LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
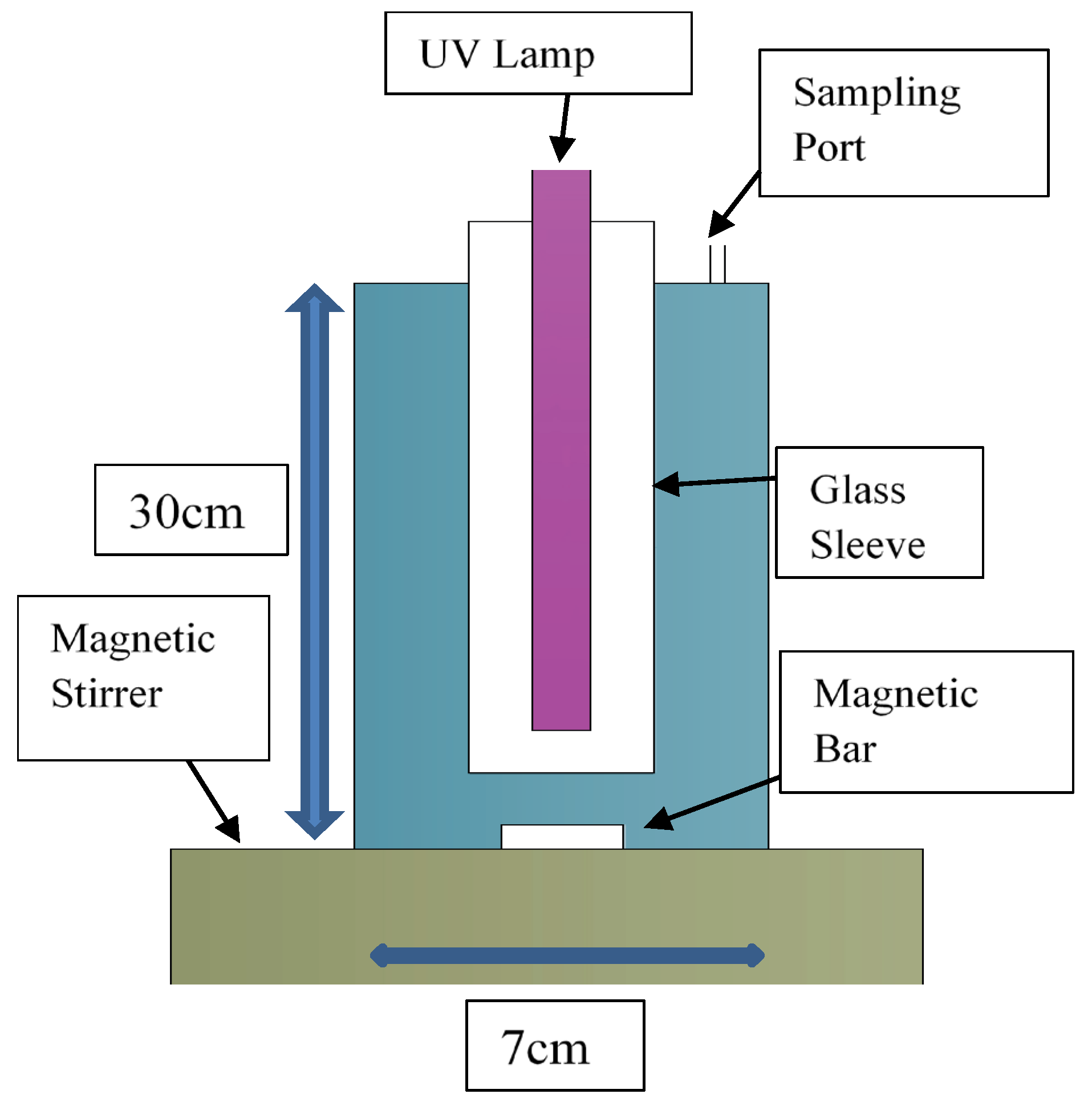
2.3. Photocatalytic Degradation (PCD) Experiments
2.4. Response Surface Methodology (RSM)
3. Results
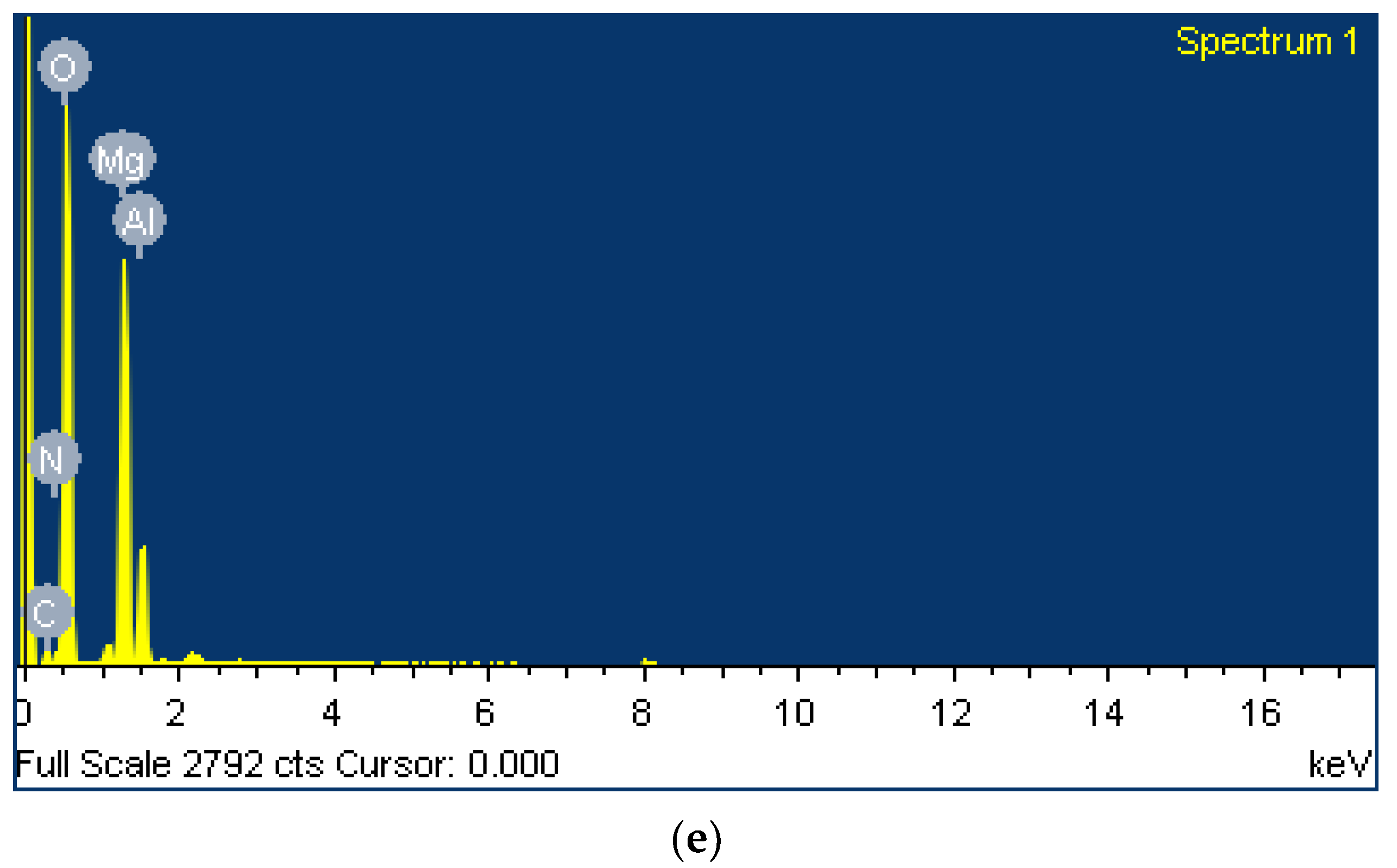
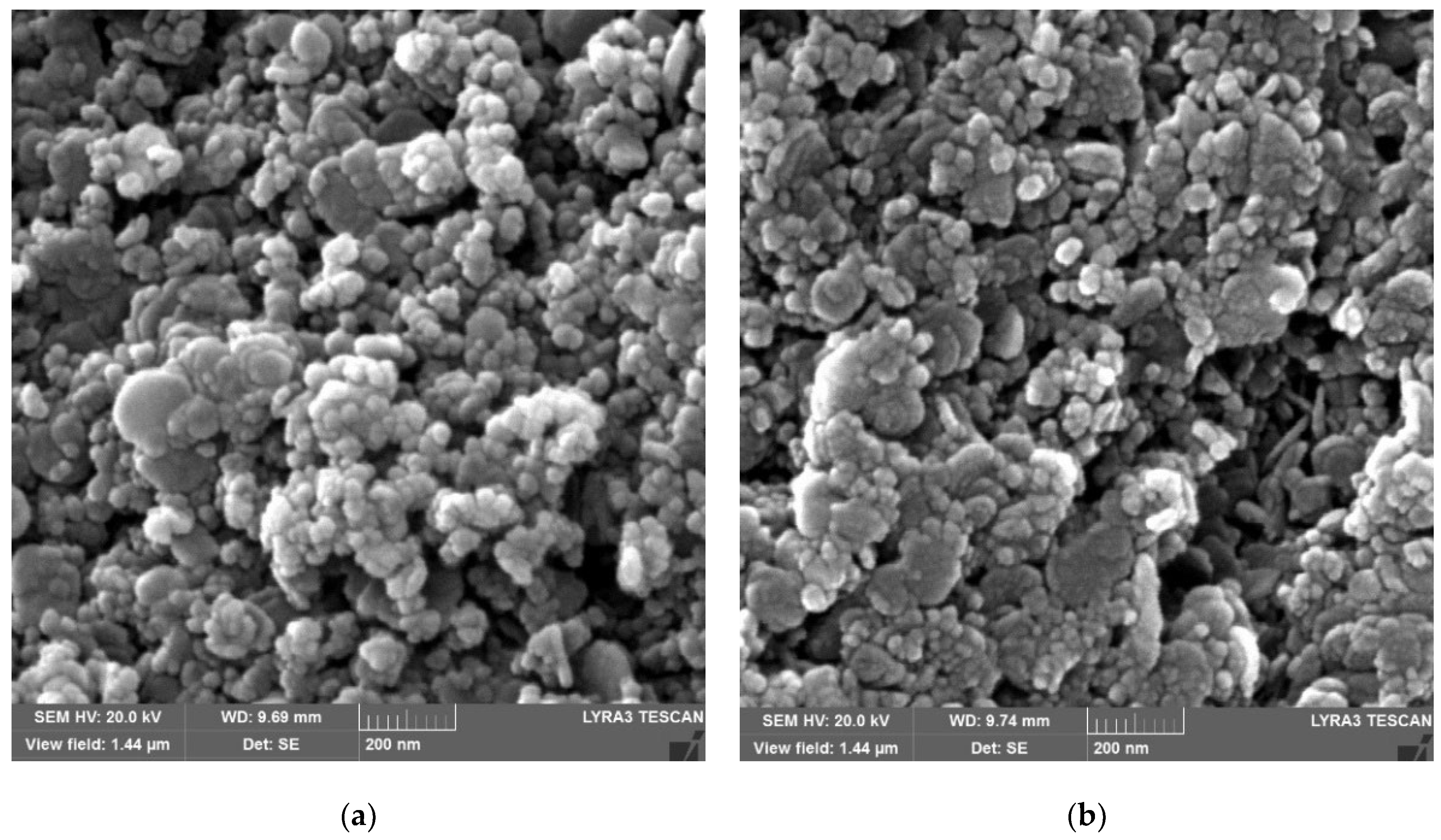
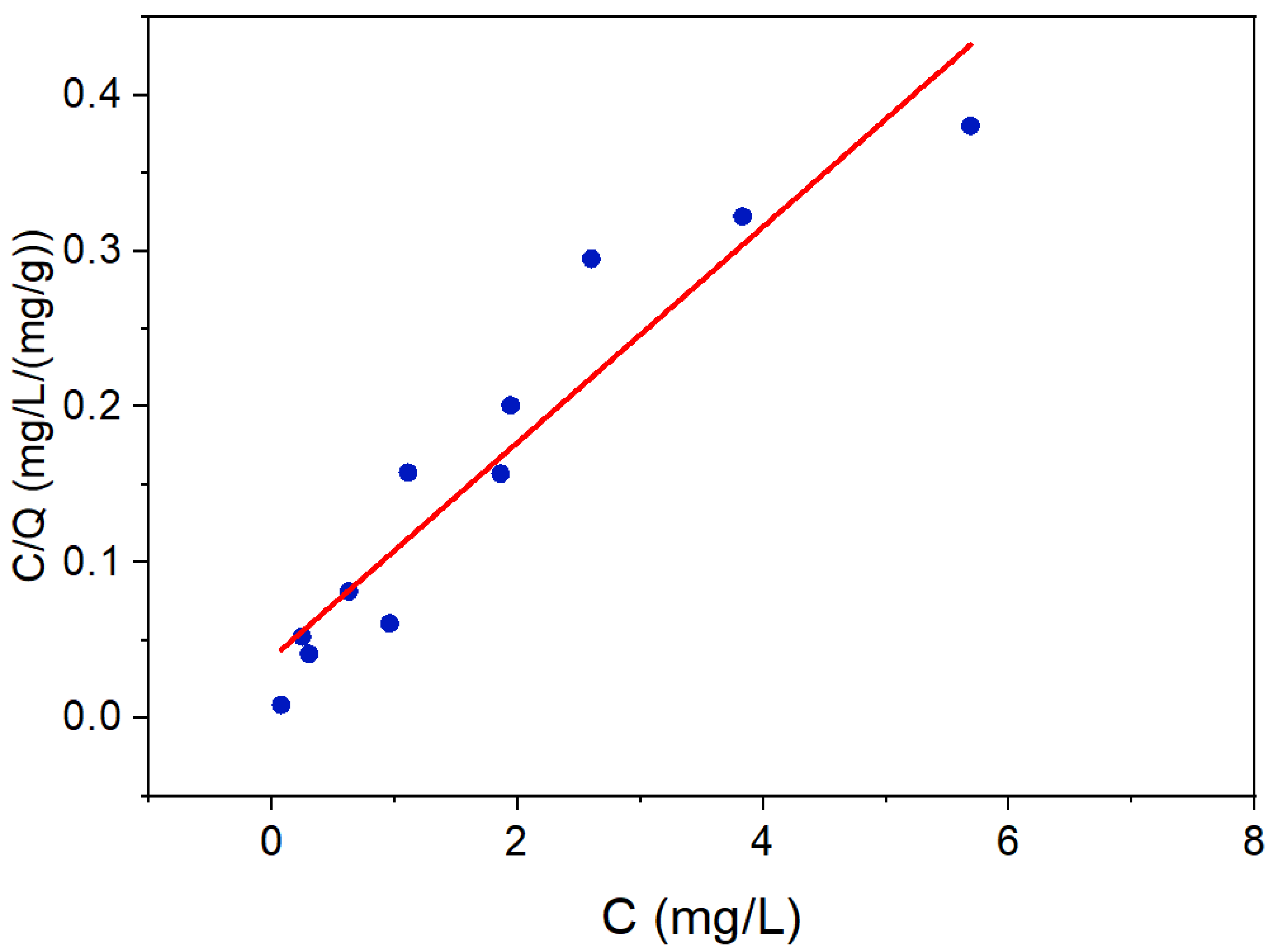
3.1. LDH and LDH-TiO2 Matrix Characterization
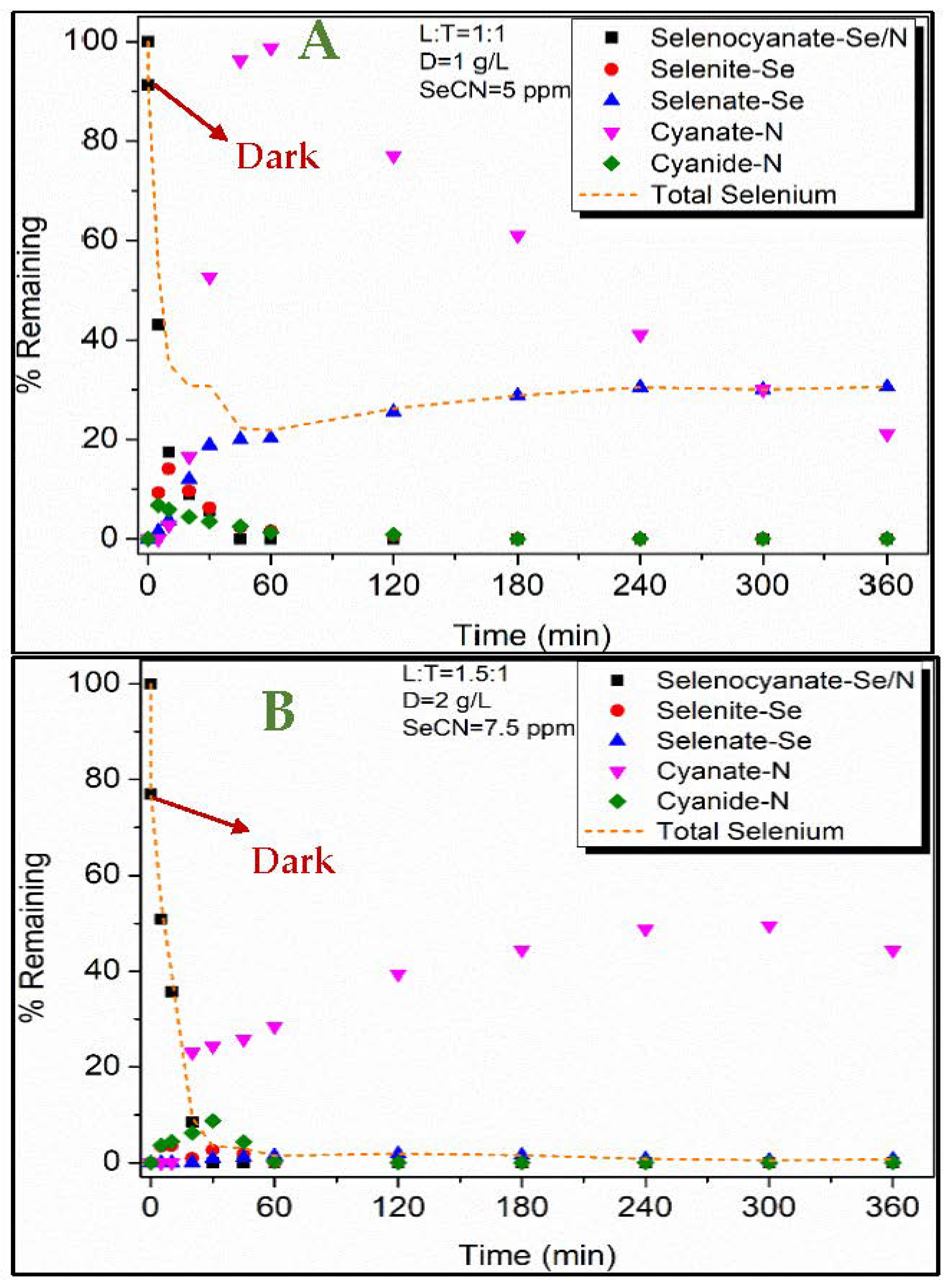
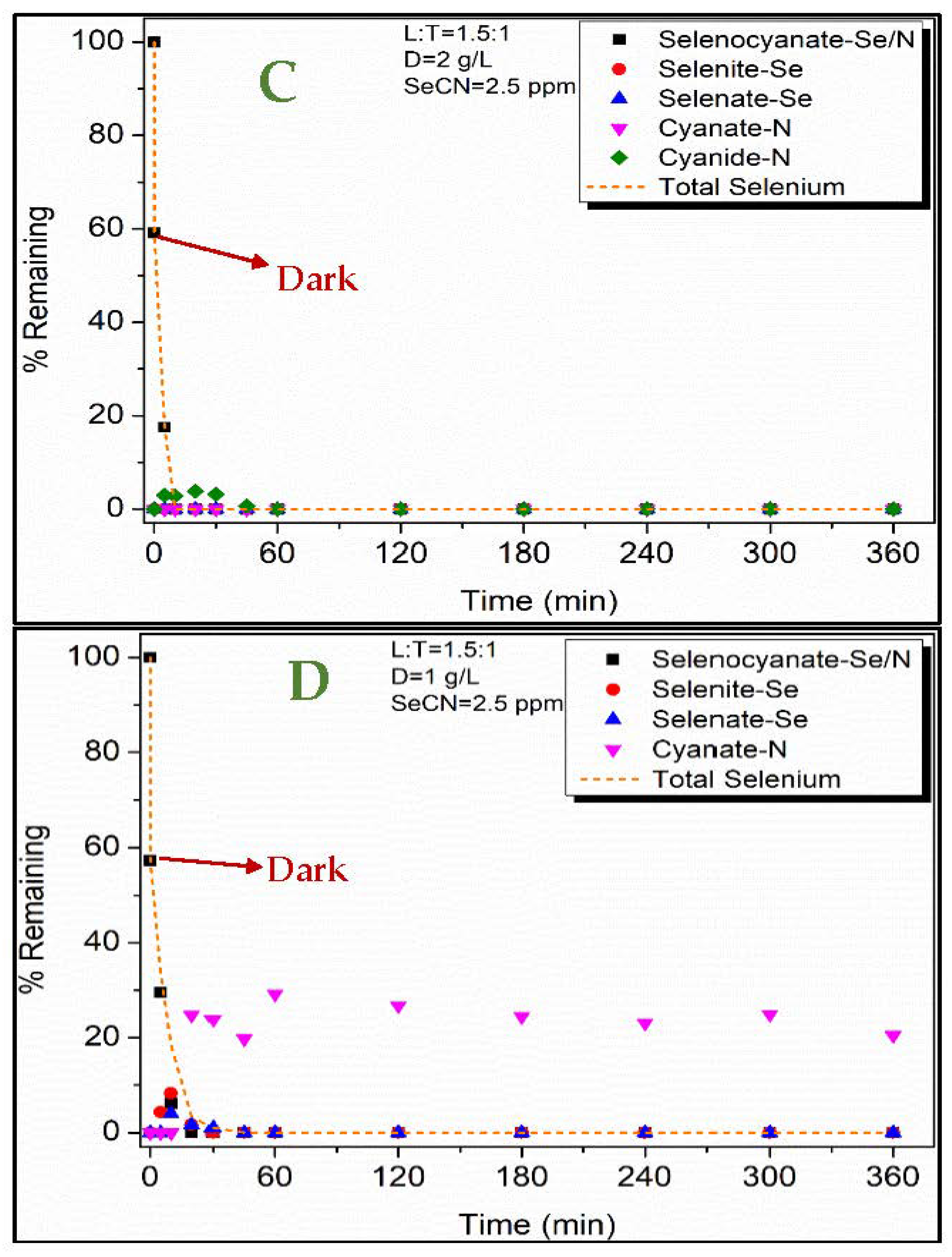
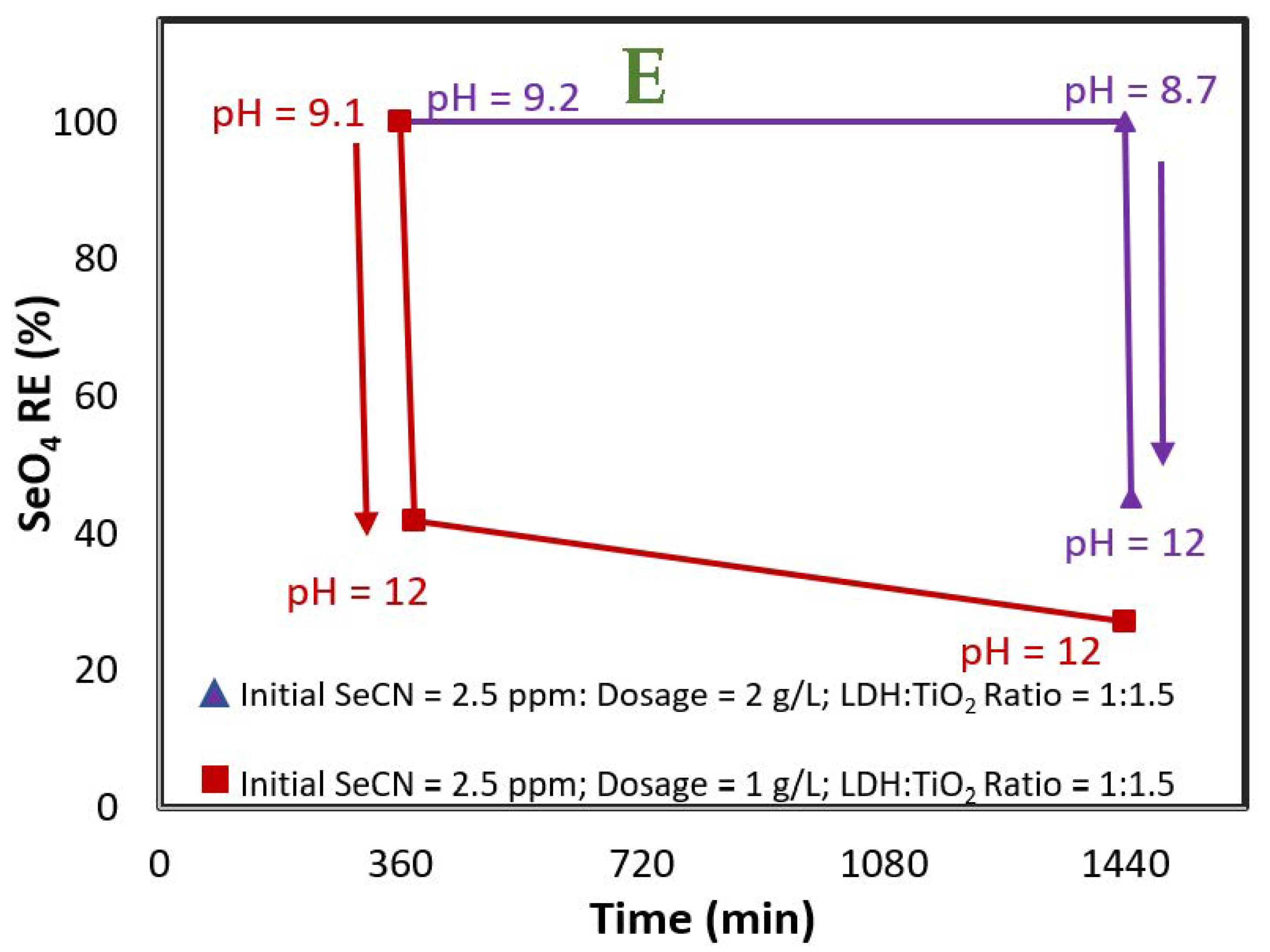
3.2. Selenocyanate Photocatalytic Degradation Using LDH:TiO2 Matrix
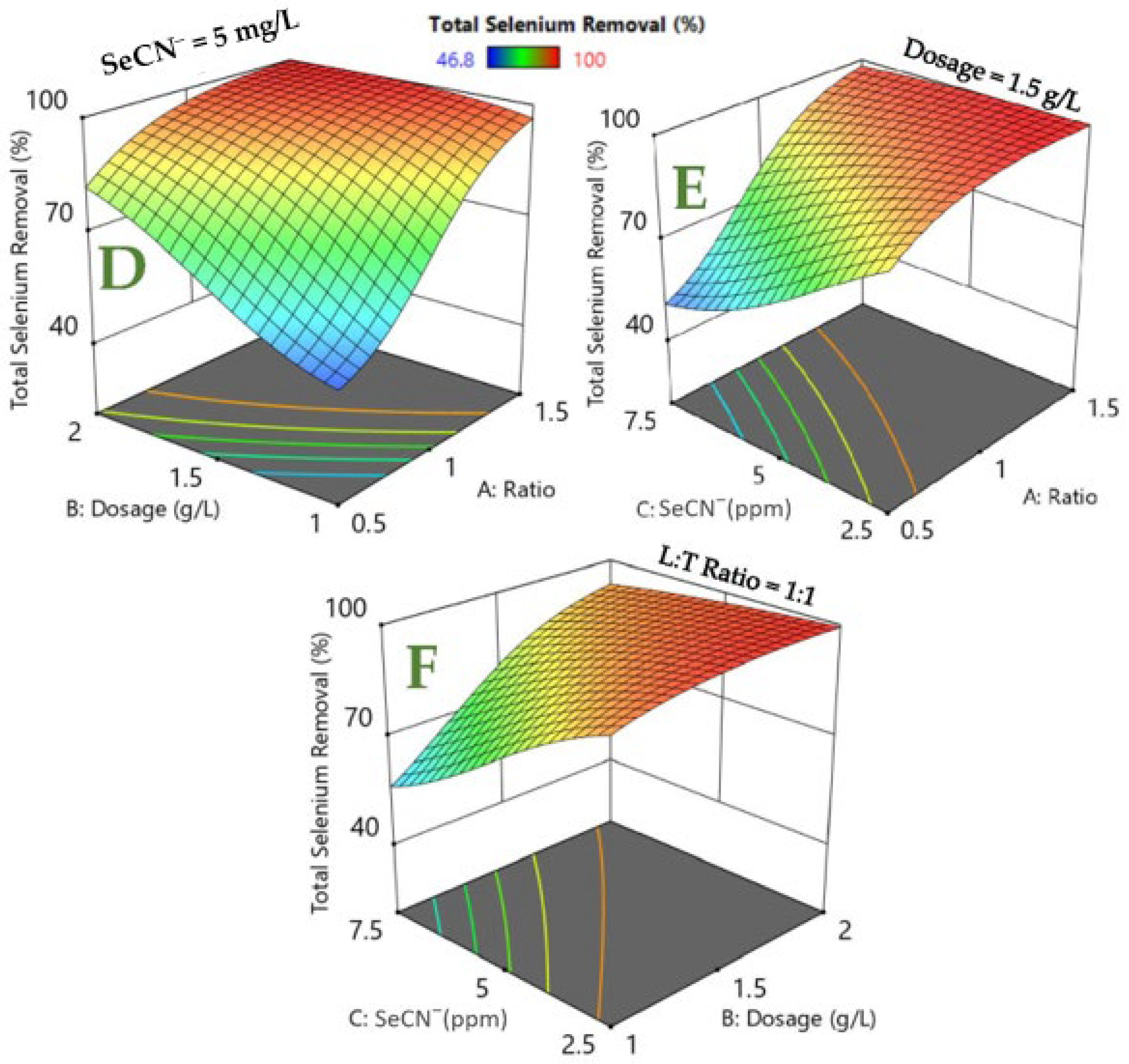
3.3. RSM Modeling of Photocatalytic Degradation Process
| A | = | LDH:TiO2 ratio (0.5:1.5); |
| B | = | adsorbent dosage (1:2 g/L); |
| C | = | selenocyanate concentration (2.5 to 7.5 mg/L); |
| Residual SeO42− | = | residual concentration of selenate in solution after 6 h of UV irradiation (mg/L); |
| TSR | = | selenocyanate removal efficiency expressed as total selenium removed (%). |
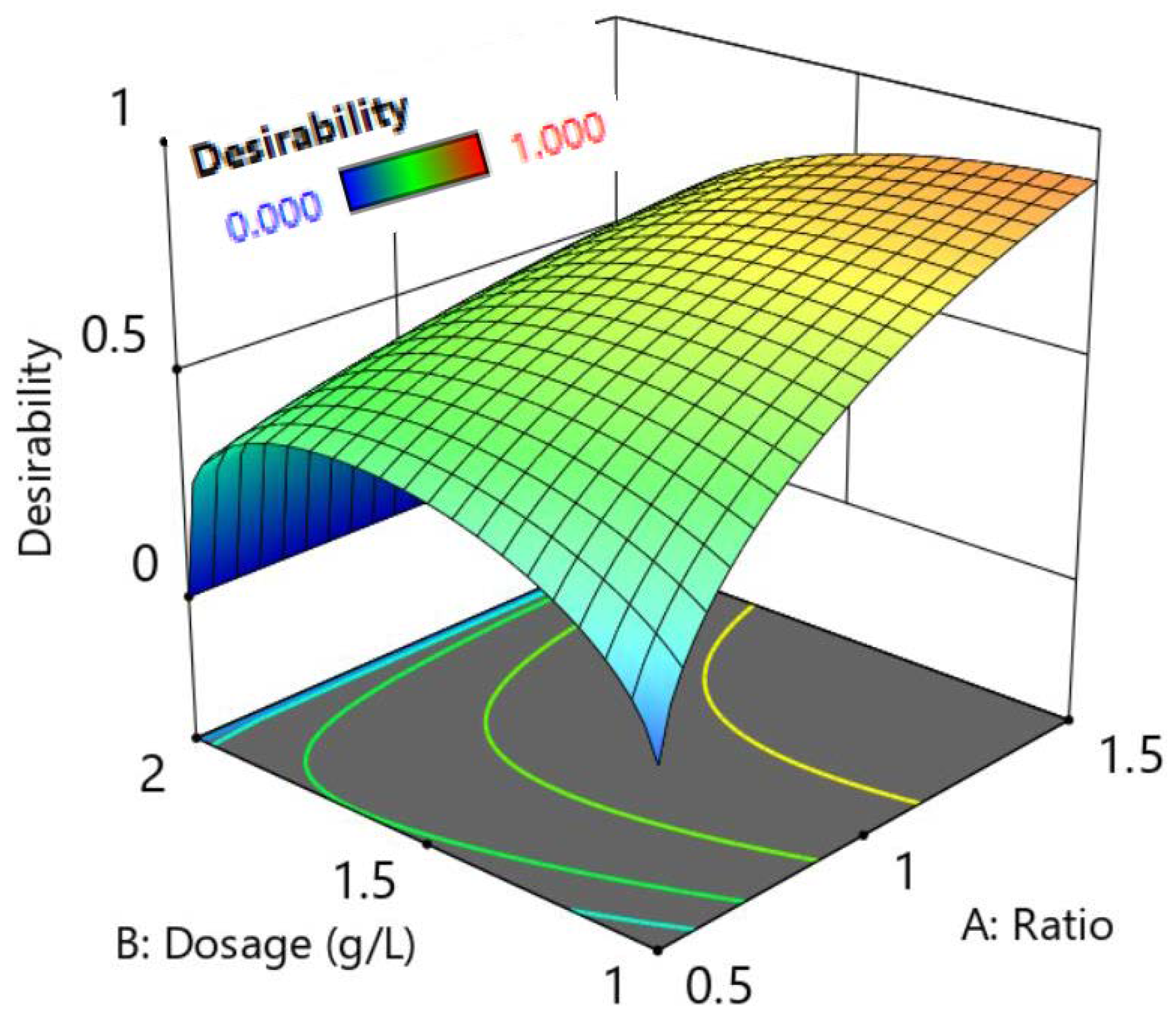
3.4. Optimization of the Photocatalytic Degradation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaji, E.; Nagaraju, A.; Sreedhar, Y.; Thejaswi, A.; Sharifi, Z. Hydrochemical characterization of groundwater in around Tirupati Area, Chittoor District, Andhra Pradesh, South India. Appl. Water Sci. 2016, 7, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, B.; Khan, M.; Antar, M.; Khalifa, A. Performance of bubble column humidification-dehumidification (HDH) desalination system. Desalin. Water Treat. 2020, 182, 101–112. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. ISBN 978-94-007-4375-5. [Google Scholar]
- Zhang, L.; Liu, N.; Yang, L.; Lin, Q. Sorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solution. J. Hazard. Mater. 2009, 170, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bang, S.; Korfiatis, G.P. Removal of selenocyanate from water using elemental iron. Water Res. 2002, 36, 3867–3873. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pickering, I.J.; Walla, M.; Terry, N. Selenium assimilation and volatilization from selenocyanate-treated Indian mustard and muskgrass. Plant Physiol. 2002, 128, 625–633. [Google Scholar] [CrossRef]
