Enhanced Photocatalytic Hydrogen Production Activity by Constructing a Robust Organic-Inorganic Hybrid Material Based Fulvalene and TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Photocatalysts
2.2.1. Synthesis of H4TTFTB-TiO2 Materials
2.2.2. Synthesis of Dye-Sensitized Composites
2.2.3. Synthesis of Composites Materials Loaded with Pt NPs
2.3. Photocatalytic Hydrogen Production
2.4. Materials Characterization
2.5. Photolectrochemical Methods
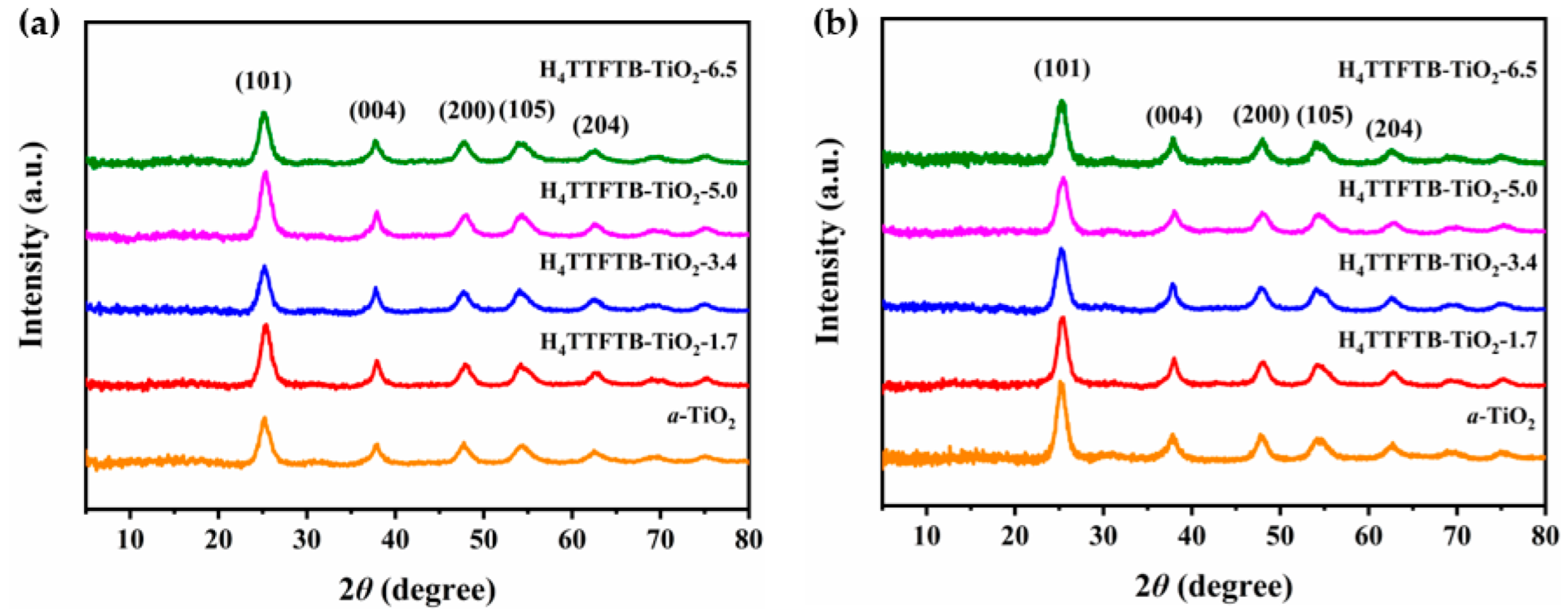
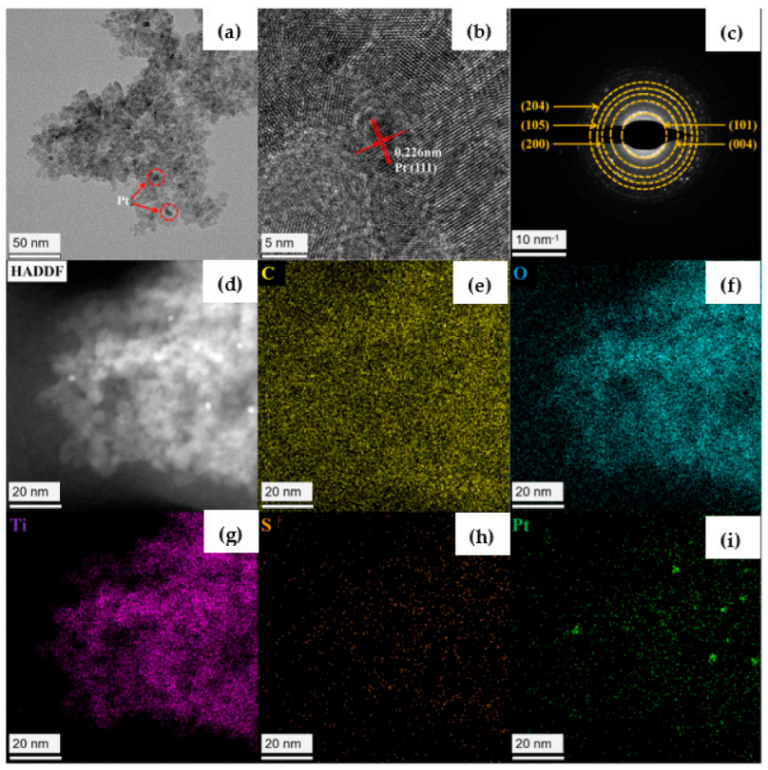
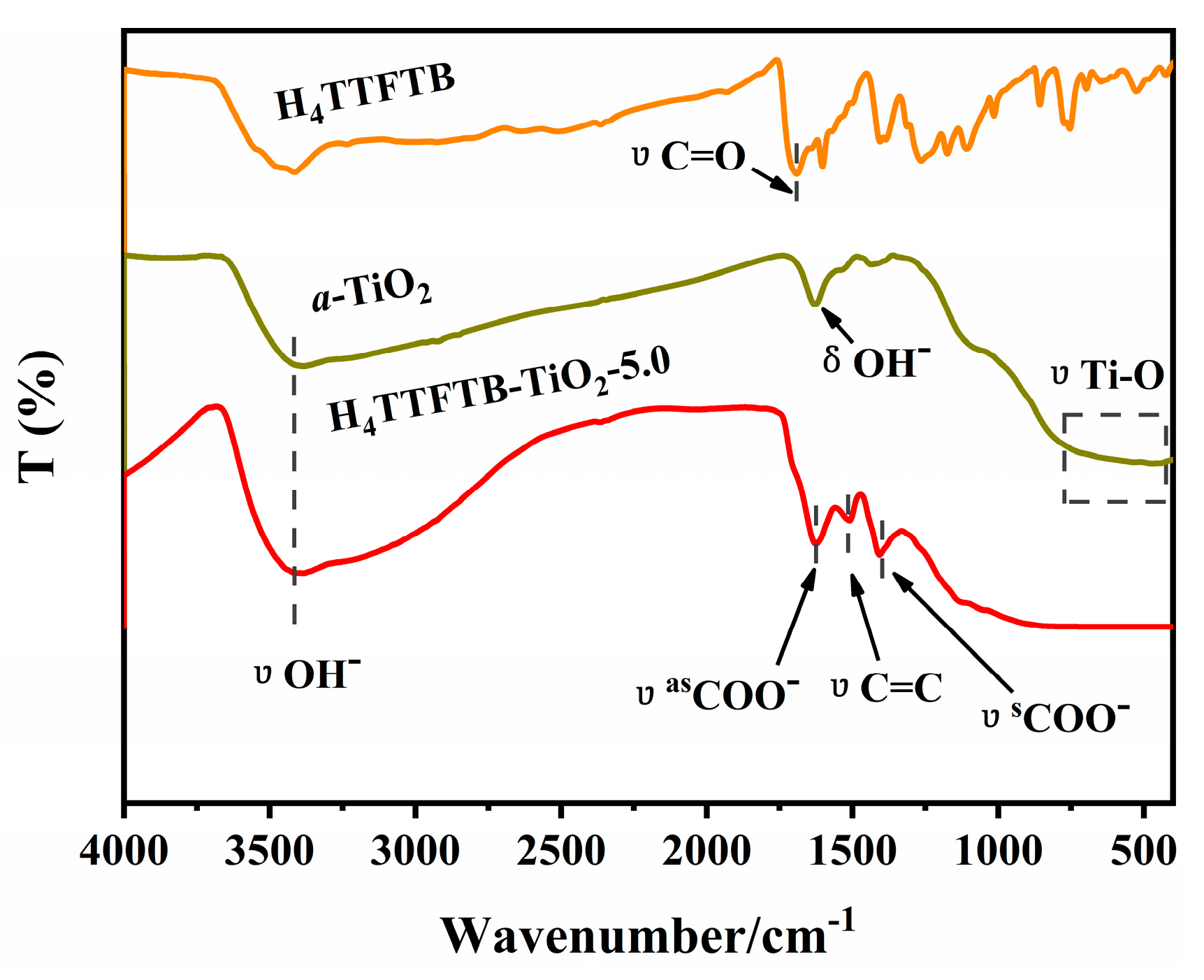
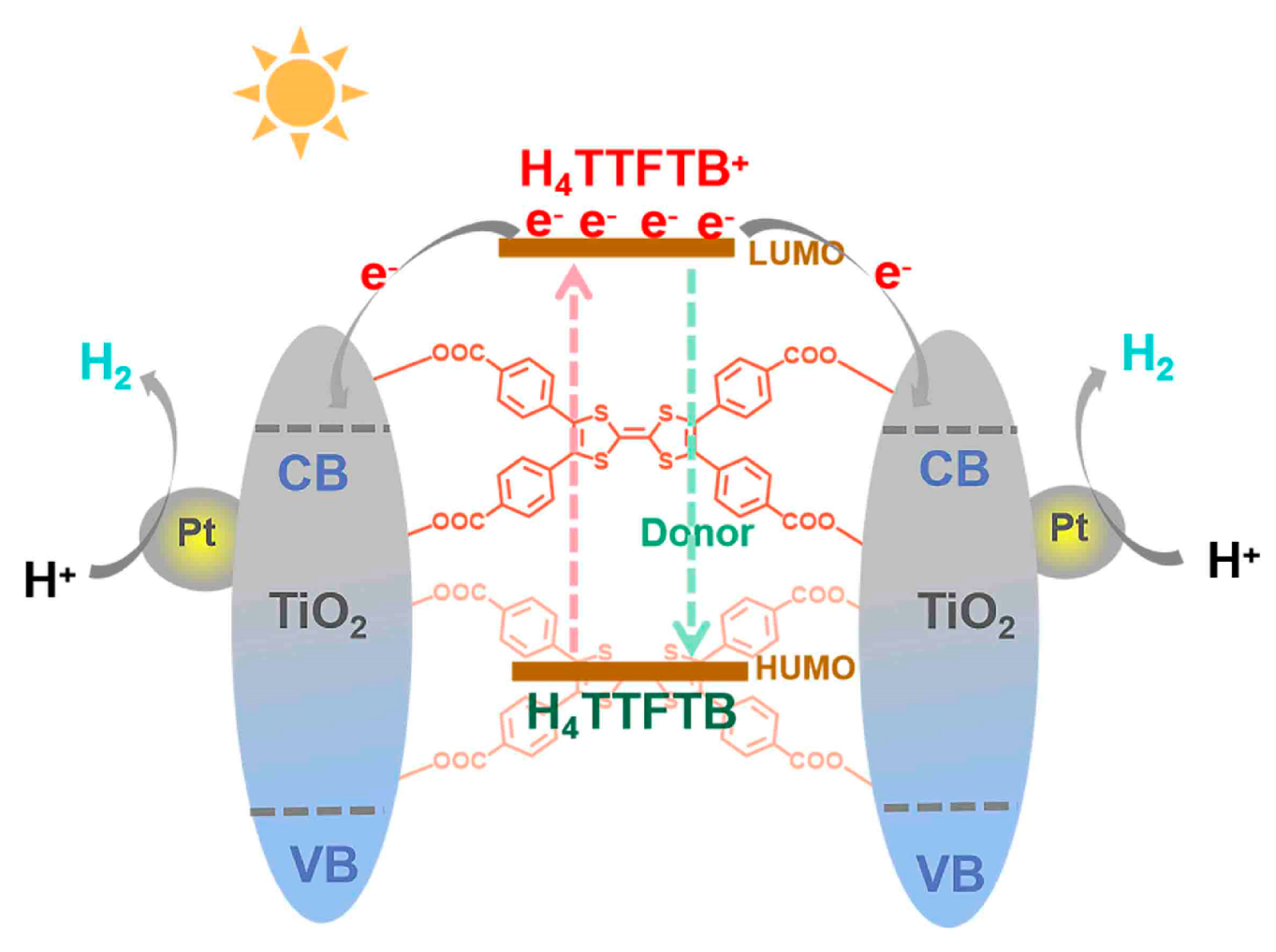
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Li, C.; Gratzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- Child, M.; Koskinen, O.; Linnanen, L.; Breyer, C. Sustainability guardrails for energy scenarios of the global energy transition. Renew. Sust. Energy Rev. 2018, 91, 321–334. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Dickson, R.; Ali Shah, S.F.; Niaz, H.; Khan, A.; Liu, J.J.; Lee, M. Availability, versatility, and viability of feedstocks for hydrogen production: Product space perspective. Renew. Sust. Energy Rev. 2021, 145, 110843. [Google Scholar] [CrossRef]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lin, L.; Zhang, R.; Yang, C.; Yang, J. Highly Efficient Photocatalytic Water Splitting over Edge-Modified Phosphorene Nanoribbons. J. Am. Chem. Soc. 2017, 139, 15429–15436. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.F.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zheng, J.; Peng, Y.K.; Prabhakaran, D.; Taylor, R.A.; Tsang, S.C.E. 2D photocatalysts with tuneable supports for enhanced photocatalytic water splitting. Mater. Today 2020, 41, 34–43. [Google Scholar] [CrossRef]
- Lin, L.; Hisatomi, T.; Chen, S.; Takata, T.; Domen, K. Visible-Light-Driven Photocatalytic Water Splitting: Recent Progress and Challenges. Trends Chem. 2020, 2, 813–824. [Google Scholar] [CrossRef]
