Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity
Abstract
:1. Introduction
2. Excremental and Methods
2.1. Materials
2.2. Preparation of Chia Extract
2.3. Synthesis of β-Ni(OH)2 Nanocluster
2.4. Characterization of β-Ni(OH)2 Nanoparticles
2.5. Anticancer Activity Studies
2.5.1. Cell Culture
2.5.2. In-Vitro Cytotoxic Activity β-Ni(OH)2 by MTT Assay
3. Results and Discussions
3.1. Characterization
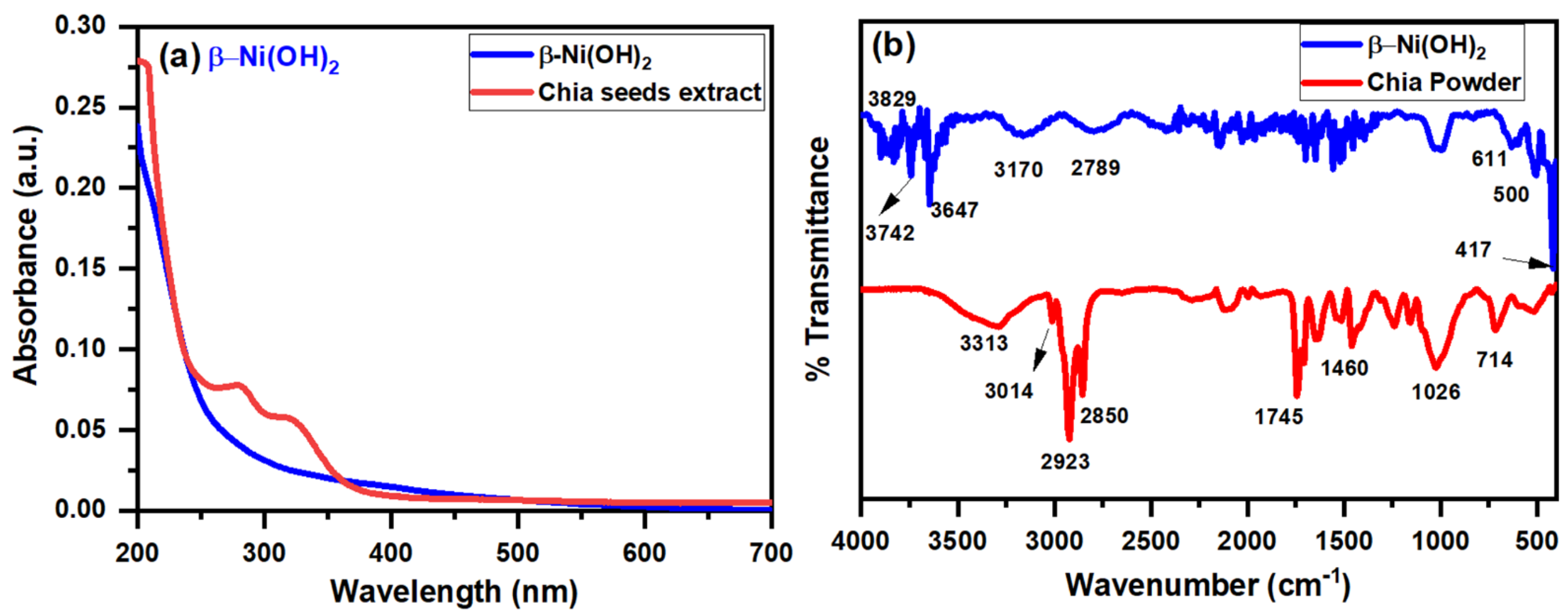
3.1.1. UV-Vis Spectroscopy
3.1.2. FT-IR Spectroscopy
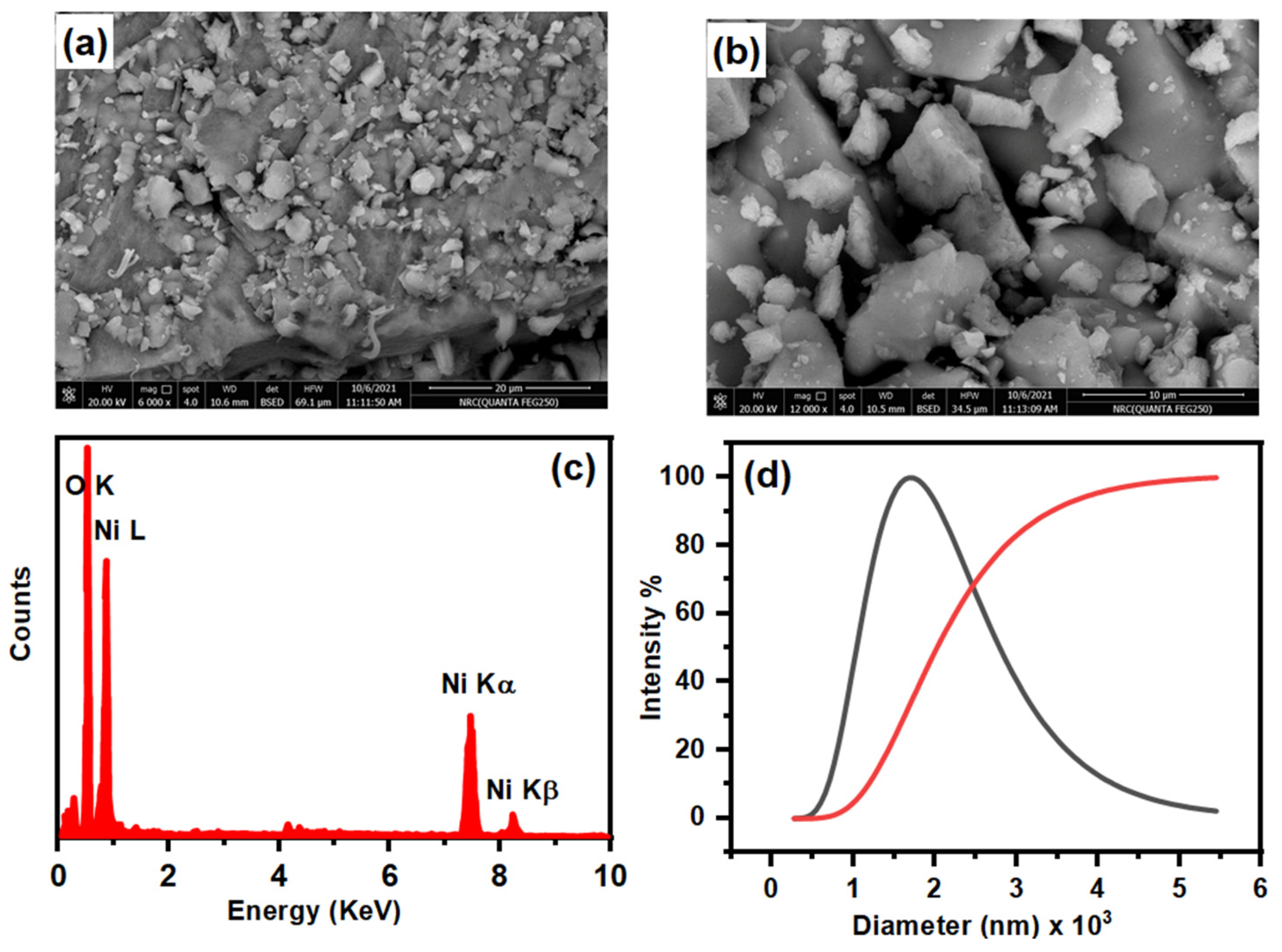
3.1.3. FE-SEM and EDS Analysis
3.1.4. HR-TEM and DLS Analysis
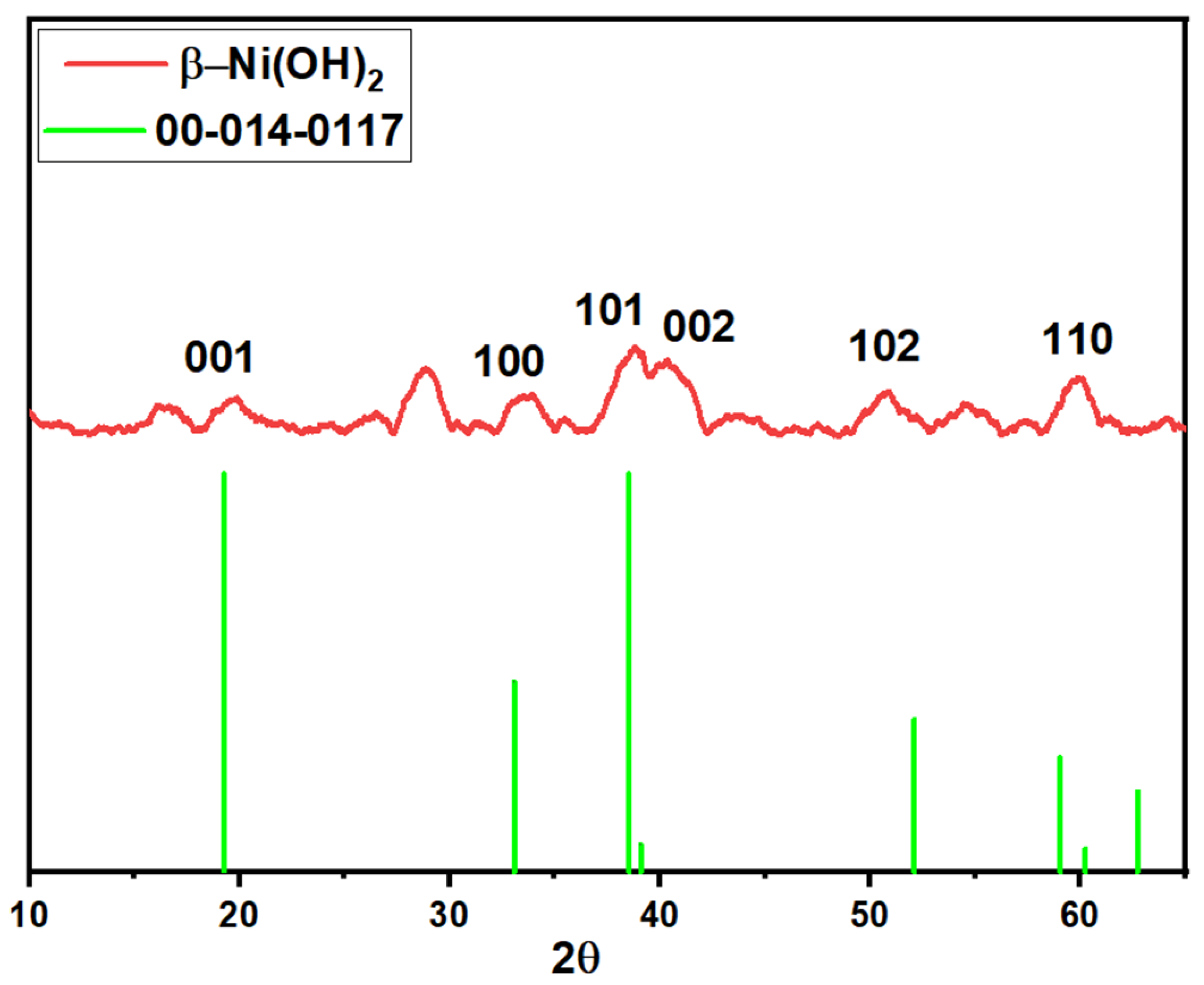
3.1.5. XRD Analysis
3.1.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Cytotoxicity Evaluation of β-Ni(OH)2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Cheng, M.Y.; Hwang, B.J. Control of uniform nanostructured α-Ni(OH)2 with self-assembly sodium dodecyl sulfate templates. J. Colloid Interface Sci. 2009, 337, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Saghatforoush, L.A.; Hasanzadeh, M.; Sanati, S.; Mehdizadeh, R. Ni(OH)2 and NiO nanostructures: Synthesis, characterization and electrochemical performance. Bull. Korean Chem. Soc. 2012, 33, 2613–2618. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Wei, Y.; Zhao, H.; Chen, S.; Bu, X.; Lu, F.; Qu, D.; Yao, L.; Zheng, J.; Zhang, J. The effect of Fe2O3 and ZnO nanoparticles on cytotoxicity and glucose metabolism in lung epithelial cells. J. Appl. Toxicol. 2015, 35, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Borah, D.; Yadav, A.K. A Novel ‘Green’ Synthesis of Antimicrobial Silver Nanoparticles (AgNPs) by using Garcinia morella (Gaertn) Desr. Fruit Extract. Nanosci. Nanotechnol.-Asia 2015, 5, 25–31. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, K.; Zhang, X.; Tan, W. Barbated Skullcup herb extract-mediated biosynthesis of gold nanoparticles and its primary application in electrochemistry. Colloids Surf. B Biointerfaces 2009, 73, 75–79. [Google Scholar] [CrossRef]