- Constantino, L.V.; Quirino, J.N.; Monteiro, A.M.; Abrão, T.; Parreira, P.S.; Urbano, A.; Santos, M.J. Sorption-desorption of selenite and selenate on Mg-Al layered double hydroxide in competition with nitrate, sulfate and phosphate. Chemosphere 2017, 181, 627–634. [Google Scholar] [CrossRef]
- Guia, M.; Pappa, J.K.; Colburna, A.S.; Meeksb, N.D.; Weaverc, B.; Wilfc, I.; Bhattacharyyaa, D. Engineered Iron/Iron Oxide Functionalized Membranes for Selenium and Other Toxic Metal Removal from Power Plant Scrubber Water. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Hu, Y.; Bird, M.I.; de Nys, R. Gracilaria waste biomass (sampah rumput laut) as a bioresource for selenium biosorption. J. Appl. Phycol. 2014, 27, 611–620. [Google Scholar] [CrossRef]
- Bakather, O.Y.; Kayvani Fard, A.; Ihsanullah; Khraisheh, M.; Nasser, M.S.; Atieh, M.A. Enhanced Adsorption of Selenium Ions from Aqueous Solution Using Iron Oxide Impregnated Carbon Nanotubes. Bioinorg. Chem. Appl. 2017, 2017, 4323619. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, T.S.; Labaran, B.A.; Ahmed, H.-R.; Mohammed, T.; Essa, M.H.; Al-Suwaiyan, M.S.; Vohra, M.S. Treatment of Aqueous Selenocyanate Anions Using Electrocoagulation. Int. J. Electrochem. Sci. 2019, 14, 10538–10564. [Google Scholar] [CrossRef]
- Murphy, A.P. Removal of selenate from water by chemical reduction. Ind. Eng. Chem. Res. 1988, 27, 187–191. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Chen, G.; Liu, H.; Qu, J. Removal of Se(IV) and Se(VI) from drinking water by coagulation. Sep. Purif. Technol. 2015, 142, 65–70. [Google Scholar] [CrossRef]
- Patwardhan, R.; Grove, L.; Ball, J.E.; Bollapragada, K.; Caskey, S.R. Removal of Selenocyanate from Industrial Water Systems with Sulfided Metal Adsorbents. US 2018/0319674 A1, 8 November 2018. [Google Scholar]
