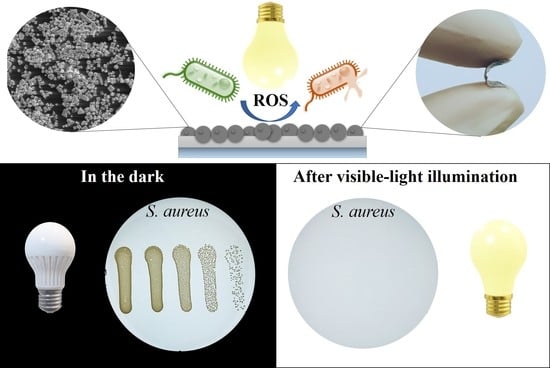
Visible-Light Active Flexible and Durable Photocatalytic Antibacterial Ethylene-co-vinyl Acetate—Ag/AgCl/α-Fe2O3 Composite Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Ag/AgCl/α-Fe2O3 Composite
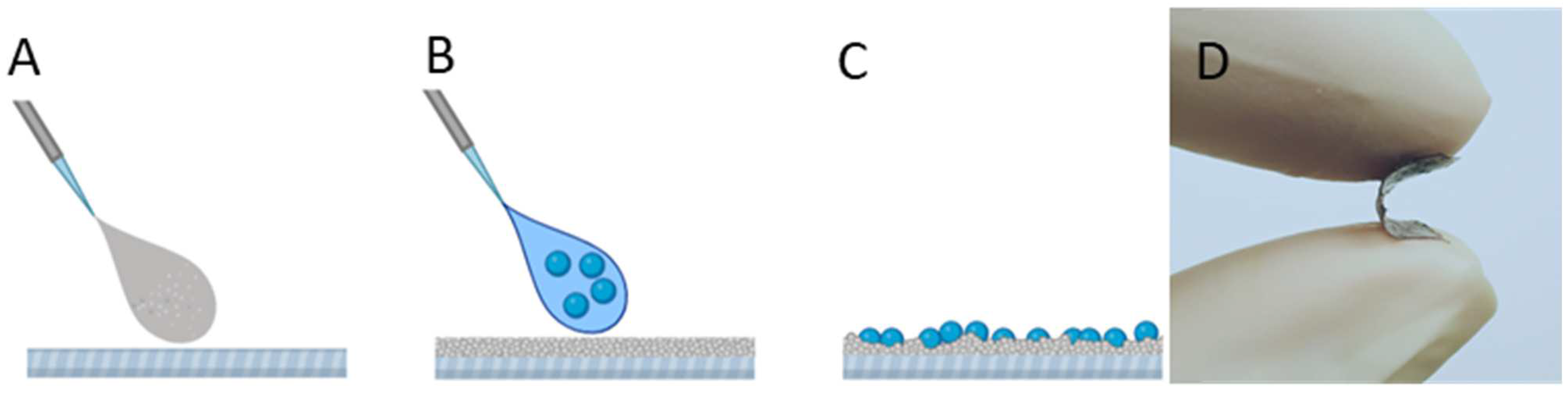
2.3. Ethylene-co-vinyl Acetate (EVA) Polymer Coating Preparation
2.4. EVA-Ag/AgCl/α-Fe2O3 Coating Preparation
2.5. Physicochemical Characterization of the EVA-Ag/AgCl/α-Fe2O3 Composite Coating
2.6. Evaluation of Antibacterial Efficacy of Photocatalytic EVA-Ag/AgCl/α-Fe2O3 Composite Coating
2.7. Analysis and Quantification of Ionic Ag Release from the Surface
2.8. Abiotic ROS Measurement
2.9. Cell Staining and Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussion
3.1. Characterization of EVA- Ag/AgCl/α-Fe2O3 Composite Coatings
3.2. Ionic Ag Release from the Surface in 1:500 Nutriend Broth (NB)
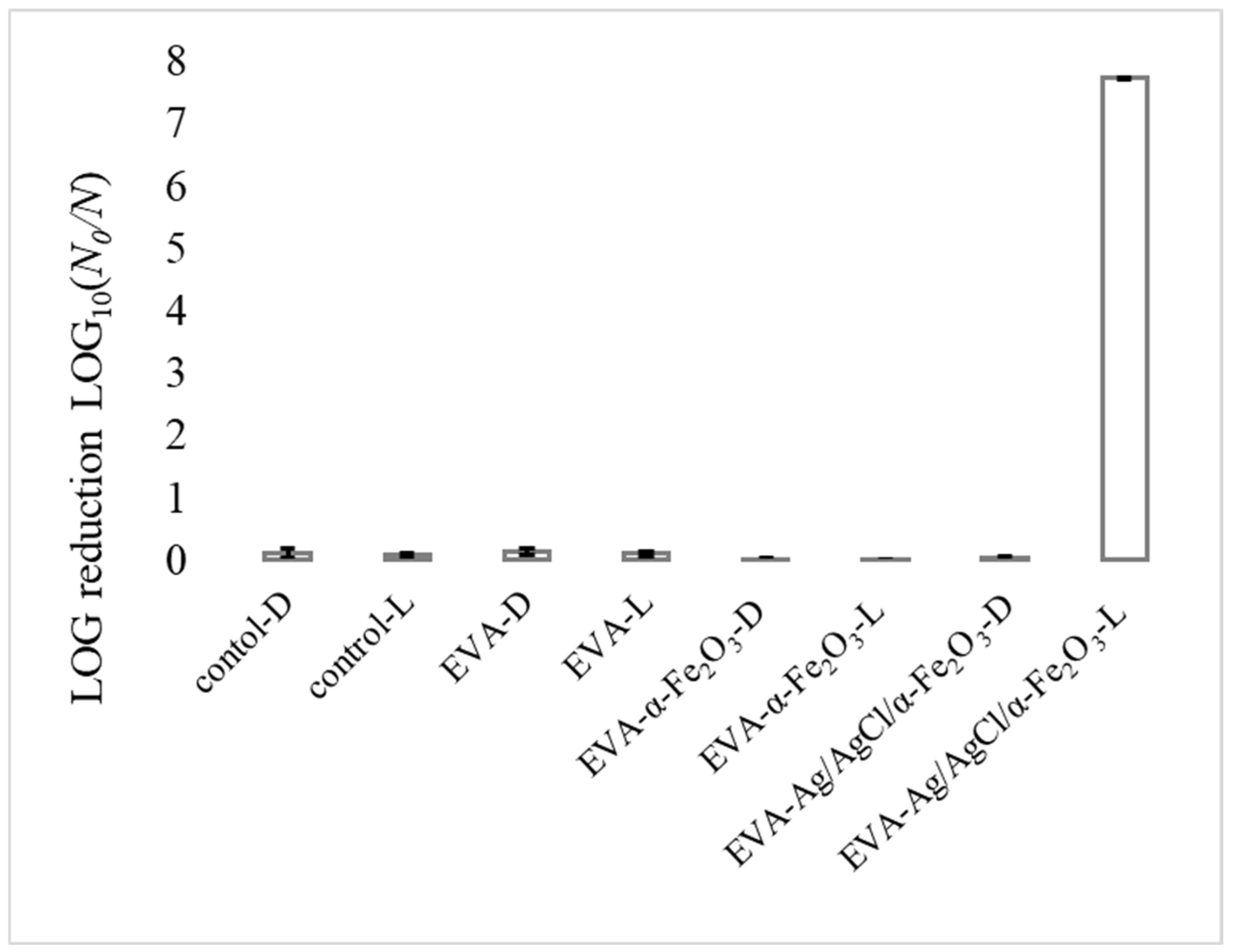
3.3. Photocatalytic Antibacterial Test Results
