Abstract
Reactions of ZnSO4∙7H2O, N-(pyridin-3-ylmethyl)-4-(pyridin-4-yl)-1,8-naphthalimide (NI-mbpy-34), and 5-bromobenzene-1,3-dicarboxylic acid (Br-1,3-H2bdc) afforded a luminescent coordination polymer, [Zn(Br-1,3-bdc)(NI-mbpy-34)]n (1), under hydro(solvo)thermal conditions. Single-crystal X-ray structure analysis revealed that 1 features a three-dimensional (3-D) 2-fold interpenetrating cds (or CdSO4) net topology with the point symbol of (65·8), where the Zn(II) centers are considered as 4-connected square-planar nodes. X-ray powder diffraction (XRPD) patterns and thermogravimetric (TG) analysis confirmed that 1 shows high chemical and thermal stabilities. Notably, 1 displayed solvent dependent photoluminescence properties; the fluorescence intensity and emission maximum of 1 in different solvent suspensions varied when a solvent was changed. Furthermore, the H2O suspension of 1 exhibited blue fluorescence emission and thus can be treated as a selective and sensitive fluorescent probe for turn-on detection of Cr3+ cations through absorbance caused enhancement (ACE) mechanism and turn-off detection of Cr2O72−/CrO42− anions through collaboration of the absorption competition and energy transfer process, with limit of detection (LOD) as low as μM scale.
1. Introduction
The monitoring and detection of chemical pollutants and/or controlled chemicals in complicated samples are very important tasks in managing the environment, water resources, and the food industry. Among various conventional instrumental techniques, fluorescence sensing responding to fluorescence turn on, turn off, or ratiometric signal, has attracted immense attention in recent years because of its particular aspects such as economics, user-friendliness, short response time, visualization, monitoring in real-time, excellent sensitivity, and high selectivity [1,2,3,4]. Various advanced fluorophore materials, including organic dyes [5,6], porous organic polymers [7], quantum dots (QDs) [8,9], carbon dots (CDs) [1,2], nanoparticles (NPs) [3,10], lanthanide organic/inorganic hybrid materials (LHMs) [11], and metal–organic frameworks/coordination polymers (MOFs/CPs) [12,13,14] have emerged.
Chromium existing as Cr(III) and Cr(VI) oxidation states in the aquatic environments can directly contaminate the soil and aquatic systems. As an essential trace biological element in humans, Cr(III) is considered to be harmless and safe. However, excessive Cr(III) may combine with DNA to cause mutations and malignant cells [10,15,16,17]. Cr(VI) shows high carcinogenicity and mutagenicity and can cause allergic reactions, hereditary genetic defects and various types of cancers that adversely affect human health [17,18,19]. The World Health Organization (WHO) has claimed a permissible limit of 50 μg/L for Cr(VI) in drinking water [20]. Lately, MOF/CP-based, fluorescence-sensory materials have been actively pursued as excellent platforms for the flourishing utilization in detection of Cr(III) and Cr(VI) ions though fluorescence quenching (turn off) effect [15,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. However, there are still rare examples to achieve the detection of Cr(III) via the fluorescence enhancement (turn on) response [15,43,44,45,46,47,48] and fluorescence shift (ratiometric) effect [41,42,43].
As part of our ongoing work in fluorescence detection of hazardous chemical contaminants [39,40,41,42,43,44,49,50,51,52], we acquired, herein, a Zn(II)-based luminescent coordination polymer, namely [Zn(Br-1,3-bdc)(NI-mbpy-34)]n (1, Br-1,3-bdc = 5-bromobenzene-1,3-dicarboxylate; NI-mbpy-34 = N-(pyridin-3-ylmethyl)-4-(pyridin-4-yl)-1,8-naphthalimide), featuring a three-dimensional (3-D) 2-fold interpenetrating cds net. Of note, coordination polymer 1 exhibited fluorescence emissions in solid-state and solvent suspensions, being a bifunctional fluorescence sensor for sensitively and selectively detecting chromium(III) cations and chromium(VI) oxyanions.
2. Experimental Section
2.1. Materials and Methods
All of the chemicals and solvents were acquired from market sources and used without further processing. Ligand NI-mbpy-34 was synthesized according to the previously reported literature [44]. The thermal analysis was conducted by a Thermo Cahn VersaTherm HS TG analyzer (Thermo, Newington, NH, USA) from 25 to 900 °C at a heating rate of 5 °C/min under a flow of nitrogen. The X-ray powder diffraction (XRPD) patterns were measured in the 2θ range of 5–50° by a Shimadzu XRD-7000 diffractometer (Shimadzu, Kyoto, Japan) using Cu Kα radiation (λ = 1.5406 Å) operating at 30 kV and 30 mA. Infrared (IR) spectroscopy was tested in a Perkin-Elmer Frontier Fourier transform infrared spectrometer (Perkin-Elmer, Taipei, Taiwan), and the region 4000–500 cm−1 was recorded with attenuated total reflection (ATR) technique. UV-Vis absorption spectra were obtained on a JASCO V-750 UV/VIS spectrophotometer (JASCO, Tokyo, Japan) at room temperature. The solid-state and solution fluorescence spectra were measured on a Hitachi F7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) at room temperature, with the excitation and emission slits of 5 nm × 5 nm and a scan rate of 1200 nm/min. A 150 W xenon arc lamp was used as an exciting light source. Elemental analyses of C, H, and N were performed on a Vario EL III elemental analyzer (Elementar, Langenselbold, Germany). X-ray photoelectron spectroscopy (XPS) was measured by an ULVAC-PHI PHI 5000 VersaProbe/Scanning ESCA Microprobe instrument (ULVACPHI Inc., Kanagawa, Japan).
