In Vitro High-Throughput Toxicological Assessment of Nanoplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical and Morphological Characterization of NPs
2.1.1. NPs Fabrication
2.1.2. Hydrodynamic Diameter (DH) Distribution and Morphological Characterization
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.2. Cell Culture Conditions
2.3. Cytotoxicity Evaluation by LDH and MTT Assay
2.4. High Content Screening (HCS)
2.4.1. Cell Culture and NPs Exposure
2.4.2. HCS Assay: Incubation of Fluorescent Staining and Imaging
2.4.3. HCS Data Analysis
2.5. Electric Cell-Substrate Impedance Sensing (ECIS) Technology
2.6. Statistical Analysis
3. Results
3.1. NP Physicochemical Characterization
3.2. Toxicological Assessment
3.3. High-Content Screening (HCS)
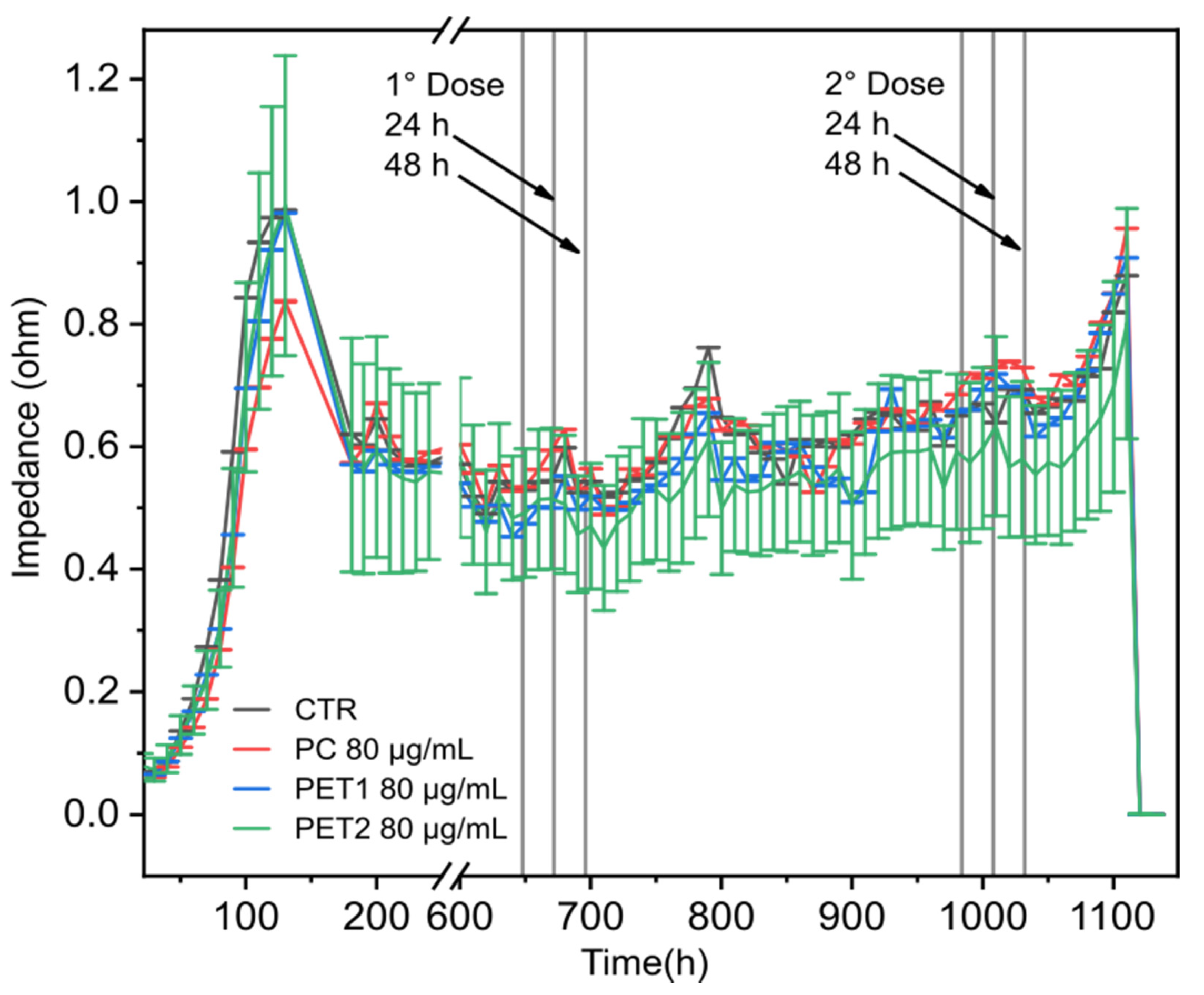
3.4. Electric Cell-Substrate Impedance Sensing (ECIS) Technology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Plastic Europe—Association of Plastics Manufactures. Plastics—The Facts 2021. Available online: file:///C:/Users/MDPI/Downloads/Plastics-the-Facts-2021-web-final.pdf (accessed on 1 June 2022).