- Prasad Ojha, G.; Muthurasu, A.; Prasad Tiwari, A.; Pant, B.; Chhetri, K.; Mukhiya, T.; Dahal, B.; Lee, M.; Park, M.; Kim, H.Y. Vapor solid phase grown hierarchical CuxO NWs integrated MOFs-derived CoS2 electrode for high-performance asymmetric supercapacitors and the oxygen evolution reaction. Chem. Eng. J. 2020, 399, 125532. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single Molecule Photocatalysis on TiO2 Surfaces. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 190199. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.J.; Zhang, X.H.; Huang, D.K.; Ding, X.; Wang, S.Y.; Yang, X.L.; Li, S.Q.; Xiang, Y.G.; Chen, H. Conjugated microporous poly(benzothiadiazole)/TiO2 heterojunction for visible-light-driven H2 production and pollutant removal. Appl. Catal. B Environ. 2017, 203, 563–571. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Zhou, Z.; Zhao, G. Highly selective aerobic oxidation of biomass alcohol to benzaldehyde by an in situ doped Au/TiO2 nanotube photonic crystal photoanode for simultaneous hydrogen production promotion. J. Mater. Chem. A 2017, 5, 12407–12415. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ti3+ TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, L.; Liu, G.; Qiu, M.; Chen, P.; Chang, Z. Pd quantum dots loading Ti3+, N co-doped TiO2 nanotube arrays with enhanced photocatalytic hydrogen production and the salt ions effects. Appl. Surf. Sci. 2021, 540, 148239. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Tiwari, A.; Mondal, I.; Pal, U.; Niveditha, S.; Bhanuprakash, K.; Singh, S.P. Dithiafulvalene functionalized diketopyrrolopyrrole based sensitizers for efficient hydrogen production. Phys. Chem. Chem. Phys. 2015, 17, 13710–13718. [Google Scholar] [CrossRef]
- Tiwari, A.; Duvva, N.; Rao, V.N.; Venkatakrishnan, S.M.; Giribabu, L.; Pal, U. Tetrathiafulvalene Scaffold-Based Sensitizer on Hierarchical Porous TiO2: Efficient Light-Harvesting Material for Hydrogen Production. J. Phys. Chem. C 2018, 123, 70–81. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, J.F.; Shen, M.H.; Liu, J.M.; Huang, L.B.; Zhong, Y.H.; Qin, S.; Guo, J.; Su, C.Y. A porous hybrid material based on calixarene dye and TiO2 demonstrating high and stable photocatalytic performance. J. Mater. Chem. A 2019, 7, 19852–19861. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Peng, C.; Xiang, Y.; Chen, H. Enhanced photocatalytic hydrogen production over conjugated polymer/black TiO2 hybrid: The impact of constructing active defect states. Appl. Surf. Sci. 2019, 465, 288–296. [Google Scholar] [CrossRef]
- Genc, E.; Yüzer, A.C.; Yanalak, G.; Harputlu, E.; Aslan, E.; Ocakoglu, K.; Ince, M.; Patir, I.H. The effect of central metal in phthalocyanine for photocatalytic hydrogen evolution via artificial photosynthesis. Renew. Energy 2020, 162, 1340–1346. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Kim, H.J. Design of ternary hybrid ATO/g-C3N4/TiO2 nanocomposite for visible-light-driven photocatalysis. J. Ind. Eng. Chem. 2018, 61, 87–96. [Google Scholar] [CrossRef]
- Nilsing, M.; Lunell, S.; Persson, P.; Ojamäe, L. Phosphonic acid adsorption at the TiO2 anatase (101) surface investigated by periodic hybrid HF-DFT computations. Surf. Sci. 2005, 582, 49–60. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tan, L.L.; Liu, J.M.; Qin, S.; Xie, Z.Q.; Huang, J.F.; Xu, Y.W.; Xiao, L.M.; Su, C.Y. Calix[4]arene based dye-sensitized Pt@UiO-66-NH2 metal-organic framework for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 206, 426–433. [Google Scholar] [CrossRef]
- Wu, H.M.; Wang, M.Y.; Jing, F.; Kong, D.R.; Chen, Y.F.; Jia, C.M.; Li, J.W. Enhanced photocatalytic hydrogen production performance of pillararene-doped mesoporous TiO2 with extended visible-light response. Chin. Chem. Lett. 2022, 33, 1983–1987. [Google Scholar] [CrossRef]
- Huang, J.F.; Liu, J.M.; Xiao, L.M.; Zhong, Y.H.; Liu, L.; Qin, S.; Guo, J.; Su, C.Y. Facile synthesis of porous hybrid materials based on Calix-3 dye and TiO2 for high photocatalytic water splitting performance with excellent stability. J. Mater. Chem. A 2019, 7, 2993–2999. [Google Scholar] [CrossRef]
