Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design
Abstract
:1. Introduction
2. Materials and Methods
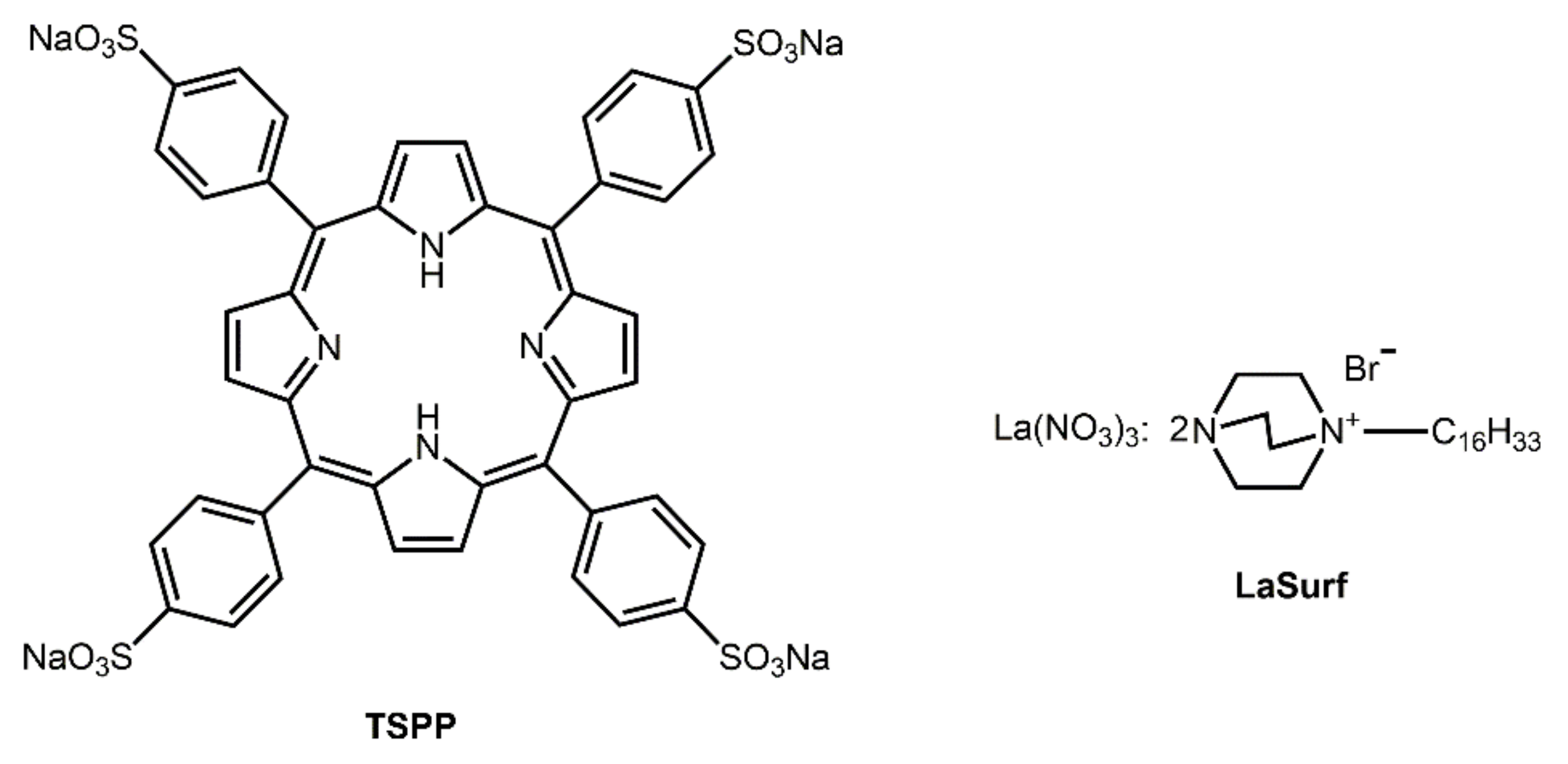
2.1. Materials
2.2. Measurements
2.3. Encapsulation of Rhodamine B
2.4. Cisplatin Complexation
2.5. Fourier Transform Pulsed-Gradient Spin-Echo NMR Measurements
2.6. Cell Viability Evaluation
2.7. Hemolytic Activity
3. Results and Discussion
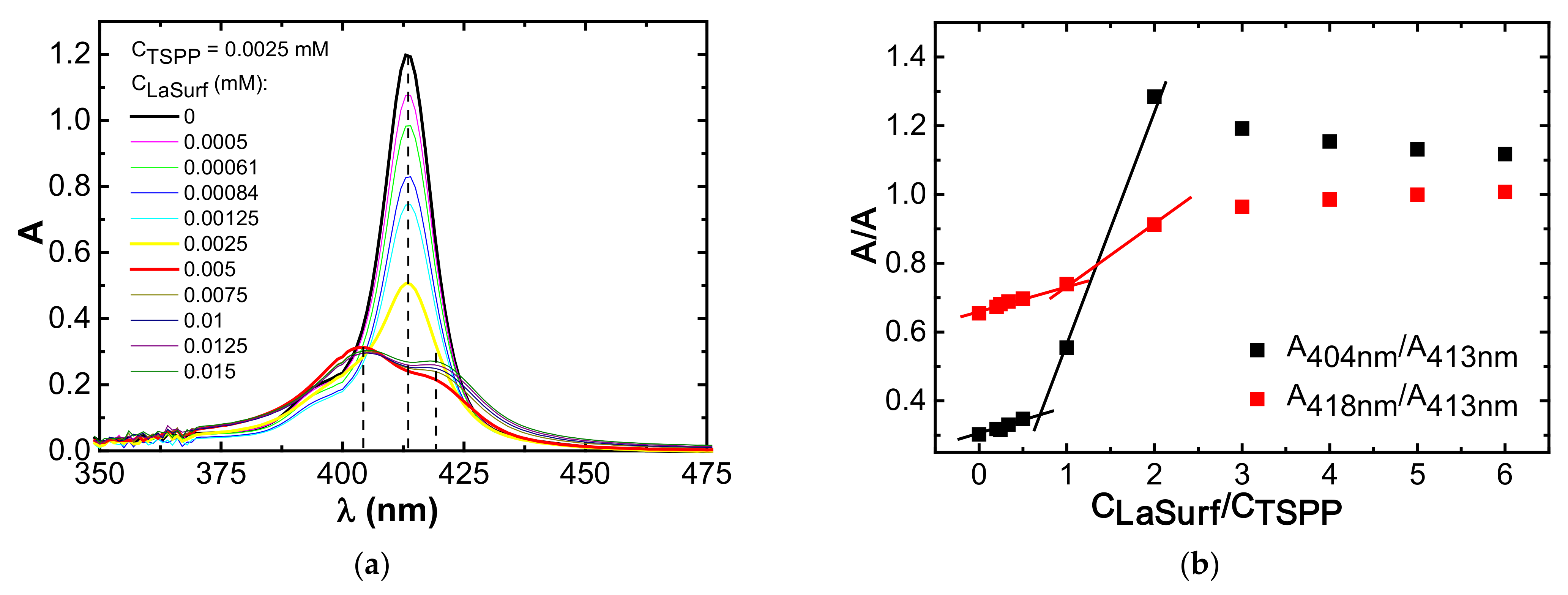
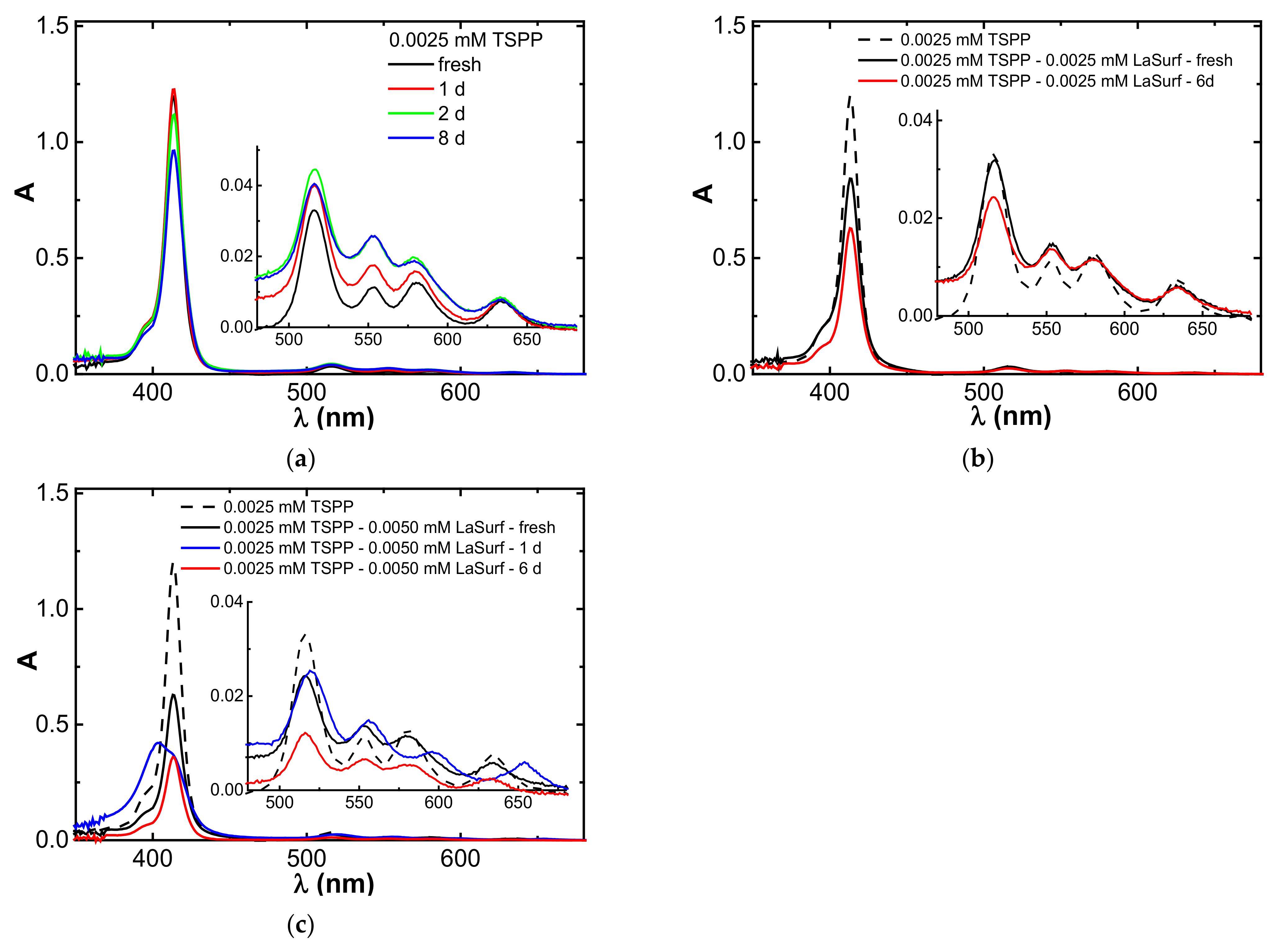
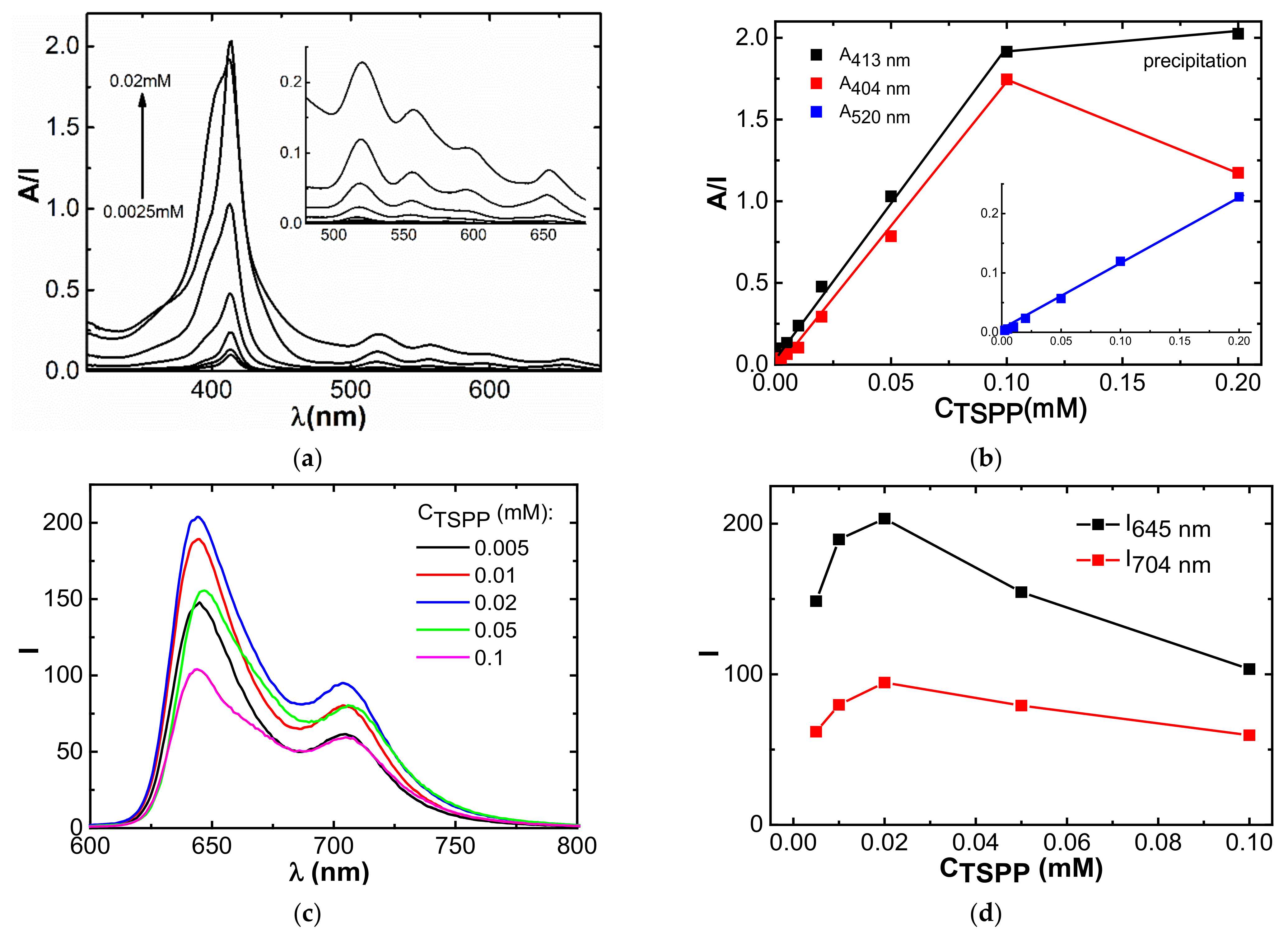
3.1. Spectrophotometry
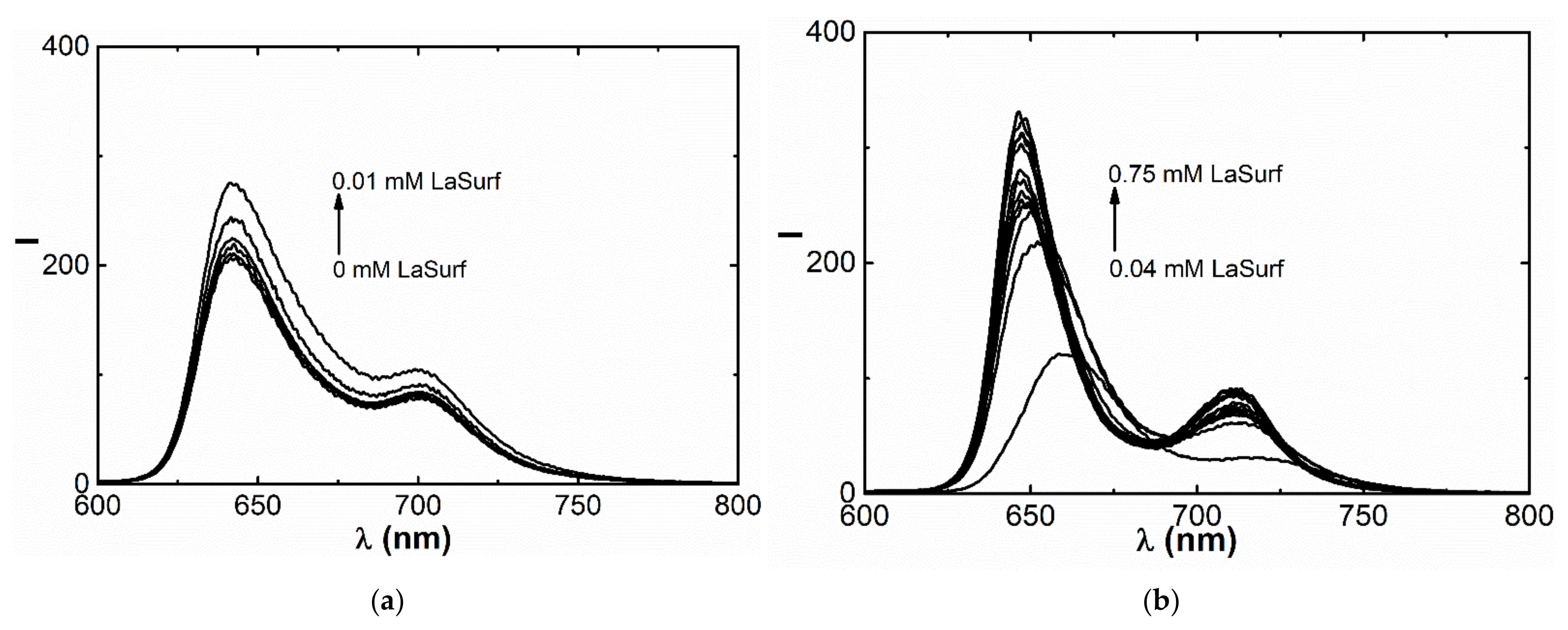
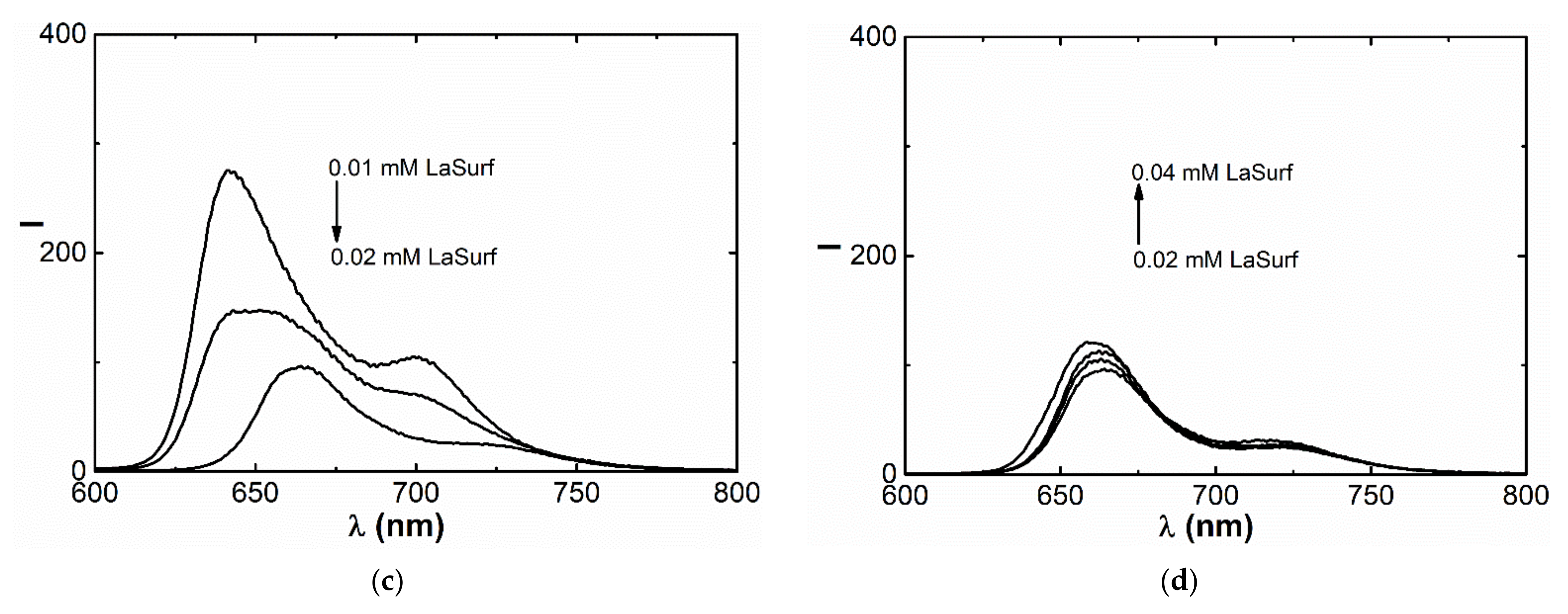
3.2. Fluorescence Spectroscopy
3.3. NMR Spectroscopy
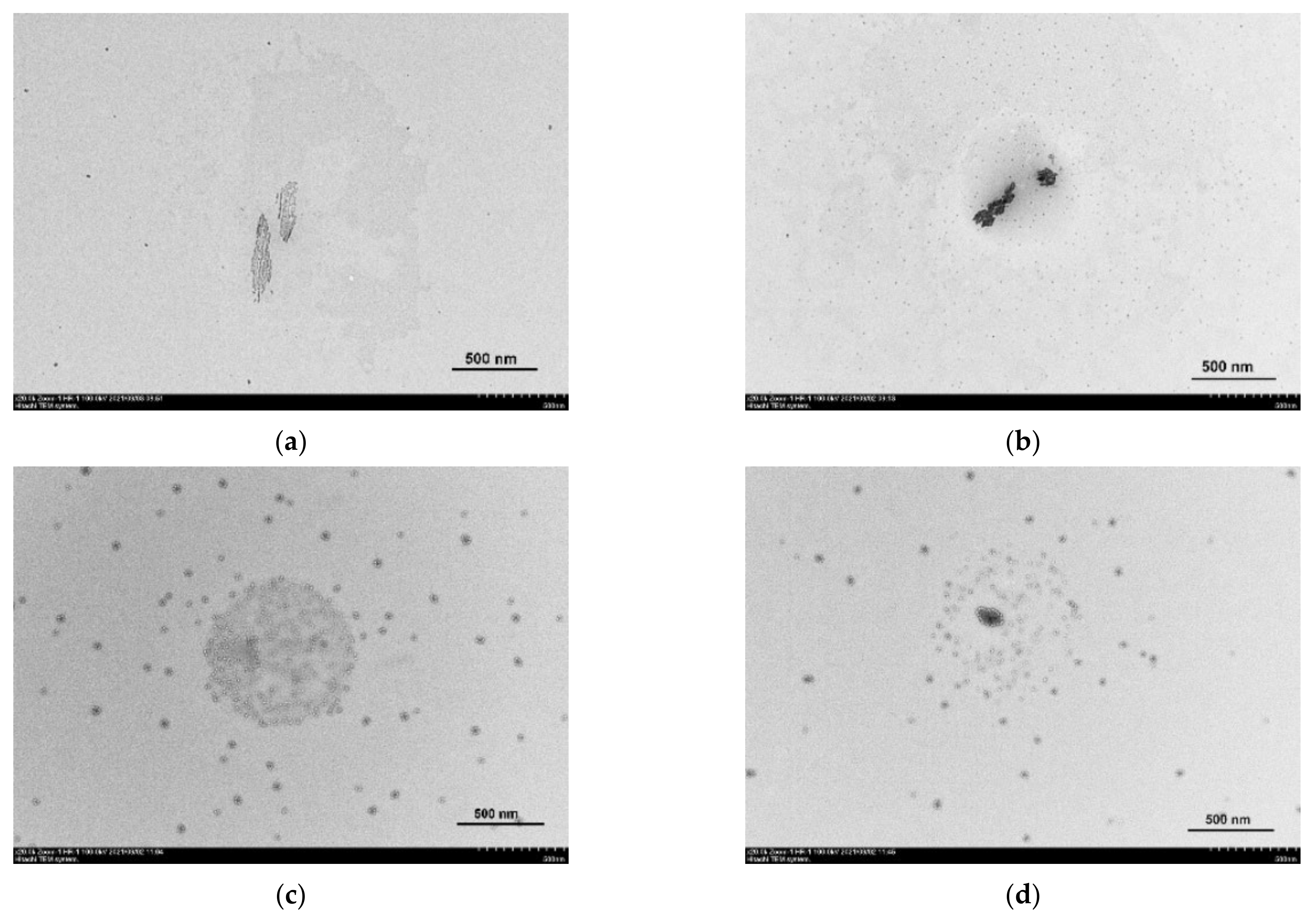
3.4. Transmission Electron Microscopy and Dynamic Light Scattering
3.5. Aggregation Stability of Stoichiometric TSPP–LaSurf Complexes
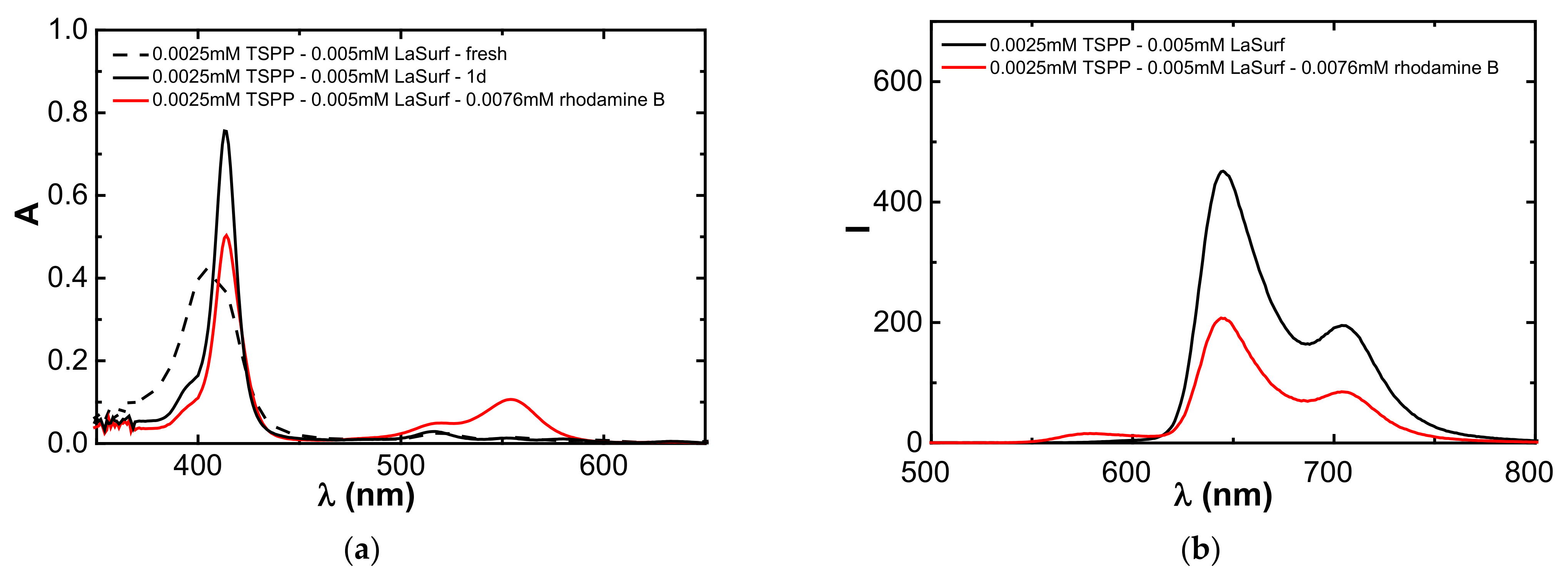
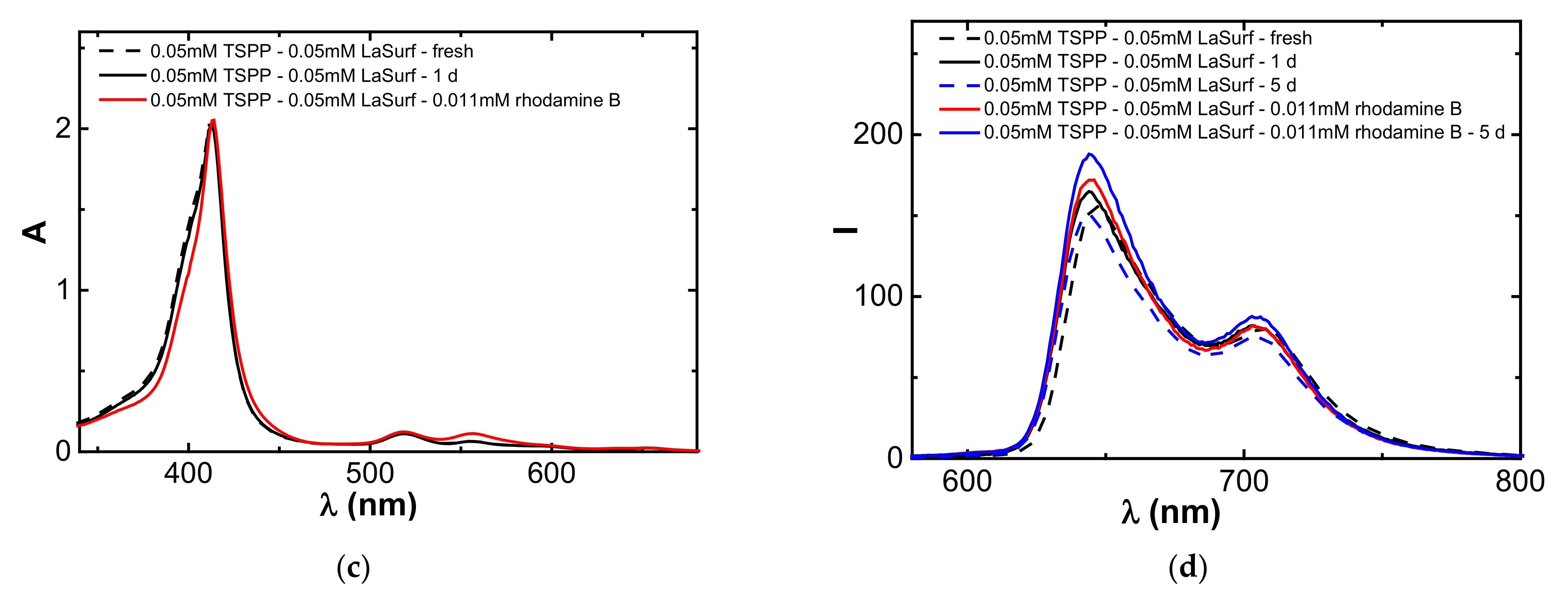
3.6. Encapsulation of Rhodamine B
3.7. Complexation with Cisplatin
3.8. Cytotoxic Effect of Cisplatin in TSPP–LaSurf Compositions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takashima, Y.; Kobayashi, Y.; Osaki, M.; Harada, A. Functional Supramolecular Materials Formed by Non-Covalent Bonds. Des. Mol. Space Mater. Sci. Catal. 2018, 567, 183–225. [Google Scholar] [CrossRef]
- Glatter, O.; Salentinig, S. Inverting Structures: From Micelles via Emulsions to Internally Self-Assembled Water and Oil Continuous Nanocarriers. Curr. Opin. Colloid Interface Sci. 2020, 49, 82–93. [Google Scholar] [CrossRef]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razuvayeva, Y.; Kashapov, R.; Zakharova, L. Calixarene-Based Pure and Mixed Assemblies for Biomedical Applications. Supramol. Chem. 2020, 32, 178–206. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly Behaviors of Calixarene-Based Amphiphile and Supra-Amphiphile and the Applications in Drug Delivery and Protein Recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Zheng, S.P.; Huang, L.B.; Sun, Z.; Barboiu, M. Self-Assembled Artificial Ion-Channels toward Natural Selection of Functions. Angew. Chemie Int. Ed. 2021, 60, 566–597. [Google Scholar] [CrossRef]
- Basílio, N.; Gómez, B.; García-Río, L.; Basilio, N.; Gómez-González, B.; Garcia-Río, L. P-Sulfonatocalix[6]Arene-Dodecyltrimethylammonium Supramolecular Amphiphilic System: Relationship between Calixarene and Micelle Concentration. Langmuir 2017, 33, 13008–13013. [Google Scholar] [CrossRef]
- Martín, V.I.; Angulo, M.; López-Cornejo, P.; López-López, M.; Marchena, M.J.; Moyá, M.L. Stoppering/Unstoppering of a Rotaxane Formed between an N-Hetorycle Ligand Containing Surfactant: β-Cyclodextrin Pseudorotaxane and Pentacyanoferrate(II) Ions. J. Colloid Interface Sci. 2017, 497, 343–349. [Google Scholar] [CrossRef]
