Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Sample Characterization
2.3. Antibacterial Activity Test
2.4. Statistical Data Analysis
3. Results and Discussion
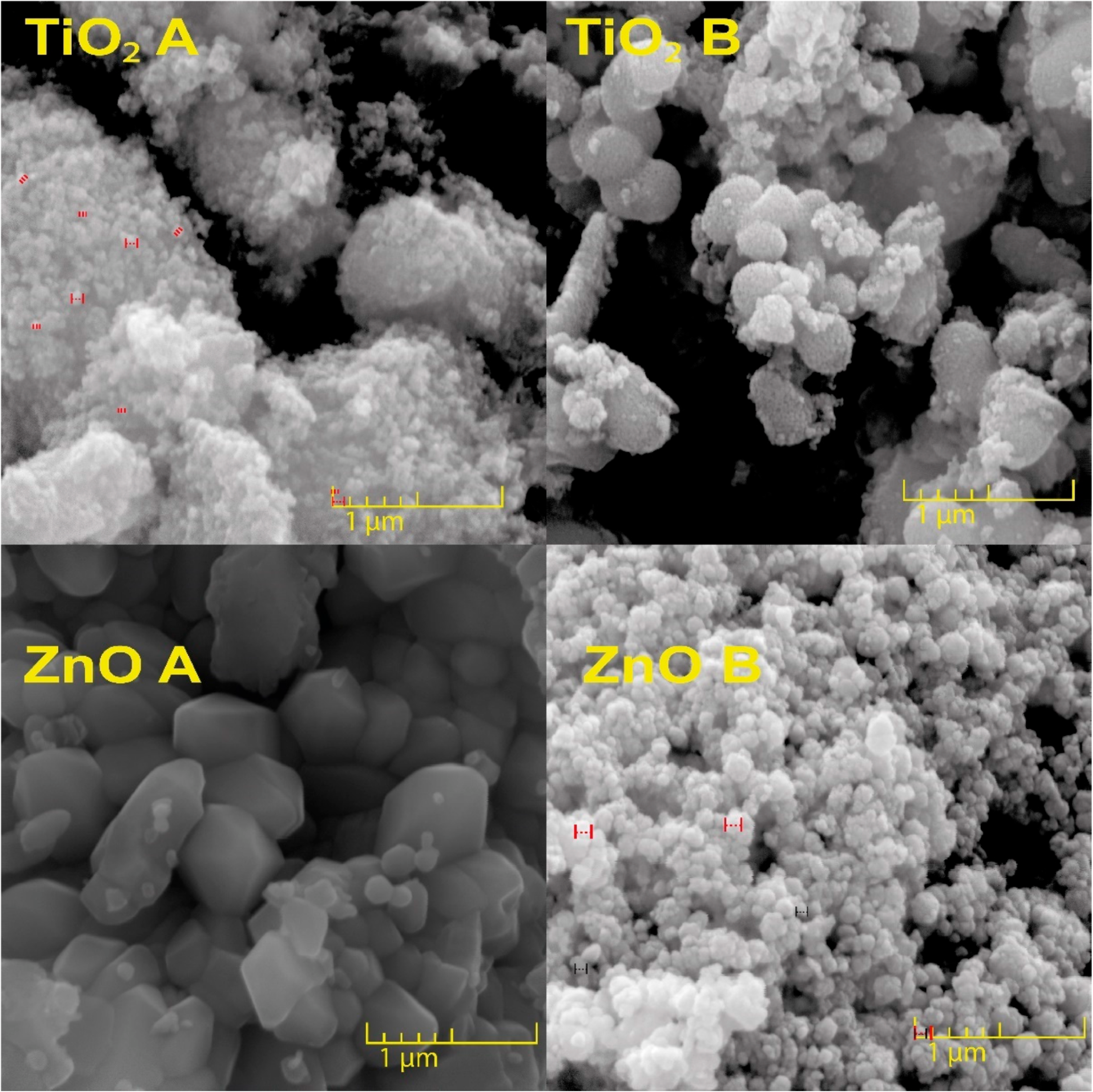
3.1. Morphological Characteristics
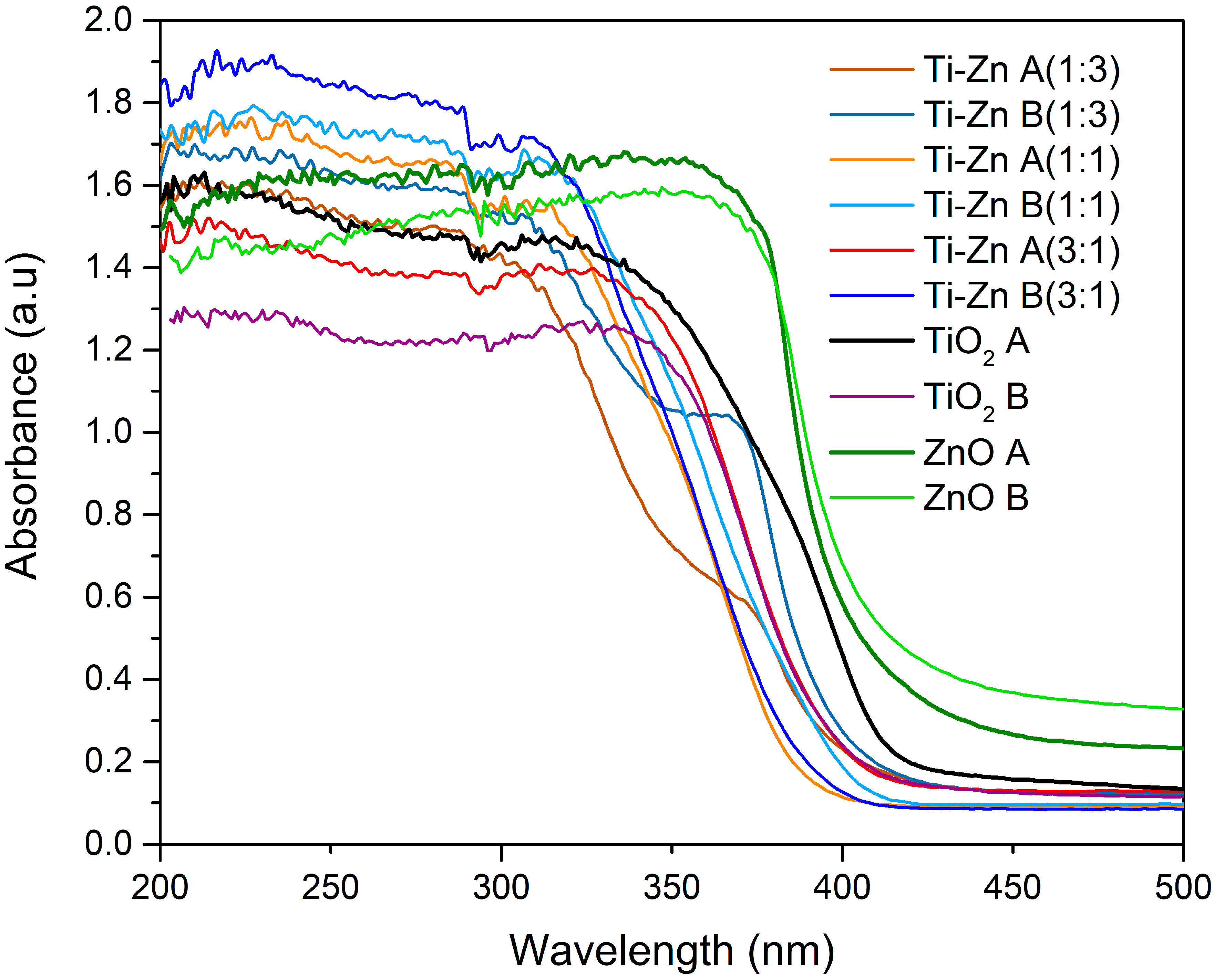
3.2. UV-Vis Analysis
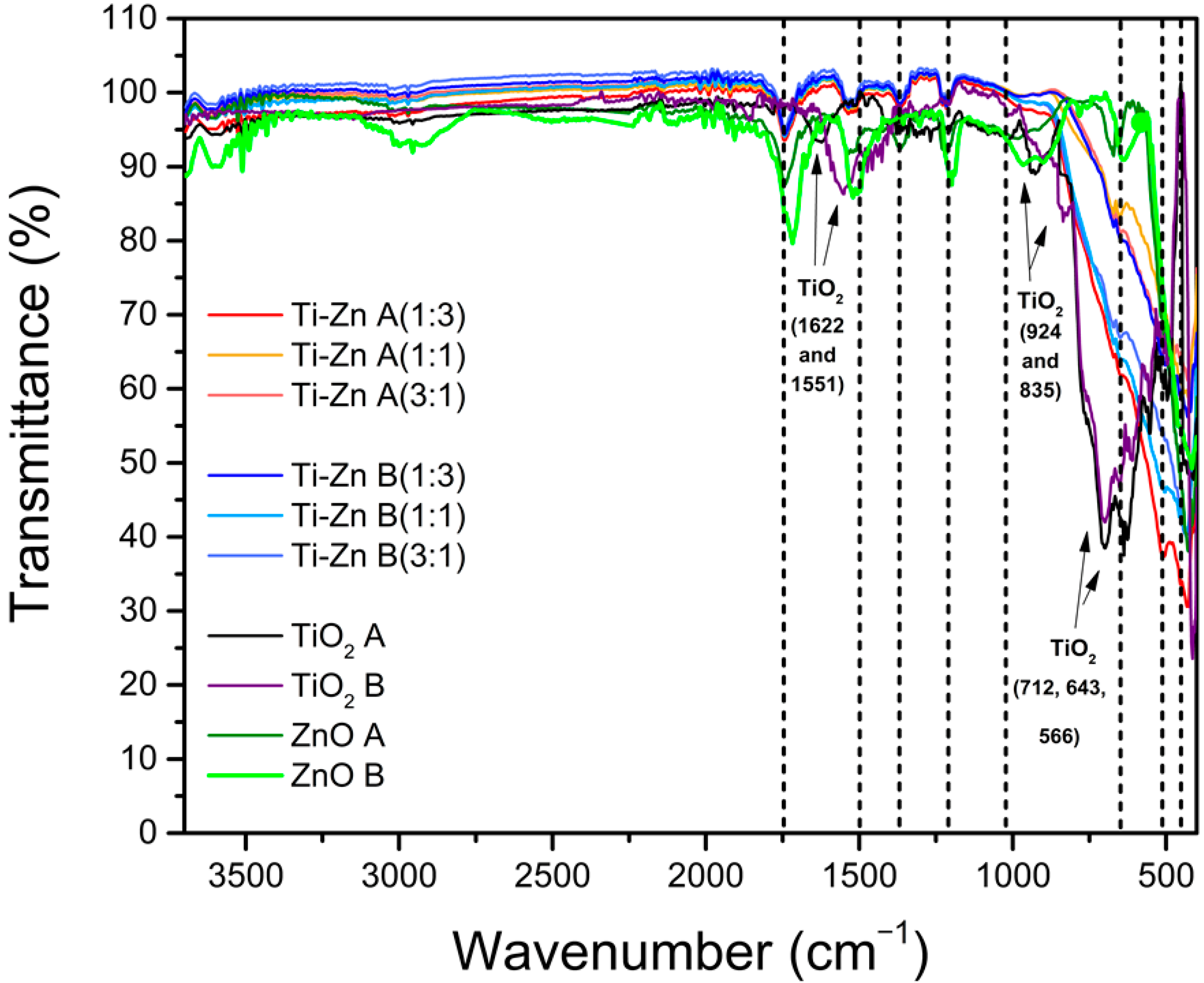
3.3. Infrared Analysis
3.4. X-ray Diffraction
3.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijayalakshmi, K.; Sivaraj, D. Synergistic antibacterial activity of barium doped TiO2 nanoclusters synthesized by microwave processing. RSC Adv. 2016, 6, 9663–9671. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; González-Silva, N.; Yahia, E.M.; González-Vargas, O.A.; Montalvo-González, E.; Pérez-Larios, A. Effect of TiO2-ZnO-MgO mixed oxide on microbial growth and toxicity against Artemia salina. Nanomaterials 2019, 9, 992. [Google Scholar] [CrossRef]
- Zhukova, L.V. Evidence for Compression of Escherichia coli K12 Cells under the Effect of TiO2 Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 27197–27205. [Google Scholar] [CrossRef]
- Carré, G.; Benhamida, D.; Peluso, J.; Muller, C.D.; Lett, M.C.; Gies, J.P.; Keller, V.; Keller, N.; André, P. On the use of capillary cytometry for assessing the bactericidal effect of TiO2. Identification and involvement of reactive oxygen species. Photochem. Photobiol. Sci. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Vladkova, T.; Staneva, A.; Albu-Kaya, M.; Martinov, B.; Ivanova, I. Collagen/(ZnTiO3 /SiO2) composites of an wide spectrum antimicrobial activity Todorka. J. Chem. Technol. Metall. 2020, 55, 60–72. [Google Scholar]
- Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-fouling and anti-bacterial modification of poly(vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Srivastava, R.S.; Misra, R.D.K. Comparative study of antimicrobial and photocatalytic activity in titania encapsulated composite nanoparticles with different dopants. Mater. Sci. Technol. 2008, 24, 589–595. [Google Scholar] [CrossRef]
- Nikpasand, A.; Parvizi, M.R. Evaluation of the Effect of Titatnium Dioxide Nanoparticles/Gelatin Composite on Infected Skin Wound Healing; An Animal Model Study. Bull. Emerg. Trauma 2019, 7, 366–372. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin-TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Suo, H.; Peng, C.; Jing, F.; Yu, S.; Cui, S.; Shen, X. Facile preparation of TiO2/ZnO composite aerogel with excellent antibacterial activities. Mater. Lett. 2019, 234, 253–256. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Aziz, W.J.; Jassim, H.A. A novel study of pH influence on Ag nanoparticles size with antibacterial and antifungal activity using green synthesis. World Sci. News 2018, 97, 139–152. [Google Scholar]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Review—Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Datteo, M.; Ferraro, L.; Seifert, G.; Di Valentin, C. TETT-functionalized TiO2 nanoparticles for DOX loading: A quantum mechanical study at the atomic scale. Nanoscale Adv. 2020, 2, 2774–2784. [Google Scholar] [CrossRef]