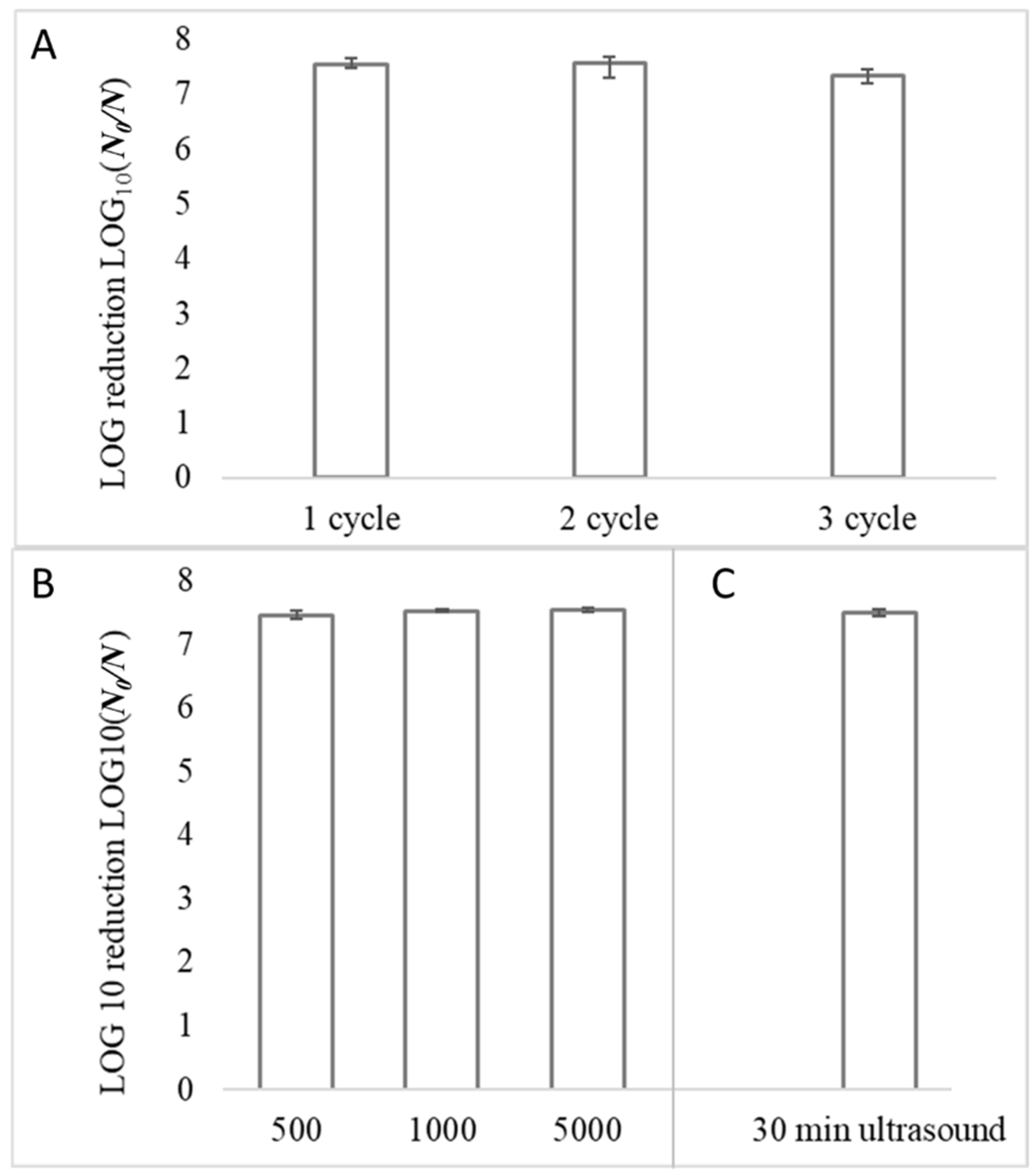
3.4. Reusability of The Photocatalytic Coatings
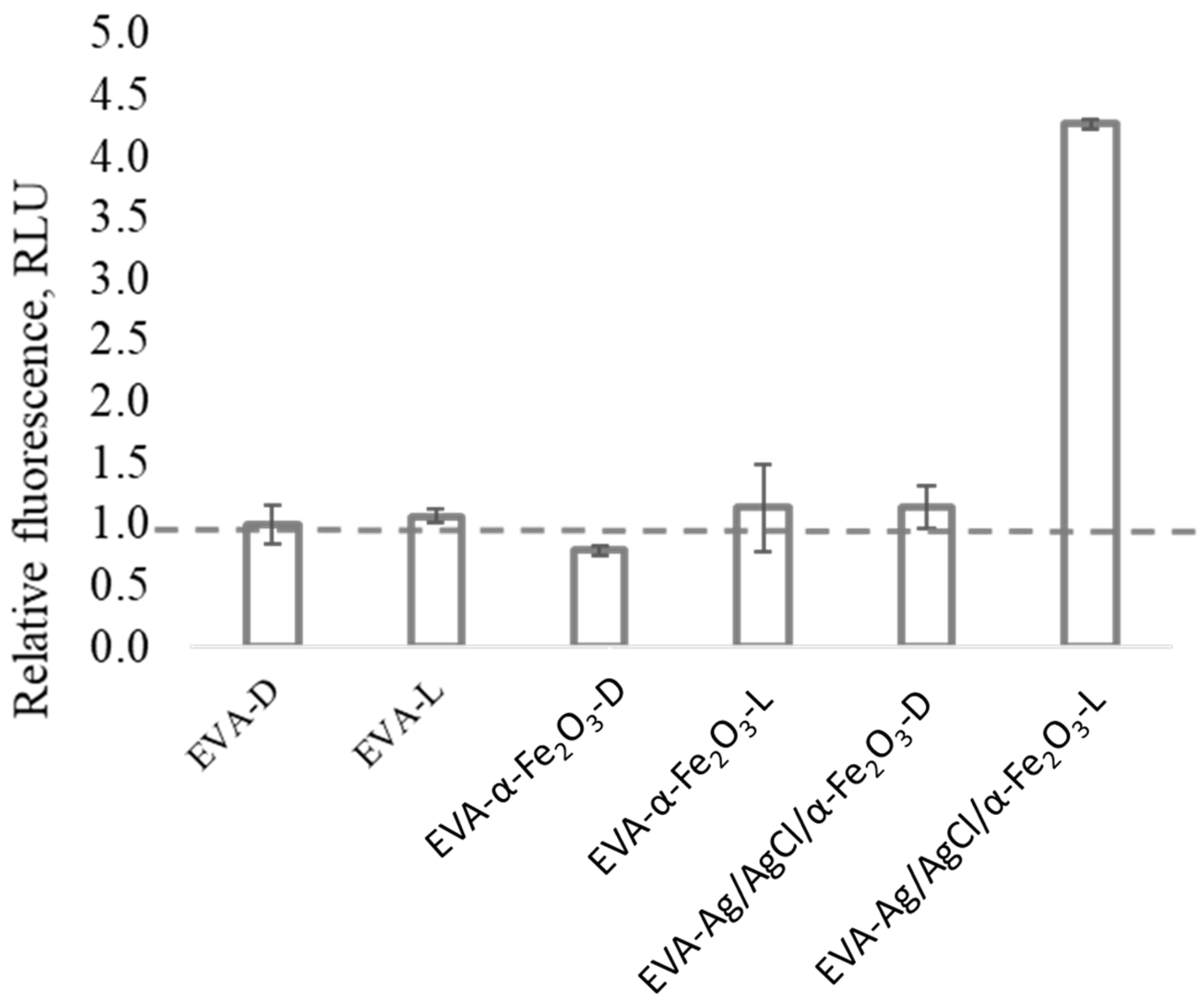
3.5. Abiotic ROS Measurement
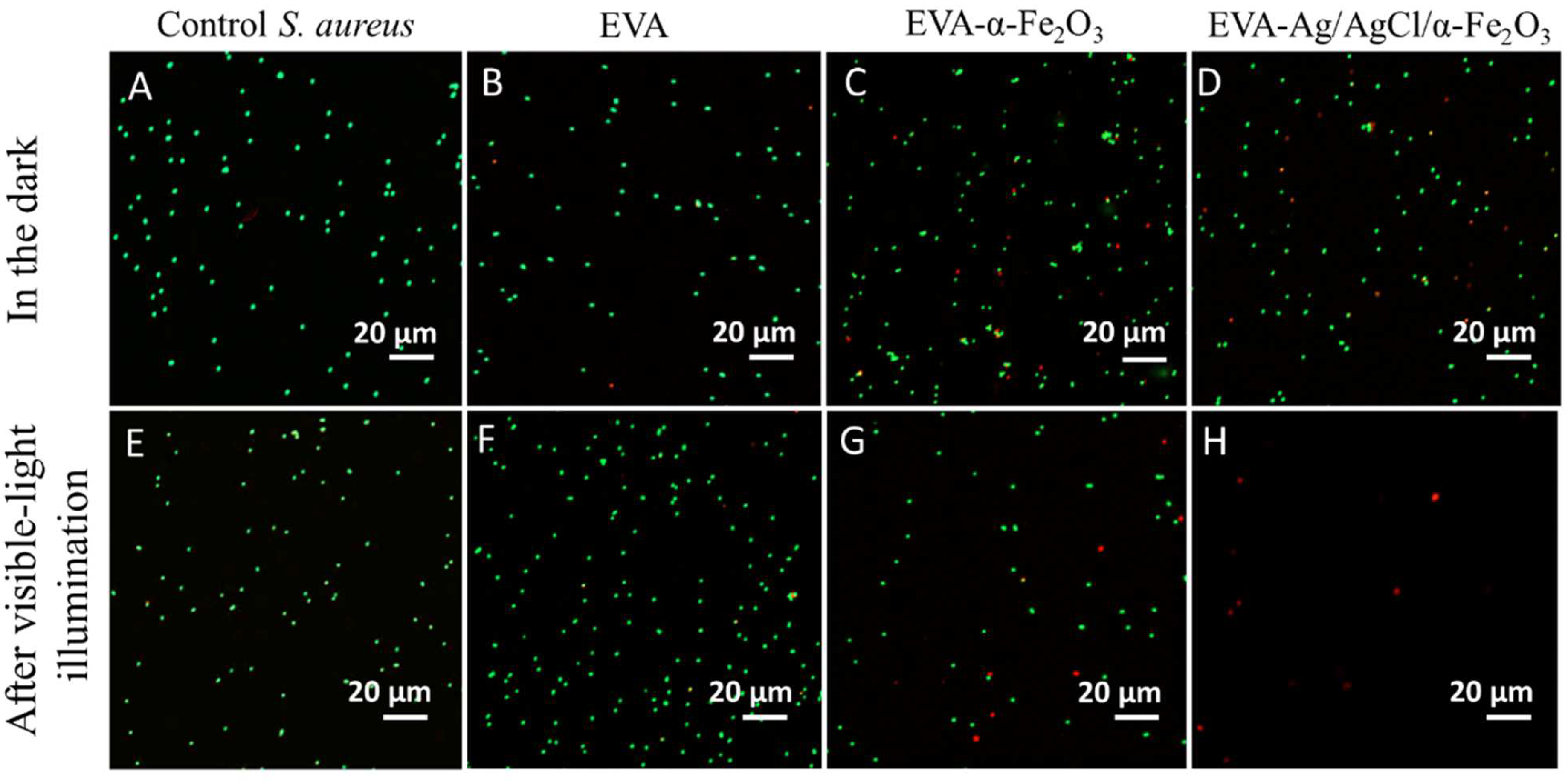
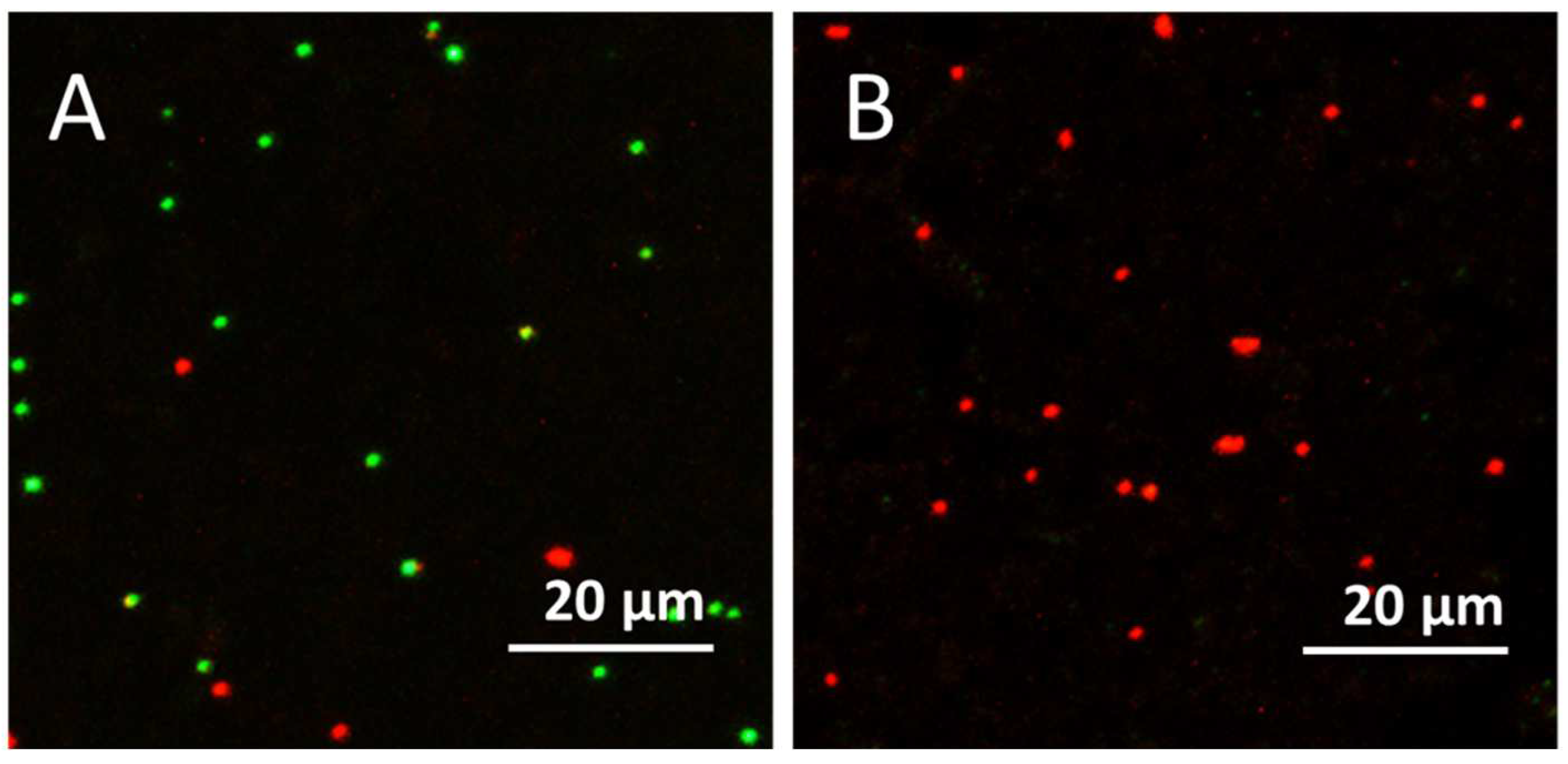
3.6. Confocal Laser Scanning Microscopy (CLSM) Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yemmireddy, V.K.; Hung, Y.-C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety-Opportunities and Challenges. Compr. Rev. Food Sci. 2017, 16, 617–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, M.; Visnapuu, M.; Saal, K.; Danilian, D.; Pärna, R.; Ivask, A.; Kisand, V. Preparation and Characterization of Photocatalytically Active Antibacterial Surfaces Covered with Acrylic Matrix Embedded Nano-ZnO and Nano-ZnO/Ag. Nanomaterials 2021, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic Antibacterial Activity of Nano-TiO2 (Anatase)-Based Thin Films: Effects on Escherichia Coli Cells and Fatty Acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nõmmiste, E.; Šutka, A.; Kahru, A.; Rähn, M.; Vija, H.; Orupõld, K.; Kisand, V.; et al. UVA-induced antimicrobial activity of ZnO/Ag nanocomposite covered surfaces. Colloids Surf. B Biointerfaces 2018, 169, 222–232. [Google Scholar] [CrossRef]
- Ramsden, J.J. Photocatalytic antimicrobial coatings. Nanotechnol. Percept. 2015, 11, 146–168. [Google Scholar] [CrossRef]
- Valenzuela, L.; Iglesias-Juez, A.; Bachiller-Baeza, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Biocide mechanism of highly efficient and stable antimicrobial surfaces based on zinc oxide–reduced graphene oxide photocatalytic coatings. J. Mater. Chem. B 2020, 8, 8294–8304. [Google Scholar] [CrossRef]
- Nistico, R.; Scalarone, D.; Magnacca, G. Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Micropor. Mesopor. Mater. 2017, 248, 18–29. [Google Scholar] [CrossRef]
- Uzum, A.; Fukatsu, K.; Kanda, H.; Kimura, Y.; Tanimoto, K.; Yoshinaga, S.; Jiang, Y.; Ishikawa, Y.; Uraoka, Y.; Ito, S. Silica-sol-based spin-coating barrier layer against phosphorous diffusion for crystalline silicon solar cells. Nanoscale Res. Lett. 2014, 9, 659. [Google Scholar] [CrossRef] [Green Version]
- Tham, D.Q.; Tuan, V.M.; Thanh, D.T.M.; Chinh, N.T.; Giang, N.V.; Trang, N.T.T.; Hang, T.T.X.; Huong, H.T.; Dung, N.T.K.; Hoang, T. Preparation and Properties of Ethylene Vinyl Acetate Copolymer/Silica Nanocomposites in Presence of EVA-g-Acrylic Acid. J. Nanosci. Nanotechnol. 2015, 15, 2777–2784. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. Engl. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of Highly Efficient Ag@AgCl Plasmonic Photocatalysts with Various Structures. Chem.—A Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef]
- Kang, S.; Fang, Y.; Huang, Y.; Cui, L.-F.; Wang, Y.; Qin, H.; Zhang, Y.; Li, X.; Wang, Y. Critical influence of g-C3N4 self-assembly coating on the photocatalytic activity and stability of Ag/AgCl microspheres under visible light. Appl. Catal. B Environ. 2015, 168–169, 472–482. [Google Scholar] [CrossRef]