- El-Kemary, M.A. Photophysical properties of 2,5-diphenyl-1,6,6a-trithiapentalene revealed by time-resolved spectroscopy. Spectrochim. Acta Part A 2001, 57, 177–183. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cui, L.; Xie, J.Z.; Leong, C.F.; D’Alessandro, D.M.; Zuo, J.L. Functional coordination polymers based on redox-active tetrathiafulvalene and its derivatives. Coordin. Chem. Rev. 2017, 345, 342–361. [Google Scholar] [CrossRef]
- Jana, A.; Bahring, S.; Ishida, M.; Goeb, S.; Canevet, D.; Salle, M.; Jeppesen, J.O.; Sessler, J.L. Functionalised tetrathiafulvalene- (TTF-) macrocycles: Recent trends in applied supramolecular chemistry. Chem. Soc. Rev. 2018, 47, 5614–5645. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Zhao, Y.; Kretschmer, K.; Wang, G. Modified Tetrathiafulvalene as an Organic Conductor for Improving Performances of Li-O2 Batteries. Angew. Chem. Int. Ed. 2017, 56, 8505–8509. [Google Scholar] [CrossRef] [Green Version]
- Hammerich, O.; Nielsen, M.B. Extended tetrathiafulvalenes with polycyclic aromatic cores. J. Mater. Chem. C 2019, 7, 2809–2822. [Google Scholar] [CrossRef]
- Mroweh, N.; Meziere, C.; Pop, F.; Auban-Senzier, P.; Alemany, P.; Canadell, E.; Avarvari, N. In Search of Chiral Molecular Superconductors: Kappa-[(S,S)-DM-BEDT-TTF]2 ClO4 Revisited. Adv. Mater. 2020, 32, 2002811. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.; Romero, J.; Calbo, J.; Vitorica-Yrezabal, I.J.; Zafra, J.L.; Casado, J.; Orti, E.; Walsh, A.; Minguez Espallargas, G. Breathing-Dependent Redox Activity in a Tetrathiafulvalene-Based Metal-Organic Framework. J. Am. Chem. Soc. 2018, 140, 10562–10569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, I.; Mundstock, A.; Treger, M.; Lange, K.; Hwang, S.; Chmelik, C.; Rusch, P.; Bigall, N.C.; Pichler, T.; Shiozawa, H.; et al. Metal-Organic Framework Co-MOF-74-Based Host-Guest Composites for Resistive Gas Sensing. ACS Appl. Mater. Interfaces 2019, 11, 14175–14181. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Kilani, M.; Siddiqui, A.; Mao, G. One-Step Synthesis of Charge-Transfer Salt Nanosensors on Microelectrode Patterns. Adv. Mater. Technol. 2020, 5, 2000554. [Google Scholar] [CrossRef]