- Wang, J.; Zhang, T.; Li, M.; Yang, Y.; Lu, P.; Ning, P.; Wang, Q. Arsenic removal from water/wastewater using layered double hydroxide derived adsorbents, a critical review. RSC Adv. 2018, 8, 22694–22709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusik, J.; Rybka, K. Removal of chromates and sulphates by Mg/Fe LDH and heterostructured LDH/halloysite materials: Efficiency, selectivity, and stability of adsorbents in single- and multi-element systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, P.; Matusik, J.; Leiviskä, T. Mg/Al LDH enhances sulfate removal and clarification of AMD wastewater in precipitation processes. Materials 2019, 12, 2334. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, P.; Matusik, J.; Strączek, T.; Kapusta, C.; Woch, W.M.; Tokarz, W.; Radziszewska, A.; Leiviskä, T. Highly effective magnet-responsive LDH-Fe oxide composite adsorbents for As(V) removal. Chem. Eng. J. 2019, 362, 207–216. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Carja, G.; Nakajima, A.; Dranca, S.; Dranca, C.; Okada, K. TiO2/ZnLDH as a self-assembled nanocomposite with photoresponsive properties. J. Phys. Chem. C 2010, 114, 14722–14728. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.Z.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Hu, T.; Gong, Q.; Wang, Q.; Sun, B.; Gao, T.; Cao, P.; Zhou, G. Spherical bi2 wo6 /bi2 s3 /mos2 n-p heterojunction with excellent visible-light photocatalytic reduction cr(Vi) activity. Nanomaterials 2020, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, J.; Liu, L.; Yang, W.; Low, J.; Gao, X.; Fu, F. Synergistic effect of oxygen defect and doping engineering on S-scheme O-ZnIn2S4/TiO2-x heterojunction for effective photocatalytic hydrogen production by water reduction coupled with oxidative dehydrogenation. Chem. Eng. J. 2022, 430, 133125. [Google Scholar] [CrossRef]
- Sun, B.; Tao, F.; Huang, Z.; Yan, W.; Zhang, Y.; Dong, X.; Wu, Y.; Zhou, G. Ti3C2 MXene-bridged Ag/Ag3PO4 hybrids toward enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2021, 535, 147354. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene co-catalyst assembled with mesoporous TiO2 for boosting photocatalytic activity of methyl orange degradation and hydrogen production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Jianna, F.; Villaluz, A.; Daniel, M.; De Luna, G.; Colades, J.I.; Garcia-segura, S.; Lu, M. Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron (II) sulfate-doped nano-titania. Process Saf. Environ. Prot. 2019, 125, 121–128. [Google Scholar] [CrossRef]