- Schmid, P.; Buchecker, T.; Khoshsima, A.; Touraud, D.; Diat, O.; Kunz, W.; Pfitzner, A.; Bauduin, P. Self-Assembly of a Short Amphiphile in Water Controlled by Superchaotropic Polyoxometalates: H4SiW12O40 vs. H3PW12O40. J. Colloid Interface Sci. 2021, 587, 347–357. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, N.; Filosa, A.; Li, X. Increasing the Size and Complexity of Discrete 2D Metallosupramolecules. Nat. Rev. Mater. 2021, 6, 145–167. [Google Scholar] [CrossRef]
- Hewson, S.W.; Mullen, K.M. Porphyrin-Containing Rotaxane Assemblies. European J. Org. Chem. 2019, 2019, 3358–3370. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Jancik, V. Coordination-Driven Assemblies Based on Meso-Substituted Porphyrins: Metal-Organic Cages and a New Type of Meso-Metallaporphyrin Macrocycles. Coord. Chem. Rev. 2020, 407, 213165. [Google Scholar] [CrossRef]
- Amado, A.M.; Uliana, J.H.; Pavan, T.Z.; Borissevitch, I. Effect of Metallization on Porphyrin Photoacoustic Response. Chem. Phys. Lett. 2020, 738, 136875. [Google Scholar] [CrossRef]
- Nakajima, S.; Hayashi, H.; Omote, Y.; Yamazaki, Y.; Hirata, S.; Maeda, T.; Kubo, Y.; Takemura, T.; Kakiuchi, Y.; Shindo, Y.; et al. The Tumour-Localizing Properties of Porphyrin Derivatives. J. Photochem. Photobiol. B Biol. 1990, 7, 189–198. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Anti-Cancer Gold, Platinum and Iridium Compounds with Porphyrin and/or N-Heterocyclic Carbene Ligand(s). Adv. Inorg. Chem. 2020, 75, 87–119. [Google Scholar] [CrossRef]
- Antoni, P.M.; Naik, A.; Albert, I.; Rubbiani, R.; Gupta, S.; Ruiz-Sanchez, P.; Munikorn, P.; Mateos, J.M.; Luginbuehl, V.; Thamyongkit, P.; et al. (Metallo)Porphyrins as Potent Phototoxic Anti-Cancer Agents after Irradiation with Red Light. Chem. A Eur. J. 2015, 21, 1179–1183. [Google Scholar] [CrossRef]
- Nifantiev, N.E.; Yashunsky, D.V. Water-Soluble Porphyrin Dervatives for Photodynamic Therapy, Their Use and Manufacture. U.S. Patent 6777,402 B2, 17 August 2004. [Google Scholar]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y.; Hou, Z.; Lin, J. An Imaging-Guided Platform for Synergistic Photodynamic/Photothermal/Chemo-Therapy with PH/Temperature-Responsive Drug Release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef]
- Ion, R.; Maresca, L.; Migoni, D.; Fanizzi, F.P.; Lecce, U.; Biologiche, T. Porphyrin—Cis-Platin Drug System for HeLa Cells Photodynamic Treatment. Int. J. Adv. Life Sci. 2009, 1, 36–45. [Google Scholar]
- Lottner, C.; Bart, K.C.; Bernhardt, G.; Brunner, H. Hematoporphyrin-Derived Soluble Porphyrin-Platinum Conjugates with Combined Cytotoxic and Phototoxic Antitumor Activity. J. Med. Chem. 2002, 45, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bao, Y.; Wang, J.; Xiao, C.; Chen, L. Construction of Carrier-Free Porphyrin-Based Drug Self-Framed Delivery System to Reverse Multidrug Resistance through Photodynamic-Chemotherapy. Dye. Pigment. 2020, 177, 107922. [Google Scholar] [CrossRef]
