Forleo, A.; Capone, S.; Epifani, M.; Taurino, A.M.; Siciliano, P. Nanostructured In2O3-SnO2 sol-gel thin film as material for NO2 detection. Sens. Actuators B Chem. 2006, 114, 646–655. [Google Scholar] [CrossRef]
- Masa, S.; Robes, D.; Hontanon, E.; Lozano, J.; Eqtestadi, S.; Narros, A. Graphene-Tin Oxide Composite Nanofibers for Low Temperature Detection of NO2 and O3. Sens. Transducers 2020, 246, 71–78. [Google Scholar]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B Chem. 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. SnO2 thin film sensor with enhanced response for NO2 gas at lower temperatures. Sens. Actuators B Chem. 2011, 156, 743–752. [Google Scholar] [CrossRef]
- Popescu, M.; Simandan, I.D.; Sava, F.; Velea, A.; Fagadar-Cosma, E. Sensor of nitrogen dioxide based on single wall carbon nanotubes and manganese-porphyrin. Dig. J. Nanomater. Biostruct. 2011, 6, 1253–1256. [Google Scholar]
- Matatagui, D.; Cruz, C.; Carrascoso, F.; Al-Enizi, A.M.; Nafady, A.; Castellanos-Gomez, A.; Horrillo, M.d.C. Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature. Nanomaterials 2022, 12, 1213. [Google Scholar] [CrossRef]
- Goutham, S.; Sadasivuni, K.K.; Kumarc, D.S.; Rao, K.V. Flexible ultra-sensitive and resistive NO2 gas sensor based on nanostructured Zn(x)Fe(1−x)2O4. RSC Adv. 2018, 8, 3243–3249. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Design of Heteronanostructures on MoS Nanosheets to Boost NO2 Room Temperature Sensing. ACS Appl. Mater. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef]
- Hewa-Rahinduwage, C.C.; Geng, X.; Silva, K.L.; Niu, X.; Zhang, L.; Brock, S.L.; Luo, L. Reversible Electrochemical Gelation of Metal Chalcogenide Quantum Dots. J. Am. Chem. Soc. 2020, 142, 12207–12215. [Google Scholar] [CrossRef]
- Zhang, J.; Leng, D.; Zhang, L.; Li, G.; Ma, F.; Gao, J.; Lu, H.; Zhu, B. Porosity and oxygen vacancy engineering of mesoporous WO3 nanofibers for fast and sensitive low-temperature NO2 sensing. J. Alloys Compd. 2021, 853, 157339. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Chen, G.; Zhan, H.; Mei, J.; Luo, N.; He, Z.; Tang, C. SnO-Sn3O4 heterostructural gas sensor with high response and selectivity to parts-per-billion-level NO2 at low operating temperature. RSC Adv. 2020, 10, 29843–29854. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Effects of Gas Adsorption Properties of an Au-Loaded Porous In2O3 Sensor on NO2-Sensing Properties. ACS Sens. 2021, 6, 4019–4028. [Google Scholar] [CrossRef] [PubMed]
- Kampitakis, V.; Gagaoudakis, E.; Zappa, D.; Comini, E.; Aperathitis, E.; Kostopoulos, A.; Kiriakidis, G.; Binas, V. Highly sensitive and selective NO2 chemical sensors based on Al doped NiO thin films. Mater. Sci. Semicond. Process. 2020, 115, 105149. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Mattson, E.C.; Wen, Z.; Chang, J.; Hou, Y.; Hirschmugl, K.J.; Chen, J. Ultrasensitive chemical sensing through facile tuning defects and functional groups in reduced graphene oxide. Anal. Chem. 2014, 86, 7516–7522. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.E.; Jung, E.Y.; Jang, H.J.; Bae, G.T.; Kim, J.Y.; Shin, B.J.; Lee, H.-K.; Tae, H.-S. Ultrafast Room Temperature Synthesis of Porous Polythiophene via Atmospheric Pressure Plasma Polymerization Technique and Its Application to NO2 Gas Sensors. Polymers 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, M.; Liu, L.; Xu, Y.; Zhang, X.; Cheng, X.; Gao, S.; Gao, Y.; Huo, L. One-step synthesis of polypyrrole/Fe2O3 nanocomposite and the enhanced response of NO2 at low temperature. J. Colloid Interface Sci. 2020, 560, 312. [Google Scholar] [CrossRef]




| № | Material (Composition, Structure) | Technology | Particle Size, nm | The Formula for Response Calculation (Gas Concentration) | Operating Temperature, °C | Response Value | Response/Recovery Time, s | Reference |
|---|---|---|---|---|---|---|---|---|
| H2 | ||||||||
| 1 | SnO2-ZnO (0.9:0.1) | Electrospinning method | 15 | Ra/Rg, (0.1–10 ppm) | 300 | 168.6 | 103/103 | [23] |
| 2 | Sn-ZnO | Spray pyrolysis | 8–14 | ∆R/R, (500 ppm) | 400 | 200 | 50/80 | [38] |
| 3 | ZnO-SnO2 | Chemical synthesis | 50–90 | (Ra − Rg) × 100% Ra (10,000 ppm) | 150 | 90% | 60/80 | [39] |
| NO2 | ||||||||
| 4 | SnO2-ZnO | Pulse laser depostion | 10–20 | Rg/Ra (3.2 ppm) | 180 | 100 | 240/480 | [40] |
| 5 | SnO2-ZnO (5:95) | Chemical technologies | 5–10 | Rg/Ra (0.5–1.0 ppm) | 150 | 48 | 100/101 | [41] |
| 6 | 7% Sb-SnO2/ZnO | Microwave hydrothermal | 10 | Rg − Ra Ra (1000 ppb) | 300 | 9.5 | 16/- | [29] |
| 7 | ZnO-SnO2 (1:1) | Wet chemical method | 11–17 | Rg − Ra Ra (500 ppb) | 20 | 13.4 | 420/480 | [42] |
| 8 | ZnO-SnO2 | Magnetron sputtering | 10 | Ra/Rg (5 ppm) | 100 | 26.4 | 20/45 | [30] |
| 9 | SnO2-ZnO (1: 99) | Solid phase pyrolysis | 13–14 | Rg/Ra (5 ppm) | 200 | 4.5 | 300/400 | [15] |
| Ethanol | ||||||||
| 10 | SnO2-ZnO (1:1) | Combined deposition, | 20–40 | Ra/Rg (200 ppm) | 300 | 4.69 | 72/- | [43] |
| 11 | Au-doped SnO2-ZnO (1:0.5; 1:1; 0.5:1) | Electrospin coating | 5–10 | Ra/Rg (100 ppm) | 300 | 90 | 130/ | [44] |
| 12 | SnO2:ZnO= (3:1; 1:1; 1:3) | Chemical deposition, | 2800 | (Ra − Rg) × 100% Ra (24 ppm) | 275 | 53% | 150/- | [45] |
| 13 | SnO2/ZnO core/shell | The thermal evaporation SnO2 NWs and the spray-coating of ZnO | 150 | Ra/Rg 100 ppm | 450 | 15.9 | 215/- | [46] |
| Materials | C 1s | O 1s | Sn3d5 | Zn2p3 |
|---|---|---|---|---|
| 0ZnO | 34.15 | 44.85 | 21.00 | 0 |
| 0.5ZnO | 27.36 | 45.64 | 26.53 | 0.47 |
| 1ZnO | 33.35 | 40.73 | 24.47 | 1.45 |
| 5ZnO | 33.39 | 41.05 | 22.55 | 3.01 |
| Materials | VBM, (eV) | Zn2p3, (eV) | Sn3d5, (eV) | ||
|---|---|---|---|---|---|
| 0ZnO | 3.49 | - | 486.65 | 0 | - |
| 0.5ZnO | 2.95 | 1021.54 | 486.55 | −0.55 | 534.99 |
| 1ZnO | 2.77 | 1021.50 | 486.45 | −0.73 | 535.05 |
| 5ZnO | 2.74 | 1021.48 | 486.43 | −0.76 | 535.05 |
| Materials of Gas Sensor | tresp. (s), When Exposed to NO2 Gas by Working Temperature | |||||