- Tan, T.T.Y.; Yip, C.K.; Beydoun, D.; Amal, R. Effects of nano-Ag particles loading on TiO2 photocatalytic reduction of selenate ions. Chem. Eng. J. 2003, 95, 179–186. [Google Scholar] [CrossRef]
- Vohra, M.S.; Al-Suwaiyan, M.S.; Essa, M.H.; Chowdhury, M.M.I.; Rahman, M.M.; Labaran, B.A. Application of Solar Photocatalysis and Solar Photo-Fenton Processes for the Removal of Some Critical Charged Pollutants: Mineralization Trends and Formation of Reaction Intermediates. Arab. J. Sci. Eng. 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Vohra, M.S.; Selimuzzaman, S.M.; Al-Suwaiyan, M.S. Aqueous phase thiosulfate removal using photo catalysis. Int. J. Environ. Res. 2011, 5, 247–254. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Beyers, E.; Mertens, M.; Zhu, H.Y.; Vansant, E.F.; Cool, P. New TiO2/MgAl-LDH nanocomposites for the photocatalytic degradation of dyes. J. Nanosci. Nanotechnol. 2010, 10, 8227–8233. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.P.; Valenzuela, M.A.; Fetter, G.; Flores, S.O. TiO2/MgAl layered double hydroxides mechanical mixtures as efficient photocatalysts in phenol degradation. J. Phys. Chem. Solids 2011, 72, 914–919. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Lu, Y.; Wang, X.; Zhu, N.; Dang, Z. Enhancement of photocatalytic degradation of dimethyl phthalate with nano-TiO2 immobilized onto hydrophobic layered double hydroxides: A mechanism study. J. Hazard. Mater. 2013, 246–247, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Hatami, M. LDH-VB9-TiO2 and LDH-VB9-TiO2/crosslinked PVA nanocomposite prepared via facile and green technique and their photo-degradation application for methylene blue dye under ultraviolet illumination. Appl. Clay Sci. 2018, 163, 235–248. [Google Scholar] [CrossRef]
- Suh, M.J.; Shen, Y.; Chan, C.K.; Kim, J.H. Titanium Dioxide-Layered Double Hydroxide Composite Material for Adsorption-Photocatalysis of Water Pollutants. Langmuir 2019, 35, 8699–8708. [Google Scholar] [CrossRef]
- Djeda, R.; Mailhot, G.; Prevot, V. Porous layered double hydroxide/TiO2 photocatalysts for the photocatalytic degradation of orange II. ChemEngineering 2020, 4, 39. [Google Scholar] [CrossRef]