- Tian, J.; Xiao, C.; Huang, B.; Wang, C.; Zhang, W. Janus Macromolecular Brushes for Synergistic Cascade-Amplified Photodynamic Therapy and Enhanced Chemotherapy. Acta Biomater. 2020, 101, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Dandash, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Nasri, S.; Brégier, F.; Granet, R.; Karam, W.; Diab-Assaf, M.; Sol, V.; Liagre, B. In Vitro Anticancer Activity of New Gold(III) Porphyrin Complexes in Colon Cancer Cells. J. Inorg. Biochem. 2017, 177, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Hong, K.I.; Lee, H.; Jang, W.D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Z.; Bai, G.; Meng, S.; Dai, H.; He, H. Interaction of Water-Soluble Cationic Porphyrin with Anionic Surfactant. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 1164–1169. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Han, M.; Chen, Z.; Zou, G. Neutral Porphyrin J-Aggregates in Premicellar SDS Solution. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 256, 151–156. [Google Scholar] [CrossRef]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-Assisted Porphyrin Based Hierarchical Nano/Micro Assemblies and Their Efficient Photocatalytic Behavior. ACS Appl. Mater. Interfaces 2014, 6, 130–136. [Google Scholar] [CrossRef]
- Chen, J.X.; Wang, H.Y.; Li, C.; Han, K.; Zhang, X.Z.; Zhuo, R.X. Construction of Surfactant-like Tetra-Tail Amphiphilic Peptide with RGD Ligand for Encapsulation of Porphyrin for Photodynamic Therapy. Biomaterials 2011, 32, 1678–1684. [Google Scholar] [CrossRef]
- Gradova, M.A.; Gradov, O.V.; Zhdanova, K.A.; Bragina, N.A.; Lobanov, A.V. Self-Assembly of Amphiphilic Meso -Aryl-Substituted Porphyrin Derivatives in the Presence of Surfactants. J. Porphyr. Phthalocyanines 2020, 24, 505–514. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, P.; Liu, M. Evolution of Various Porphyrin Nanostructures via an Oil/Aqueous Medium: Controlled Self-Assembly, Further Organization, and Supramolecular Chirality. J. Am. Chem. Soc. 2010, 132, 9644–9652. [Google Scholar] [CrossRef] [PubMed]
- Gradova, M.A.; Artemov, V.V.; Lobanov, A.V. Aggregation Behavior of Tetraphenylporphyrin in Aqueous Surfactant Solutions: Chiral Premicellar J-Aggregate Formation. J. Porphyr. Phthalocyanines 2015, 19, 845–851. [Google Scholar] [CrossRef]