2.2. Synthesis of [Zn(Br-1,3-bdc)(NI-mbpy-34)]n (1)
NI-mbpy-34 (9.1 mg, 0.025 mmol) was dissolved in 2 mL of N,N′-dimethylformamide (DMF); ZnSO4∙7H2O (14.3 mg, 0.050 mmol) was dissolved in 2 mL of H2O; Br-1,3-H2bdc (12.3 mg, 0.050 mmol) was dissolved in 1 mL of DMF. The above-mentioned solutions were sequentially added to a 23 mL Teflon-lined stainless steel reactor placed in an autoclave. This was sealed and then heated to 80 °C for 6 h and kept at 80 °C for 48 h. After slowly cooling to 30 °C for 36 h, the mixture was washed with distilled water and ethanol, and yellowish crystals were filtered off and dried. The yield based on NI-mbpy-34 was about 60%. IR (ATR, cm−1): 3071, 1617, 1322, 1462, 990, 884, 723. Anal. Calcd for C31H18BrN3O6Zn: C, 55.21; H, 2.67; N, 6.23%. Found: C, 54.90; H, 2.65; N, 6.20%.
2.3. Single-Crystal X-ray Structure Determinations
The single-crystal data taken at 150(2) K for 1 were collected on a Bruker D8 Venture diffractometer with a graphite monochromated Mo Kα radiation (λ = 0.71073 Å) and a PHOTO100 CMOS detector. The structures were solved by direct methods using SHELXTL [53] and refined on F2 by the full-matrix least-squares using the SHELXL-2014/7 [54] and WINGX [55]. Non-hydrogen atoms were confirmed by successive difference Fourier syntheses and were refined with anisotropic displacement parameters. The hydrogen atoms were produced theoretically on their calculated positions and refined with isotropic displacement parameters set to 1.2Ueq of the attached atom. The single-crystal data and refinement parameters of 1 are summarized in Table 1. CCDC 1991626 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (23 December 2022).

Table 1.
Crystallographic data for 1.
2.4. Fluorescence Measurements
Finely ground powders of 1 (1 mg) were suspended in various solvents (3 mL) including dichloromethane (CH2Cl2), N,N′-dimethylacetamide (DMAc), N,N′-dimethylformamide (DMF), H2O, methanol (CH3OH), and toluene. The prepared suspensions were ultrasonicated via pulsed ultrasound for 10 min and then agitated for further 30 min to yield more stable suspensions.
The H2O suspensions of 1 were utilized to conduct fluorescence sensing experiments. Aqueous solutions of metal ions, including AgNO3, Al(NO3)3, Mg(NO3)2, Ca(NO3)2, Co(NO3)2, Cr(NO3)3, Cu(NO3)2, Fe(NO3)3, NaNO3, KNO3, Mn(NO3)2, Ni(NO3)2, and Pb(NO3)2, and anions, including NaF, KCl, KBr, KI, KClO4, K2CO3, K2Cr2O7, K2CrO4, KNO3, and K3PO4 were prepared with concentration of 0.10 M for fluorescence sensing studies.
Qualitative studies were carried out by adding 0.10 M analyte (30 μL) into the well-prepared H2O suspensions of 1; then, the fluorescence spectra were recorded after waiting for 3 min. Anti-interference studies were conducted on a series of competition experiments with addition of the solution of different perturbed analytes (0.10 M, 30 μL) followed by the targeted analyte (0.10 M, 30 μL) into the H2O suspensions. In each step, the fluorescence spectra were recorded.
The fluorescence quantitative titration experiments were performed with the gradual addition of analytes in aqueous solutions (0.10 M), and then the fluorescence spectra were monitored. The Stern–Volmer equation: I0/I = 1 + Ksv[Q], where I0 and I denote the fluorescence intensities before and after the addition of analytes, respectively, Ksv is the Stern–Volmer quenching constant (M−1), and [Q] is the concentration of analyte (mM), was applied to quantitatively analyze the fluorescence quenching effect.
Limit of detection (LOD) determinations were performed at low concentrations of analyte. Prior to the fluorescence titration, five blank measurements of fluorescence for the H2O suspensions of 1 were carried out for determining the standard deviation (σ). LODs were calculated using the equation: LOD = 3σ/k, where k represents the absolute value of the slope of the calibration curve.
3. Results and Discussion
3.1. Crystal Structure of [Zn(Br-1,3-bdc)(NI-mbpy-34)]n (1)
Single-crystal X-ray structure analysis reveals that the crystal structure of 1 belongs to the monoclinic space group C2/c. There is one cationic Zn(II) center, one fully-deprotonated Br-1,3-bdc2− anion, and one neutral NI-mbpy-34 ligand in the asymmetric unit. The Zn(II) center is surrounded by two oxygen atoms of two carboxylate groups from two distinct Br-1,3-bdc2− ligands and two nitrogen atoms of one 3-pyridyl (imide end) and one 4-pyridyl (naphthalene end) groups from two distinct NI-mbpy-34 ligands to adopt a {ZnO2N2} tetrahedral geometry (Figure 1a). The anionic Br-1,3-bdc2− ligand has a μ2-Br-1,3-bdc-κO:κO mode to bridge two Zn(II) centers; each of the two carboxylate groups is in a monodentate-κO coordination mode (Figure 1b). The Zn(II) centers are connected by the anionic Br-1,3-bdc2− and the neutral NI-mbpy-34 ligands to form a three-dimensional (3-D) porous framework (Figure 1c). If the Zn(II) centers are considered as 4-connected square-planar nodes and both the Br-1,3-bdc2− and NI-mbpy-34 ligands are considered as linear linkers (Figure 1a), the 3-D framework of 1 can be simplified as a 4-connected cds (or CdSO4) net topology with the point symbol of (65·8) (Figure 1d). The potential voids of the single cds network are occupied by the other independent identical framework via interpenetration in opposite orientation to generate a 2-fold interpenetrating net (Figure 1e), leaving insufficient solvent accessible voids. Notably, two neighboring naphthalimide skeletons in the two independent equivalent cds frameworks are nearly parallel in a head-to-tail manner and the distance between them is about 3.50 Å (Figure S1), suggesting significant π–π interactions.

Figure 1.
Crystal structure of 1: (a) the coordination environment around the Zn(II) center and schematic representation of the 4-connected node; (b) the coordination mode of Br-1,3-bdc2− dianion; (c) a single 3-D framework; (d) schematic representation of the 4-connected cds network with the point symbol of (65·8); (e) 2-fold interpenetrating cds networks.
