The Other Dimension—Tuning Hole Extraction via Nanorod Width
Abstract
1. Introduction
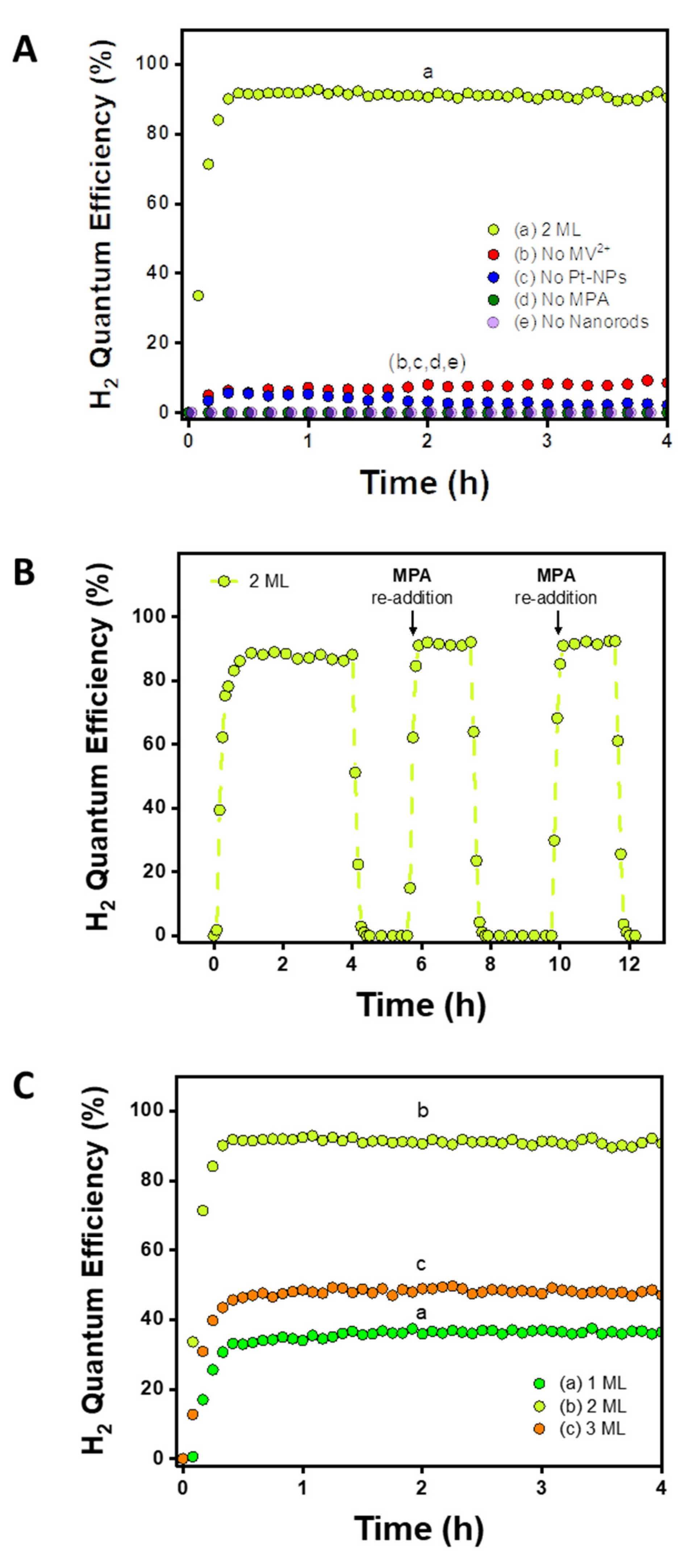
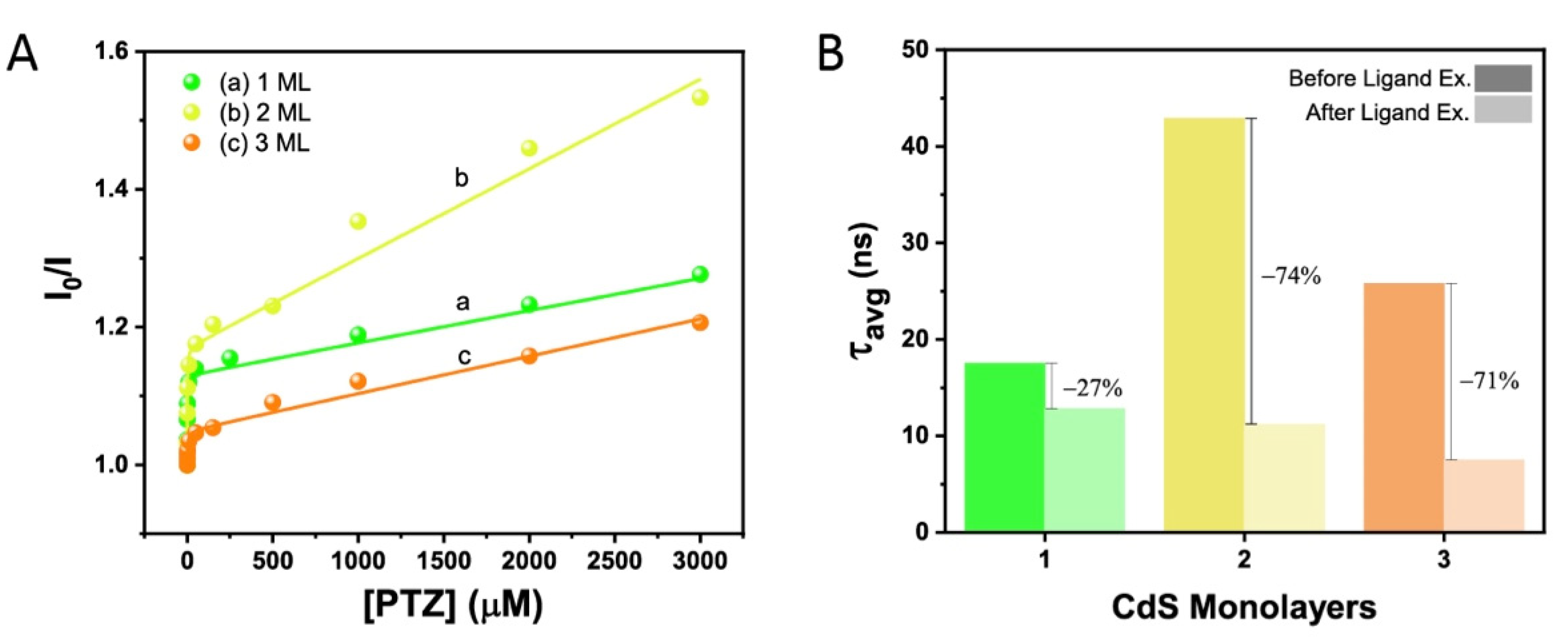
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalisman, P.; Nakibli, Y.; Amirav, L. Perfect Photon-to-Hydrogen Conversion Efficiency. Nano Lett. 2016, 16, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Wächtler, M.; Kalisman, P.; Amirav, L. Charge-Transfer Dynamics in Nanorod Photocatalysts with Bimetallic Metal Tips. J. Phys. Chem. C 2016, 120, 24491–24497. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Luminescence Studies of Individual Quantum Dot Photocatalysts. J. Am. Chem. Soc. 2013, 135, 13049–13053. [Google Scholar] [CrossRef][Green Version]
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.; Adrovic, A.; Volbers, D.; Wyrwich, R.; Doblinger, M.; Susha, A.S.; Rogach, A.L.; et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Berr, M.J.; Wagner, P.; Fischbach, S.; Vaneski, A.; Schneider, J.; Susha, A.S.; Rogach, A.L.; Jackel, F.; Feldmann, J. Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation. Appl. Phys. Lett. 2012, 100, 223903. [Google Scholar] [CrossRef]
- Acharya, K.P.; Khnayzer, R.S.; O’Connor, T.; Diederich, G.; Kirsanova, M.; Klinkova, A.; Roth, D.; Kinder, E.; Imboden, M.; Zamkov, M. The Role of Hole Localization in Sacrificial Hydrogen Production by Semiconductor–Metal Heterostructured Nanocrystals. Nano Lett. 2011, 11, 2919–2926. [Google Scholar] [CrossRef]
- Wu, K.F.; Chen, Z.Y.; Lv, H.J.; Zhu, H.M.; Hill, C.L.; Lian, T.Q. Hole Removal Rate Limits Photodriven H2 Generation Efficiency in CdS-Pt and CdSe/CdS-Pt Semiconductor Nanorod-Metal Tip Heterostructures. J. Am. Chem. Soc. 2014, 136, 7708–7716. [Google Scholar] [CrossRef]
- Agosti, A.; Nakibli, Y.; Amirav, L.; Bergamini, G. Photosynthetic H2 generation and organic transformations with CdSe@CdS-Pt nanorods for highly efficient solar-to-chemical energy conversion. Nano Energy 2020, 70, 104510. [Google Scholar] [CrossRef]
- Agosti, A.; Natali, M.; Amirav, L.; Bergamini, G. Towards Solar Factories: Prospects of Solar-to-Chemical Energy Conversion using Colloidal Semiconductor Photosynthetic Systems. ChemSusChem 2020, 13, 4894–4899. [Google Scholar] [CrossRef]
