Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review
Abstract
:1. Introduction
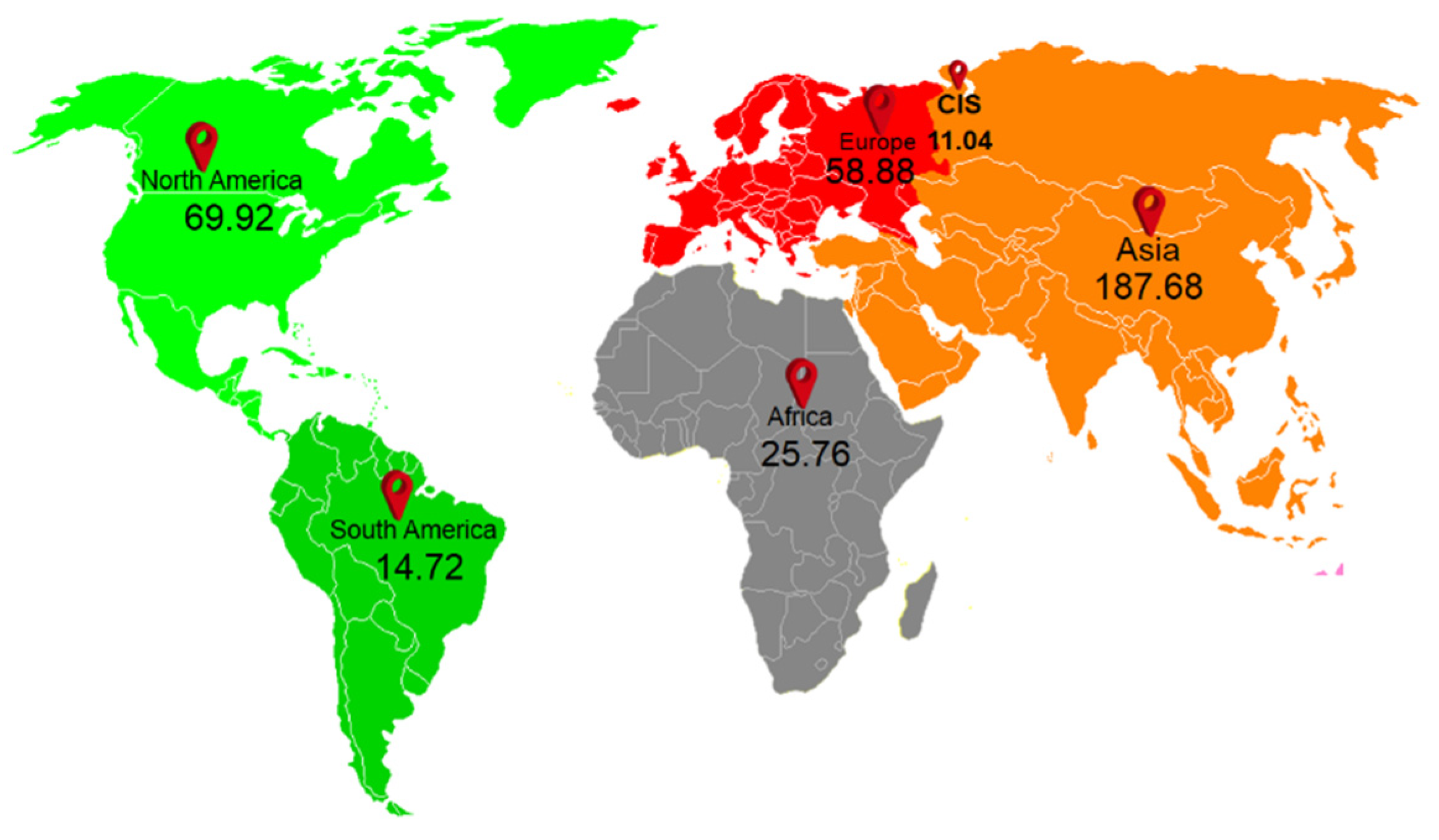
2. Sources of Plastic in Agriculture Systems
3. The Fate of Plastic in Agricultural Soil
3.1. Bioavailability
3.2. Behaviour in Rhizospheric Soil
3.3. Interaction with Soil Microbes
4. Fate and Uptake in Agricultural Plants
4.1. Transport of Plastic in Root Tissue
4.2. Translocation of Plastic from Root to Leaves
5. Effects of MPs and NPs on Plants
5.1. Effects of MPs and NPs on Plant Physiology
5.2. Effects of MPs and NPs on Plant Biochemical Indicators
6. Conclusions and Future Prospective
- More clarifications are required for the quantification of decomposition and understanding the mechanisms behind the degradation rate in agricultural soil.
- Long-term batch studies are required to understand the sorption/bioavailability potential of plastic in soil environments.
- More data are required for a better understanding of plastic behavior in different soils types and their interaction with soil microorganisms, especially in rhizosphere soil.
- Root exudates and mucilage are major barriers to contaminant entry in the plants; detailed studies are required for a better understanding of how plastic deals with entry into the plants.