- Borrelle, S.B.; Ringma, J.; Lavender Law, K.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted Growth in Plastic Waste Exceeds Efforts to Mitigate Plastic Pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef] [PubMed]
- Wayman, C.; Niemann, H. The Fate of Plastic in the Ocean Environment—A Minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Hasselerharm, P.E.; Gort, G.; Peeters, E.T.H.M.; Koelmans, A.A. Nano- and Microplastics Affect the Composition of Freshwater Benthic Communities in the Long Term. Sci. Adv. 2020, 6, eaay4054. [Google Scholar] [CrossRef] [Green Version]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First Study of Its Kind on the Microplastic Contamination of Soft Drinks, Cold Tea and Energy Drinks—Future Research and Environmental Considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating Scenarios toward Zero Plastic Pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Arp, H.P.H.; Kühnel, D.; Rummel, C.; Macleod, M.; Potthoff, A.; Reichelt, S.; Rojo-Nieto, E.; Schmitt-Jansen, M.; Sonnenberg, J.; Toorman, E.; et al. Weathering Plastics as a Planetary Boundary Threat: Exposure, Fate, and Hazards. Environ. Sci. Technol. 2021, 55, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO) Plastics—Environmental Aspects—State of Knowledge and Methodologies. Available online: https://www.iso.org/standard/72300.html (accessed on 16 March 2022).
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in Wild Fish from North East Atlantic Ocean and Its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Ockenden, A.; Tremblay, L.A.; Dikareva, N.; Simon, K.S. Towards More Ecologically Relevant Investigations of the Impacts of Microplastic Pollution in Freshwater Ecosystems. Sci. Total Environ. 