- Almeida, L.M.; Zílio, S.C.; De Boni, L.; Borissevitch, I.E.; Franzen, P.L.; Corrêa, D.S.; Gonçalves, P.J.; Mendonça, C.R. Effect of Interaction with Micelles on the Excited-State Optical Properties of Zinc Porphyrins and J-Aggregates Formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 309–317. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Jiang, J. Controlling the Growth of Porphyrin Based Nanostructures for Tuning Third-Order NLO Properties. Nanoscale 2014, 6, 1871–1878. [Google Scholar] [CrossRef]
- Te Wang, Y.; Jin, W.J. H-Aggregation of Cationic Palladium-Porphyrin as a Function of Anionic Surfactant Studied Using Phosphorescence, Absorption and RLS Spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 871–877. [Google Scholar] [CrossRef]
- Carmona, T.; Pineiro, M.; Monteiro, C.J.P.; Pereira, M.M.; Valente, A.J.M. Interactions between Cationic Surfactants and 5,10,15,20-Tetrakis(4-Sulfonatophenyl)Porphyrin Tetrasodium Salt as Seen by Electric Conductometry and Spectroscopic Techniques. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 288–296. [Google Scholar] [CrossRef]
- Yaffe, O.; Korin, E.; Bettelheim, A. Interaction of Fe(III) Tetrakis(4-N-Methylpyridinium)Porphyrin with Sodium Dodecyl Sulfate at Submicellar Concentrations. Langmuir 2008, 24, 11514–11517. [Google Scholar] [CrossRef]
- Roldán-Carmona, C.; González-Delgado, A.M.; Guerrero-Martínez, A.; De Cola, L.; Giner-Casares, J.J.; Pérez-Morales, M.; Martín-Romero, M.T.; Camacho, L. Molecular Organization and Effective Energy Transfer in Iridium Metallosurfactant-Porphyrin Assemblies Embedded in Langmuir-Schaefer Films. Phys. Chem. Chem. Phys. 2011, 13, 2834–2841. [Google Scholar] [CrossRef]
- González-Delgado, A.M.; Pérez-Morales, M.; Giner-Casares, J.J.; Muũoz, E.; Martín-Romero, M.T.; Camacho, L. Reversible Collapse of Insoluble Monolayers: New Insights on the Influence of the Anisotropic Line Tension of the Domain. J. Phys. Chem. B 2009, 113, 13249–13256. [Google Scholar] [CrossRef]
- Pérez-Morales, M.; Pedrosa, J.M.; Martín-Romero, M.T.; Möbius, D.; Camacho, L. Reversible Trilayer Formation at the Air-Water Interface from a Mixed Monolayer Containing a Cationic Lipid and an Anionic Porphyrin. J. Phys. Chem. B 2004, 108, 4457–4465. [Google Scholar] [CrossRef] [Green Version]
- El-Hachemi, Z.; Mancini, G.; Ribó, J.M.; Sorrenti, A. Role of the Hydrophobic Effect in the Transfer of Chirality from Molecules to Complex Systems: From Chiral Surfactants to Porphyrin/Surfactant Aggregates. J. Am. Chem. Soc. 2008, 130, 15176–15184. [Google Scholar] [CrossRef] [PubMed]