- Haigh, P.A. Visible Light; IOP Publishing: Bristol, UK, 2020. [Google Scholar] [CrossRef]
- Lozano, T.; Rey, M.; Rojas, E.; Moya, S.; Fleddermann, J.; Estrela-Lopis, I.; Donath, E.; Wang, B.; Mao, Z.; Gao, C.; et al. Cytotoxicity effects of metal oxide nanoparticles in human tumor cell lines. J. Phys. Conf. Ser. 2011, 304, 012046. [Google Scholar] [CrossRef] [Green Version]
- Cambre, M.H.; Holl, N.J.; Wang, B.; Harper, L.; Lee, H.J.; Chusuei, C.C.; Hou, F.Y.S.; Williams, E.T.; Argo, J.D.; Pandey, R.R.; et al. Cytotoxicity of NiO and Ni(OH)2 nanoparticles is mediated by oxidative stress-induced cell death and suppression of cell proliferation. Int. J. Mol. Sci. 2020, 21, 2355. [Google Scholar] [CrossRef] [Green Version]
- Forest, V.; Leclerc, L.; Hochepied, J.-F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol. Vitr. 2017, 38, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Boskabadi, S.H.; Balanezhad, S.Z.; Neamati, A.; Tabrizi, M.H. The green-synthesized zinc oxide nanoparticle as a novel natural apoptosis inducer in human breast (MCF7 and MDA-MB231) and colon (HT-29) cancer cells. Inorg. Nano-Metal Chem. 2021, 51, 733–743. [Google Scholar] [CrossRef]
- Di Bucchianico, S.; Gliga, A.R.; Åkerlund, E.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Calcium-dependent cyto- and genotoxicity of nickel metal and nickel oxide nanoparticles in human lung cells. Part. Fibre Toxicol. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 2011, 6, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tientong, J.; Garcia, S.; Thurber, C.R.; Golden, T.D. Synthesis of nickel and nickel hydroxide nanopowders by simplified chemical reduction. J. Nanotechnol. 2014, 2014, 193162. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.S.; Gillespie, P.A.; Chen, L.C. Inhalation exposure to nickel hydroxide nanoparticles induces systemic acute phase response in mice. Toxicol. Res. 2011, 27, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovalenko, V.; Kotok, V. Synthesis OF Ni(OH)2, Suitable For Supercapacitor Application, By The Cold Template Homogeneous Precipitation Method. East.-Eur. J. Enterp. Technol. 2021, 2, 45–51. [Google Scholar] [CrossRef]