2021, 792, 148507. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene Nanoplastics Alter the Cytotoxicity of Human Pharmaceuticals on Marine Fish Cell Lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65. [Google Scholar] [CrossRef]
- Anguissola, S.; Garry, D.; Salvati, A.; O’Brien, P.J.; Dawson, K.A. High Content Analysis Provides Mechanistic Insights on the Pathways of Toxicity Induced by Amine-Modified Polystyrene Nanoparticles. PLoS ONE 2014, 9, 108025. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic Effects of Polystyrene Nanoplastics with Different Surface Functionalization on Human HepG2 Cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal Efficiency of Micro- and Nanoplastics (180 Nm–125 Μm) during Drinking Water Treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Déniel, M.; Nicolai, T.; Chassenieux, C.; Lagarde, F. Towards More Realistic Reference Microplastics and Nanoplastics: Preparation of Polyethylene Micro/Nanoparticles with a Biosurfactant. Environ. Sci. Nano 2019, 6, 315–324. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Beltzung, A.; Frehland, S.; Schmiedgruber, M.; Cingolani, A.; Schmidt, F. Synthesis of Metal-Doped Nanoplastics and Their Utility to Investigate Fate and Behaviour in Complex Environmental Systems. Nat. Nanotechnol. 2019, 14, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Pessoni, L.; Veclin, C.; El Hadri, H.; Cugnet, C.; Davranche, M.; Pierson-Wickmann, A.C.; Gigault, J.; Grassl, B.; Reynaud, S. Soap- and Metal-Free Polystyrene Latex Particles as a Nanoplastic Model. Environ. Sci. Nano 2019, 6, 2253–2258. [Google Scholar] [CrossRef]