- Yehezkeli, O.; de Oliveira, D.R.B.; Cha, J.N. Electrostatically Assembled CdS–Co3O4 Nanostructures for Photo-assisted Water Oxidation and Photocatalytic Reduction of Dye Molecules. Small 2015, 11, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Kalisman, P.; Kauffmann, Y.; Amirav, L. Photochemical oxidation on nanorod photocatalysts. J. Mater. Chem. A 2015, 3, 3261–3265. [Google Scholar] [CrossRef]
- Andrews, J.L.; Cho, J.; Wangoh, L.; Suwandaratne, N.; Sheng, A.; Chauhan, S.; Nieto, K.; Mohr, A.; Kadassery, K.J.; Popeil, M.R.; et al. Hole Extraction by Design in Photocatalytic Architectures Interfacing CdSe Quantum Dots with Topochemically Stabilized Tin Vanadium Oxide. J. Am. Chem. Soc. 2018, 140, 17163–17174. [Google Scholar] [CrossRef] [PubMed]
- Becker-Koch, D.; Albaladejo-Siguan, M.; Hofstetter, Y.J.; Solomeshch, O.; Pohl, D.; Rellinghaus, B.; Tessler, N.; Vaynzof, Y. Doped Organic Hole Extraction Layers in Efficient PbS and AgBiS2 Quantum Dot Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 18750–18757. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Ghosh, H.N. Hot-Hole Extraction from Quantum Dot to Molecular Adsorbate. Chem.-A Eur. J. 2015, 21, 4405–4412. [Google Scholar] [CrossRef]
- Tarafder, K.; Surendranath, Y.; Olshansky, J.H.; Alivisatos, A.P.; Wang, L.W. Hole Transfer Dynamics from a CdSe/CdS Quantum Rod to a Tethered Ferrocene Derivative. J. Am. Chem. Soc. 2014, 136, 5121–5131. [Google Scholar] [CrossRef]
- Tseng, H.W.; Wilker, M.B.; Damrauer, N.H.; Dukovic, G. Charge Transfer Dynamics between Photoexcited CdS Nanorods and Mononuclear Ru Water-Oxidation Catalysts. J. Am. Chem. Soc. 2013, 135, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Pearce, O.M.; Duncan, J.S.; Damrauer, N.H.; Dukovic, G. Ultrafast Hole Transfer from CdS Quantum Dots to a Water Oxidation Catalyst. J. Phys. Chem. C 2018, 122, 17559–17565. [Google Scholar] [CrossRef]
- Dong, K.; Le, T.-A.; Nakibli, Y.; Schleusener, A.; Wächtler, M.; Amirav, L. Molecular Metallocorrole - Nanorod Photocatalytic System for Sustainable Hydrogen Production. ChemSusChem 2022, 15, e202200804. [Google Scholar] [CrossRef]
- Bridewell, V.L.; Alam, R.; Karwacki, C.J.; Kamat, P.V. CdSe/CdS Nanorod Photocatalysts: Tuning the Interfacial Charge Transfer Process through Shell Length. Chem. Mater. 2015, 27, 5064–5071. [Google Scholar] [CrossRef]
