Silica Aerogel Hybridized with Melamine-Terephthalaldehyde Polymer for In-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environment Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
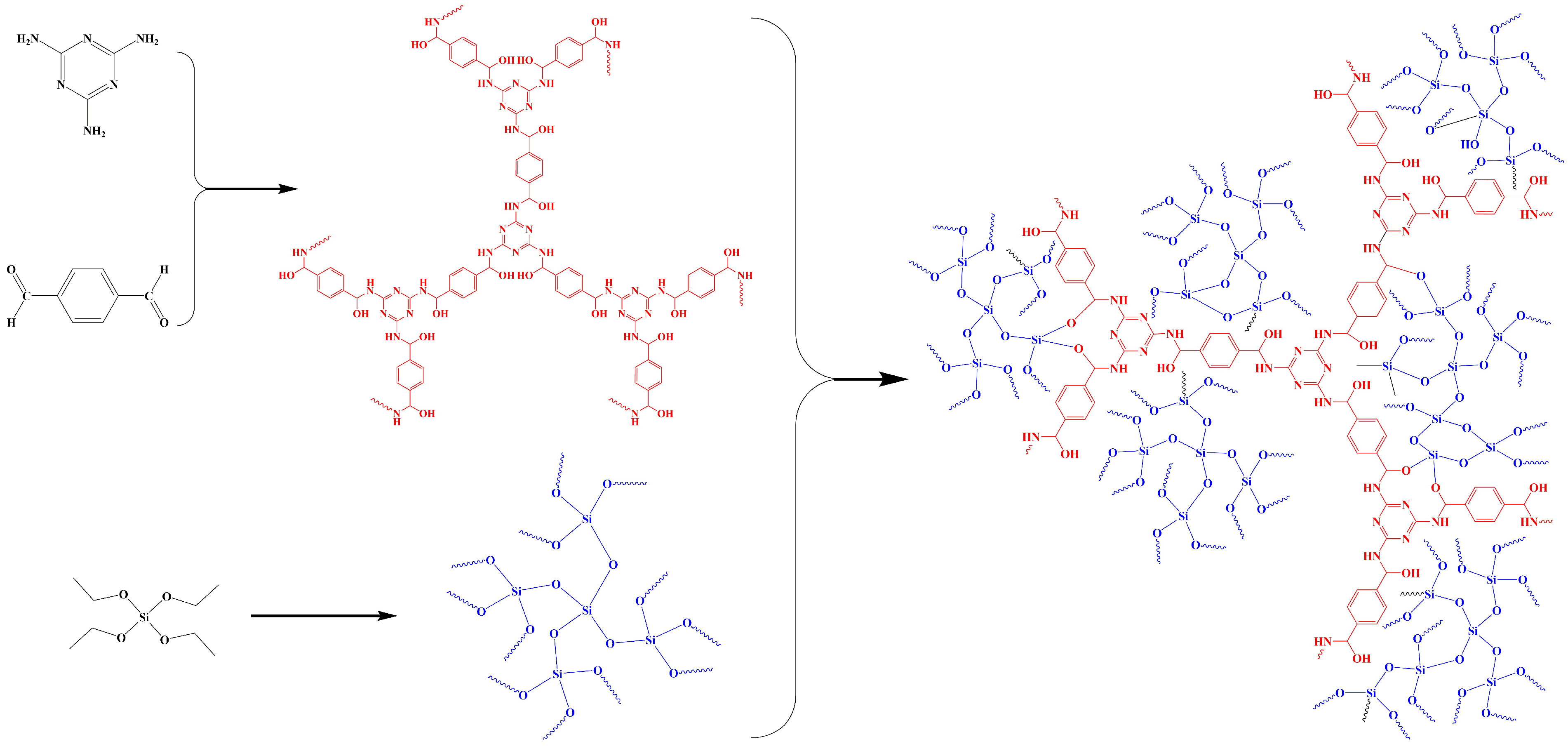
2.3. Preparation of Extraction Tube
2.4. Online Analytical Procedure
2.5. Sample Preparation
3. Results
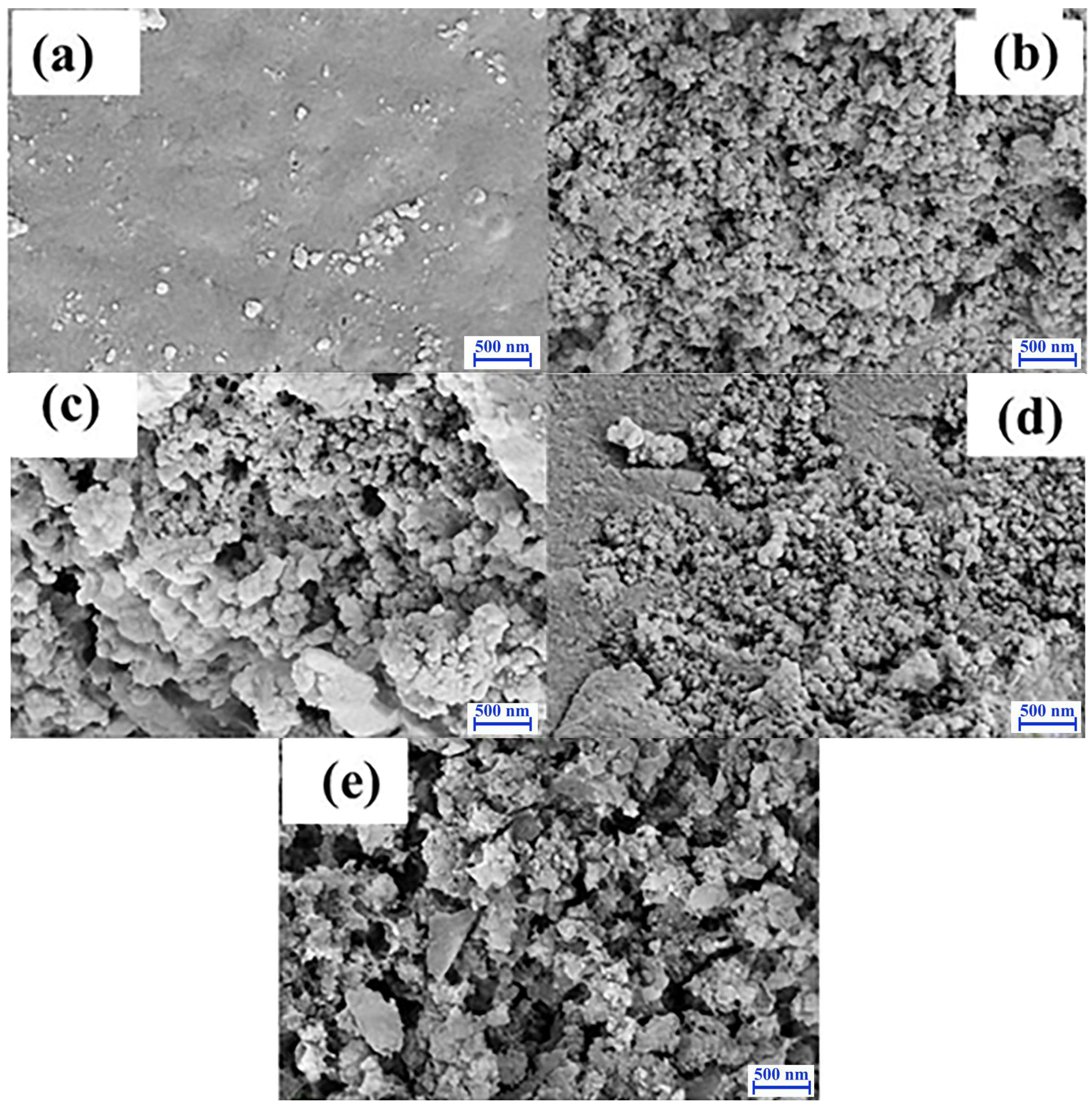
3.1. Characterization
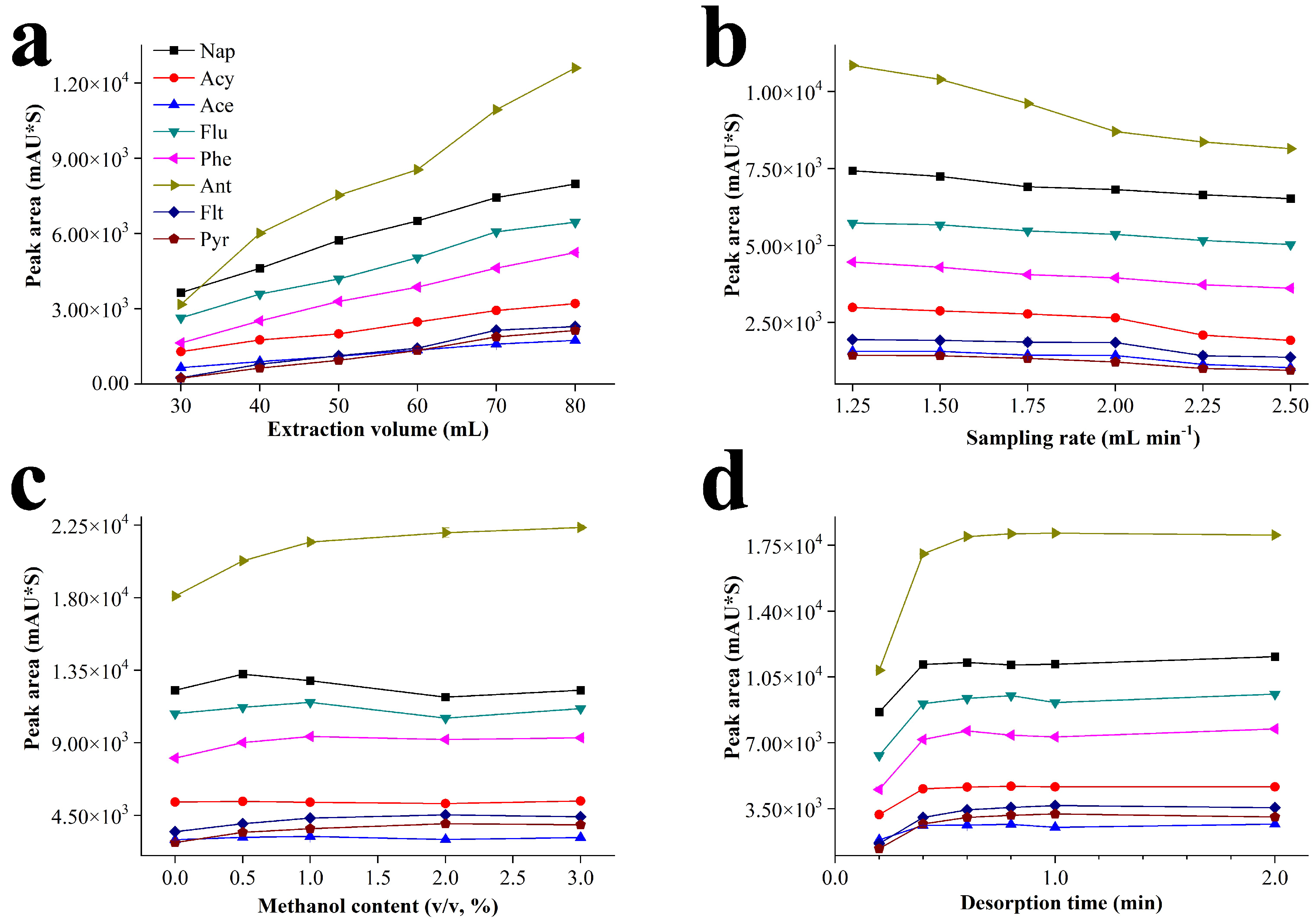
3.2. Investigation of Extraction and Desorption Conditions
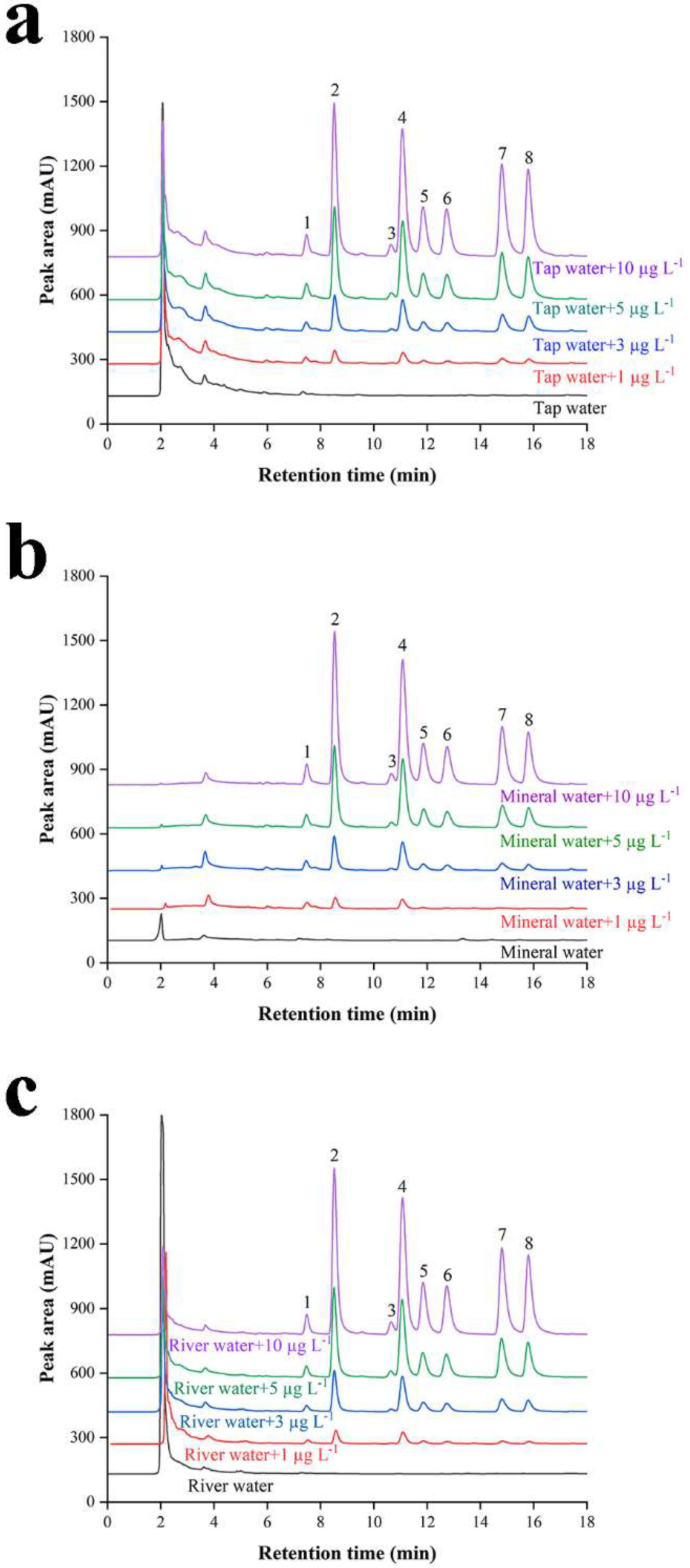
3.3. Method Evaluation and Sample Analysis
3.4. Comparison with Other Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moliner-Martinez, Y.; Herraez-Hernandez, R.; Verdu-Andres, J.; Molins-Legua, C.; Campins-Falco, P. Recent advances of in-tube solid-phase microextraction. TrAC-Trend. Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef]
- Li, C.; Sun, M.; Ji, X.; Han, S.; Feng, J.; Guo, W.; Feng, J. Triazine-based organic polymers@SiO2 nanospheres for sensitive solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2020, 43, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Oliveira, I.G.C.; Queiroz, M.E.C. Innovative extraction materials for fiber-in-tube solid phase microextraction: A review. Anal. Chim. Acta 2021, 1165, 238110. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Rodriguez, H.D.; Verdu-Andres, J.; Herráez-Hernández, R.; Campins-Falco, P. Innovations in extractive phases for in-tube solid-phase microextraction coupled to miniaturized liquid chromatography: A critical review. Molecules 2020, 25, 2460. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ji, X.; Li, C.; Sun, M.; Han, S.; Feng, J.; Sun, H.; Feng, Y.; Sun, M. Recent advance of new sample preparation materials in the analysis and detection of environmental pollutants. Chin. J. Chromatogr. 2021, 39, 781–801. [Google Scholar]
- Lashgari, M.; Yamini, Y. An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, S.; Wang, C.; Wang, Z. Advances in construction of triazine-based porous organic polymers and their applications in solid phase microextraction. Chin. J. Chromatogr. 2021, 39, 125–129. [Google Scholar] [CrossRef]
- Gao, W.; Cheng, J.; Yuan, X.; Tian, Y. Covalent organic framework-graphene oxide composite: A superior adsorption material for solid phase microextraction of bisphenol A. Talanta 2021, 222, 121501. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Ji, X.; Li, C.; Han, S.; Sun, H.; Sun, M. Recent advances of covalent organic frameworks for solid-phase microextraction. TrAC-Trend. Anal. Chem. 2021, 137, 116208. [Google Scholar] [CrossRef]
- Rocio-Bautista, P.; Pacheco-Fernandez, I.; Pasan, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Fernandez, I.; Rentero, M.; Ayala, J.H.; Pasan, J.; Pino, V. Green solid-phase microextraction fiber coating based on the metal-organic framework CIM-80(Al): Analytical performance evaluation in direct immersion and headspace using gas chromatography and mass spectrometry for the analysis of water, urine and brewed coffee. Anal. Chim. Acta 2020, 1133, 137–149. [Google Scholar] [PubMed]