- Blancho, F.; Davranche, M.; Fumagalli, F.; Ceccone, G.; Gigault, J. A Reliable Procedure to Obtain Environmentally Relevant Nanoplastic Proxies. Environ. Sci. Nano 2021, 8, 3211–3219. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool to Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Veronesi, M.; Sánchez-Moreno, P.; Tolardo, V.; Bandiera, T.; Pompa, P.P.; Athanassiou, A.; Fragouli, D. PET Nanoplastics Interactions with Water Contaminants and Their Impact on Human Cells. Environ. Pollut. 2020, 271, 116262. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and Nano-Plastics in Marine Environment: Source, Distribution and Threats—A Review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Diepens, M.; Gijsman, P. Outdoor and Accelerated Weathering Studies of Bisphenol A Polycarbonate. Polym. Degrad. Stab. 2011, 96, 649–652. [Google Scholar] [CrossRef] [Green Version]
- Garvey, C.J.; Impéror-Clerc, M.; Rouzière, S.; Gouadec, G.; Boyron, O.; Rowenczyk, L.; Mingotaud, A.F.; Ter Halle, A. Molecular-Scale Understanding of the Embrittlement in Polyethylene Ocean Debris. Environ. Sci. Technol. 2020, 54, 11173–11181. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [CrossRef] [Green Version]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of Polyethylene Terephthalate (PET) Nanoparticles with Fluorescent Tracers for Studies in Mammalian Cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- ISO/TR 10993–22:2017; Biological Evaluation of Medical Devices—Part 22: Guidance on Nanomaterials. International Organization for Standardization (ISO). 2017. Available online: https://www.iso.org/standard/65918.html (accessed on 1 June 2022).