- Asif, A.H.; Wang, S.; Sun, H. Hematite-based nanomaterials for photocatalytic degradation of pharmaceuticals and personal care products (PPCPs): A short review. Curr. Opin. Green Sustain. Chem. 2021, 28, 100447. [Google Scholar] [CrossRef]
- Šutka, A.; Šutka, A.; Vanags, M.; Spule, A.; Eglītis, R.; Vihodceva, S.; Šmits, K.; Tamm, A.; Mežule, L. Identifying Iron-Bearing Nanoparticle Precursor for Thermal Transformation into the Highly Active Hematite Photo-Fenton Catalyst. Catalysts 2020, 10, 778. [Google Scholar] [CrossRef]
- Chang, N.; Chen, Y.-R.; Xie, F.; Liu, Y.-P.; Wang, H.-T. A promising Z-scheme heterojunction via loading Ag/AgCl into porous Co3O4 derived from ZIF-67 for visible light driven photocatalysis. Micropor. Mesopor. Mater. 2020, 307, 110530. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A-Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S.; Xie, M.; Li, Y.; Jing, L.; Xu, H.; Zhang, Q.; Li, H. Core–shell magnetic Ag/AgCl@Fe2O3 photocatalysts with enhanced photoactivity for eliminating bisphenol A and microbial contamination. New J. Chem. 2016, 40, 3413–3422. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Zhang, L. New Insight into Daylight Photocatalysis of AgBr@Ag: Synergistic Effect between Semiconductor Photocatalysis and Plasmonic Photocatalysis. Chem.–A Eur. J. 2012, 18, 6360–6369. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, X.; Cheng, Z.; Liao, Y.; Wang, Z.; Pu, Q.; Duan, M. Synthesis of Pp-16@Ag/AgCl of high performance photocatalyst particles for decomposition of Rhodamine B and fast green dyes. Mater. Chem. Phys. 2018, 218, 98–107. [Google Scholar] [CrossRef]
- Ai, C.; Wu, S.; Li, L.; Lei, Y.; Shao, X. Novel magnetically separable γ-Fe2O3/Ag/AgCl/g-C3N4 composite for enhanced disinfection under visible light. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 583, 123981. [Google Scholar] [CrossRef]