- Hou, J.L.; Weng, Y.G.; Liu, P.Y.; Cui, L.N.; Zhu, Q.Y.; Dai, J. Effects of the Ligand Structures on the Photoelectric Activities, a Model Study Based on Titanium-Oxo Clusters Anchored with S-Heterocyclic Ligands. Inorg. Chem. 2019, 58, 2736–2743. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, S.; Zhao, C.; Jiu, T.; Bi, F.; Jian, H.; Zhao, M.; Zhang, G.; Wang, L.; Li, F.; et al. TTA as a potential hole transport layer for application in conventional polymer solar cells. J. Energy Chem. 2020, 42, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Li, Y.; Liu, L.; Zhao, C.; Jiu, T.; Hu, M.; Xiao, X. Non-planar tetrathiafulvalene derivative modified hole transporting layer for efficient organic solar cells with improved fill factor. Solar Energy 2021, 224, 883–888. [Google Scholar] [CrossRef]
- Su, J.; Cai, P.; Yan, T.; Yang, Z.M.; Yuan, S.; Zuo, J.L.; Zhou, H.C. Enhancing the photothermal conversion of tetrathiafulvalene-based MOFs by redox doping and plasmon resonance. Chem. Sci. 2022, 13, 1657–1664. [Google Scholar] [CrossRef]
- Lu, M.; Liu, J.; Li, Q.; Zhang, M.; Liu, M.; Wang, J.L.; Yuan, D.Q.; Lan, Y.Q. Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O. Angew. Chem. Int. Ed. 2019, 58, 12392–12397. [Google Scholar] [CrossRef]
- Ge, C.Y.; Hou, J.L.; Zhou, Z.Y.; Zhu, Q.Y.; Dai, J. A Cyclic Titanium-Oxo Cluster with a Tetrathiafulvalene Connector as a Precursor for Highly Efficient Adsorbent of Cationic Dyes. Inorg. Chem. 2022, 61, 486–495. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Su, Z.; Zhang, W.; Wang, N.; Mele, G.; Li, J. New porphyrin/Cu(II) porphyrin-TiO2 nanohybrids for improved photocatalytic oxidation and reduction activities. Mater. Chem. Phys. 2020, 252, 123228. [Google Scholar] [CrossRef]
- Yu, M.M.; Li, J.; Sun, W.J.; Jiang, M.; Zhang, F.X. Preparation, characterization, and photocatalytic properties of composite materials of copper(II) porphyrin/TiO2. J. Mater. Sci. 2014, 49, 5519–5528. [Google Scholar] [CrossRef]
- Verma, P.; Singh, A.; Rahimi, F.A.; Sarkar, P.; Nath, S.; Pati, S.K.; Maji, T.K. Charge-transfer regulated visible light driven photocatalytic H2 production and CO2 reduction in tetrathiafulvalene based coordination polymer gel. Nat. Commun. 2021, 12, 7313. [Google Scholar] [CrossRef]
