Effect of Amorphous Crosslinker on Phase Behavior and Electro-Optic Response of Polymer-Stabilized Blue Phase Liquid Crystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
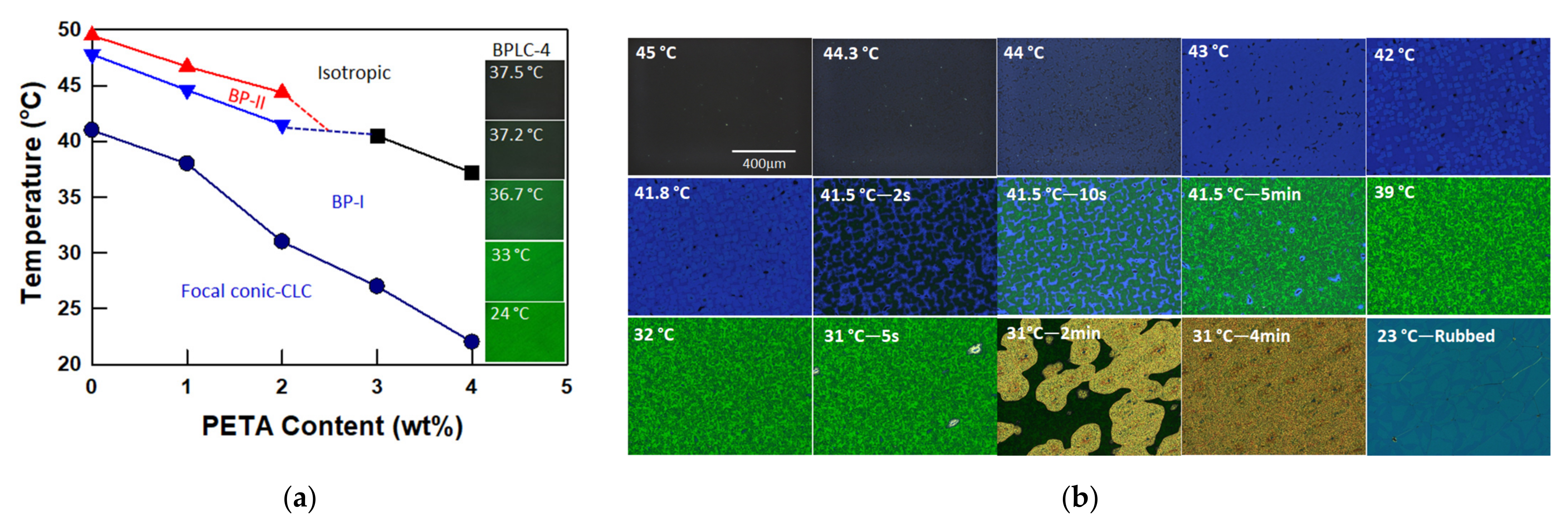
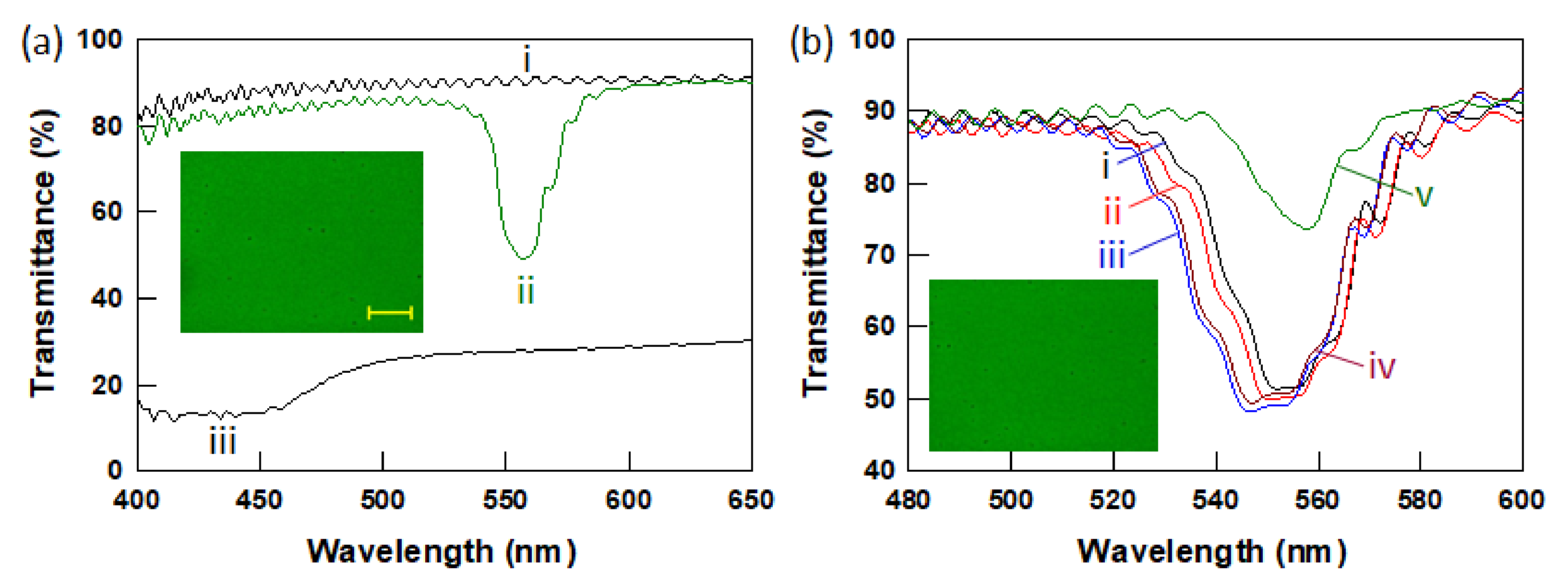
3.1. Phase Behavior of BPLCs with Various PETA Concentrations
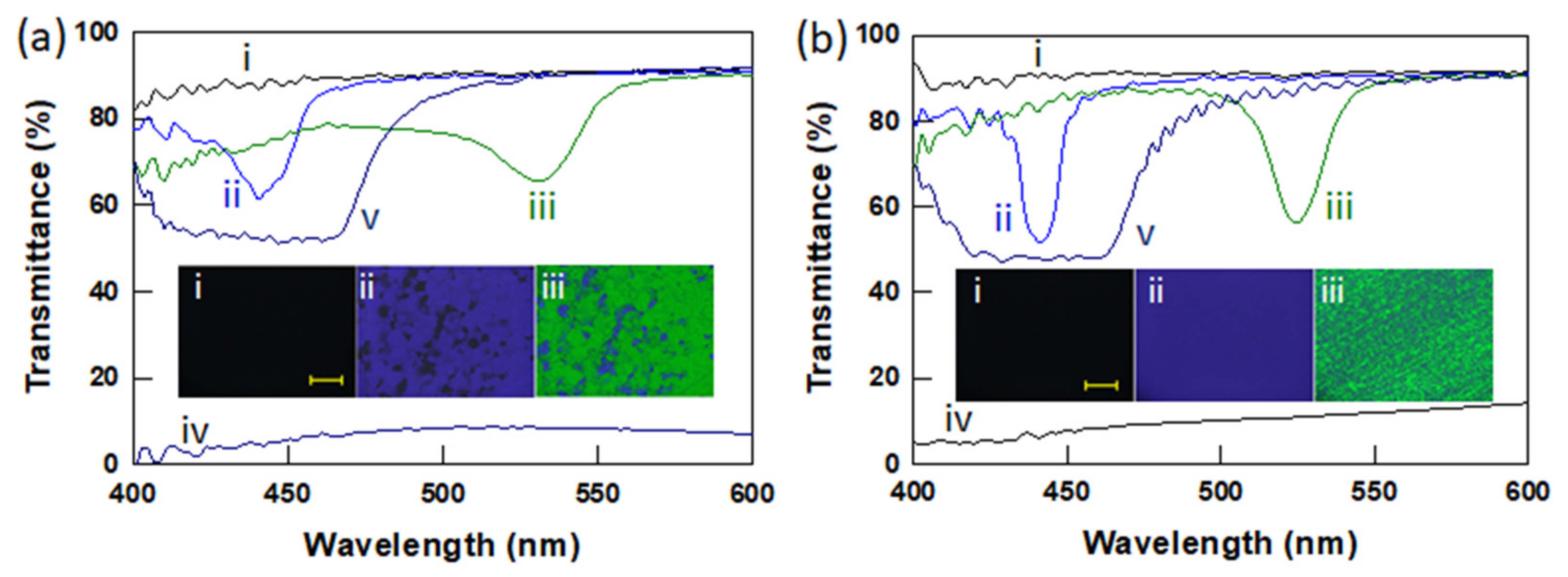
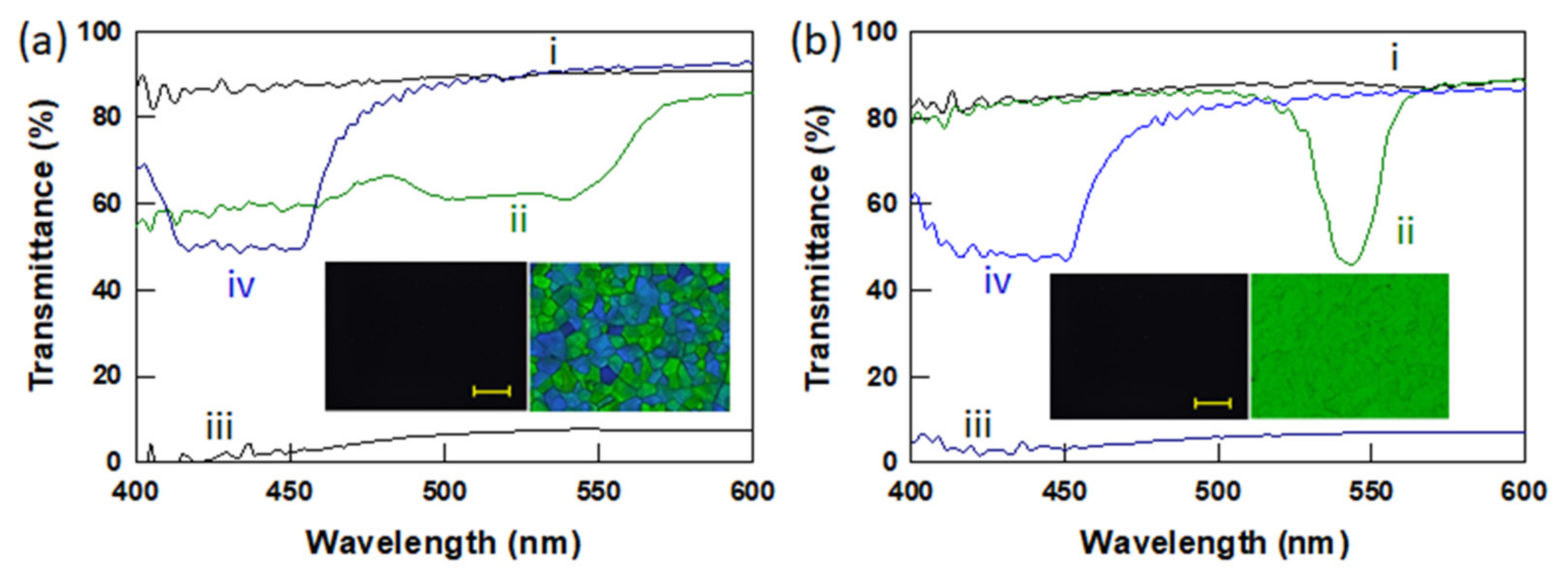
3.2. Polymer Stabilized BPLCs via In Situ Photopolymerization
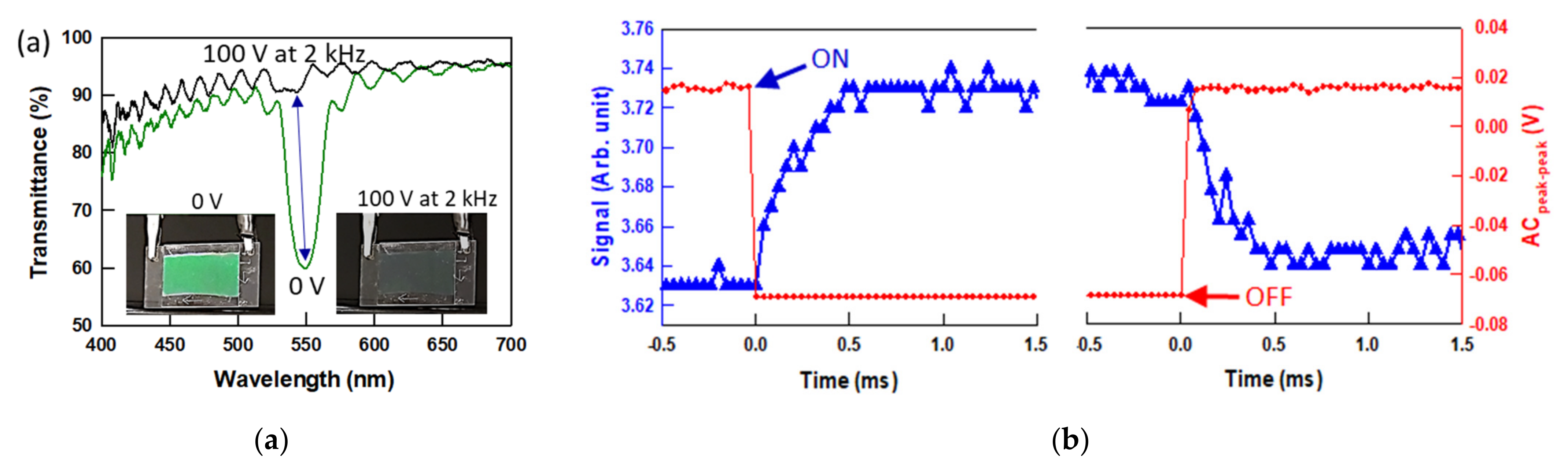
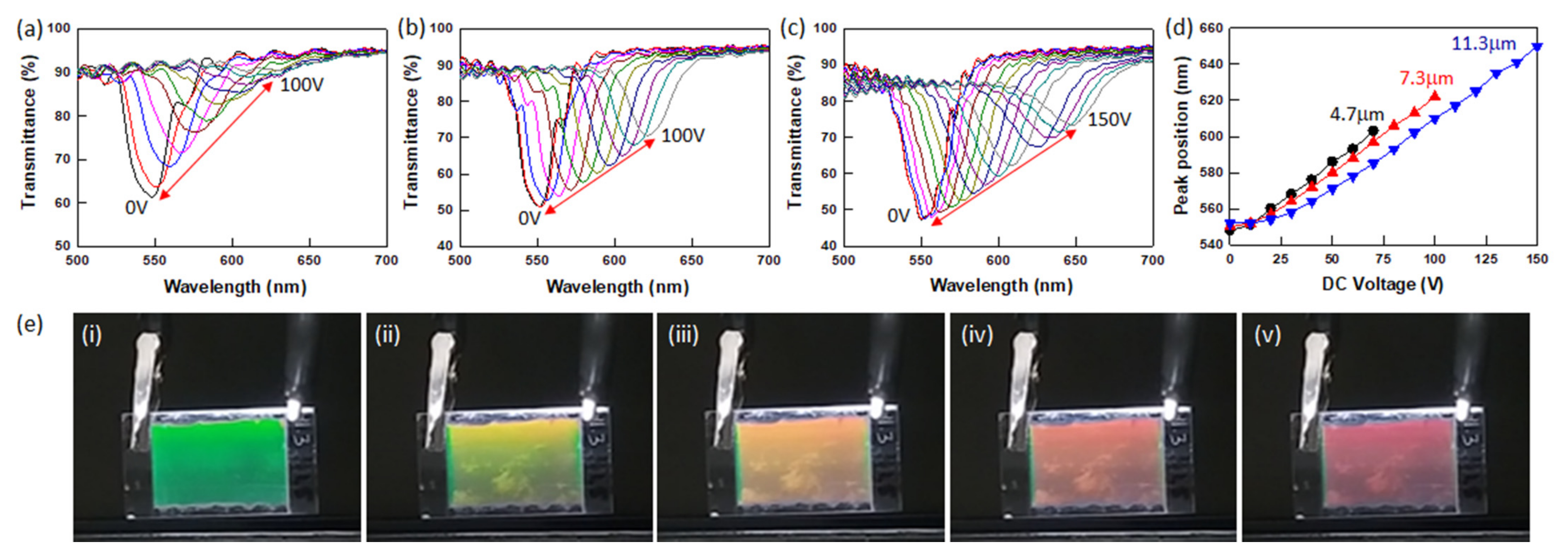
3.3. Electro-Optic Response of Polymer-Stabilized BPLCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castles, F.; Day, F.V.; Morris, S.M.; Ko, D.-H.; Gardiner, D.J.; Qasim, M.M.; Nosheen, S.; Hands, P.J.W.; Choi, S.S.; Friend, R.H.; et al. Blue-phase templated fabrication of three-dimensional nanostructures for photonic applications. Nat. Mater. 2012, 11, 599–603. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Coles, H.J.; Pivnenko, M.N. Liquid crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Lin, T.-H.; Li, Y.; Wang, C.-T.; Jau, H.-C.; Chen, C.-W.; Li, C.-C.; Bisoyi, H.K.; Bunning, T.J.; Li, Q. Red, green and blue reflections enabled in an optically tunable self-organized 3D cubic nanostructured thin film. Adv. Mater. 2013, 25, 5050–5054. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.