- ASTM E2526; Standard Test Method for Evaluation of Cytotoxicity of Nanoparticulate Materials in Porcine Kidney Cells and Human Hepatocarcinoma Cells. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2013; pp. 4–9. Available online: https://www.astm.org/e2526-08r13.html (accessed on 1 June 2022).
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicol. Vitr. 2012, 26, 1243–1246. [Google Scholar] [CrossRef]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of Acute Effects of Polystyrene and Polyvinyl Chloride Micro- and Nanoplastics in an Advanced In Vitro Triple Culture Model of the Healthy and Inflamed Intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Rubio, L.; Marcos, R.; Cortés, C. Interactions of Polystyrene Nanoplastics with In Vitro Models of the Human Intestinal Barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, S.; Aynard, A.; Grassl, B.; Gigault, J. Nanoplastics: From Model Materials to Colloidal Fate. Curr. Opin. Colloid Interface Sci. 2022, 57, 101528. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into Chain Scission and Release Profiles from Photodegradation of Polycarbonate Microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef]
- Gniadek, M.; Dąbrowska, A. The Marine Nano- and Microplastics Characterisation by SEM-EDX: The Potential of the Method in Comparison with Various Physical and Chemical Approaches. Mar. Pollut. Bull. 2019, 148, 210–216. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, Tissue Distribution, and Toxicity of Polystyrene Nanoparticles in Developing Zebrafish (Danio Rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Gioria, S.; Caputo, F.; Urbán, P.; Maguire, C.M.; Bremer-Hoffmann, S.; Prina-Mello, A.; Calzolai, L.; Mehn, D. Are Existing Standard Methods