- Pradines, V.; Bijani, C.; Stigliani, J.L.; Blanzat, M.; Rico-Lattes, I.; Pratviel, G. Cationic Porphyrin-Anionic Surfactant Mixtures for the Promotion of Self-Organized 1:4 Ion Pairs in Water with Strong Aggregation Properties. Chem. Phys. Chem. 2015, 16, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Durgo, K.; Halec, I.; Šola, I.; Franekić, J. Cytotoxic and Genotoxic Effects of the Quercetin/Lanthanum Complex on Human Cervical Carcinoma Cells in Vitro. Arh. Hig. Rada Toksikol. 2011, 62, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Lan, Z.; Sun, X.; Shi, L.; Liu, Q.; Ni, J. Proteomic Analysis of Lanthanum Citrate-Induced Apoptosis in Human Cervical Carcinoma SiHa Cells. BioMetals 2010, 23, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S. Lanthanum and Its Rapidly Emerging Role as an Anti-Carcinogenic Agent. J. Cell. Biochem. 2009, 106, 193. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Huang, Z. Proteomic Detection of Changes in Protein Synthesis Induced by Lanthanum in BGC-823 Human Gastric Cancer Cells. BioMetals 2005, 18, 89–95. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Ziganshina, A.; Sergeeva, T.; Lukashenko, S.; Sapunova, A.; Voloshina, A.; Kashapova, N.; Nizameev, I.; Salnikov, V.; et al. Supraamphiphilic Systems Based on Metallosurfactant and Calix[4]Resorcinol: Self-Assembly and Drug Delivery Potential. Inorg. Chem. 2020, 59, 18276–18286. [Google Scholar] [CrossRef]
- Lottner, C.; Knuechel, R.; Bernhardt, G.; Brunner, H. Combined Chemotherapeutic and Photodynamic Treatment on Human Bladder Cells by Hematoporphyrin-Platinum(II) Conjugates. Cancer Lett. 2004, 203, 171–180. [Google Scholar] [CrossRef]
- Zhiltsova, E.P.; Ibatullina, M.R.; Lukashenko, S.S.; Valeeva, F.G.; Pashirova, T.N.; Kutyreva, M.P.; Zakharova, L.Y. Complexes of 1-Hexadecyl-4-Aza-1-Azoniabicyclo[2.2.2]Octane Bromide with Transition Metal Nitrates. Micelle-Forming, Solubilizing, and Adsorption Properties. Colloid J. 2017, 79, 621–629. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Kashapov, R.R.; Zhil’Tsova, E.P.; Gaisin, N.K.; Gnezdilov, O.I.; Konov, A.B.; Lukashenko, S.S.; Magdeev, I.M. Catalytic Properties of Micellar Systems Based on 4-Aza-1-Alkyl-1- Azoniabicyclo[2.2.2]Octane Bromides. Kinet. Catal. 2011, 52, 179–185. [Google Scholar] [CrossRef]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. In Macro to Nano Spectroscopy; IntechOpen: London, UK, 2012; pp. 87–108. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Ma, R.; Li, J.; Li, Y.; An, Y.; Shi, L. J- and H-Aggregates of 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)-Porphyrin and Interconversion in PEG-b-P4VP Micelles. Biomacromolecules 2008, 9, 2601–2608. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Gelamo, E.L.; Itri, R.; Tabak, M. Small Angle X-ray Scattering Study of Meso-Tetrakis (4-Sulfonatophenyl) Porphyrin in Aqueous Solution: A Self-Aggregation Model. Biophys. J. 2003, 85, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Kiss, M.P.; Valicsek, Z.; Horváth, O. Formation, Photophysics, and Photochemistry of Anionic Lanthanide(III) Mono- And Bisporphyrins. Molecules 2019, 24, 1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheinin, V.B.; Kulikova, O.M.; Koifman, O.I. Invertion and Methylation of Pyrrole Ring in Tetrasulfophenylporphyrin: Basicity, Aggregation Properties, Chirality. J. Mol. Liq. 2019, 277, 397–408. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Wei, Y.; Duan, M.; Chen, X.; Hu, Y. Transformation of H-Aggregates and J-Dimers of Water-Soluble Tetrakis (4-Carboxyphenyl) Porphyrin in Polyion Complex Micelles. Polymers 2018, 10, 494. [Google Scholar] [CrossRef] [Green Version]
- Cabrer, A.; Ribó, J.M.; El-Hachemi, Z.; Crusats, J. 5,10,15,20-Tetrasulfonatophenylporphyrin Regioisomers: How the Location of the Sulfonato Groups Determines the Formation of Their Supramolecular Aggregates. J. Porphyr. Phthalocyanines 2015, 19, 852–857. [Google Scholar] [CrossRef]
- Egawa, Y.; Hayashida, R.; Anzai, J.I. PH-Induced Interconversion between J-Aggregates and H-Aggregates of 5,10,15,20-Tetrakis(4-Sulfonatophenyl)Porphyrin in Polyelectrolyte Multilayer Films. Langmuir 2007, 23, 13146–13150. [Google Scholar] [CrossRef]
- Flores, M.E.; Sano, N.; Araya-Hermosilla, R.; Shibue, T.; Olea, A.F.; Nishide, H.; Moreno-Villoslada, I. Self-Association of 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)-Porphyrin Tuned by Poly(Decylviologen) and Sulfobutylether-β-Cyclodextrin. Dye. Pigment. 2015, 112, 262–273. [Google Scholar] [CrossRef]
- Pino-Pinto, J.P.; Oyarzun-Ampuero, F.; Orellana, S.L.; Flores, M.E.; Nishide, H.; Moreno-Villoslada, I. Aerogels Containing 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)-Porphyrin with Controlled State of Aggregation. Dye. Pigment. 2017, 139, 193–200. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. Controlled J-Aggregation of Porphyrins by Cationic Surfactants. Curr. Sci. 1996, 70, 997–999. [Google Scholar]
- Gandini, S.C.M.; Yushmanov, V.E.; Tabak, M. Interaction of Fe(III)- and Zn(II)-Tetra(4-Sulfonatophenyl) Porphyrins with Ionic and Nonionic Surfactants: Aggregation and Binding. J. Inorg. Biochem. 2001, 85, 263–277. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Yushmanov, V.E.; Borissevitch, I.E.; Tabak, M. Interaction of the Tetra(4-Sulfonatophenyl)Porphyrin with Ionic Surfactants: Aggregation and Location in Micelles. Langmuir 1999, 15, 6233–6243. [Google Scholar] [CrossRef]
- Kadish, K.M.; Maiya, G.B.; Araullo, C.; Guilard, R. Micellar Effects on the Aggregation of Tetraanionic Porphyrins. Spectroscopic Characterization of Free-Base Meso-Tetrakis(4-Sulfonatophenyl)Porphyrin, (TPPS)H2, and (TPPS)M (M = Zinc(II), Copper(II), and Vanadyl) in Aqueous Micellar Media. Inorg. Chem. 1989, 28, 2725–2731. [Google Scholar] [CrossRef]