-T.; Lee, B.R.; Gim, M.-J.; Park, K.-W.; Song, M.H.; Choi, S.-W. Liquid-crystalline blue phase laser with widely tunable wavelength. Adv. Mater. 2013, 25, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Castles, F.; Morris, S.M.; Hung, J.M.C.; Qasim, M.M.; Wright, A.D.; Nosheen, S.; Choi, S.S.; Outram, B.I.; Elston, S.J.; Burgess, C.; et al. Stretchable liquid-crystal blue-phase gels. Nat. Mater. 2014, 13, 817–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-W.; Li, C.-C.; Jau, H.-C.; Yu, L.-C.; Hong, C.-L.; Guo, D.-Y.; Wang, C.-T.; Lin, T.-H. Electric field-driven shifting and expansion of photonic band gaps in 3D liquid photonic crystals. ACS Photonics 2015, 2, 1524–1531. [Google Scholar] [CrossRef]
- Khoo, I.C.; Chen, C.-W.; Ho, T.-J. High efficiency holographic Bragg grating with optically prolonged memory. Sci. Rep. 2016, 6, 36148. [Google Scholar] [CrossRef]
- Cao, W.; Munoz, A.; Palffy-Muhoray, P.; Taheri, B. Lasing in a three-dimensional photonic crystal of the liquid crystal blue phase II. Nat. Mater. 2002, 1, 111–113. [Google Scholar] [CrossRef]
- Khoo, I.C.; Hong, K.L.; Zhao, S.; Ma, D.; Lin, T.-H. Blue-phase liquid crystal cored optical fiber array with photonic bandgaps and nonlinear transmission properties. Opt. Express 2013, 21, 4319–4327. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, S.