- Wu, K.; Li, K.; Chen, S.; Hou, Y.J.; Lu, Y.L.; Wang, J.S.; Wei, M.J.; Pan, M.; Su, C.Y. The Redox Coupling Effect in a Photocatalytic Ru(II)-Pd(II) Cage with TTF Guest as Electron Relay Mediator for Visible-Light Hydrogen-Evolving Promotion. Angew. Chem. Int. Ed. 2020, 59, 2639–2643. [Google Scholar] [CrossRef]
- Le, T.T.; Akhtar, M.S.; Park, D.M.; Lee, J.C.; Yang, O.B. Water splitting on Rhodamine-B dye sensitized Co-doped TiO2 catalyst under visible light. Appl. Catal. B Environ. 2012, 111–112, 397–401. [Google Scholar] [CrossRef]
- Jing, F.; Guo, Y.; Li, B.; Chen, Y.F.; Jia, C.; Li, J. Enhanced photocatalytic hydrogen production under visible light of an organic-inorganic hybrid material based on enzo[1,2-b:4,5-b’]dithiophene polymer and TiO2. Chin. Chem. Lett. 2022, 33, 1303–1307. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Zeng, F.; Peng, G.; Li, J.; Yi, Z. Multicarbazole-Based D-pi-A Dyes Sensitized Hydrogen Evolution under Visible Light Irradiation. ACS Omega 2020, 5, 2027–2033. [Google Scholar] [CrossRef]
- Shen, X.F.; Watanabe, M.; Takagaki, A.; Song, J.T.; Ishihara, T. Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts 2020, 10, 535. [Google Scholar] [CrossRef]
- Liu, X.; Lai, H.; Li, J.; Peng, G.; Yi, Z.; Zeng, R.; Wang, M.; Liu, Z. Polyaniline sensitized Pt@TiO2 for visible-light-driven H2 generation. Int. J. Hydrogen Energy 2019, 44, 4698–4706. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.F.; Chen, G.; Zhan, C.H.; Kitao, O.; Tamiaki, H.; Sasaki, S.i. Near-infrared absorption carboxylated chlorophyll-a derivatives for biocompatible dye-sensitized hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 15731–15738. [Google Scholar] [CrossRef]
- Yan, Z.; Yu, X.; Zhang, Y.; Jia, H.; Sun, Z.; Du, P. Enhanced visible light-driven hydrogen production from water by a noble-metal-free system containing organic dye-sensitized titanium dioxide loaded with nickel hydroxide as the cocatalyst. Appl. Catal. B Environ. 2014, 160–161, 173–178. [Google Scholar] [CrossRef]