- Gjuroski, I.; Furrer, J.; Vermathen, M. Probing the Interactions of Porphyrins with Macromolecules Using NMR Spectroscopy Techniques. Molecules 2021, 26, 1942. [Google Scholar] [CrossRef]
- Murphy, R.B.; Pham, D.T.; Johnston, M.R. A Tetra-Porphyrin Host Exhibiting Interannular Cooperativity. Chem. A Eur. J. 2019, 25, 13037–13043. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Koch, A.; Seidl, P.R. Visualization and Quantification of the Anisotropic Effect of C=C Double Bonds on 1H NMR Spectra of Highly Congested Hydrocarbons-Indirect Estimates of Steric Strain. J. Phys. Chem. A 2008, 112, 4989–4995. [Google Scholar] [CrossRef]
- Momenteau, M.; Mispelter, J.; Loock, B.; Bisagni, E. Both-Faces Hindered Porphyrins. Part 1. Synthesis and Characterization of Basket-Handle Porphyrins and Their Iron Complexes. J. Chem. Soc. Perkin Trans. 1 1983, 189–196. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Ramakrishna, B.; Venkatesan, R.; Almeida Paz, F.A.; Tomé, J.P.C. Facile Synthesis of Highly Stable BF3-Induced Meso-Tetrakis (4-Sulfonato Phenyl) Porphyrin (TPPS4)-J-Aggregates: Structure, Photophysical and Electrochemical Properties. New J. Chem. 2013, 37, 3745. [Google Scholar] [CrossRef]
- Wishart, D.S.; Bigam, C.G.; Yao, J.; Abildgaard, F.; Dyson, H.J.; Oldfield, E.; Markley, J.L.; Sykes, B.D. 1H, 13C and 15N Chemical Shift Referencing in Biomolecular NMR. J. Biomol. NMR 1995, 6, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Stilbs, P. Fourier Pulsed-Gradient Studies of Molecular Diffusion. Prog. NMR Spectrosc. 1987, 19, 1–45. [Google Scholar] [CrossRef]
- Chen, H.; Pazicni, S.; Krett, N.L.; Ahn, R.W.; Penner-Hahn, J.E.; Rosen, S.T.; O’Halloran, T.V. Coencapsulation of Arsenic-and Platinum-Based Drugs for Targeted Cancer Treatment. Angew. Chemie Int. Ed. 2009, 48, 9295–9299. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shtemenko, N.I.; Yegorova, D.Y.; Babiy, S.O.; Brown, A.J.; Yang, T.; Shtemenko, A.V.; Dunbar, K.R. Liposomes Loaded with a Dirhenium Compound and Cisplatin: Preparation, Properties and Improved in Vivo Anticancer Activity. J. Liposome Res. 2015, 25, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.V.; Richard, A.J.; Vicente, M.G.H.; Russo, P.S. Characterization of the Self-Assembly of Meso-Tetra(4-Sulfonatophenyl)Porphyrin (H2TPPS4–) in Aqueous Solutions. Biomacromolecules 2012, 13, 60–72. [Google Scholar] [CrossRef]
- Valicsek, Z.; Horváth, O. Application of the Electronic Spectra of Porphyrins for Analytical Purposes: The Effects of Metal Ions and Structural Distortions. Microchem. J. 2013, 107, 47–62. [Google Scholar] [CrossRef] [Green Version]
- Dechan, P.; Bajju, G.D. Synthesis and Spectroscopic Properties of Axial Phenoxide and Para Amino Phenoxide Incorporated Indium (III) Porphyrins. J. Mol. Struct. 2019, 1195, 140–152. [Google Scholar] [CrossRef]