3.2. X-ray Powder Diffraction (XRPD) Patterns and Chemical Stability
X-ray powder diffraction (XRPD) patterns of as-synthesized 1 are in agreement with the simulated patterns calculated from single-crystal X-ray diffraction data (Figure 2), confirming the phase purity of bulky samples. Further, the chemical stability of 1 in different solvents was checked. After immersing in dichloromethane (CH2Cl2), N,N′-dimethylacetamide (DMAc), N,N′-dimethylformamide (DMF), H2O, methanol (CH3OH), and toluene for 24 h, the XRPD patterns of the solvent-treated samples showed that the characteristic peaks match well with those of the XRPD pattern of as-synthesized 1 and that simulated from the single crystal data, although the peak intensities are somewhat different (Figure 2). This demonstrates that the original framework of 1 can retain a high crystallinity after immersion in solvents, confirming its high stability.
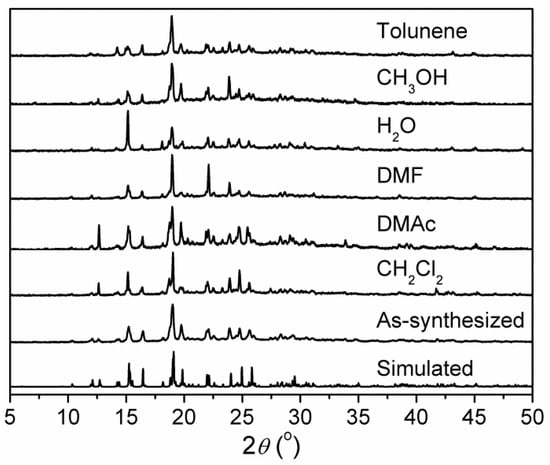
Figure 2.
Simulated XRPD pattern of 1 and XRPD patterns of as-synthesized 1 and 1 immersed in different solvents for 24 h.
3.3. Thermal Properties
The thermal properties of 1 were evaluated from the thermogravimetric (TG) analysis. As a representative, the TG analysis plot of 1 shows no weight loss before 378 °C (Figure S2), indicating high thermal stability. Then a two-step decomposition of the framework occurred, which was ended upon heating to ca. 640 °C. During the decomposition, bromide might react with divalent zinc to generate ZnBr2 (b.p. = 697 °C), which escaped at higher temperature. The remaining residue of 6.2% was reasonably assigned to the ZnO component (calcd 6.0%).
3.4. Photoluminescence Properties
Previous research has shown that NI-mbpy-34 is highly emissive and can be a luminescence source for coordination polymers due to its highly conjugated π-electron system [44]. In solid-state, NI-mbpy-34 showed emission band(s) in the region of 400–600 nm with maximum at 462 nm upon excitation at λex = 370 nm, while Br-1,3-H2bdc displayed only an extremely weak emission band upon excitation at λex = 360 nm (Figure S3). When excited at λex = 306 nm, 1 exhibited solid-state fluorescence with two emission peaks centered at 444 nm and 504 nm. From the band position and shape, the emissions were tentatively attributed to the ligand-centered emission of NI-mbpy-34 perturbed by metal coordination.
Subsequently, the fluorescence properties of 1 in different solvent suspensions, such as CH2Cl2, DMAc, DMF, H2O, CH3OH, and toluene were also investigated (Figure 3). We observed that the fluorescence intensity and emission maximum of 1 in different solvent suspensions varied as the solvent was changed, implying solvent-dependent photoluminescence properties. Upon excitation, 1 emitted strong fluorescence emissions in CH3OH and DMF suspensions, moderate emissions in H2O and DMAc suspensions, and weak emissions in CH2Cl2 and toluene suspensions. In addition, the emission maxima of these suspensions varied from 384 nm to 432 nm, showing remarkable blue shift compared to the solid-state fluorescence. The phenomena can most likely be attributed to the different collision interactions rather than crystal structure change [56,57], since that 1 is highly stable in all chosen solvents. Additionally, it is noted that the fluorescence emission intensities are nearly directly proportional to the concentrations of 1 in H2O suspensions (Figure S4).

Figure 3.
Fluorescence emission spectra of 1 in suspension-phase of different solvents.
3.5. Fluorescence Sensing of Metal Ions
The fluorescence sensing properties of 1 toward metal ions have been explored, and the fluorescence sensing measurements were carried out in water. Aqueous solutions of nitrate salt of thirteen different metal ions, including Ag+, Al3+, Mg2+, Ca2+, Co2+, Cr3+, Cu2+, Fe3+, Na+, K+, Mn2+, Ni2+, and Pb2+, were separately added into the H2O suspensions of 1 in a quartz cuvette with the concentration at 1.0 mM. The photoluminescence measurements were obtained at an excitation wavelength of 306 nm before and after addition of metal ions under the same experimental conditions (Figure 4a). Upon addition of the different metal ions, the mono- and divalent metal ions exerted a relatively weak effect (intensity change ≤ 10%) on the emission of 1, and the Fe3+ ion addition led to a weak enhancement effect with ca. 20-nm blue shift. Interestingly, the trivalent metal ions of Cr3+ and Al3+ resulted in a remarkable fluorescence enhancement by 8.7 and 3.3 times, respectively, along with ca. 20-nm blue shift. The results demonstrate that 1 may be an excellent fluorescence sensor for Cr3+ detection with efficient selectivity. To confirm our assumption, interference experiments were carried out to examine the ability of 1 to selectively detect Cr3+ ions in the co-existence of interfering metal ions with equal concentrations of 1.0 mM. Experimental results clearly indicated that in sensing Cr3+ by 1, Al3+ displayed strong competitive effect while other selected perturbed metal ions showed insignificant interference (Figure 5), suggesting that 1 has good selectivity along with anti-interference ability for Cr3+ sensing in water. Briefly stated, 1 is highly selective for Cr3+ detection over other perturbed metal ions with the exception of Al3+. Further studies on Cr3+ detection by varying the concentrations of 1 in H2O suspensions showed almost unchanged fluorescence enhancement ratios (Figure S5), suggesting specific Cr3+ sensing performances in water.