|---|---|---|---|---|---|---|
| 200 °C | 250 °C | |||||
| 5 ppm | 10 ppm | 50 ppm | 5 ppm | 10 ppm | 50 ppm | |
| 0ZnO | 67 | 60 | 58 | 87 | 70 | 86 |
| 0.5ZnO | 144 | 144 | 240 | 80 | 86 | 244 |
| 1ZnO | 151 | 126 | 108 | 85 | 90 | 162 |
| 5ZnO | 394 | 388 | 87 | 126 | 170 | 552 |
| Material | Method | Gas Concentration, ppm | Operating Temperature, °C | Sensitivity | Response/Recovery Time | Reference |
|---|---|---|---|---|---|---|
| Single wall carbon nanotubes—Mn-porphyrin | Chemical technologies and Langmuir–Blodgett technique | 2.5 | 100 | 38% | 9 min/-- | [82] |
| WS2 | Drawing on paper | 0.8 | Room temperature | 42% | 5.2 min/19 min | [83] |
| Zn(0.5)Fe(0.5)2O4 | Method of sol–gel auto combustion | 5 | 90 | 0.54% | 100 s/100 s | [84] |
| MoS2/ZnO | Wet chemical method | 5 | Room temperature | 3050% | 211 s/1000 s | [85] |
| Thioglycolate- capped CdS quantum dots | Electrochemical method | 0.011 | Room temperature | 17% | <30 s/<30 s | [86] |
| WO3 nanofiber | Electrospinning | 3 | 90 | 100 | 125 s/231 s | [87] |
| SnO–Sn3O4 | Solvothermal process | 0.5 | 75 | 63.4 | 87 s/178 s | [88] |
| Au/pr-In2O3 | Ultrasonic-Spray Pyrolysis | 5 | 100 | ~300 | 30 min/doesn’t recover | [89] |
| Al-doped NiO | RF-sputtered | 0.2 | 200 | 2.7 | 20 min/40 min | [90] |
| voltage activation rGO | Chemically reducing GO water dispersion | 0.05 | Room temperature | 5 | 2.1 min/28 min | [91] |
| porous polythiophene (PTh) films | Plasma jets polymerization technique | 0.25 | Room temperature | 21% | 1250 s/2500 s | [92] |
| polypyrrole/Fe2O3 | One-step hydrothermal technique | 0.1 | 50 | 128% | 150 s/879 s | [93] |
| 0.5ZnO-99.5SnO2 | Solid-phase low-temperature pyrolysis | 0.5 | 200 | 2.5 | 80 s/90 s | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, V.V.; Ivanishcheva, A.P.; Volkova, M.G.; Storozhenko, V.Y.; Gulyaeva, I.A.; Pankov, I.V.; Volochaev, V.A.; Khubezhov, S.A.; Bayan, E.M. High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field. Nanomaterials 2022, 12, 2025. https://doi.org/10.3390/nano12122025
Petrov VV, Ivanishcheva AP, Volkova MG, Storozhenko VY, Gulyaeva IA, Pankov IV, Volochaev VA, Khubezhov SA, Bayan EM. High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field. Nanomaterials. 2022; 12(12):2025. https://doi.org/10.3390/nano12122025
Chicago/Turabian StylePetrov, Victor V., Alexandra P. Ivanishcheva, Maria G. Volkova, Viktoriya Yu. Storozhenko, Irina A. Gulyaeva, Ilya V. Pankov, Vadim A. Volochaev, Soslan A. Khubezhov, and Ekaterina M. Bayan. 2022. "High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field" Nanomaterials 12, no. 12: 2025. https://doi.org/10.3390/nano12122025
APA StylePetrov, V. V., Ivanishcheva, A. P., Volkova, M. G., Storozhenko, V. Y., Gulyaeva, I. A., Pankov, I. V., Volochaev, V. A., Khubezhov, S. A., & Bayan, E. M. (2022). High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field. Nanomaterials, 12(12), 2025. https://doi.org/10.3390/nano12122025