- Vinichenko, Y.P.; Sidorova, E.N. Synthesis and characterization of nickel hydroxide nanoparticles obtained by chemical deposition method under different precipitation conditions. J. Phys. Conf. Ser. 2016, 741, 12194. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, B.; Alsalme, A.; Alharthi, F.A.; Mani, D.; Anandan, K.; Amutha, P.; Sobral, A.J.F.N. Synthesis, characterization of gelatin assisted ZnO and its effective utilization of toxic azo dye degradation under direct sunlight. Opt. Mater. 2021, 113, 110854. [Google Scholar] [CrossRef]
- Hua, J. Synthesis and characterization of gold nanoparticles (AuNPs) and ZnO decorated zirconia as a potential adsorbent for enhanced arsenic removal from aqueous solution. J. Mol. Struct. 2021, 1228, 129482. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S. Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3+2] cycloaddition of azides and alkynes at room temperature. J. Colloid Interface Sci. 2015, 457, 141–147. [Google Scholar] [CrossRef]
- Salmani, M.H.; Abedi, M.; Mozaffari, S.A.; Mahvi, A.H.; Sheibani, A.; Jalili, M. Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J. Environ. Health Sci. Eng. 2021, 19, 603–612. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.Y.; Song, K.Y.; Park, J.S.; Lee, H.J. Hydrothermal synthesis of mesoporous ZnO microspheres as NOX gas sensor materials—Calcination effects on microstructure and sensing performance. Ceram. Int. 2020, 46, 19354–19364. [Google Scholar] [CrossRef]
- Baykal, A.; Kavas, H.; Durmuş, Z.; Demir, M.; Kazan, S.; Topkaya, R.; Toprak, M.S. Sonochemical synthesis and chracterization of Mn3O4 nanoparticles. Cent. Eur. J. Chem. 2010, 8, 633–638. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Kalaivani, S.S.; Manikandan, A.; Kavitha, H.P.; Srinivasan, R.; Balasubramanian, N. Sonochemical synthesis and visible light induced photocatalytic property of reduced graphene oxide@ZnO hexagonal hollow rod nanocomposite. J. Alloys Compd. 2020, 836, 155377. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green synthesis of iron nanoparticles by Acacia nilotica pods extract and its catalytic, adsorption, and antibacterial activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef] [Green Version]