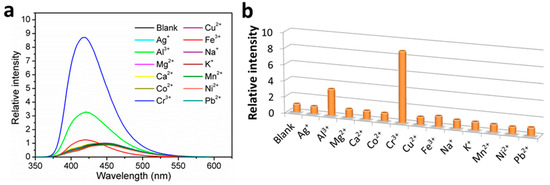
Figure 4.
(a) Fluorescence emission spectra, and; (b) fluorescence relative ratio responses of 1 in H2O suspensions containing various metal ions at 1.0 mM.

Figure 5.
Fluorescence relative ratio responses of 1 in H2O suspensions containing various metal ions before and after addition of Cr3+ ions with equal concentrations at 1.0 mM.
To further investigate the sensitivity of 1 toward Cr3+ ions, the fluorescence titration experiments were executed. As expected, gradually increasing fluorescence emission intensities were observed at around 420 nm with increasing concentrations of Cr3+ ions. As shown in Figure 6b, there exists a nonlinear relationship between the fluorescence intensity and the Cr3+ ion concentration, with the formula of I = −2823.98 × exp(−[Cr3+]/0.83) + 2906.06 (R2 = 0.9929), suggesting a saturation behavior at high concentrations. On the basis of quantitative titrations (Figure S6), the LOD for Cr3+ was determined to be 3.13 μM (corresponding to 162.9 ppb). This proves that 1 can effectively detect Cr3+ ions with remarkable sensitivity.
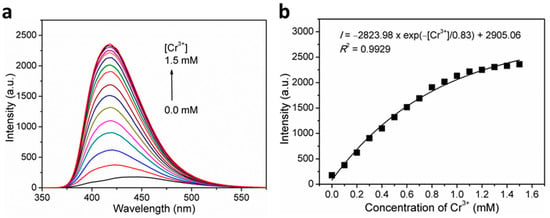
Figure 6.
(a) Concentration-dependent fluorescence emission spectra of 1 in H2O suspensions upon incremental addition of Cr3+ ions when excited at λex = 306 nm, and; (b) Plot of fluorescence intensity versus Cr3+ ion concentration for 1 in H2O suspensions.
The possible fluorescence sensing mechanism toward Cr3+ was investigated. The XRPD patterns of 1 recovered from Cr3+ aqueous solutions showed high consistency with the XRPD patterns of as-synthesized 1 in peak positions (Figure S7), which suggested that the framework of 1 keeps its integrity after Cr3+ detection. Thus, the turn-on sensing mechanism can exclude the possibility of framework collapse. However, small but appreciable changes in the relative intensity of the XRPD peaks were observed, so it seems that some changes in the crystal structure occurred. Indeed, X-ray photoelectron spectroscopy (XPS) analysis on 1 indicated the existence of Cr3+ cation in the framework of 1 after immersion as the observation of the Cr 2p3/2 and Cr 2p1/2 peaks at around 577.1 and 586.6 eV, respectively (Figure S8a). This might alter the intensity of the XRPD peaks. Notably, the O 1s peak in the XPS spectra did not shift after Cr3+ immersion (Figure S8b), and also the IR spectra did not change significantly (Figure S9). These phenomena imply that the influence of Cr3+ is not through bonding or there might be extremely weak interactions only between Cr3+ and the framework of 1 instead of the ligand-containing system [47]. Furthermore, the UV−vis absorption spectra of 1 were further checked, which demonstrated that 1 has an absorption band at around 350 nm corresponded to the excitation wavelength applied. Obviously, the absorbance increased remarkably after the addition of Cr3+ but exhibited no significant change after the addition of other different metal ions, such as Al3+ and Fe3+ (Figure S10), which implied that the turn-on effect of 1 toward Cr3+ can be properly explained by the absorbance caused enhancement (ACE) mechanism [46,58].
3.6. Fluorescence Sensing of Anions
The fluorescence sensing properties of 1 toward anions were also explored, and ten different anions, including F−, Cl−, Br−, I−, ClO4−, CO32−, Cr2O72−, CrO42−, NO3−, and PO43−, were chosen. Similar to the procedures used for metal ion sensing, the fluorescence sensing measurements were carried out in water; each individual aqueous solution of anion was added to the well-prepared H2O suspension of 1, and the photoluminescence measurements were obtained at an excitation wavelength of 306 nm before and after addition of anion. As can be seen, most of the chosen anions exerted a relatively weak effect (intensity change ≤ 10%) on the emission of 1 (Figure 7). The strongest fluorescence quenching effect was observed in the cases of the two chromium(VI) oxyanions, Cr2O72− and CrO42−, which showed quenching efficiencies of about 90% and 74%, respectively (quenching efficiency (%) = (I0 − I)/I0 × 100%, where I0 and I are the maximum fluorescence intensity of 1 before and after addition of analytes). Notably, when different concentrations of 1 in H2O suspensions were utilized, the high fluorescence quenching efficiencies are almost retained (Figure S5). Hence, the concentration of 1 in H2O suspension has no significant effect on the detection performances toward Cr2O72− and CrO42−. Furthermore, interference experiments have shown that the quenching efficiencies of 1 toward Cr2O72− and CrO42− anions are hardly affected by other competitive anions (Figure 8), confirming the excellent anti-interference ability and thus the high selectivity of 1 as a fluorescence probe for detection of Cr2O72− and CrO42− anions in water.
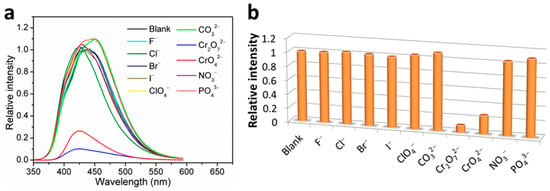
Figure 7.
(a) Fluorescence emission spectra and (b) fluorescence relative ratio responses of 1 in H2O suspensions containing various anions at 1.0 mM.