- Li, Z.; Ou-Yang, Y.; Liu, Y.; Wang, Y.Q.; Zhu, X.L.; Zhang, Z.Z. Folic acid-conjugated TiO2-doped mesoporous carbonaceous nanocomposites loaded with Mitoxantrone HCl for chemo-photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 1197–1206. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.S.; Cho, Y.K. Significantly enhanced antibacterial activity of TiO2 nanofibers with hierarchical nanostructures and controlled crystallinity. Analyst 2015, 140, 616–622. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef]
- Faraji, M.; Mohaghegh, N.; Abedini, A. TiO2 nanotubes/Ti plates modified by silver-benzene with enhanced photocatalytic antibacterial properties. New J. Chem. 2018, 42, 2058–2066. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Dobrowolska, A.; Czaczyk, K.; Motylenko, M.; Rafaja, D.; Ehrlich, H.; Jesionowski, T. Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 2019, 463, 791–801. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, W.; Yao, L.; Wang, J.; Han, H.; Zhu, T.; Liang, Y.; Fu, J.; Wang, Y. 3D Ag/ZnO microsphere SERS substrate with ultra-sensitive, recyclable and self-cleaning performances: Application for rapid in site monitoring catalytic dye degradation and insight into the mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125507. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Singh, J.; Rawat, M.; Kumar, S. Expanding horizon: Green synthesis of TiO2 nanoparticles using Carica papaya leaves for photocatalysis application. Mater. Res. Express 2019, 6, 095034. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Jose, M. Streptomycin loaded TiO2 nanoparticles: Preparation, characterization and antibacterial applications. J. Nanostruct. Chem. 2017, 7, 47–53. [Google Scholar] [CrossRef]