- Usman Ahme, M.; Noseh Dahi, J.; Yada Sudi, I.; Gabriel, S.; Kulini Joh, I. Green Synthesis of Manganese Oxide Nanoparticles from Cassia tora Leaves and its Toxicological Evaluation. Asian J. Appl. Sci. 2020, 13, 60–67. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Das, B.; Mohanty, A.; Mohapatra, S. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Appl. Nanosci. 2017, 7, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Hrnčič, M.K.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview-phytochemical profile, isolation methods, and application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajadi, S.M. Synthesis and characterization of titanium dioxide nanoparticles using Euphorbia heteradena Jaub root extract and evaluation of their stability. Ceram. Int. 2015, 41, 14435–14439. [Google Scholar] [CrossRef]
- Joshi, N.; Pathak, A.; Anupam, R.; Jain, N.; Singh, J.; Upadhyaya, C.P. A Rapid and Efficient Biosynthesis of Metallic Nanoparticles Using Aqueous Extract of Chia (Salvia hispanica L.) Seeds. Bionanoscience 2019, 9, 893–902. [Google Scholar] [CrossRef]
- de Falco, B.; Amato, M.; Lanzotti, V. Chia seeds products: An overview. Phytochem. Rev. 2017, 16, 745–760. [Google Scholar] [CrossRef]
- Divyapriya, G.K.; Veeresh, D.J.; Yavagal, P.C. Evaluation of Antibacterial Efficacy of Chia (Salvia hispanica) Seeds Extract Against Porphyromonas Gingivalis, Fusobacterium Nucleatum and Aggregatibacter Actinomycetemcomitans-an in-Vitro Study. Int. J. Ayurveda Pharma Res. 2016, 4, 2322–2902. [Google Scholar]
- Hernández-Morales, L.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Espinoza, K.A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci. 2019, 489, 952–961. [Google Scholar] [CrossRef]
- Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using chia seeds extract and evaluation of its electrochemical activity. Processes 2021, 9, 2027. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Khalaf, H.S.; Mohamed, S.F.; Hussien, H.A.; Kutkat, O.M.; Amr, A.E. Synthesis and antiviral activity of 1,2,3-triazole glycosides based substituted pyridine via click cycloaddition. Russ. J. Gen. Chem. 2017, 87, 2444–2453. [Google Scholar] [CrossRef]
- Hassan, A.S.; Mady, M.F.; Awad, H.M.; Hafez, T.S. Synthesis and antitumor activity of some new pyrazolo[1,5-a]pyrimidines. Chin. Chem. Lett. 2017, 28, 388–393. [Google Scholar] [CrossRef]
- Samrot, A.V.; Lavanya Agnes Angalene, J.; Roshini, S.M.; Stefi, S.M.; Preethi, R.; Raji, P.; Madan Kumar, A.; Suresh Kumar, S. Purification, characterization and exploitation of Azadirachta indica gum for the production of drug loaded nanocarrier. Mater. Res. Express 2020, 7, 055007. [Google Scholar] [CrossRef]
- Jia, S.; Yu, H.; Lin, Y.; Dai, Y. Characterization of extracellular polysaccharides from Nostoc flagelliforme cells in liquid suspension culture. Biotechnol. Bioprocess Eng. 2007, 12, 271–275. [Google Scholar] [CrossRef]
- Barakat, M.A.; Anjum, M.; Kumar, R.; Alafif, Z.O.; Oves, M.; Ansari, M.O. Design of ternary Ni(OH)2/graphene oxide/TiO2 nanocomposite for enhanced photocatalytic degradation of organic, microbial contaminants, and aerobic digestion of dairy wastewater. J. Clean. Prod. 2020, 258, 120588. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Khalifa, R.E.; El Sohaimy, S.A. Functional Properties of Chia Seed Mucilage Supplemented In Low Fat Yoghurt. Alex. Sci. Exch. J. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Hassanin, H.A.; Taha, A.; Afkar, E. Novel bio-mediated Ag/Co3O4 nanocomposites of different weight ratios using aqueous neem leaf extract: Catalytic and microbial behaviour. Ceram. Int. 2020, 47, 3099–3107. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Muralidharan, G. Electrochemical supercapacitor behaviour of α-Ni(OH)2 nanoparticles synthesized via green chemistry route. J. Electroanal. Chem. 2014, 727, 53–58. [Google Scholar] [CrossRef]