- Yang, L.; Shahrivari, Z.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. Removal of Trace Levels of Arsenic and Selenium from Aqueous Solutions by Calcined and Uncalcined Layered Double Hydroxides (LDH). Ind. Eng. Chem. Res. 2005, 44, 6804–6815. [Google Scholar] [CrossRef]
- Li, M.; Chowdhury, T.; Kraetz, A.; Jing, H.; Dopilka, A.; Farmen, L.; Sinha, S.; Chan, C. Layered Double Hydroxide Sorbents for Removal of Selenium from Power Plant Wastewaters. ChemEngineering 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Tian, N.; Zhou, Z.; Tian, X.; Yang, C.; Li, Y. Superior capability of MgAl2O4 for selenite removal from contaminated groundwater during its reconstruction of layered double hydroxides. Sep. Purif. Technol. 2017, 176, 66–72. [Google Scholar] [CrossRef]
- Li, M.; Farmen, L.M.; Chan, C.K. Selenium Removal from Sulfate-Containing Groundwater Using Granular Layered Double Hydroxide Materials. Ind. Eng. Chem. Res. 2017, 56, 2458–2465. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; Mccarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Pang, X.; Chen, L.; Liu, Y.; Chi, M.; Li, Z.; Plank, J. Growth behavior of water dispersed MgAl layered double hydroxide nanosheets. RSC Adv. 2017, 7, 14989–14997. [Google Scholar] [CrossRef] [Green Version]
- Seftel, E.M.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.F.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Han, E.; Vijayarangamuthu, K.; Youn, J.-S.; Park, Y.K.; Jung, S.C.; Jeon, K.J. Degussa P25 TiO2 modified with H2O2 under microwave treatment to enhance photocatalytic properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellaoui, K.; Pavlovic, I.; Barriga, C. Nanohybrid Layered Double Hydroxides Used to Remove Several Dyes from Water. ChemEngineering 2019, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Nejati, K.; Mokhtari, A.; Khodam, F.; Rezvani, Z. Syntheses of Mg-Al-NO3 layered double hydroxides with high crystallinity in the presence of amines. Can. J. Chem. 2015, 94, 66–71. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Aşçı, Y.S. Removal of textile dye mixtures by using modified Mg–Al–Cl layered double hydroxide (LDH). J. Dispers. Sci. Technol. 