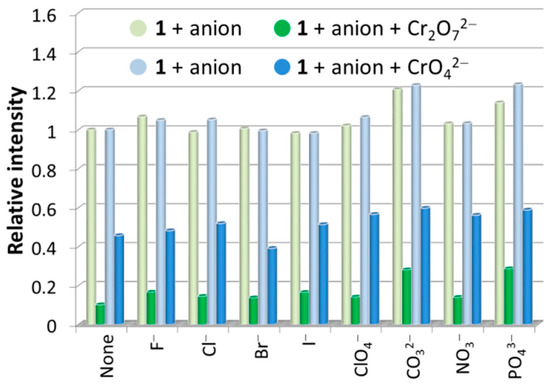
Figure 8.
Fluorescence relative ratio responses of 1 in H2O suspensions containing various anions before and after addition of Cr2O72−/CrO42− ions with equal concentrations at 1.0 mM.
Since Cr3+ enhances fluorescence of 1 in H2O suspension and Cr(VI) anions quench it, and both species can coexist in environmental conditions, it is of interest to study the influence of Cr3+ detection in the coexistence of Cr(VI) anions and vice versa. Experimental results clearly indicate that Cr(VI) anions strongly interfere with Cr3+ detection while Cr3+ ions cause no interference on the detection of Cr2O72− and CrO42− anions (Figure S11). Again, this confirms that 1 is highly selective for Cr2O72−/CrO42− detection.
The detection sensitivity can be determined by quantitative analysis and LOD. Hence, fluorescent titration experiments were performed. As expected, the recorded fluorescence intensities gradually decreased with the gradual increase in the volume concentrations of Cr2O72− and CrO42− in the H2O suspensions of 1 (Figure 9a,b). Furthermore, the dependence of the fluorescence intensity on Cr2O72− or CrO42− ion concentration was investigated, which can be well fitted to I = 180.62 × exp(−[Cr2O72−]/0.56) − 2.86 (R2 = 0.99717) for Cr2O72− and I = 154.46 × exp(−[CrO42−]/1.06) + 15.61 (R2 = 0.99789) for Cr2O72− (Figure S12). The quantification of fluorescence quenching effect was further examined through the Stern–Volmer equation. As observed, the Stern–Volmer plots for sensing Cr2O72− and CrO42− analytes by 1 both exhibited upward curves of I0/I against the analyte concentration over the titration concentrations (Figure 9c,d), implying the cooperation of dynamic and static quenching processes [34,59,60]. On the basis of quantitative titrations, the good linear regression analyses on Stern–Volmer plots gave the Ksv value of 2.52 × 103 M−1 (R2 = 0.99259) in the range of 0–0.5 mM for sensing Cr2O72− and 1.42 × 103 M−1 (R2 = 0.99672) in the range of 0–2.0 mM for sensing CrO42− (inset in Figure 9c,d). The LOD was determined to be 43.36 μM (corresponding to 9.36 ppm) for Cr2O72− and 25.57 μM (corresponding to 2.97 ppm) for CrO42− (Figure S13).
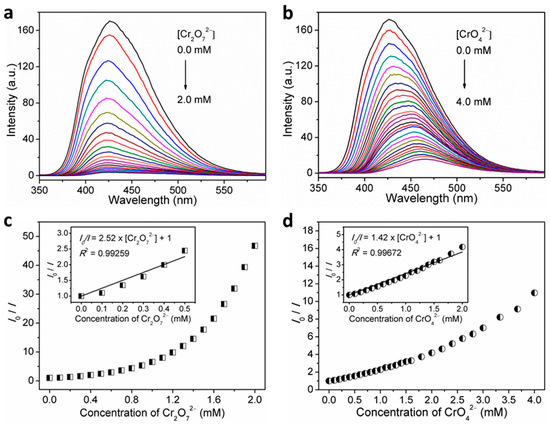
Figure 9.
Concentration-dependent fluorescence spectra of 1 in H2O suspensions by incremental addition of (a) Cr2O72−, and; (b) CrO42− upon excitation at λex = 306 nm, and Stern–Volmer plot of I0/I versus concentration of; (c) Cr2O72−, and; (d) CrO42− for 1 in H2O suspensions (inset: linear Stern–Volmer plot).
The plausible fluorescence-quenching mechanisms have been investigated. The XRPD patterns of 1 before and after treatment of Cr2O72− and CrO42− showed a high degree of similarity (Figure S7), suggesting the maintenance of framework integrity, thus ruling out framework collapse as being the fluorescence quenching mechanism. However, the excitation wavelength to irradiate 1 was greatly overlapped with the absorbance band of Cr2O72− and CrO42−, implying that the competitive absorption of excitation energy might serve dominant influence on the fluorescence quenching detection of 1 toward Cr2O72− and CrO42−. Further, energy transfer process might also contribute efforts in quenching the fluorescence of 1 because the fluorescence-emission band of 1 in H2O suspension was partially overlapped and the absorbance band of Cr2O72− and CrO42− in aqueous solutions (Figure S14).