- Soils are probably the major sink for NPs. However, the fate and behavior of NPs in soil environments remains poorly understood. Additionally, full-lifecycle studies on the interactions between NPs and plants are quite scarce. There is an urgent need to explore the mechanisms behind the toxicity of NPs in plants.
- The current knowledge about the bioaccumulation of NPs in plants and understandings of dietary uptake are limited, even though less information about their fate and behavior are inside the food web. Critically the main question in this context is if and how the uptake, bioaccumulation, and trophic transfer of NPs differ respectively to other pollutants.
- Interactions of NPs with other engineered nanoparticles under different growth media have not been explored yet. Thus, more conclusive data is required for a better understanding of NPs in agriculture systems to overcome this emerging issue.
Author Contributions
Funding
Conflicts of Interest
References
- Zainudin, B.H.; Wong, T.W.; Hamdan, H. Pectin as oral colon-specific nano-and microparticulate drug carriers. Polym. Sci. Innov. Appl. 2020, 257–286. [Google Scholar] [CrossRef]
- Chris, W. Plastics. 2020. Available online: https://www.explainthatstuff.com/plastics.html (accessed on 10 October 2020).
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastic and Nanoplastic in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Sci. Total Environ. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, eaba9475. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics Europe Market Research Group (PEMRG) and Conversion Market & Strategy GmbH, Final Report. 2020. Available online: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020 (accessed on 21 June 2020).
- Barcelo, D.J.M. Microplastics analysis. MethodsX 2020, 7, 100884. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Ma, D.; Wu, J.; Chai, C.; Shi, Y. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere 2016, 164, 314–321. [Google Scholar] [CrossRef]
- He, L.; Wu, D.; Rong, H.; Li, M.; Tong, M.; Kim, H. Influence of nano-and microplastic particles on the transport and deposition behaviors of bacteria in quartz sand. Sci. Total Environ. 2018, 52, 11555–11563. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Mai, L.; Bao, L.J.; Wong, C.S.; Zeng, E.Y.; Zeng, E.Y. Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency; Elsevier: Amsterdam, The Netherlands, 2018; p. 365. [Google Scholar]
- Jianping, L.; Minrong, L.; Jinnan, W.; Jianjian, L.; Hongwen, S.; Maoxing, H. Theoretical and Reality Basis of GEC Research. In Report on Global Environmental Competitiveness; Springer: Berlin/Heidelberg, Germany, 2013; pp. 23–35. [Google Scholar]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Westerhoff, P. We Should Expect More out of Our Sewage Sludge. Environ. Sci. Technol. 2015, 49, 8271–8276. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, Q.; Zhang, H.-B.; Pan, X.; Tu, C.; Li, L.; Yang, J. Pay attention to research on microplastic pollution in soil for prevention of ecological and food chain risks. Bull. Chin. Acad. Sci. 2018, 33, 1021–1030. [Google Scholar]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.; Ulke, J.; Font, A.; Chan, K.; Kelly, F. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.; Branco, V.; Carvalho, C.; Guilhermino, L. Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci. Rep. 2018, 8, 15655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Cho, H.-J.; Kim, E.; Huh, Y.H.; Kim, H.-J.; Kim, B.; Kang, T.; Lee, J.-S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, J.; Lee, S.; Choi, I. Identification of adverse outcome pathway related to high-density polyethylene microplastics exposure: Caenorhabditis elegans transcription factor RNAi screening and zebrafish study. J. Hazard. Mater. 2020, 388, 121725. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Zhao, Y.; Xiang, Y.; He, D.; Pan, X. Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environ. Pollut. 2020, 257, 113475. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sheng, G.D.; Chen, Q.-L.; O’Connor, P. Do combined nanoscale polystyrene and tetracycline impact on the incidence of resistance genes and microbial community disturbance in Enchytraeus crypticus? J. Hazard. Mater. 2020, 387, 122012. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A.S. Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020, 173, 115577. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Xiao, X.; Wu, J.; Wei, S.; Liu, Y.; Muir, D.C.; Ouyang, G. Joint effect of nanoplastics and humic acid on the uptake of PAHs for Daphnia magna: A model study. J. Hazard. Mater. 2020, 391, 122195. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Huang, G.; Zhou, T.; Zhao, Y.; Ma, J. Interfacial interaction between diverse microplastics and tetracycline by adsorption in an aqueous solution. Sci. Total Environ. 2020, 721, 137729. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef]
- Nolte, T.M.; Hartmann, N.B.; Kleijn, M.; Garnæs, J.; van de Meent, D.; Hendriks, A.J.; Baun, A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 2017, 183, 11–20. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Redondo-Hasselerharm, P.; Leslie, H.A.; Kraak, M.H.S.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhou, J.; Wang, G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ. Sci. Pollut. Res. 2021, 28, 16042–16053. [Google Scholar] [CrossRef]
- Schwab, F.; Rothen-Rutishauser, B.; Petri-Fink, A. When plants and plastic interact. Nat. Nanotechnol. 2020, 15, 729–730. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Redondo-Hasselerharm, P.E.; Diepens, N.J.; Koelmans, A.A. Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes. Sci. Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Kalčíková, G.; Gotvajn, A.Z.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Zeb, A.; Zheng, S.; Ma, T.; Peng, F.; Tang, J.; Liu, W. Do polystyrene nanoplastics affect the toxicity of cadmium to wheat (Triticum aestivum L.)? Environ. Pollut. 2020, 263, 114498. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.M.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef] [Green Version]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: Part Two of a Global Assessment; GESAMP: Madrid, Spain, 2016; p. 220. [Google Scholar]
- Sepúlveda, A.; Schluep, M.; Renaud, F.; Streicher, M.; Kuehr, R.; Hagelüken, C.; Gerecke, A.C. A review of the environmental fate and effects of hazardous substances released from electrical and electronic equipments during recycling: Examples from China and India. Environ. Impact Assess. Rev. 2010, 30, 28–41. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Weinstein, S. Main Ingredient in Marine Soup: Eliminating Plastic Bag Pollution through Consumer Disincentive. Cal. W. Int′l LJ 2009, 40, 291. [Google Scholar]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Heo, N.W.; Han, G.M.; Lee, M.J.; Kang, D.; et al. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef]
- Outlook. 2015. Available online: https://www.iea.org/reports/world-energy-outlook-2015 (accessed on 25 July 2021).