- Shamish, Z.; Zohar, M.; Shamir, D.; Burg, A. Controlling the size and pattern pitch of ni(OH)2 nanoclusters using dip-pen nanolithography to improve water oxidation. Molecules 2020, 25, 2937. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, D.G.; Zhu, H.L.; Qi, Y.X.; Li, H.; Lun, N.; Bai, Y.J. Mn3O4/Ni(OH)2 nanocomposite as an applicable electrode material for pseudocapacitors. Electrochim. Acta 2017, 249, 155–165. [Google Scholar] [CrossRef]
- Parsaee, Z. Synthesis of novel amperometric urea-sensor using hybrid synthesized NiO-NPs/GO modified GCE in aqueous solution of cetrimonium bromide. Ultrason. Sonochem. 2018, 44, 120–128. [Google Scholar] [CrossRef]
- MacCuspie, R.I.; Rogers, K.; Patra, M.; Suo, Z.; Allen, A.J.; Martin, M.N.; Hackley, V.A. Challenges for physical characterization of silver nanoparticles under pristine and environmentally relevant conditions. J. Environ. Monit. 2011, 13, 1212–1226. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.K.; Srivastava, P.; Ameen, S.; Akhtar, M.S.; Singh, G.; Yadava, S. Azadirachta indica plant-assisted green synthesis of Mn3O4 nanoparticles: Excellent thermal catalytic performance and chemical sensing behavior. J. Colloid Interface Sci. 2016, 472, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Karthik, P.E.; Kundu, S. Petal-like hierarchical array of ultrathin Ni(OH)2 nanosheets decorated with Ni(OH)2 nanoburls: A highly efficient OER electrocatalyst. Catal. Sci. Technol. 2017, 7, 882–893. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef] [Green Version]
- Stern, L.A.; Hu, X. Enhanced oxygen evolution activity by NiOx and Ni(OH)2 nanoparticles. Faraday Discuss. 2014, 176, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Ted Kroon, R.E. Nanoscience and the scherrer equation versus the “scherrer-gottingen equation”. S. Afr. J. Sci. 2013, 109, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Li, W.; Liu, L. Vertically aligned porous Nickel(II) hydroxide nanosheets supported on carbon paper with long-term oxygen evolution