2017, 38, 923–929. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Q.; Hou, J.; Pei, L.; Li, Y.; Ai, S. Platinum nanocrystals supported on CoAl mixed metal oxide nanosheets derived from layered double hydroxides as catalysts for selective hydrogenation of cinnamaldehyde. J. Catal. 2015, 331, 193–202. [Google Scholar] [CrossRef]
- Hayashi, A.; Kubota, M.; Okamura, M.; Nakayama, H. Complex formation with layered double hydroxides for the remediation of hygroscopicity. Chem. Pharm. Bull. 2015, 63, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikhwivhilu, L.M.; Sinha Ray, S.; Coville, N.J. Influence of bases on hydrothermal synthesis of titanate nanostructures. Appl. Phys. A Mater. Sci. Process. 2009, 94, 963–973. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Nope, E.; Sathicq, Á.G.; Martínez, J.J.; Rojas, H.A.; Luque, R.; Romanelli, G.P. Ternary hydrotalcites in the multicomponent synthesis of 4H-pyrans. Catalysts 2020, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, T.P.F.; Aquino, S.F.; Pereira, S.I.; Dias, A. Use of calcined layered double hydroxides for the removal of color and organic matter from textile effluents: Kinetic, equilibrium and recycling studies. Braz. J. Chem. Eng. 2014, 31, 19–26. [Google Scholar] [CrossRef]
- Anbia, M.; Amirmahmoodi, S. Removal of Hg (II) and Mn (II) from aqueous solution using nanoporous carbon impregnated with surfactants. Arab. J. Chem. 2016, 9, S319–S325. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, M.F.; Bellato, C.R.; Mounteer, A.H.; Ferreira, S.O.; Milagres, J.L.; Miranda, L.D.L. Enhanced photocatalytic activity of TiO2-impregnated with MgZnAl mixed oxides obtained from layered double hydroxides for phenol degradation. Appl. Surf. Sci. 2015, 357, 1765–1775. [Google Scholar] [CrossRef]
- Paikaray, S.; Hendry, M.J.; Essilfie-Dughan, J. Controls on arsenate, molybdate, and selenate uptake by hydrotalcite-like layered double hydroxides. Chem. Geol. 2013, 345, 130–138. [Google Scholar] [CrossRef]