4. Conclusions
In this research, we have successfully synthesized a 2-fold interpenetrated coordination polymer 1 featuring a 4-connected cds network topology with the point symbol of (65·8). Coordination polymer 1 emits fluorescence in both solid-state and suspension-phase of different solvents, making it a potential candidate to be employed in detection of Cr(III) cations via remarkable fluorescence enhancement response due to ACE mechanism, and in sensing of Cr(VI) oxyanions (Cr2O72− and CrO42−) via fluorescence-quenching effect due to collaboration of absorption competition and energy transfer process, with high sensitivity and selectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12010158/s1. Figure S1: Plot of the inter-net π–π interactions between two neighboring naphthalimide skeletons in the two independent identical cds frameworks in the crystal structure of 1. Figure S2: TG curve of 1. Figure S3: Solid-state excitation (solid lines) and emission spectra (solid lines with symbols) of NI-mbpy-34, Br-1,3-H2bdc, and 1 at room temperature. Figure S4: Fluorescence emission spectra of 1 in H2O suspensions of different concentrations at room temperature upon excitation at 306 nm. Inset: Fluorescence relative ratio responses of 1 in H2O suspensions of different concentrations. Figure S5: Effect of the concentration of 1 in H2O suspension on the fluorescence intensity responses upon addition of Cr3+ ions (enhancement) and Cr(VI) anions (quenching) at 1.0 mM. Figure S6: Linear region of fluorescence intensity for the H2O suspensions of complex 1 upon incremental addition of Cr3+ ions. The following table lists the relevant parameters of LOD for the H2O suspensions of complex 1 toward Cr3+ ions. Conditions: λem = 414 nm (λex = 280 nm). Figure S7: XRPD patterns of 1 before and after immersing in Cr3+, Cr2O72−, and CrO42− aqueous solutions for 24 h. Figure S8: (a) XPS high resolution spectra of Cr 2p for 1 after sensing Cr3+. (b) XPS high resolution spectra of O 1s for 1 before and after sensing Cr3+. Figure S9: IR spectra of 1 before and after immersing in Cr3+ aqueous solution for 24 h. Figure S10: UV-vis spectra of 1 before and after immersing in Cr3+, Al3+, Fe3+ aqueous solutions for 24 h. Figure S11: Fluorescence emission spectra of 1 in H2O suspension (1 mg/3 mL) before and after addition of interfering/analyte ions at room temperature upon excitation at 306 nm. Figure S12: Fluorescence intensity traces (λem = 438 nm) for the H2O suspensions of 1 upon incremental addition of Cr2O72− and CrO42− ions when excited at 306 nm, following the first-order exponential decay. Figure S13: Linear region of fluorescence intensity (λem = 438 nm) for the H2O suspensions of 1 upon incremental addition of Cr2O72− and CrO42− ions when excited at 306 nm. The following table lists the relevant parameters of LOD for the H2O suspensions of 1 toward Cr2O72− and CrO42− ions. Figure S14: Spectral overlap between the normalized emission spectra of 1 in H2O suspensions and the normalized absorption spectra of Cr2O72− and CrO42− in aqueous solutions.
Author Contributions
J.-Y.W. conceived and designed the experiments; K.-S.L. and M.-J.T. performed the experiments; M.-J.T. and J.-Y.W. analyzed the data; J.-Y.W. contributed reagents/materials/analysis tools; J.-Y.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported the Ministry of Science and Technology of Taiwan (MOST 106-2113-M-260-007-, MOST 107-2113-M-260-001-, and MOST 108-2113-M-260-002-) and National Chi Nan University.
Data Availability Statement
All required data is provided within the manuscript.
Acknowledgments
The authors gratefully acknowledge the Advanced High-Tech Research Center of National Chi Nan University (MOST 110-2731-M-260-001-, NMR004800, MS004800, XRD003300, TA000400) and the Instrument Center of National Chung Hsing University (MOST 110-2731-M-005-001-, XRD001300, EA000100, ESCA00003100) for providing valuable assistance on research support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. Trends Analyt. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Ebrahim, S.; Shokry, A.; Khalil, M.M.A.; Ibrahim, H.; Soliman, M. Polyaniline/Ag nanoparticles/graphene oxide nanocomposite fluorescent sensor for recognition of chromium (VI) ions. Sci. Rep. 2020, 10, 13617. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Cao, D. Amino-Functionalized Luminescent Metal–Organic Framework Test Paper for Rapid and Selective Sensing of SO2 Gas and Its Derivatives by Luminescence Turn-On Effect. Anal. Chem. 2018, 90, 3608–3614. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef] [PubMed]
- Sunnapu, O.; Kotla, N.G.; Maddiboyina, B.; Asthana, G.S.; Shanmugapriya, J.; Sekar, K.; Singaravadivel, S.; Sivaraman, G. Rhodamine based effective chemosensor for Chromium(III) and their application in live cell imaging. Sens. Actuators B Chem. 2017, 246, 761–768. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Li, X.; Li, X.; Wang, R. Imidazolium-based porous organic polymers: Anion exchange-driven capture and luminescent probe of Cr2O72−. ACS Appl. Mater. Interfaces 2016, 8, 18904–18911. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Mou, Z.-L.; Zhang, R.-L.; Liang, S.-S.; Zhang, Z.-Q. An efficient ratiometric fluorescence sensor based on metal–organic frameworks and quantum dots for highly selective detection of 6-mercaptopurine. Biosens. Bioelectron. 2017, 91, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Huang, R.F.; Ma, X.G.; Guo, L.H.; Wang, Y.; Fan, Y.M. Selective fluorescence sensor based on ion-imprinted polymer-modified quantum dots for trace detection of Cr(VI) in aqueous solution. Anal. Bioanal. Chem. 2019, 411, 7165–7175. [Google Scholar] [CrossRef]
- Chen, D.; Wu, G.H.; Wang, Z.Q.; Ren, W.Z.; Zhang, Y.J.; Wu, A.G. Selective colorimetric detection of Cr(III) and Cr(VI) using gallic acid capped gold nanoparticles. Dalton Trans. 2016, 45, 8347–8354. [Google Scholar]
- Chen, X.; Xu, Y.; Li, H. Lanthanide organic/inorganic hybrid systems: Efficient sensors for fluorescence detection. Dyes Pigments 2020, 178, 108386. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Lustig, W.P.; Li, J. Functionalizing Luminescent Metal–Organic Frameworks for Enhanced Photoluminescence. ACS Energy Lett. 2020, 5, 2671–2680. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.C. Luminescent sensors based on metal–organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Karmakar, A.; Samanta, P.; Dutta, S.; Ghosh, S.K. Fluorescent “Turn-on” Sensing Based on Metal–Organic Frameworks (MOFs). Chem. Asian J. 2019, 14, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Wang, J.; Zhang, Y.; Li, H.; Yang, L.; Liao, S.; Gu, W.; Liu, X. An amino-decorated dual-functional metal–organic framework for highly selective sensing of Cr(III) and Cr(VI) ions and detection of nitroaromatic explosives. J. Mater Chem. A 2016, 4, 15494–15500. [Google Scholar] [CrossRef]
- Calevro, F.; Campani, S.; Ragghianti, M.; Bucci, S.; Mancino, G. Tests of toxicity and teratogenicity in biphasic vertebrates treated with heavy metals (Cr3+, A13+, Cd2+). Chemosphere 1998, 37, 3011–3017. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Dayan, A.; Paine, A. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef]
- WHO/SDE/WSH/03.04/4; Chromium in Drinking-Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality. WHO: Geneva, Switzerland, 2003.