- Coropceanu, I.; Rossinelli, A.; Caram, J.R.; Freyria, F.S.; Bawendi, M.G. Slow-Injection Growth of Seeded CdSe/CdS Nanorods with Unity Fluorescence Quantum Yield and Complete Shell to Core Energy Transfer. ACS Nano 2016, 10, 3295–3301. [Google Scholar] [CrossRef]
- Khon, E.; Lambright, K.; Khnayzer, R.S.; Moroz, P.; Perera, D.; Butaeva, E.; Lambright, S.; Castellano, F.N.; Zamkov, M. Improving the Catalytic Activity of Semiconductor Nanocrystals through Selective Domain Etching. Nano Lett. 2013, 13, 2016–2023. [Google Scholar] [CrossRef][Green Version]
- Olshansky, J.H.; Ding, T.X.; Lee, Y.V.; Leone, S.R.; Alivisatos, A.P. Hole Transfer from Photoexcited Quantum Dots: The Relationship between Driving Force and Rate. J. Am. Chem. Soc. 2015, 137, 15567–15575. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Zhang, J.; Li, C.; Xu, X.; Jin, Y. Shell Thickness Engineering Significantly Boosts the Photocatalytic H2 Evolution Efficiency of CdS/CdSe Core/Shell Quantum Dots. ACS Appl. Mater. Interfaces 2017, 9, 35712–35720. [Google Scholar] [CrossRef]
- Brown, K.A.; Song, Q.; Mulder, D.W.; King, P.W. Diameter Dependent Electron Transfer Kinetics in Semiconductor–Enzyme Complexes. ACS Nano 2014, 8, 10790–10798. [Google Scholar] [CrossRef]
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Nelson, J.H.; Shevchenko, E.V.; Aloni, S.; Sadtler, B.; Alivisatos, A.P. Seeded growth of highly luminescent CdSe/CdS nanoheterostructures with rod and tetrapod morphologies. Nano Lett. 2007, 7, 2951–2959. [Google Scholar] [CrossRef]
- Hill, L.J.; Bull, M.M.; Sung, Y.; Simmonds, A.G.; Dirlam, P.T.; Richey, N.E.; DeRosa, S.E.; Shim, I.B.; Guin, D.; Costanzo, P.J.; et al. Directing the Deposition of Ferromagnetic Cobalt onto Pt-Tipped CdSe@CdS Nanorods: Synthetic and Mechanistic Insights. ACS Nano 2012, 6, 8632–8645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef]
- Wu, K.F.; Rodriguez-Cordoba, W.E.; Liu, Z.; Zhu, H.M.; Lian, T.Q. Beyond Band Alignment: Hole Localization Driven Formation of Three Spatially Separated Long-Lived Exciton States in CdSe/CdS Nanorods. Acs Nano 2013, 7, 7173–7185. [Google Scholar] [CrossRef]
- Sitt, A.; Hadar, I.; Banin, U. Band-gap engineering, optoelectronic properties and applications of colloidal heterostructured semiconductor nanorods. Nano Today 2013, 8, 494–513. [Google Scholar] [CrossRef]