Suitable for the Evaluation of Nanomedicines: Some Case Studies. Nanomedicine 2018, 13, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Monteiro-Riviere, N.A.; Inman, A.O.; Zhang, L.W. Limitations and Relative Utility of Screening Assays to Assess Engineered Nanoparticle Toxicity in a Human Cell Line. Toxicol. Appl. Pharmacol. 2009, 234, 222–235. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH Assay for Assessing Nanoparticle Toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Guadagnini, R.; Kenzaoui, B.H.; Walker, L.; Pojana, G.; Magdolenova, Z.; Bilanicova, D.; Saunders, M.; Juillerat-Jeanneret, L.; Marcomini, A.; Huk, A.; et al. Toxicity Screenings of Nanomaterials: Challenges due to Interference with Assay Processes and Components of Classic In Vitro Tests. Nanotoxicology 2015, 9, 13–24. [Google Scholar] [CrossRef]
- Liang, L.; Cui, M.; Zhang, M.; Zheng, P.; Deng, Z.; Gao, S.; Wang, X.; Zhang, X.; Wang, C.; Liu, Y.; et al. Nanoparticles’ Interference in the Evaluation of in Vitro Toxicity of Silver Nanoparticles. RSC Adv. 2015, 5, 67327–67334. [Google Scholar] [CrossRef]
- Liu, H.; Tian, L.; Wang, S.; Wang, D. Size-Dependent Transgenerational Toxicity Induced by Nanoplastics in Nematode Caenorhabditis Elegans. Sci. Total Environ. 2021, 790, 148217. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.; Wang, Y.; Fu, L.; Man, M.; Chen, L. Realistic Polyethylene Terephthalate Nanoplastics and the Size- and Surface Coating-Dependent Toxicological Impacts on Zebrafish Embryos. Environ. Sci. Nano 2020, 7, 2313–2324. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-Dependent Cellular Toxicity of Silver Nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100A, 1033–1043. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold Nanoparticles: Distribution, Bioaccumulation and Toxicity. In Vitro and In Vivo Studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y. Microplastics as Both a Sink and a Source of Bisphenol A in the Marine Environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Greven, A.C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zheng, X.; Luo, X.; Mai, B. Leaching of Brominated Flame Retardants (BFRs) from BFRs-Incorporated Plastics in Digestive Fluids and the Influence of Bird Diets. J. Hazard. Mater. 2020, 393, 122397. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Tong, W.; Li, Y.; Xie, Y.; Chen, Y.; Wen, Z.; Feng, S.; Wang, X.; Li, P.; Wang, Y.; et al. Hydrothermal Route-Enabled Synthesis of Sludge-Derived Carbon with Oxygen Functional Groups for Bisphenol A Degradation through Activation of Peroxymonosulfate. J. Hazard. Mater. 2020, 388, 121801. [Google Scholar] [CrossRef]
- Anwer, S.; Szászi, K. Measuring Cell Growth and Junction Development in Epithelial Cells Using Electric Cell-Substrate Impedance Sensing (ECIS). Bio Protoc. 2020, 10, e3729. [Google Scholar] [CrossRef] [PubMed]
- Anchan, A.; Kalogirou-Baldwin, P.; Johnson, R.; Kho, D.T.; Joseph, W.; Hucklesby, J.; Finlay, G.J.; O’Carroll, S.J.; Angel, C.E.; Graham, E.S. Real-Time Measurement of Melanoma Cell-Mediated Human Brain Endothelial Barrier Disruption Using Electric Cell-Substrate Impedance Sensing Technology. Biosensors 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric Cell-Substrate Impedance Sensing for the Quantification of Endothelial Proliferation, Barrier Function, and Motility. J. Vis. Exp. 2014, 85, e51300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a Potential Environmental Health Factor: Effects of Polystyrene Nanoparticles on Human Intestinal Epithelial Caco-2 Cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]