-T. Electric field-induced monodomain blue phase liquid crystals. Appl. Phys. Lett. 2013, 102, 171110. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-T.; Yang, D.-K. Reflective Liquid Crystal Displays; Wiley: Chichester, UK, 2001. [Google Scholar]
- Tanaka, S.; Yoshida, H.; Kawata, Y.; Kuwahara, R.; Nishi, R.; Ozaki, M. Double-twist cylinders in liquid crystalline cholesteric blue phases observed by transmission electron microscopy. Sci. Rep. 2015, 5, 16180. [Google Scholar] [CrossRef] [Green Version]
- Kobashi, J.; Yoshida, H.; Ozaki, M. Planar optics with patterned chiral liquid crystals. Nat. Photonics 2016, 10, 389–392. [Google Scholar] [CrossRef]
- Jo, S.-Y.; Jeon, S.-W.; Kim, B.-C.; Bae, J.-H.; Araoka, F.; Choi, S.-W. Polymer stabilization of liquid-crystal blue phase II toward photonic crystals. ACS Appl. Mater. Interfaces 2017, 9, 8941–8947. [Google Scholar] [CrossRef]
- Kim, K.; Hur, S.-T.; Kim, S.; Jo, S.-Y.; Lee, B.R.; Song, M.H.; Choi, S.-W. A well-aligned simple cubic blue phase for a liquid crystal laser. J. Mater. Chem. C 2015, 3, 5383–5388. [Google Scholar] [CrossRef] [Green Version]
- Meiboom, S.; Sammon, M.; Brinkman, W.F. Lattice of disclinations: The structure of the blue phases of cholesteric liquid crystals. Phys. Rev. A 1983, 27, 438–454. [Google Scholar] [CrossRef]
- Kitzerow, H.S.; Bahr, C. Chirality in Liquid Crystals; Springer: New York, NY, USA, 2001. [Google Scholar]