- Jia, X.-X.; Yao, R.-X.; Zhang, F.-Q.; Zhang, X.-M. A Fluorescent Anionic MOF with Zn4(trz)2 Chain for Highly Selective Visual Sensing of Contaminants: Cr(III) Ion and TNP. Inorg. Chem. 2017, 56, 2690–2696. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Zhao, F.; Liu, J.-J.; Liu, Z.-L.; Wang, Y.-Q. An ultrastable zinc(II)–organic framework as a recyclable multi-responsive luminescent sensor for Cr(III), Cr(VI) and 4-nitrophenol in the aqueous phase with high selectivity and sensitivity. J. Mater. Chem. A 2017, 5, 20035–20043. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, M.; Ma, Y.; Li, L. Multi-Responsive Luminescent Sensors Based on Two-Dimensional Lanthanide–Metal Organic Frameworks for Highly Selective and Sensitive Detection of Cr(III) and Cr(VI) Ions and Benzaldehyde. Cryst. Growth Des. 2017, 17, 4326–4335. [Google Scholar] [CrossRef]
- Dong, J.; Xu, H.; Hou, S.-L.; Wu, Z.-L.; Zhao, B. Metal–Organic Frameworks with Tb4 Clusters as Nodes: Luminescent Detection of Chromium(VI) and Chemical Fixation of CO2. Inorg. Chem. 2017, 56, 6244–6250. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.-Z.; Kong, X.-J.; Yu, J.; Lv, X.-L.; Wu, Y.; Guo, Z.-J.; Li, J.-R. Zr(IV)-Based Metal–Organic Framework with T-Shaped Ligand: Unique Structure, High Stability, Selective Detection, and Rapid Adsorption of Cr2O72− in Water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+, Cr2O72−/CrO42− in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shi, J.-J.; Ding, B.; Liu, Z.-Y.; Wang, X.-G.; Zhao, X.-J.; Yang, E.-C. A heterometallic sodium(I)–europium(III)–organic layer exhibiting dual-responsive luminescent sensing for nitrofuran antibiotics, Cr2O72− and MnO4− anions. Dalton Trans. 2019, 48, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. A Hydrolytically Stable Luminescent Cationic Metal-organic Framework for Highly Sensitive and Selective Sensing of Chromate Anion in Natural Water Systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Laiya, R.; Suresh, E. Conventional Synthesis of Zn(II)/Cd(II) Luminescent Coordination Polymers: Dual Sensing Probe for Selective Detection of Chromate Anions and TNP in Aqueous Phase. Inorg. Chem. 2017, 56, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Q.; Li, G.Y.; Xu, J.; Hu, T.L.; Bu, X.H. A Water-Stable Luminescent ZnII Metal-Organic Framework as Chemosensor for High-Efficiency Detection of CrVI-Anions (Cr2O72− and CrO42−) in Aqueous Solution. Chem. Eur. J. 2018, 24, 3192–3198. [Google Scholar] [CrossRef]
- Lv, R.; Li, H.; Su, J.; Fu, X.; Yang, B.; Gu, W.; Liu, X. Zinc Metal–Organic Framework for Selective Detection and Differentiation of Fe(III) and Cr(VI) Ions in Aqueous Solution. Inorg. Chem. 2017, 56, 12348–12356. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, Y.-X.; Cao, C.; Ni, C.-Y.; Ren, Z.-G.; Young, D.J.; Lang, J.-P. Nickel(II)-Based Two-Dimensional Coordination Polymer Displaying Superior Capabilities for Selective Sensing of Cr(VI) Ions in Water. Cryst. Growth Des. 2019, 19, 3518–3528. [Google Scholar] [CrossRef]
- Wu, X.-X.; Fu, H.-R.; Han, M.-L.; Zhou, Z.; Ma, L.-F. Tetraphenylethylene Immobilized Metal–Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72− and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Sun, X.; Yao, S.; Yu, C.; Li, G.; Liu, C.; Huo, Q.; Liu, Y. An ultrastable Zr-MOF for fast capture and highly luminescence detection of Cr2O72− simultaneously in an aqueous phase. J. Mater. Chem. A 2018, 6, 6363–6369. [Google Scholar] [CrossRef]
- Xiao, Q.-Q.; Dong, G.-Y.; Li, Y.-H.; Cui, G.-H. Cobalt(II)-Based 3D Coordination Polymer with Unusual 4,4,4-Connected Topology as a Dual-Responsive Fluorescent Chemosensor for Acetylacetone and Cr2O72−. Inorg. Chem. 2019, 58, 15696–15699. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.-Q.; Li, Y.-H.; Liu, D.; Cui, G.-H. A water-stable luminescent Co(II) coordination polymer as probe for efficient detection of Cr(VI)-anions (Cr2O72− and CrO42−) in aqueous solution. Inorg. Chem. Commun. 2020, 111, 107665. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, S.-D.; Cao, K.-Z.; Zou, G.-D.; Wang, S.-Y. Novel Zn(II) coordination polymer based on a semi-rigid tricarboxylate acid ligand: Synthesis, structure, and fluorescence recognition of acetylacetone and chromium(VI) anions. J. Solid State Chem. 2020, 302, 122380. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, S.-D.; Cao, K.-Z.; Zou, G.-D.; Liu, H.-Q. A new fluorescent Cu(I) coordination polymer for selective detection of oxo-anion chromium(VI) in water. Inorg. Chem. Commun. 2021, 132, 108844. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Chuang, P.-M.; Wu, J.-Y. Solvent-Induced Controllable Supramolecular Isomerism: Phase Transformation, CO2 Adsorption, and Fluorescence Sensing toward CrO42−, Cr2O72−, MnO4− and Fe3+. Inorg. Chem. 2020, 59, 9095–9107. [Google Scholar] [CrossRef]