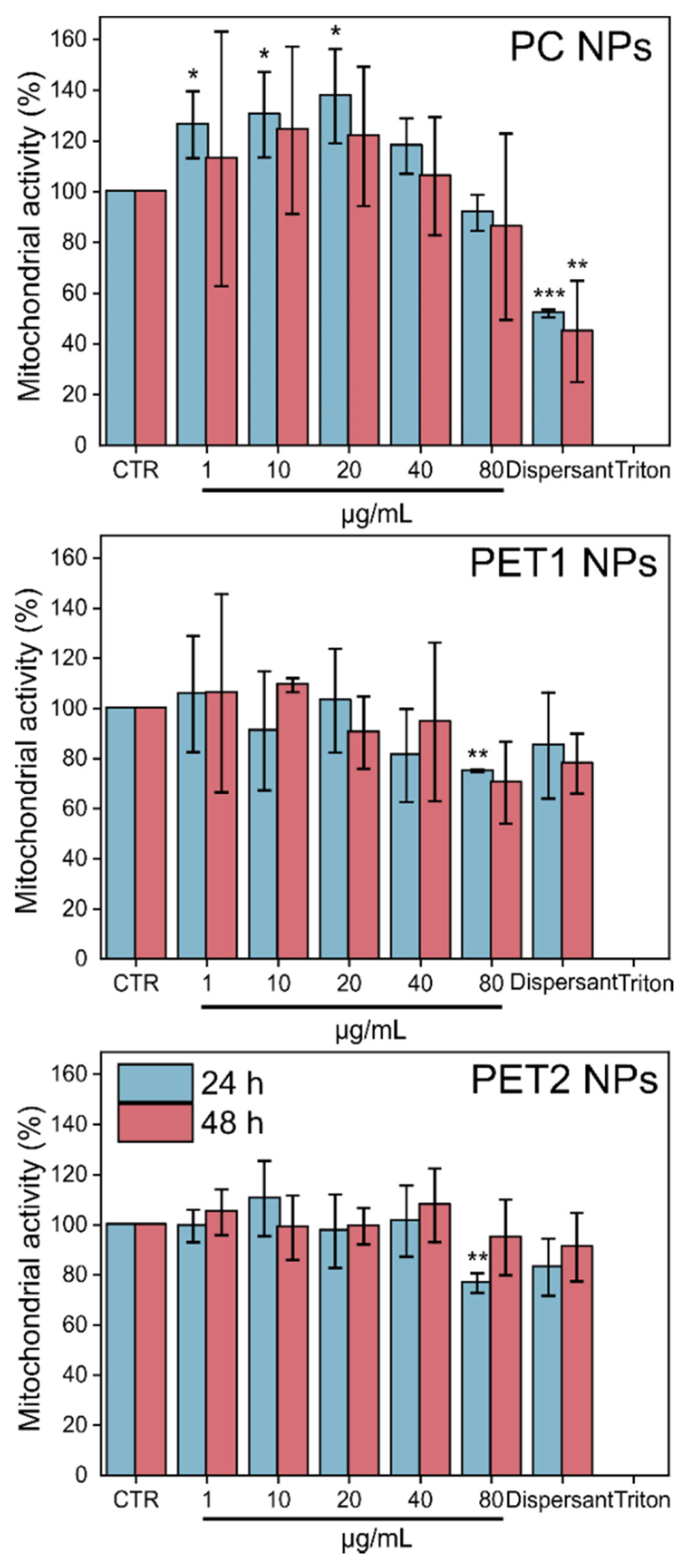



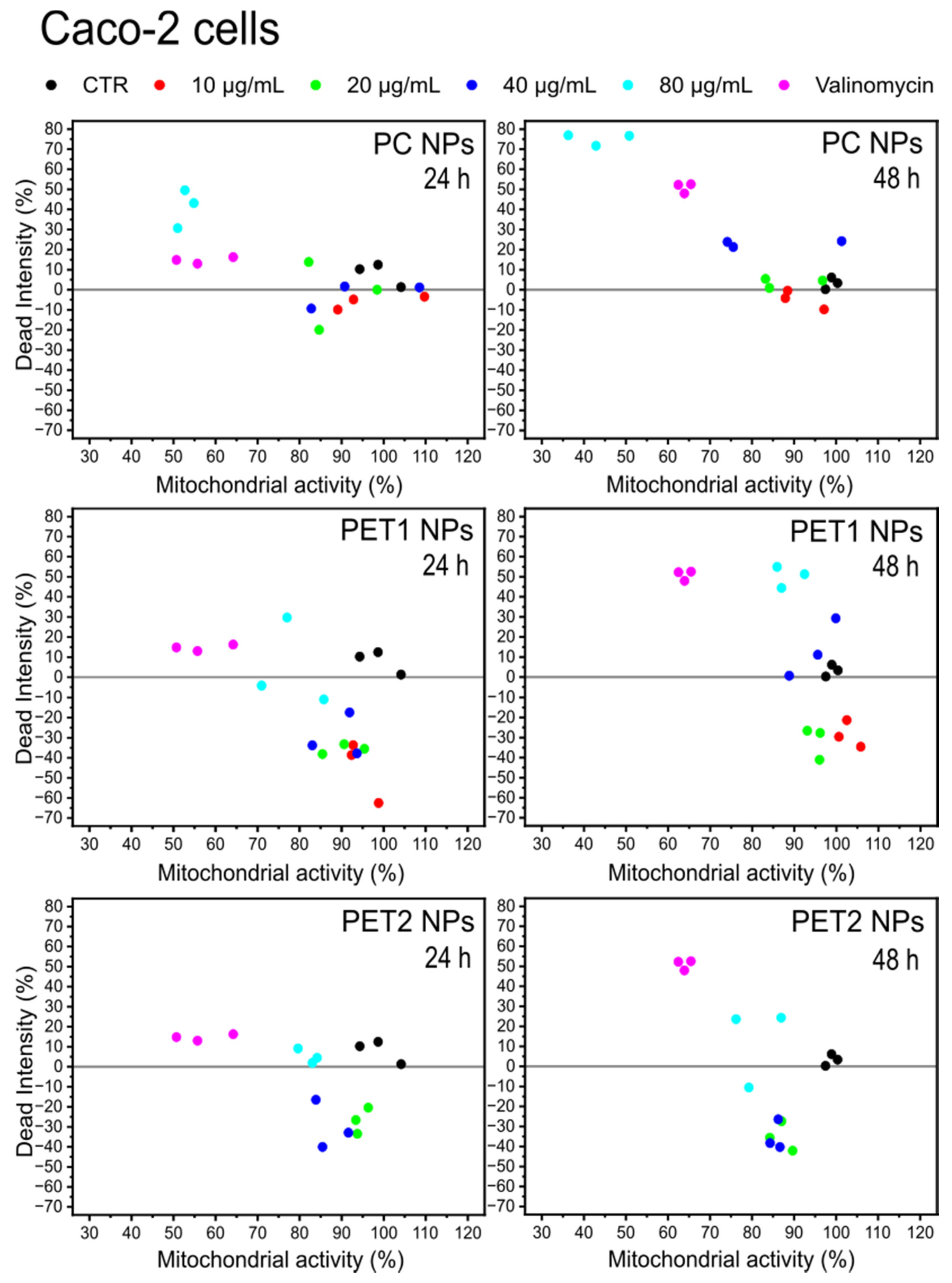
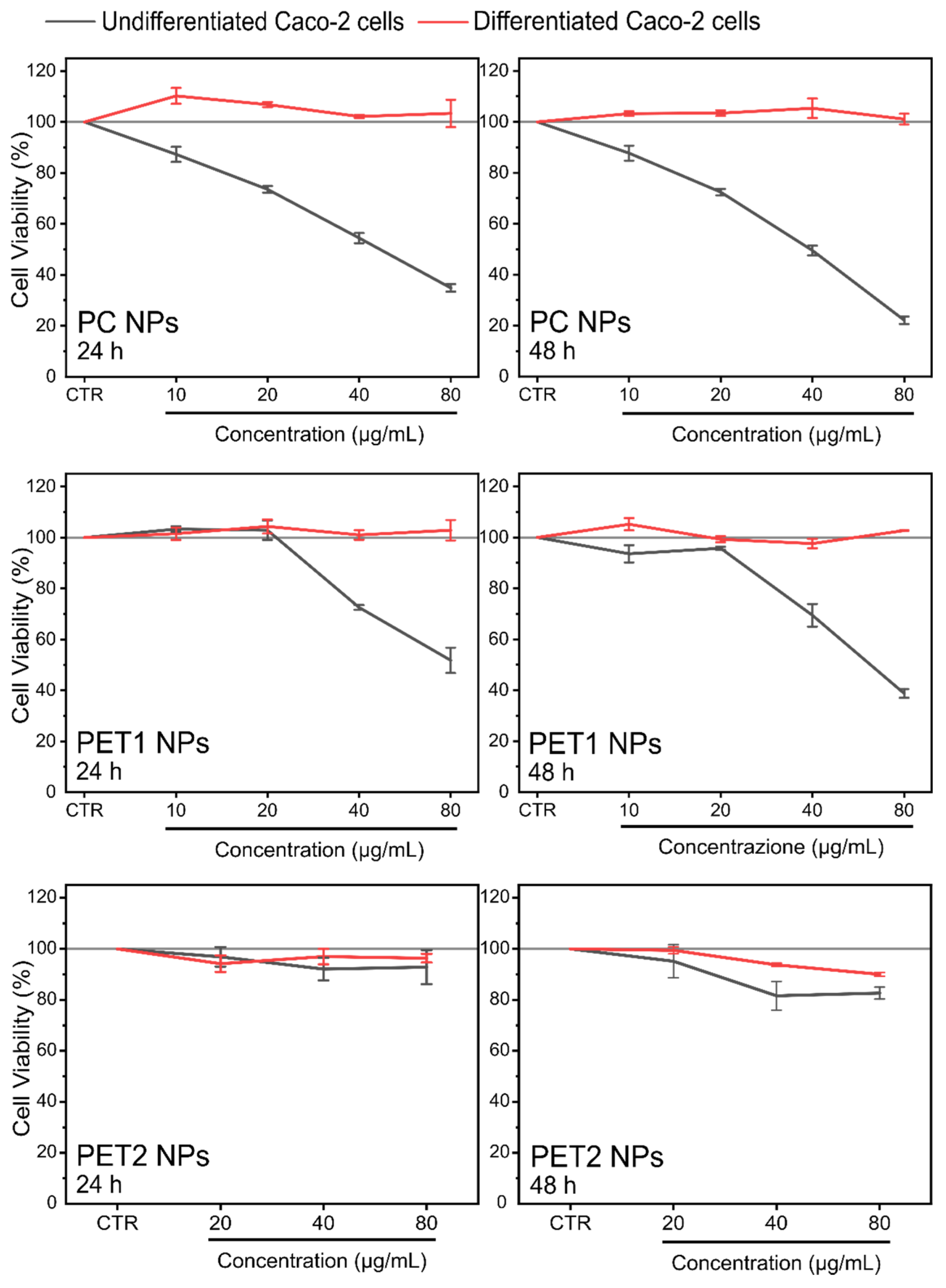
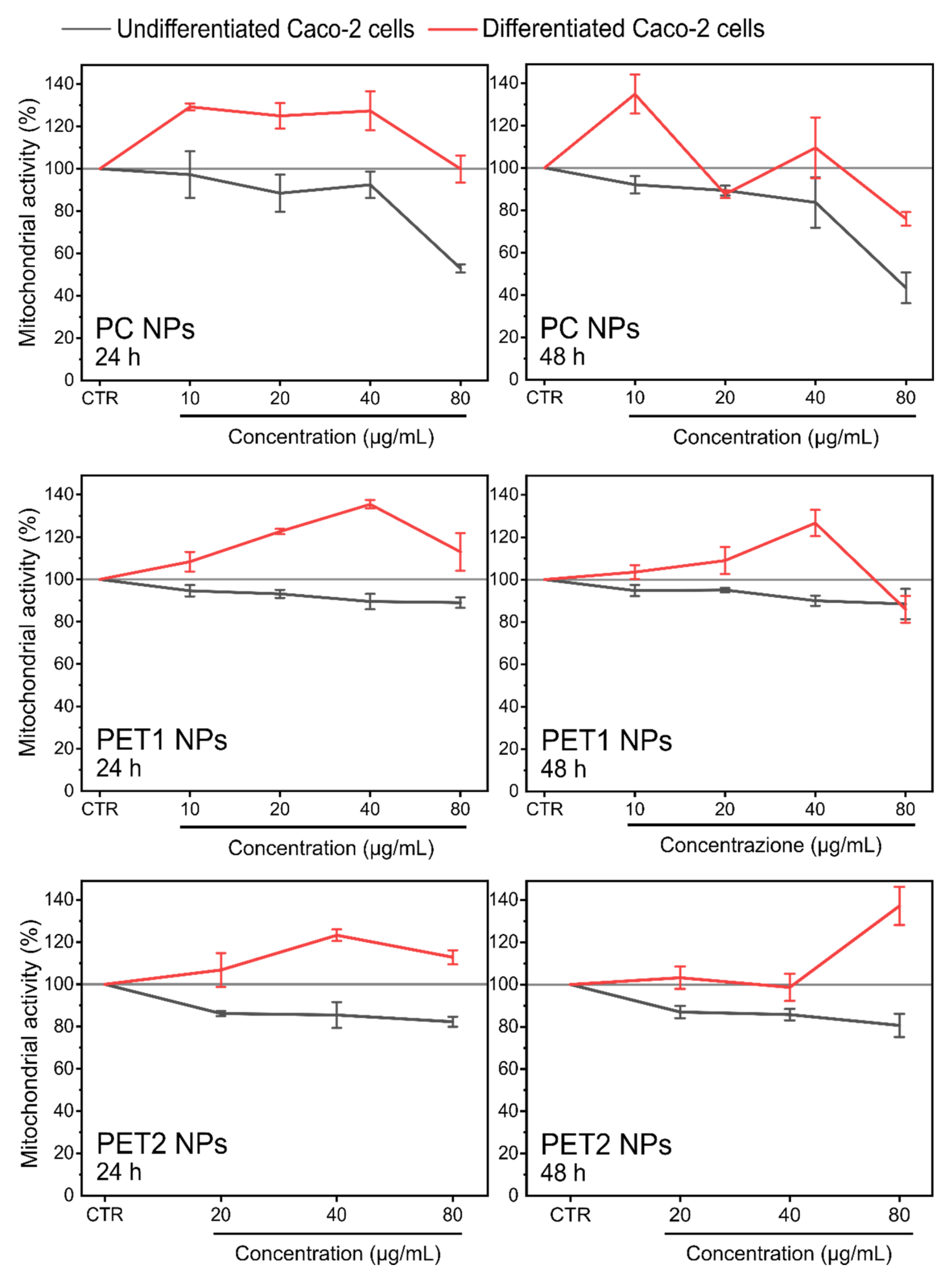
| 24 h | 48 h | |
|---|---|---|
| HePG2 | EC50 (µg/mL) | EC50 (µg/mL) |
| PC NPs | 73.12 | 38.15 |
| PET1 NPs | 69.03 | 68.86 |
| PET2 NPs | 74.15 | 48 |
| Caco-2 | EC50 (µg/mL) | EC50 (µg/mL) |
| PC NPs | 55.31 | 44.62 |
| PET1 NPs | 79.41 | 40.06 |
| PET2 NPs | 92.45 | 82.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolardo, V.; Magrì, D.; Fumagalli, F.; Cassano, D.; Athanassiou, A.; Fragouli, D.; Gioria, S. In Vitro High-Throughput Toxicological Assessment of Nanoplastics. Nanomaterials 2022, 12, 1947. https://doi.org/10.3390/nano12121947
Tolardo V, Magrì D, Fumagalli F, Cassano D, Athanassiou A, Fragouli D, Gioria S. In Vitro High-Throughput Toxicological Assessment of Nanoplastics. Nanomaterials. 2022; 12(12):1947. https://doi.org/10.3390/nano12121947
Chicago/Turabian StyleTolardo, Valentina, Davide Magrì, Francesco Fumagalli, Domenico Cassano, Athanassia Athanassiou, Despina Fragouli, and Sabrina Gioria. 2022. "In Vitro High-Throughput Toxicological Assessment of Nanoplastics" Nanomaterials 12, no. 12: 1947. https://doi.org/10.3390/nano12121947
APA StyleTolardo, V., Magrì, D., Fumagalli, F., Cassano, D., Athanassiou, A., Fragouli, D., & Gioria, S. (2022). In Vitro High-Throughput Toxicological Assessment of Nanoplastics. Nanomaterials, 12(12), 1947. https://doi.org/10.3390/nano12121947