- Song, X.-Y.; Chen, J.; Shi, Y.-P. Different configurations of carbon nanotubes reinforced solid-phase microextraction techniques and their applications in the environmental analysis. TrAC-Trend. Anal. Chem. 2017, 86, 263–275. [Google Scholar] [CrossRef]
- Ji, X.; Feng, J.; Li, C.; Han, S.; Sun, M.; Feng, J.; Sun, H.; Fan, J.; Guo, W. Application of biocharcoal aerogel sorbent for solid-phase microextraction of polycyclic aromatic hydrocarbons in water samples. J. Sep. Sci. 2020, 43, 4364–4373. [Google Scholar] [CrossRef]
- Aziz-Zanjani, M.O.; Mehdinia, A. A review on procedures for the preparation of coatings for solid phase microextraction. Microchim. Acta 2014, 181, 1169–1190. [Google Scholar] [CrossRef]
- Dietz, C.; Sanz, J.; Camara, C. Recent developments in solid-phase microextraction coatings and related techniques. J. Chromatogr. A 2006, 1103, 183–192. [Google Scholar] [CrossRef]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namiesnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef]
- Li, J.; Zhang, E.; Liu, Y.; Huang, H.; Su, Y.; Li, W. Preparation of the ultralow density aerogel and its application. Prog. Chem. 2020, 32, 713–726. [Google Scholar]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [Green Version]
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non-Cryst. Solids 2021, 562, 12770. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Han, S.; Ji, X.; Li, C.; Feng, J.; Sun, H.; Fan, J. Poly (ionic liquid)-hybridized silica aerogel for solid-phase microextraction of polycyclic aromatic hydrocarbons prior to gas chromatography-flame ionization detection. Microchim. Acta 2021, 188, 96. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, J.; Wang, X.; Luo, C.; Loussala, H.M.; Sun, M. An organic-inorganic hybrid silica aerogel prepared by co-precursor method for solid-phase microextraction coating. Talanta 2019, 194, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, J.; Ji, X.; Li, C.; Han, S.; Sun, M.; Feng, Y.; Feng, J.; Sun, H. Polyaniline/titanium dioxide nanorods functionalized carbon fibers for in-tube solid-phase microextraction of phthalate esters prior to high performance liquid chromatography-diode array detection. J. Chromatogr. A 2021, 1642, 462003. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Tang, M.; Xu, L. C-12-Ag wire as solid-phase microextraction fiber for determination of benzophenone ultraviolet filters in river water. J. Chromatogr. A 2013, 1298, 1–8. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; Wang, S.; Yu, J.; Wang, X.; Xie, F.; Liu, H.; Xie, J. Simultaneous determination of 11 monohydroxylated PAHs in human urine by stir bar sorptive extraction and liquid chromatography/tandem mass spectrometry. Talanta 2013, 116, 822–826. [Google Scholar] [CrossRef]
- Yazdi, M.N.; Yamini, Y.; Asiabi, H. Multiwall carbon nanotube- zirconium oxide nanocomposite hollow fiber solid phase microextraction for determination of polyaromatic hydrocarbons in water, coffee and tea samples. J. Chromatogr. A 2018, 1554, 8–15. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Bao, T.; Chen, Z. Polydopamine-based immobilization of zeolitic imidazolate framework-8 for in-tube solid-phase microextraction. J. Chromatogr. A 2015, 1388, 9–16. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Bu, Y.; Tian, Y.; Luo, C.; Sun, M. Mesoporous titanium oxide with high-specific surface area as a coating for in-tube solid-phase microextraction combined with high-performance liquid chromatography for the analysis of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2017, 40, 2474–2481. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Feng, J.; Tian, Y.; Luo, C.; Sun, M. Basalt fibers coated with nano-calcium carbonate for in-tube solid-phase microextraction and online analysis of estrogens coupled with high-performance liquid chromatography. Anal. Methods 2018, 10, 2234–2241. [Google Scholar] [CrossRef]
| Analytes | Linear Ranges (μg L−1) | LODs (ng L−1) | Linear Coefficients | EFs a | Repeatability (n = 5, RSD%) | |
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||
| Nap | 0.010–15.0 | 3.0 | 0.9962 | 2055 | 0.61 | 7.0 |
| Acy | 0.016–20.0 | 5.0 | 0.9997 | 2061 | 1.5 | 6.8 |
| Ace | 0.016–15.0 | 5.0 | 0.9997 | 2393 | 1.3 | 15 |
| Flu | 0.010–20.0 | 3.0 | 0.9990 | 2289 | 2.3 | 12 |
| Phe | 0.016–10.0 | 5.0 | 0.9989 | 2271 | 3.1 | 18 |
| Ant | 0.016–20.0 | 5.0 | 0.9993 | 1951 | 3.3 | 12 |
| Flt | 0.016–10.0 | 5.0 | 0.9937 | 2052 | 8.3 | 12 |
| Pyr | 0.016–10.0 | 5.0 | 0.9920 | 1724 | 8.2 | 9.5 |