- Xu, M.; Iglesia, E.; Apestegu, C.R.; Cosimo, D.I.; Al, E.T. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1−xAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Alanis, C.; Natividad, R.; Barrera-Diaz, C.; Martínez-Miranda, V.; Prince, J.; Valente, J.S. Photocatalytically enhanced Cr(VI) removal by mixed oxides derived from MeAl (Me: Mg and/or Zn) layered double hydroxides. Appl. Catal. B Environ. 2013, 140–141, 546–551. [Google Scholar] [CrossRef]
- Forano, C. Environmental remediation involving layered double hydroxides. Clay Surf. Fundam. Appl. 2004, 425–458. [Google Scholar]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Vohra, M.S. Selenocyanate (SeCN−) contaminated wastewater treatment using TiO2 photocatalysis: SeCN− complex destruction, intermediates formation, and removal of selenium species. Fresenius Environ. Bull. 2015, 24, 8–11. [Google Scholar]
- Vohra, M.S.; Labaran, B.A. Photocatalytic treatment of mixed selenocyanate and phenol streams: Process modeling, optimization, and kinetics. Environ. Prog. Sustain. Energy 2020, 39, e13401. [Google Scholar] [CrossRef]
- You, Y.; Vance, G.F.; Zhao, H. Selenium adsorption on Mg-Al and Zn-Al layered double hydroxides. Appl. Clay Sci. 2001, 20, 13–25. [Google Scholar] [CrossRef]
- Isaacs-Paez, E.D.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Martinez-Rosales, J.M.; Flores-Cano, J.V. Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions. Mechanism and effect of operating conditions. Chem. Eng. J. 2014, 245, 248–257. [Google Scholar] [CrossRef]
- Raissi, S.; Farsani, R.E. Statistical process optimization Through multi-response surface methodology. World Acad. Sci. Eng. Technol. 2009, 39, 280–284. [Google Scholar]






| Factors | Level −1 | Level 0 | Level 1 |
|---|---|---|---|
| A (w/w ratio of LDH:TiO2 matrix) | 0.5 | 1 | 1.5 |
| B (dosage of LDH:TiO2 matrix (g/L)) | 1.0 | 1.50 | 2.0 |
| C (selenocyanate (mg/L)) | 2.50 | 5.0 | 7.50 |
| Exp No. | LDH:TiO2 Ratio | Dosage (g/L) | SeCN− (mg/L) | Residual SeO42− (mg/L) | Total Selenium Removal (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1.5 | 5 | 0.63 | 90.2 |
| 2 | 1 | 2 | 5 | 0.15 | 98 |
| 3 | 1 | 1 | 5 | 1.94 | 71.4 |
| 4 | 1 | 1.5 | 7.5 | 1.86 | 82.7 |
| 5 | 1.5 | 2 | 7.5 | 0.07 | 99.3 |