- Šutka, A.; Järvekülg, M.; Gross, A.K.; Kook, M.; Käämbre, T.; Visnapuu, M.; Trefalt, G.; Šutka, A. Visible light to switch-on desorption from goethite. Nanoscale 2019, 11, 3794. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Wang, P.; Zhu, C.; Zhai, Y.; Dong, S. Facile solvothermal synthesis of cube-like Ag@AgCl: A highly efficient visible light photocatalyst. Nanoscale 2011, 3, 2931. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Pfanner, K.; Gfeller, N.; Calzaferri, G. Photochemical oxidation of water with thin AgCl layers. J. Photochem. Photobiol. A Chem. 1996, 95, 175–180. [Google Scholar] [CrossRef]
- Suppi, S.; Kasemets, K.; Ivask, A.; Künnis-Beres, K.; Sihtmäe, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeast and algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Che, H.; Zhang, X.; Han, J.; Mao, Y.; Wang, Y.; Liu, C.; Dong, H. Fabricationof Z-Scheme heterojunction by anchoring mesoporous γ-Fe2O3 nanospheres on g-C3N4 for degrading tetracycline hydrochloride in water. ACS Sustain. Chem. Eng. 2018, 6, 16437–16447. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vihodceva, S.; Šutka, A.; Otsus, M.; Vija, H.; Grase, L.; Kahru, A.; Kasemets, K. Visible-Light Active Flexible and Durable Photocatalytic Antibacterial Ethylene-co-vinyl Acetate—Ag/AgCl/α-Fe2O3 Composite Coating. Nanomaterials 2022, 12, 1984. https://doi.org/10.3390/nano12121984
Vihodceva S, Šutka A, Otsus M, Vija H, Grase L, Kahru A, Kasemets K. Visible-Light Active Flexible and Durable Photocatalytic Antibacterial Ethylene-co-vinyl Acetate—Ag/AgCl/α-Fe2O3 Composite Coating. Nanomaterials. 2022; 12(12):1984. https://doi.org/10.3390/nano12121984
Chicago/Turabian StyleVihodceva, Svetlana, Andris Šutka, Maarja Otsus, Heiki Vija, Liga Grase, Anne Kahru, and Kaja Kasemets. 2022. "Visible-Light Active Flexible and Durable Photocatalytic Antibacterial Ethylene-co-vinyl Acetate—Ag/AgCl/α-Fe2O3 Composite Coating" Nanomaterials 12, no. 12: 1984. https://doi.org/10.3390/nano12121984