| Real Samples | Analytes | Detection Results | Recovery (%) a | Recovery (%) b | Recovery (%) c | Recovery (%) d |
|---|---|---|---|---|---|---|
| Tap water | Nap | ND | 100 | 84.4 | 119 | 97.2 |
| Acy | ND | 83.8 | 96.6 | 102 | 101 | |
| Ace | ND | 84.4 | 81.9 | 113 | 112 | |
| Flu | ND | 87.2 | 80.5 | 112 | 106 | |
| Phe | ND | 89.4 | 89.3 | 116 | 126 | |
| Ant | ND | 99.8 | 89.2 | 117 | 121 | |
| Flt | ND | 102 | 96.9 | 118 | 118 | |
| Pyr | ND | 118 | 111 | 117 | 112 | |
| Mineral water | Nap | ND | 99.3 | 83.8 | 111 | 93.4 |
| Acy | ND | 87.9 | 93.0 | 97.6 | 94.1 | |
| Ace | ND | 98.9 | 91.4 | 95.0 | 97.9 | |
| Flu | ND | 83.0 | 92.5 | 99.9 | 96.1 | |
| Phe | ND | 83.2 | 87.8 | 100 | 105 | |
| Ant | ND | 86.1 | 86.9 | 103 | 106 | |
| Flt | ND | 87.8 | 81.9 | 93.1 | 107 | |
| Pyr | ND | 96.5 | 99.6 | 95.9 | 111 | |
| River water | Nap | NQ | 85.7 | 83.3 | 112 | 95.6 |
| Acy | ND | 85.6 | 89.7 | 107 | 91.8 | |
| Ace | ND | 80.8 | 84.6 | 117 | 104 | |
| Flu | ND | 90.0 | 93.1 | 116 | 98.3 | |
| Phe | ND | 97.5 | 86.6 | 114 | 121 | |
| Ant | ND | 90.4 | 94.8 | 119 | 104 | |
| Flt | ND | 121 | 114 | 106 | 113 | |
| Pyr | ND | 116 | 121 | 102 | 114 |
| Methods | Extraction Materials | LODs (ng L−1) | Linear Ranges (μg L−1) | Extraction Time (min) | Analytical Mode | References |
|---|---|---|---|---|---|---|
| In-tube SPME-HPLC-DAD | MT-SiO2 aerogel | 3.0–5.0 | 0.01–20 | 35 | Online | This work |
| Fiber SPME-HPLC-UVD | C12-Ag wire | 580–1860 | 5–200 | 60 | Offline | [24] |
| SBSE-HPLC-MS/MS | Polydimethylsiloxane | 1–22 | 0.01–100 | 180 | Offline | [25] |