| 6 | 1.5 | 2 | 2.5 | 0 | ~100 |
| 7 | 0.5 | 1.5 | 5 | 2.6 | 63 |
| 8 | 1.5 | 1 | 7.5 | 0.96 | 90.8 |
| 9 | 0.5 | 1 | 7.5 | 5.69 | 46.8 |
| 10 | 1.5 | 1 | 2.5 | 0 | ~100 |
| 11 | 0.5 | 1 | 2.5 | 1.11 | 67.7 |
| 12 | 1 | 1.5 | 2.5 | 0 | ~100 |
| 13 | 1.5 | 1.5 | 5 | 0 | ~100 |
| 14 | 0.5 | 2 | 7.5 | 3.43 | 67.6 |
| 15 | 0.5 | 2 | 2.5 | 0.25 | 92.8 |
| Element | Weight% | Atomic% | Mg/Al Ratio |
|---|---|---|---|
| C | 5.56 | 7.9 | - |
| N | 5.27 | 6.4 | - |
| O | 64.57 | 68.9 | - |
| Mg | 18.55 | 13.0 | 3.4 |
| Al | 6.05 | 3.8 | |
| Totals | 100.00 | - | - |
| Models | Significance Values for the Model Terms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | A: L:T Ratio | B: Dosage | C: SeCN− | AB | AC | BC | A2 | B2 | C2 | |
| RS | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.021 | <0.0001 | 0.0187 | 0.0115 | -- | -- |
| TS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.0017 | 0.0035 | 0.0468 | 0.0512 | -- |
| Statistic | RS Model | TS Model |
|---|---|---|
| R2 | 0.9868 | 0.9917 |
| Adjusted R2 | 0.9736 | 0.9806 |
| Predicted R2 | 0.9265 | 0.9188 |
| Adequate Precision | 29.99 | 34.46 |
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| A: LDH:TiO2 | within range | 0.5 | 1.5 |
| B:Dose (g/L) | Reduce | 1 | 2 |
| C: Selenocyanate (mg/L) | 7.5 | 2.5 | 7.5 |
| Selenate remaining (mg/L) | reduce | 0 | 5.7 |
| Removal of selenate (percentage) | increase | 47 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussaini, M.; Vohra, M. LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials 2022, 12, 2035. https://doi.org/10.3390/nano12122035
Hussaini M, Vohra M. LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials. 2022; 12(12):2035. https://doi.org/10.3390/nano12122035
Chicago/Turabian StyleHussaini, Minaam, and Muhammad Vohra. 2022. "LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling" Nanomaterials 12, no. 12: 2035. https://doi.org/10.3390/nano12122035
APA StyleHussaini, M., & Vohra, M. (2022). LDH-TiO2 Composite for Selenocyanate (SeCN−) Photocatalytic Degradation: Characterization, Treatment Efficiency, Reaction Intermediates and Modeling. Nanomaterials, 12(12), 2035. https://doi.org/10.3390/nano12122035