performance. Chem. Asian J. 2017, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, G.H.; Li, L.H.; Liu, L.; Xu, Y.; Ding, H.Y.; Zhang, T. Large-Scale Self-Assembly of 3D Flower-like Hierarchical Ni/Co-LDHs Microspheres for High-Performance Flexible Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Kim, S.J.; Jang, J.S.; Kim, D.H.; Kim, I.D. Catalytic Metal Nanoparticles Embedded in Conductive Metal–Organic Frameworks for Chemiresistors: Highly Active and Conductive Porous Materials. Adv. Sci. 2019, 6, 1900250. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Zinc oxide quantum dots: A potential candidate to detain liver cancer cells. Bioprocess Biosyst. Eng. 2015, 38, 155–163. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanwal, S.; Rizwan, K.; Shahid, S. Enhanced antimicrobial, antioxidant, in vivo antitumor and in vitro anticancer effects against breast cancer cell line by green synthesized un-doped SnO2 and Co-doped SnO2 nanoparticles from Clerodendrum inerme. Microb. Pathog. 2018, 125, 366–384. [Google Scholar] [CrossRef] [PubMed]

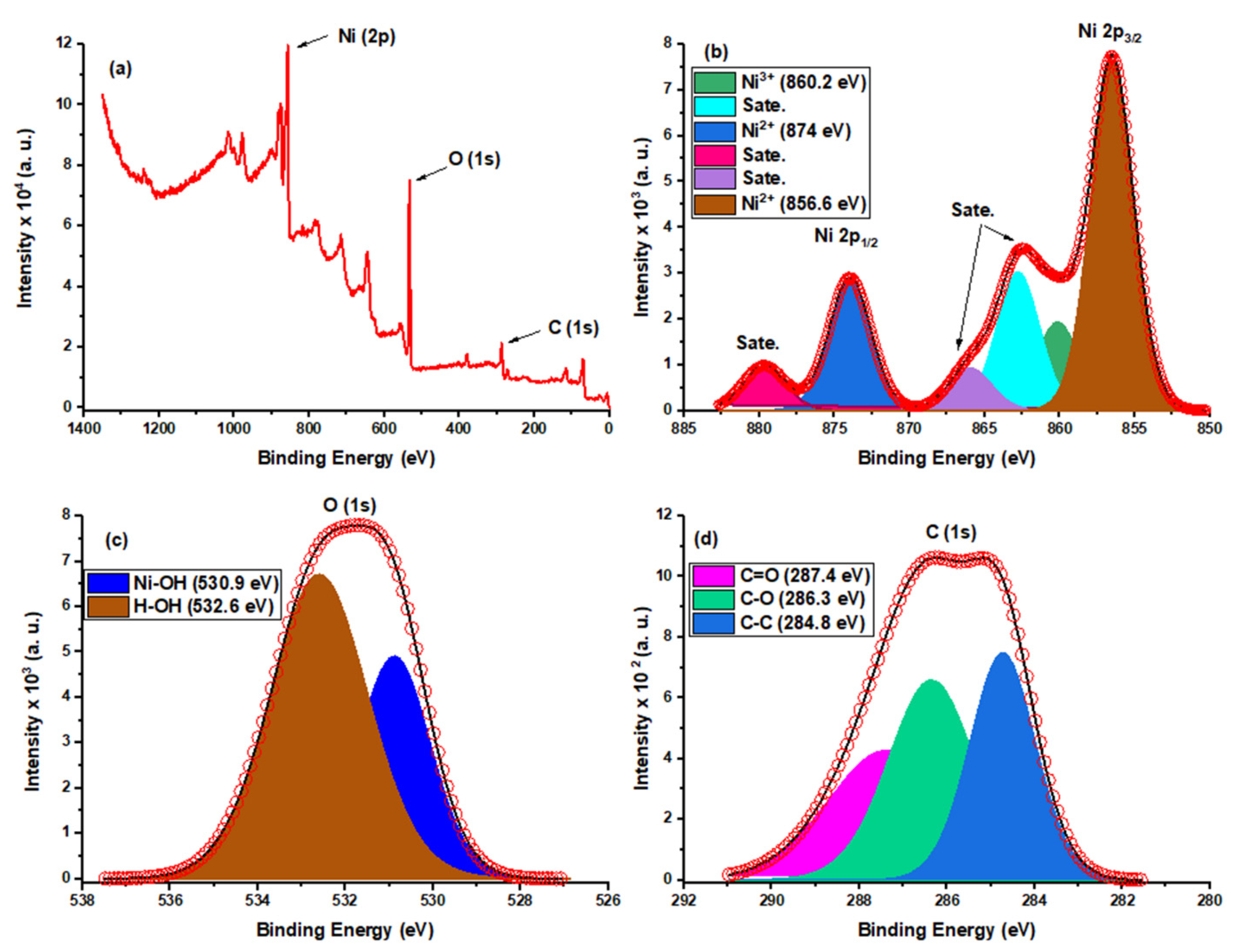


| β-Ni(OH)2 | Peak (BE) | FWHM (eV) | Atomic% |
|---|---|---|---|
| Ni 2p | 857.27 | 5.52 | 22.95 |
| O 1s | 532.77 | 4.26 | 58.95 |
| C 1s | 286.51 | 4.35 | 18.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanin, H.A.; Taha, A. Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity. Nanomaterials 2022, 12, 1919. https://doi.org/10.3390/nano12111919
Hassanin HA, Taha A. Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity. Nanomaterials. 2022; 12(11):1919. https://doi.org/10.3390/nano12111919
Chicago/Turabian StyleHassanin, Hanaa A., and Amel Taha. 2022. "Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity" Nanomaterials 12, no. 11: 1919. https://doi.org/10.3390/nano12111919
APA StyleHassanin, H. A., & Taha, A. (2022). Sonochemical-Assisted Biogenic Synthesis of Theophrasite β-Ni(OH)2 Nanocluster Using Chia Seeds Extract: Characterization and Anticancer Activity. Nanomaterials, 12(11), 1919. https://doi.org/10.3390/nano12111919