- Bronstein, N.D.; Li, L.; Xu, L.; Yao, Y.; Ferry, V.E.; Alivisatos, A.P.; Nuzzo, R.G. Luminescent Solar Concentration with Semiconductor Nanorods and Transfer-Printed Micro-Silicon Solar Cells. ACS Nano 2014, 8, 44–53. [Google Scholar] [CrossRef]
- Nakibli, Y.; Mazal, Y.; Dubi, Y.; Wächtler, M.; Amirav, L. Size Matters: Cocatalyst Size Effect on Charge Transfer and Photocatalytic Activity. Nano Lett. 2018, 18, 357–364. [Google Scholar] [CrossRef]
- Zhu, H.; Song, N.; Lv, H.; Hill, C.L.; Lian, T. Near Unity Quantum Yield of Light-Driven Redox Mediator Reduction and Efficient H2 Generation Using Colloidal Nanorod Heterostructures. J. Am. Chem. Soc. 2012, 134, 11701–11708. [Google Scholar] [CrossRef]
- Wu, K.; Du, Y.; Tang, H.; Chen, Z.; Lian, T. Efficient Extraction of Trapped Holes from Colloidal CdS Nanorods. J. Am. Chem. Soc. 2015, 137, 10224–10230. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001. [Google Scholar]
- Landes, C.; Burda, C.; Braun, M.; El-Sayed, M.A. Photoluminescence of CdSe Nanoparticles in the Presence of a Hole Acceptor: n-Butylamine. J. Phys. Chem. B 2001, 105, 2981–2986. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Leppert, V.; Kelley, D.F. Static and Dynamic Emission Quenching in Core/Shell Nanorod Quantum Dots with Hole Acceptors. J. Phys. Chem. C 2009, 113, 19161–19171. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Tuning the Emission of CdSe Quantum Dots by Controlled Trap Enhancement. Langmuir 2010, 26, 11272–11276. [Google Scholar] [CrossRef]
- Micheel, M.; Liu, B.; Wächtler, M. Influence of Surface Ligands on Charge-Carrier Trapping and Relaxation in Water-Soluble CdSe@CdS Nanorods. Catalysts 2020, 10, 1143. [Google Scholar] [CrossRef]
- Kelestemur, Y.; Cihan, A.F.; Guzelturk, B.; Demir, H.V. Type-tunable amplified spontaneous emission from core-seeded CdSe/CdS nanorods controlled by exciton–exciton interaction. Nanoscale 2014, 6, 8509–8514. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shahar, Y.; Scotognella, F.; Waiskopf, N.; Kriegel, I.; Dal Conte, S.; Cerullo, G.; Banin, U. Effect of Surface Coating on the Photocatalytic Function of Hybrid CdS-Au Nanorods. Small 2015, 11, 462–471. [Google Scholar] [CrossRef]
- Diroll, B.T.; Turk, M.E.; Gogotsi, N.; Murray, C.B.; Kikkawa, J.M. Ultrafast Photoluminescence from the Core and the Shell in CdSe/CdS Dot-in-Rod Heterostructures. ChemPhysChem 2016, 17, 759–765. [Google Scholar] [CrossRef]