- Kikuchi, H. Liquid crystalline blue phases. Struct. Bonding 2008, 128, 99. [Google Scholar]
- Manda, R.; Pagidi, S.; Heo, Y.; Lim, Y.J.; Kim, M.; Lee, S.H. Electrically tunable photonic band gap structure in monodomain blue-phase liquid crystals. NPG Asia Mater. 2020, 12, 42. [Google Scholar] [CrossRef]
- Takahashi, M.; Ohkawa, T.; Yoshida, H.; Fukuda, J.-I.; Kikuchi, H.; Ozaki, M. Orientation of liquid crystalline blue phases on unidirectionally orienting surfaces. J. Phys. D Appl. Phys. 2018, 51, 104003. [Google Scholar] [CrossRef]
- Martínez-González, J.A.; Li, X.; Sadati, M.; Zhou, Y.; Zhang, R.; Nealey, P.F.; de Pablo, J.J. Directed self-assembly of liquid crystalline blue phases into ideal single-crystals. Nat. Commun. 2017, 8, 15854. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Martínez-González, J.A.; Hernández-Ortiz, J.P.; Ramírez-Hernández, A.; Zhou, Y.; Sadati, M.; Zhang, R.; Nealey, P.F.; de Pablo, J.J. Mesoscale martensitic transformation in single crystals of topolo gical defects. Proc. Natl. Acad. Sci. USA 2017, 114, 10011–10016. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Hou, C.-T.; Li, C.-C.; Jau, H.-C.; Wang, C.-T.; Hong, C.-L.; Guo, D.-Y.; Wang, C.-Y.; Chiang, S.-P.; Bunning, T.J.; et al. Large three-dimensional photonic crystals based on monocrystalline liquid crystal blue phases. Nat. Commun. 