- Venugopal, G.; Thangavel, S.; Vasudevan, V.; Zoltán, K. Efficient visible-light piezophototronic activity of ZnO-Ag8S hybrid for degradation of organic dye molecule. J. Phys. Chem. Solids 2020, 143, 109473. [Google Scholar] [CrossRef]
- Rani, S.; Suri, P.; Shishodia, P.K.; Mehra, R.M. Synthesis of nanocrystalline ZnO powder via sol-gel route for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1639–1645. [Google Scholar] [CrossRef]
- Jing, F.; Suo, H.; Cui, S.; Tang, X.; Zhang, M.; Shen, X.; Lin, B.; Jiang, G.; Wu, X. Facile synthesis of TiO2/Ag composite aerogel with excellent antibacterial properties. J. Sol-Gel Sci. Technol. 2018, 86, 590–598. [Google Scholar] [CrossRef]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2018, 25, 10328–10339. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.H.; Van Le, Q.; Peng, W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Baruah, R.; Yadav, A.; Das, A.M. Livistona jekinsiana fabricated ZnO nanoparticles and their detrimental effect towards anthropogenic organic pollutants and human pathogenic bacteria. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 251, 119459. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Lopez, R.; Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2–ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Hu, S.; Li, J. Preparation and enhanced photoelectrochemical performance of coupled bicomponent ZnO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.U.; Tahir, M.B.; Nadeem Riaz, K.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2020, 102, 434–442. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnO nanocomposites for solar energy driven photocatalysis. Mater. Sci. Semicond. Process. 2017, 61, 114–124. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Morales-Luna, M. Enhanced photocatalytic activity of TiO2-ZnO thin films deposited by dc reactive magnetron sputtering. Ceram. Int. 2017, 43, 8831–8838. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Taha, S.Y. Synthesis and Characterization of TiO2 Nanoparticles via Sol-Gel Method by Pulse Laser Ablation Synthesis and Characterization of TiO2 Nanoparticles via Sol-Gel Method by Pulse Laser Ablation. Eng. Tech. J. 2015, 33, 761–771. [Google Scholar]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO nanoparticles-modified dressings to inhibit wound pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Baldeón-Apaestegui, S.; Hernández-Gorritti, W.R. Identification of capsaicin and dihydrocapsaicin in the oleoresin extract obtained from panca chili (Capsicum chinense). Ing. Ind. 2017, 35, 223. [Google Scholar] [CrossRef][Green Version]
- Borcan, L.C.; Dudas, Z.; Len, A.; Fuzi, J.; Borcan, F.; Tomescu, M.C. Synthesis and characterization of a polyurethane carrier used for a prolonged transmembrane transfer of a chili pepper extract. Int. J. Nanomed. 2018, 13, 7155–7166. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arreguin, A.; Carriles, R.; Ochoa-Alejo, N.; López, M.G.; Sánchez-Segura, L. Generation of BSA-capsaicin Nanoparticles and Their Hormesis Effect on the Rhodotorula mucilaginosa Yeast. Molecules 2019, 24, 2800. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wei, M.M.; Song, C.; Ma, Y.F.; Zheng, X.J.; Xiong, D.C.; Ye, X.S. Chemical synthesis and biological evaluation of penta- to octa-saccharide fragments of Vi polysaccharide from: Salmonella typhi. Org. Chem. Front. 2018, 5, 2179–2188. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 nanoparticles via sol-gel method. Adv. Mater. Res. 2011, 173, 184–189. [Google Scholar] [CrossRef]
- Yusuf, Y.; Ghazali, M.J.; Otsuka, Y.; Ohnuma, K.; Morakul, S.; Nakamura, S.; Abdollah, M.F. Antibacterial properties of laser surface-textured TiO2/ZnO ceramic coatings. Ceram. Int. 2020, 46, 3949–3959. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B. Influence of acidic pH on the formulation of TiO2 nanocrystalline powders with enhanced photoluminescence property. Heliyon 2017, 3, e00246. [Google Scholar] [CrossRef]
- Stoyanova, A.; Hitkova, H.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Ivanova, N.; Sredkova, M. Synthesis and antibacterial activity of TiO2/ZnO nanocomposites prepared via nonhydrolytic route. J. Chem. Technol. Metall. 2013, 48, 154–161. [Google Scholar]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef]
- Wu, D.; Wei, D.; Du, M.; Ming, S.; Ding, Q.; Tan, R. Targeting Antibacterial Effect and Promoting of Skin Wound Healing after Infected with Methicillin-Resistant Staphylococcus aureus for the Novel Polyvinyl Alcohol Nanoparticles. Int. J. Nanomed. 2021, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]

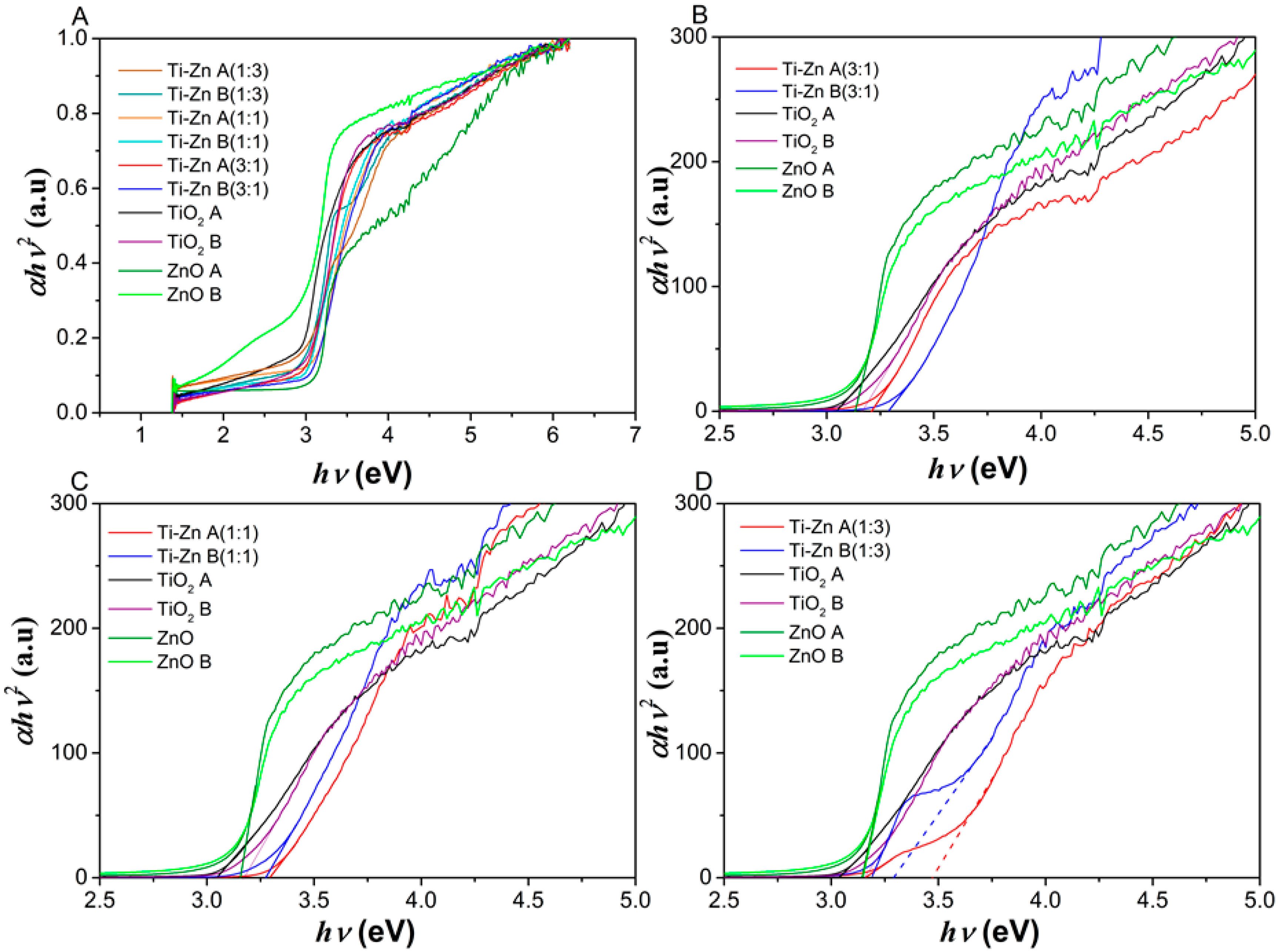
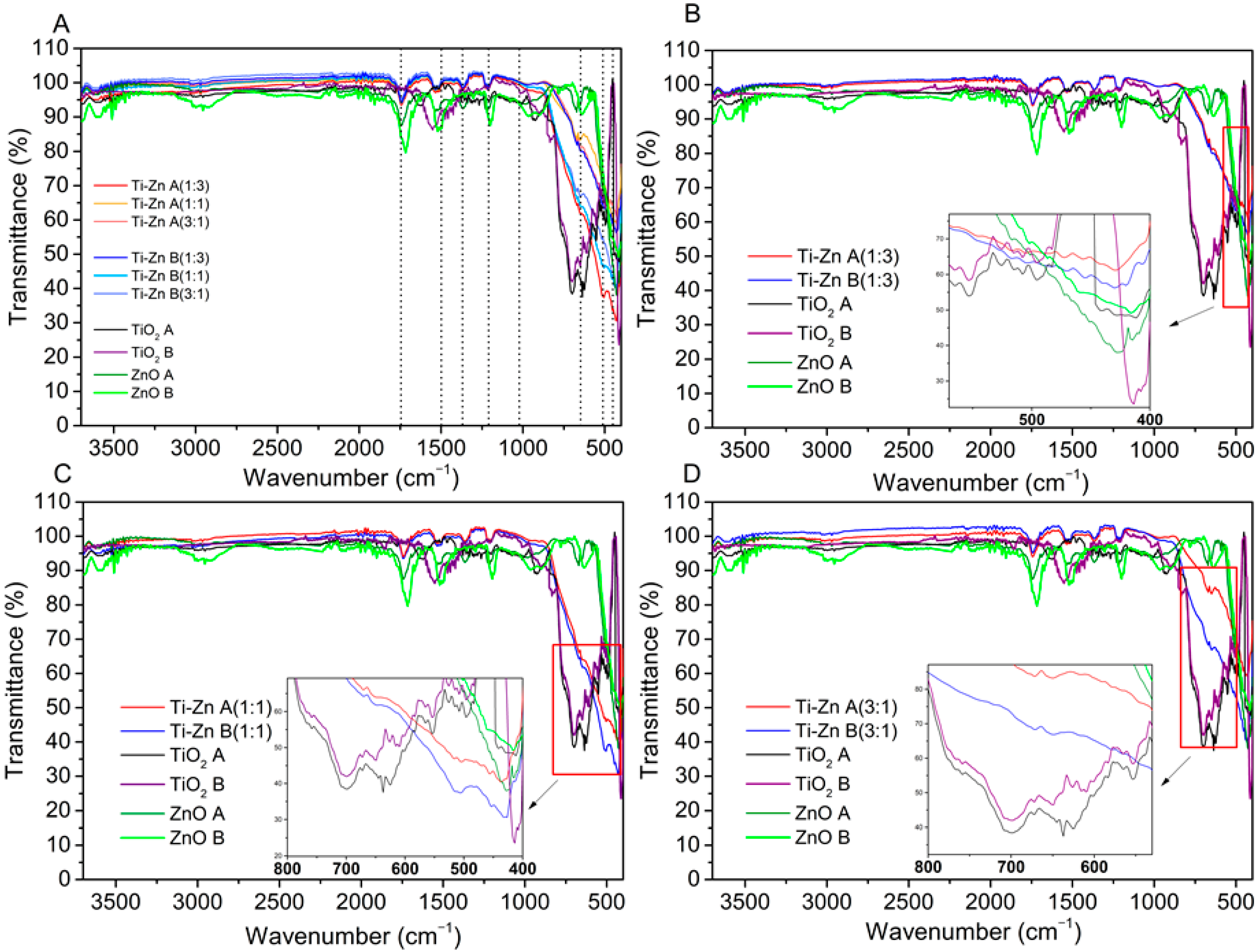
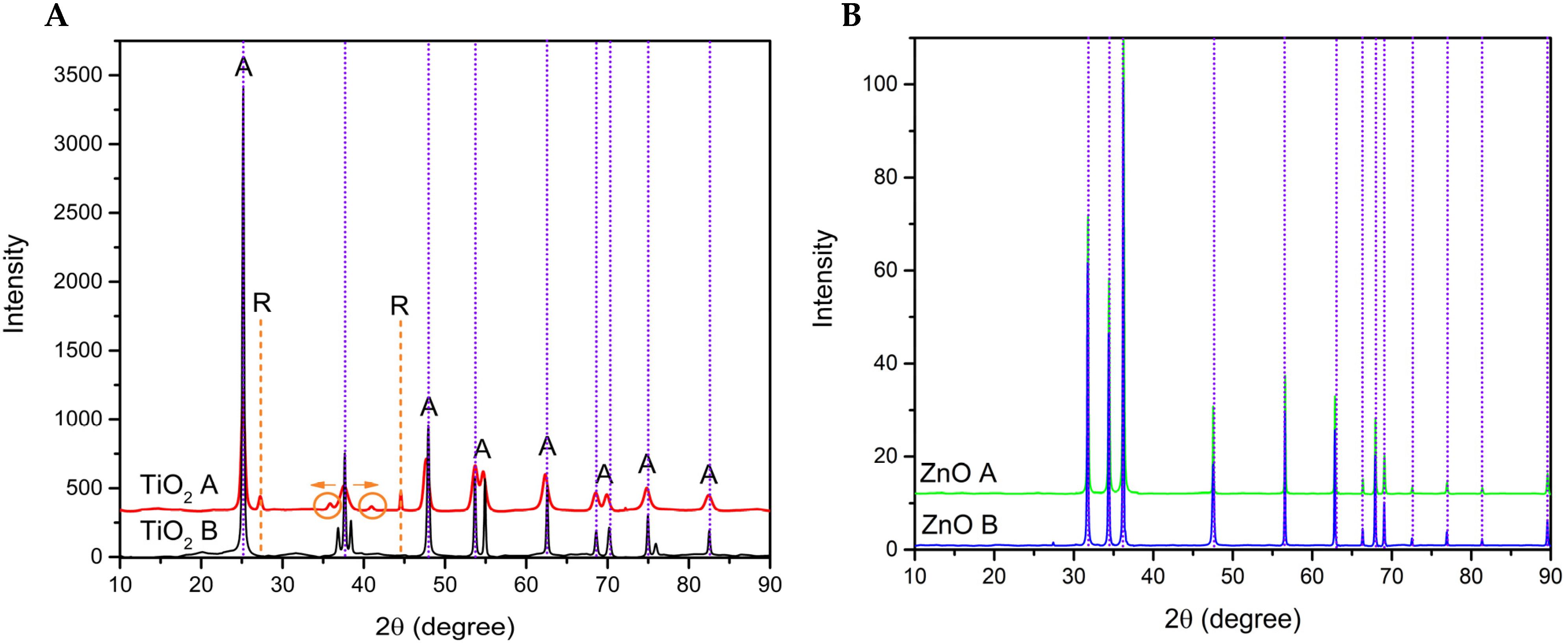
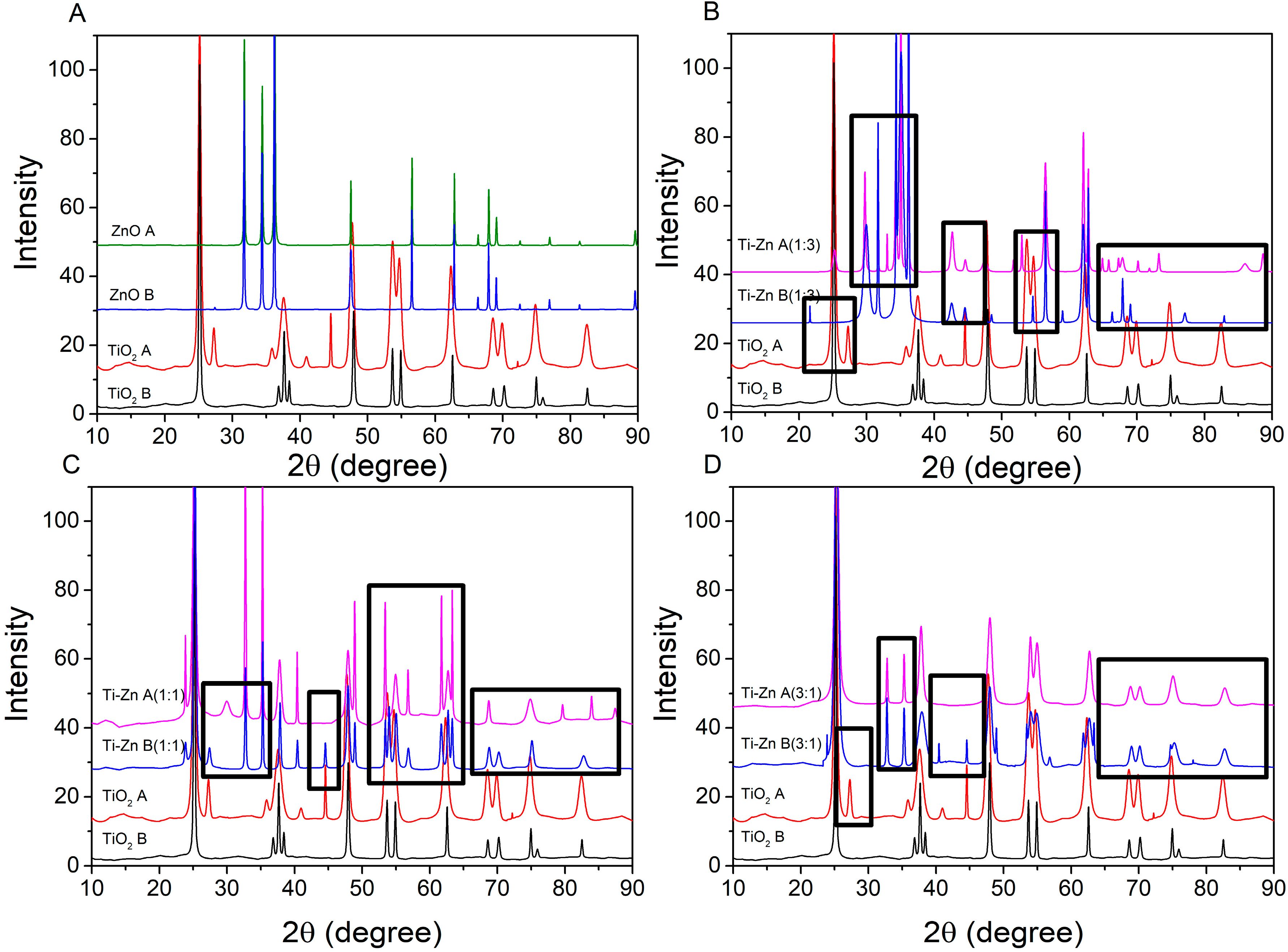