- Jiang, Q.-J.; Lin, J.-Y.; Hu, Z.-J.; Hsiao, V.K.S.; Chung, M.-Y.; Wu, J.-Y. Luminescent Zinc(II) Coordination Polymers of Bis(pyridin-4-yl)benzothiadiazole and Aromatic Polycarboxylates for Highly Selective Detection of Fe(III) and High Valent Oxyanions. Cryst. Growth Des. 2021, 21, 2056–2067. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Lee, J.-J.; Su, C.-H.; Tsai, M.-J.; Li, C.-Y.; Wu, J.-Y. From lamellar net to bilayered-lamella and to porous pillared-bilayer: Reversible crystal-to-crystal transformation, CO2 adsorption, and fluorescence detection of Fe3+, Al3+, Cr3+, MnO4−, and Cr2O72− in water. Dalton Trans. 2020, 49, 14201–14215. [Google Scholar] [CrossRef]
- Chuang, P.-M.; Wu, J.-Y. A highly stable Zn coordination polymer exhibiting pH-dependent fluorescence and as a visually ratiometric and on−off fluorescence sensor. CrystEngComm 2021, 23, 5226–5240. [Google Scholar] [CrossRef]
- Chuang, P.-M.; Huang, Y.-W.; Liu, Y.-L.; Wu, J.-Y. Influence of linker substitution on fluorescence responsive sensing of isostructural coordination polymers: Visual turn-on, ratiometric, and turn-off sensing in water. CrystEngComm 2021, 23, 2222–2234. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Liao, K.-S.; Hsu, L.-J.; Wu, J.-Y. A luminescent Cd(II) coordination polymer as a fluorescence-responsive sensor for enhancement sensing of Cr3+ and Al3+ ions and quenching detection of chromium(VI) oxyanions. J. Solid State Chem. 2021, 304, 122564. [Google Scholar] [CrossRef]
- Liang, X.; Jia, Y.; Zhan, Z.; Hu, M. A highly selective multifunctional Zn-coordination polymer sensor for detection of Cr (III), Cr (VI) ion, and TNP molecule. Appl. Organomet. Chem. 2019, 33, e4988. [Google Scholar] [CrossRef]
- Tian, X.-M.; Yao, S.-L.; Qiu, C.-Q.; Zheng, T.-F.; Chen, Y.-Q.; Huang, H.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Turn-On Luminescent Sensor toward Fe3+, Cr3+, and Al3+ Based on a Co(II) Metal–Organic Framework with Open Functional Sites. Inorg. Chem. 2020, 59, 2803–2810. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Yan, H.; Lu, J.; Liu, H.; Li, Y.; Wang, S.; Li, D.; Dou, J.; Yang, L.; et al. Multiresponsive Luminescent Sensitivities of a 3D Cd-CP with Visual Turn-on and Ratiometric Sensing toward Al3+ and Cr3+ as Well as Turn-off Sensing toward Fe3+. Inorg. Chem. 2020, 59, 3828–3837. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Qin, B.; Li, W.; Zheng, H.; Zhang, X.; Zhang, J. Turn-on fluorescence in a stable Cd(II) metal–organic framework for highly sensitive detection of Cr3+ in water. Dyes Pigments 2020, 178, 108359. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Li, C.-Y.; Wu, J.-Y. Luminescent Zn(II) coordination polymers as efficiently fluorescent sensors for highly sensitive detection of explosive nitroaromatics. CrystEngComm 2018, 20, 6762–6774. [Google Scholar] [CrossRef]
- Chen, T.-C.; Tsai, M.-J.; Wu, J.-Y. Fluorescent Cadmium Bipillared-Layer Open Frameworks: Synthesis, Structures, Sensing of Nitro Compounds, and Capture of Volatile Iodine. Chem. Eur. J. 2019, 25, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Li, C.-Y.; Wu, J.-Y. A highly stable luminescent coordination polymer for sensing of volatile iodine and its metal-ion exchange properties with Cu2+ ions. J. Photochem. Photobiol. A Chem. 2020, 389, 112256. [Google Scholar] [CrossRef]
- Su, C.-H.; Tsai, M.-J.; Wang, W.-K.; Li, Y.-Y.; Wu, J.-Y. Engineering Tailored Bifunctional Luminescent Pillared-Layer Frameworks for Adsorption of CO2 and Sensitive Detection of Nitrobenzene in Water Media. Chem. Eur. J. 2021, 27, 6529–6537. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Wang, S.; Cao, T.; Yan, H.; Li, Y.; Lu, J.; Ma, R.; Li, D.; Dou, J.; Bai, J. Functionalization of Microporous Lanthanide-Based Metal–Organic Frameworks by Dicarboxylate Ligands with Methyl-Substituted Thieno[2,3-b]thiophene Groups: Sensing Activities and Magnetic Properties. Inorg. Chem. 2016, 55, 5139–5151. [Google Scholar] [CrossRef]
- Song, J.-F.; Luo, J.-J.; Jia, Y.-Y.; Xin, L.-D.; Lin, Z.-Z.; Zhou, R.-S. Solvent-induced construction of two zinc supramolecular isomers: Synthesis, framework flexibility, sensing properties, and adsorption of dye molecules. RSC Adv. 2017, 7, 36575–36584. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Guo, L.; Cao, D.P. Metal–Organic Framework as Luminescence Turn-on Sensor for Selective Detection of Metal Ions: Absorbance Caused Enhancement Mechanism. Sens. Actuators B 2018, 256, 839–845. [Google Scholar] [CrossRef]
- Sharma, V.; De, D.; Pal, S.; Saha, P.; Bharadwaj, P.K. A 2D Coordination Network That Detects Nitro Explosives in Water, Catalyzes Baylis–Hillman Reactions, and Undergoes Unusual 2D→3D Single-Crystal to Single-Crystal Transformation. Inorg. Chem. 2017, 56, 8847–8855. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.H.; Shi, F.; Zhang, W.M.; Zang, S.Q.; Mak, T.C.W. Selective Sensing of Fe3+ and Al3+ Ions and Detection of 2,4,6-Trinitrophenol by a Water-Stable Terbium-Based Metal–Organic Framework. Chem. Eur. J. 2015, 21, 15705–15712. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).