2017, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Lin, J.; Li, Q.; Li, R.Z. Influence of long-lasting electric field on the formation of monodomain polymer stabilized blue phase liquid crystals. J. Appl. Phys. 2019, 125, 024501. [Google Scholar] [CrossRef]
- Kitzerow, H.S.; Schmid, H.; Ranft, A.; Heppke, G.; Hikmet, R.A.M.; Lub, J. Observation of blue phases in chiral net-works. Liq. Cryst. 1993, 14, 911–916. [Google Scholar] [CrossRef]
- Heppke, G.; Jérôme, B.; Kitzerow, H.-S.; Pieranski, P. Electrostriction of the cholesteric blue phases BPI and BPII in mixtures with positive dielectric anisotropy. J. Phys. 1989, 50, 2991–2998. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Rao, L.; Jiao, M.; Li, Y.; Cheng, H.C.; Wu, S.T. Polymer-stabilized optically-isotropic liquid crystals for next-generation display and photonics applications. J. Mater. Chem. 2011, 21, 7870–7877. [Google Scholar] [CrossRef]
- Heppke, G.; Kitzerow, H.-S.; Krumrey, M. Electric field induced variation of the refractive index in cholesteric blue phases. Mol. Cryst. Liq. Cryst. Lett. 1985, 2, 59–65. [Google Scholar]
- Xu, D.; Yan, J.; Yuan, J.; Peng, F.; Chen, Y.; Wu, S.-T. Electro-optic response of polymer-stabilized blue phase liquid crystals. Appl. Phys. Lett. 2014, 105, 011119. [Google Scholar] [CrossRef] [Green Version]