- Lupo, M.G.; Della Sala, F.; Carbone, L.; Zavelani-Rossi, M.; Fiore, A.; Lüer, L.; Polli, D.; Cingolani, R.; Manna, L.; Lanzani, G. Ultrafast Electron−Hole Dynamics in Core/Shell CdSe/CdS Dot/Rod Nanocrystals. Nano Lett. 2008, 8, 4582–4587. [Google Scholar] [CrossRef]
- Morgan, D.P.; Kelley, D.F. What Does the Transient Absorption Spectrum of CdSe Quantum Dots Measure? J. Phys. Chem. C 2020, 124, 8448–8455. [Google Scholar] [CrossRef]
- Grennell, A.N.; Utterback, J.K.; Pearce, O.M.; Wilker, M.B.; Dukovic, G. Relationships between Exciton Dissociation and Slow Recombination within ZnSe/CdS and CdSe/CdS Dot-in-Rod Heterostructures. Nano Lett. 2017, 17, 3764–3774. [Google Scholar] [CrossRef]
- Wang, L.; Nonaka, K.; Okuhata, T.; Katayama, T.; Tamai, N. Quasi-Type II Carrier Distribution in CdSe/CdS Core/Shell Quantum Dots with Type I Band Alignment. J. Phys. Chem. C 2018, 122, 12038–12046. [Google Scholar] [CrossRef]
- Wu, K.F.; Hill, L.J.; Chen, J.Q.; McBride, J.R.; Pavlopolous, N.G.; Richey, N.E.; Pyun, J.; Lian, T.Q. Universal Length Dependence of Rod-to-Seed Exciton Localization Efficiency in Type I and Quasi-Type II CdSe@CdS Nanorods. Acs Nano 2015, 9, 4591–4599. [Google Scholar] [CrossRef]
- Müller, C.; Pascher, T.; Eriksson, A.; Chabera, P.; Uhlig, J. KiMoPack: A python Package for Kinetic Modeling of the Chemical Mechanism. J. Phys. Chem. A 2022, 126, 4087–4099. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosner, T.; Pavlopoulos, N.G.; Shoyhet, H.; Micheel, M.; Wächtler, M.; Adir, N.; Amirav, L. The Other Dimension—Tuning Hole Extraction via Nanorod Width. Nanomaterials 2022, 12, 3343. https://doi.org/10.3390/nano12193343
Rosner T, Pavlopoulos NG, Shoyhet H, Micheel M, Wächtler M, Adir N, Amirav L. The Other Dimension—Tuning Hole Extraction via Nanorod Width. Nanomaterials. 2022; 12(19):3343. https://doi.org/10.3390/nano12193343
Chicago/Turabian StyleRosner, Tal, Nicholas G. Pavlopoulos, Hagit Shoyhet, Mathias Micheel, Maria Wächtler, Noam Adir, and Lilac Amirav. 2022. "The Other Dimension—Tuning Hole Extraction via Nanorod Width" Nanomaterials 12, no. 19: 3343. https://doi.org/10.3390/nano12193343
APA StyleRosner, T., Pavlopoulos, N. G., Shoyhet, H., Micheel, M., Wächtler, M., Adir, N., & Amirav, L. (2022). The Other Dimension—Tuning Hole Extraction via Nanorod Width. Nanomaterials, 12(19), 3343. https://doi.org/10.3390/nano12193343