| Code | Relation Molar | pH (HNO3) | pH (NH3·H2O) | Direct Bandgap (Eg in eV) | Indirect Bandgap (Eg in eV) | ||
|---|---|---|---|---|---|---|---|
| TiO2 | 1 | 2.56 | 8.37 | 2.77 a | 2.82 b | 3.02 a | 3.20 b |
| Ti–Zn3 | 3:1 | 2.51 | 8.26 | 2.83 a | 2.92 b | 3.22 a | 3.31 b |
| Ti–Zn2 | 1:1 | 2.27 | 8.28 | 2.86 a | 3.03 b | 3.29 a | 3.30 b |
| Ti–Zn1 | 1:3 | 2.68 | 8.18 | 2.87 a | 2.94 b | 3.16 a | 3.19 b |
| ZnO c | 1 | 2.38 c | 8.34 | 2.96 | 2.90 | 3.17 | 3.15 |
| Material | Crystal Size (nm) | Principal Peak Position (°2θ) | d-Spacing (Å) | (hkl) | a (Å) | c (Å) |
|---|---|---|---|---|---|---|
| TiO2A | 12.26 ± 0.22 | 25.153 | 3.5376 | [101] | 3.85 | 10.50 |
| TiO2B | 23.87 ± 0.78 | 25.215 | 3.5290 | [101] | 3.78 | 9.52 |
| ZnOA | 69.05 ± 5.81 | 36.216 | 2.4780 | [101] | 3.25 | 5.20 |
| ZnOB | 70.82 ± 5.50 | 36.178 | 2.4808 | [101] | 3.14 | 5.21 |
| Ti–Zn A (3:1) | 17.19 ± 0.51 | 25.308, 35.288 | 3.5163, 2.5413 | [101] [212] | 7.95 | 6.151 |
| Ti–Zn B (3:1) | 41.30 ± 3.60 | 25.278, 35.829 | 3.5204, 2.5413 | [101] [021] | 9.93 | 8.19 |
| Ti–Zn A (1:1) | 36.34 ± 2.20 | 25.163, 35.258 | 3.5362, 2.5434 | [101] [300] | 7.91 | 10.93 |
| Ti–Zn B (1:1) | 34.55 ± 1.57 | 25.223, 35.265 | 3.5279, 2.5429 | [101] [205] | 9.62 | 5.93 |
| Ti–Zn A (1:3) | 21.57 ± 0.68 | 25.156, 36.637 | 3.5371, 2.4508 | [101] [004] | 5.94 | 6.39 |
| Ti–Zn B (1:3) | 29.71 ± 1.30 | 29.930, 36.204 | 2.9829, 2.6039 | [220] [302] | 5.99 | 8.42 |
| Treatment | E. coli (mm) | S. paratyphi (mm) | S. aureus (mm) | L. monocytogenes (mm) |
|---|---|---|---|---|
| Ampicillin (C+) a | 19.78 ± 1.09 | 28.56 ± 1.24 | 24.44 ± 0.88 | 26.22 ± 1.09 |