- Stegemeyer, H.; Porsch, F. Electric field effect on phase transitions in liquid-crystalline blue-phase systems. Phys. Rev. A 1984, 30, 3369–3371. [Google Scholar] [CrossRef]
- Kitzerow, H.-S. The effect of electric fields on blue phases. Mol. Cryst. Liq. Cryst. 1992, 202, 51–83. [Google Scholar] [CrossRef]
- Chen, P.-J.; Chen, M.; Ni, S.-Y.; Chen, H.-S.; Lin, Y.-H. Influence of alignment layers on crystal growth of polymer-stabilized blue phase liquid crystals. Opt. Mater. Express 2016, 6, 1003. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Guan, B.; He, W.; Yang, Z.; Wang, J.; Ikeda, T.; Jiang, L. Fabrication and photonic applications of large-domain blue phase films. J. Mater. Chem. C 2019, 7, 9460. [Google Scholar] [CrossRef]
- Chen, K.M.; Gauza, S.; Xianyu, H.; Wu, S.T. Submillisecond gray-level response time of a polymer stabilized blue-phase liquid crystal. J. Disp. Technol. 2010, 6, 49–51. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Y.; Wu, S.T. Low voltage and high transmittance blue-phase liquid crystal displays with corrugated electrodes. Appl. Phys. Lett. 2010, 96, 011102. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.Y.; Chien, L.C. Electrically switched color with polymer-stabilized blue-phase liquid crystals. Opt. Lett. 2010, 35, 562. [Google Scholar] [CrossRef]