- Andrianov, D.S.; Levitskiy, O.A.; Rybakov, V.B.; Magdesieva, T.V.; Cheprakov, A.V. Metal Complexes of Diaryltetrabenzodiazaporphyrins. ChemistrySelect 2016, 1, 360–374. [Google Scholar] [CrossRef]
- Sarkar, S.; Sruthi, P.K.; Ramanathan, N.; Sundararajan, K. Experimental Evidence of N–H⋯N Hydrogen Bonding in the Heterodimers of Pyrrole with Nitrogen Bases. J. Mol. Struct. 2020, 1204, 127511. [Google Scholar] [CrossRef]
- Záruba, K.; Králová, J.; Øezanka, P.; Pouèková, P.; Veverková, L.; Král, V. Modified Porphyrin-Brucine Conjugated to Gold Nanoparticles and Their Application in Photodynamic Therapy. Org. Biomol. Chem. 2010, 8, 3202–3206. [Google Scholar] [CrossRef]



| System | Ds (TSPP), 10−10 m2 s−1 | Ds (LaSurf), 10−10 m2 s−1 |
|---|---|---|
| Pure solutions | 3.35 (0.1 mM) | 4.62 (0.3 mM), 1.44 (1 mM) |
| 0.1 mM TSPP–0.3 mM LaSurf | 0.82 | 1.31 |
| 0.1 mM TSPP–0.4 mM LaSurf | 0.80 | 1.46 |
| 0.1 mM TSPP–1 mM LaSurf | 0.80 | 1.36 |
| System | IC50, μM | |
|---|---|---|
| Chang Liver | M-HeLa | |
| cisplatin [49] | 900 ± 71 | 60 ± 4.8 |
| LaSurf:cisplatin = 3:200 [49] | 900 ± 69 | 60 ± 5.1 |
| TSPP:LaSurf:cisplatin = 1:2:1 | 2.3 ± 0.11 | 1.85 ± 0.09 |
| TSPP:LaSurf:cisplatin = 1:3:1 | 2.6 ± 0.13 | 2.1 ± 0.11 |
| TSPP:LaSurf:cisplatin = 1:3:10 | 20.7 ± 1.00 | 4.0 ± 0.20 |
| TSPP:LaSurf:cisplatin = 1:3:25 | 12.0 ± 0.60 | 7.1 ± 0.36 |
| TSPP:LaSurf:cisplatin = 1:3:50 | 21.1 ± 1.05 | 15.0 ± 0.75 |
| TSPP:LaSurf:cisplatin = 1:3:100 | 28.0 ± 1.40 | 11.0 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashapov, R.R.; Razuvayeva, Y.S.; Lukashenko, S.S.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Syakaev, V.V.; Salnikov, V.V.; Zakharova, L.Y. Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design. Nanomaterials 2022, 12, 1986. https://doi.org/10.3390/nano12121986
Kashapov RR, Razuvayeva YS, Lukashenko SS, Amerhanova SK, Lyubina AP, Voloshina AD, Syakaev VV, Salnikov VV, Zakharova LY. Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design. Nanomaterials. 2022; 12(12):1986. https://doi.org/10.3390/nano12121986
Chicago/Turabian StyleKashapov, Ruslan R., Yuliya S. Razuvayeva, Svetlana S. Lukashenko, Syumbelya K. Amerhanova, Anna P. Lyubina, Alexandra D. Voloshina, Victor V. Syakaev, Vadim V. Salnikov, and Lucia Y. Zakharova. 2022. "Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design" Nanomaterials 12, no. 12: 1986. https://doi.org/10.3390/nano12121986
APA StyleKashapov, R. R., Razuvayeva, Y. S., Lukashenko, S. S., Amerhanova, S. K., Lyubina, A. P., Voloshina, A. D., Syakaev, V. V., Salnikov, V. V., & Zakharova, L. Y. (2022). Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design. Nanomaterials, 12(12), 1986. https://doi.org/10.3390/nano12121986