| Distilled water (C−) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| TiO2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| ZnO | 0 ± 0 | 0 ± 0 | 7.89 ± 0.60 b | 9.22 ± 1.09 b |
| Ti–Zn A (3:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn B (3:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn A (1:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn B (1:1) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ti–Zn A (1:3) | 0 ± 0 | 0 ± 0 | 7.67 ± 0.58 b | 10.17 ± 1.04 b |
| Ti–Zn B (1:3) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Barajas, N.; Becerra-Solano, L.; Gutiérrez-Mercado, Y.K.; Macías-Carballo, M.; M. Gómez, C.; Pérez-Larios, A. Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials 2022, 12, 1948. https://doi.org/10.3390/nano12121948
Rodríguez-Barajas N, Becerra-Solano L, Gutiérrez-Mercado YK, Macías-Carballo M, M. Gómez C, Pérez-Larios A. Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials. 2022; 12(12):1948. https://doi.org/10.3390/nano12121948
Chicago/Turabian StyleRodríguez-Barajas, Noé, Luis Becerra-Solano, Yanet Karina Gutiérrez-Mercado, Monserrat Macías-Carballo, Claudia M. Gómez, and Alejandro Pérez-Larios. 2022. "Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties" Nanomaterials 12, no. 12: 1948. https://doi.org/10.3390/nano12121948
APA StyleRodríguez-Barajas, N., Becerra-Solano, L., Gutiérrez-Mercado, Y. K., Macías-Carballo, M., M. Gómez, C., & Pérez-Larios, A. (2022). Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials, 12(12), 1948. https://doi.org/10.3390/nano12121948