- Choi, H.; Higuchi, H.; Ogawa, Y.; Kikuchi, H. Polymer-stabilized supercooled blue phase. Appl. Phy. Lett. 2012, 101, 131904. [Google Scholar] [CrossRef]
- Sridurai, V.; Mathews, M.; Yelamaggad, C.V.; Nair, G.G. Electrically Tunable Soft Photonic Gel Formed by Blue Phase Liquid Crystal for Switchable Color-Reflecting Mirror. ACS Appl. Mater. Interfaces 2017, 9, 39569–39575. [Google Scholar] [CrossRef]
- Guo, D.-Y.; Chen, C.-W.; Li, C.-C.; Jau, H.-C.; Lin, K.-H.; Feng, T.-M.; Wang, C.-T.; Bunning, T.J.; Khoo, I.C.; Lin, T.-H. Reconfiguration of three-dimensional liquid crystalline photonic crystals by electrostriction. Nat. Mater. 2020, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-W.; Hou, D.-S.; Li, X.; Sun, D.-P.; Lan, J.-F.; Zhu, J.-L.; Ye, W.-J. Symmetric continuously tunable photonic band gaps in blue-phase liquid crystals switched by an alternating current field. ACS Appl. Mater. Interfaces 2019, 11, 22015. [Google Scholar] [CrossRef]
- Wang, M.; Zou, C.; Sun, J.; Zhang, L.; Wang, L.; Xiao, J.; Li, F.; Song, P.; Yang, H. Asymmetric tunable photonic band gaps in self-organized 3D nanostructure of polymer stabilized blue phase I modulated by voltage polarity. Adv. Funct. Mater. 2017, 27, 1702261. [Google Scholar] [CrossRef]
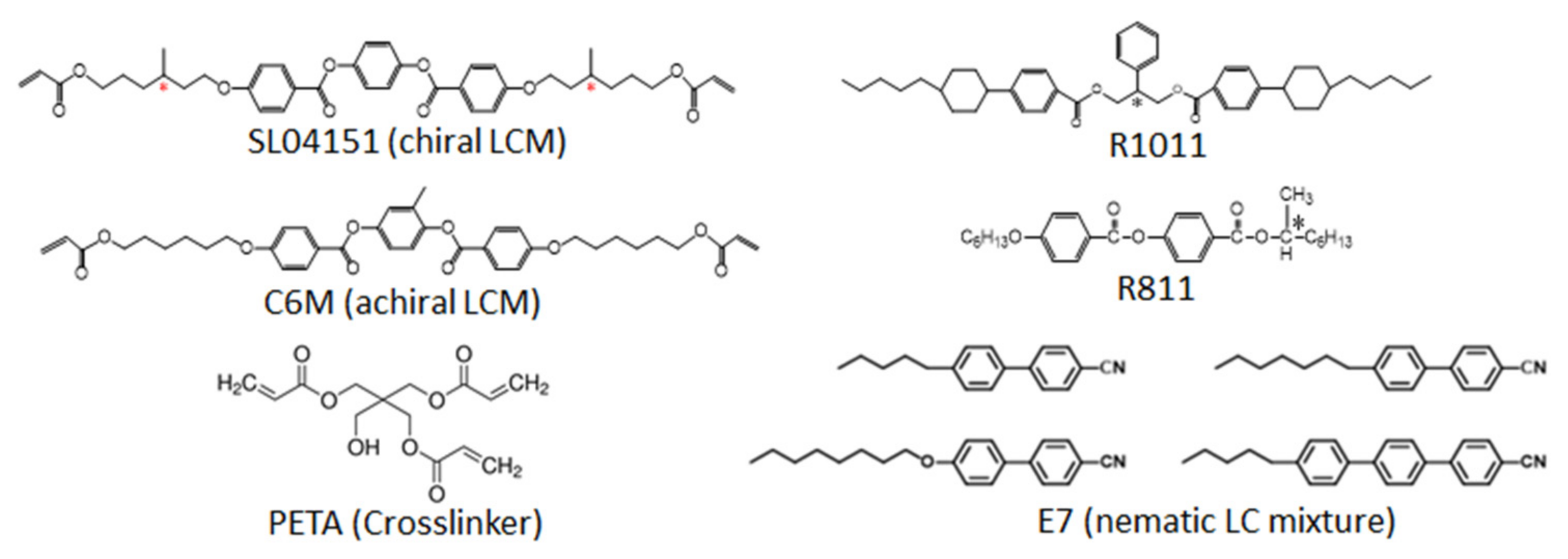

| Sample | SL04151 (wt%) | PETA (wt%) | R1011 (wt%) | R811 (wt%) | E7 (wt%) |
|---|---|---|---|---|---|