| Fiber SPME-HPLC-UVD | Multiwall carbon nanotube/ZrO2 | 33–160 | 0.1–200 | 30 | Offline | [26] |
| In-tube SPME-HPLC-FLD | Zeolitic imidazolate framework-8 polydopamine | 5–50 | 0.01–5 | 25 | Online | [27] |
| In-tube SPME-HPLC-DAD | Mesoporous titanium oxide | 10–100 | 0.03–30 | 36 | Online | [28] |
| In-tube SPME-HPLC-DAD | Nano-calcium carbonate | 50–300 | 0.15–20 | 26 | Online | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Zhang, S.; Feng, J.; Sun, M. Silica Aerogel Hybridized with Melamine-Terephthalaldehyde Polymer for In-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environment Water. Nanomaterials 2022, 12, 1766. https://doi.org/10.3390/nano12101766
Jiang Q, Zhang S, Feng J, Sun M. Silica Aerogel Hybridized with Melamine-Terephthalaldehyde Polymer for In-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environment Water. Nanomaterials. 2022; 12(10):1766. https://doi.org/10.3390/nano12101766
Chicago/Turabian StyleJiang, Qiong, Shuwu Zhang, Juanjuan Feng, and Min Sun. 2022. "Silica Aerogel Hybridized with Melamine-Terephthalaldehyde Polymer for In-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environment Water" Nanomaterials 12, no. 10: 1766. https://doi.org/10.3390/nano12101766
APA StyleJiang, Q., Zhang, S., Feng, J., & Sun, M. (2022). Silica Aerogel Hybridized with Melamine-Terephthalaldehyde Polymer for In-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environment Water. Nanomaterials, 12(10), 1766. https://doi.org/10.3390/nano12101766