- Aslan, E.; Gonce, M.K.; Yigit, M.Z.; Sarilmaz, A.; Stathatos, E.; Ozel, F.; Can, M.; Patir, I.H. Photocatalytic H2 evolution with a Cu2WS4 catalyst on a metal free D-π-A organic dye-sensitized TiO2. Appl. Catal. B Environ. 2017, 210, 320–327. [Google Scholar] [CrossRef]
- Veikko, U.; Zhang, X.; Peng, T.; Cai, P.; Cheng, G. The synthesis and characterization of dinuclear ruthenium sensitizers and their applications in photocatalytic hydrogen production. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 105, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.P.; Chen, X.L.; Huang, Z.J.; Lei, Y.; Xiao, L.M.; Huang, J.F.; Li, S.Y.; Liu, J.M. Robust Heterogeneous Photocatalyst for Visible-Light-Driven Hydrogen Evolution Promotion: Immobilization of a Fluorescein Dye-Encapsulated Metal-Organic Cage on TiO2. ACS Appl. Mater. Interfaces 2021, 13, 57230–57240. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Su, S.; Zhong, X.; Kong, D.; Li, B.; Song, Y.; Jia, C.; Chen, Y. Enhanced Photocatalytic Hydrogen Production Activity by Constructing a Robust Organic-Inorganic Hybrid Material Based Fulvalene and TiO2. Nanomaterials 2022, 12, 1918. https://doi.org/10.3390/nano12111918
Wang M, Su S, Zhong X, Kong D, Li B, Song Y, Jia C, Chen Y. Enhanced Photocatalytic Hydrogen Production Activity by Constructing a Robust Organic-Inorganic Hybrid Material Based Fulvalene and TiO2. Nanomaterials. 2022; 12(11):1918. https://doi.org/10.3390/nano12111918
Chicago/Turabian StyleWang, Mengyuan, Shizhuo Su, Xin Zhong, Derui Kong, Bo Li, Yujie Song, Chunman Jia, and Yifan Chen. 2022. "Enhanced Photocatalytic Hydrogen Production Activity by Constructing a Robust Organic-Inorganic Hybrid Material Based Fulvalene and TiO2" Nanomaterials 12, no. 11: 1918. https://doi.org/10.3390/nano12111918
APA StyleWang, M., Su, S., Zhong, X., Kong, D., Li, B., Song, Y., Jia, C., & Chen, Y. (2022). Enhanced Photocatalytic Hydrogen Production Activity by Constructing a Robust Organic-Inorganic Hybrid Material Based Fulvalene and TiO2. Nanomaterials, 12(11), 1918. https://doi.org/10.3390/nano12111918