| BPLC-0 | 9 | 0 | 4 | 21 | 66 |
| BPLC-1 | 8 | 1 | 4 | 21 | 66 |
| BPLC-2 | 7 | 2 | 4 | 21 | 66 |
| BPLC-3 | 6 | 3 | 4 | 21 | 66 |
| BPLC-4 | 5 | 4 | 4 | 21 | 66 |
| BPLC-4-C6M | 5 | 4 (C6M) 1 | 4 | 21 | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.M.; Tohgha, U.; Bunning, T.J.; McConney, M.E.; Godman, N.P. Effect of Amorphous Crosslinker on Phase Behavior and Electro-Optic Response of Polymer-Stabilized Blue Phase Liquid Crystals. Nanomaterials 2022, 12, 48. https://doi.org/10.3390/nano12010048
Lee KM, Tohgha U, Bunning TJ, McConney ME, Godman NP. Effect of Amorphous Crosslinker on Phase Behavior and Electro-Optic Response of Polymer-Stabilized Blue Phase Liquid Crystals. Nanomaterials. 2022; 12(1):48. https://doi.org/10.3390/nano12010048
Chicago/Turabian StyleLee, Kyung Min, Urice Tohgha, Timothy J. Bunning, Michael E. McConney, and Nicholas P. Godman. 2022. "Effect of Amorphous Crosslinker on Phase Behavior and Electro-Optic Response of Polymer-Stabilized Blue Phase Liquid Crystals" Nanomaterials 12, no. 1: 48. https://doi.org/10.3390/nano12010048
APA StyleLee, K. M., Tohgha, U., Bunning, T. J., McConney, M. E., & Godman, N. P. (2022). Effect of Amorphous Crosslinker on Phase Behavior and Electro-Optic Response of Polymer-Stabilized Blue Phase Liquid Crystals. Nanomaterials, 12(1), 48. https://doi.org/10.3390/nano12010048