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Thermal Degradation of Real-World Waste Plastics and Simulated Mixed Plastics in a Two-Stage Pyrolysis–Catalysis Reactor for Fuel Production. Energy Fuels 2015, 29, 2601–2609. [Google Scholar] [CrossRef]
- Liu, E.; He, W.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef] [Green Version]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Ziajahromi, S.; Neale, P.A.; Silveira, I.T.; Chua, A.; Leusch, F.D. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Mintenig, S.; Int-Veen, I.; Löder, M.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy considerations for limiting unintended residual plastic in agricultural soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Xu, B.; He, Y.; Brookes, P.C.; Tang, C.; Xu, J. Differences in transport behavior of natural soil colloids of contrasting sizes from nanometer to micron and the environmental implications. Sci. Total Environ. 2018, 634, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. 2015, 22, 2584–2598. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. Critical Review. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar]
- Da Costa, G.A.C.; Akutsu, R.D.C.; Gallo, L.R.D.R.; Araújo, W.M.C. Knowledge and Consumer Behavior Related to Safe Practices of Food Handling. J. Saf. Stud. 2016, 2, 15–33. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; Van Der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ingraffia, R.; Machado, A.A.D.S. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [Green Version]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic Contamination in Karst Groundwater Systems. Ground Water 2019, 57, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Baumhardt, R.; Jones, O.R.; Schwartz, R.C. Long-Term Effects of Profile-Modifying Deep Plowing on Soil Properties and Crop Yield. Soil Sci. Soc. Am. J. 2008, 72, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Azeem, I.; Khan, A.A.; Bibi, H.; Azeem, K.; Naz, F.; Khan, K.; Shah, S.A.A.; Shah, K.J.P. Nitrogen sources and Bioaab inclined yield and nutrients uptake specifically in Triticum aestivum. Pure Appl. Biol. PAB 2019, 8, 2311–2325. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef]
- Cey, E.E.; Rudolph, D.L.; Passmore, J. Influence of macroporosity on preferential solute and colloid transport in unsaturated field soils. J. Contam. Hydrol. 2009, 107, 45–57. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Shakoor, N.; Shafiq, M.; Pavlicek, A.; Part, F.; Zafiu, C.; Raza, A.; Ahmad, M.A.; Jilani, G.; White, J.C.; et al. A critical review of the environmental impacts of manufactured nano-objects on earthworm species. Environ. Pollut. 2021, 290, 118041. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; Van Der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.-L.; An, X.-L.; Yang, X.-R.; Christie, P.; Ke, X.; Wu, L.-H.; Zhu, Y.-G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Yu, M.; van der Ploeg, M.; Lwanga, E.H.; Yang, X.; Zhang, S.; Ma, X.; Geissen, V. Leaching of microplastics by preferential flow in earthworm (Lumbricus terrestris) burrows. Environ. Chem. 2019, 16, 31–40. [Google Scholar] [CrossRef]
- Heinze, W.M. Nanoplastic Transport in Soils by Advection and Bioturbation. Mater’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2019. [Google Scholar]
- Adeel, M.; Tingting, J.; Hussain, T.; He, X.; Ahmad, M.A.; Irshad, M.K.; Rui, Y. Bioaccumulation of ytterbium oxide nanoparticles insinuate oxidative stress, inflammatory, and pathological lesions in ICR mice. Environ. Sci. Pollut. Res. 2020, 27, 32944–32953. [Google Scholar] [CrossRef]
- Adeel, M.; Ma, C.; Ullah, S.; Rizwan, M.; Hao, Y.; Chen, C.; Jilani, G.; Shakoor, N.; Li, M.; Wang, L.; et al. Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: A life cycle study on Eisenia fetida. Environ. Pollut. 2019, 254, 113032. [Google Scholar] [CrossRef]
- Adeel, M.; Shakoor, N.; Ahmad, M.A.; White, J.C.; Jilani, G.; Rui, Y. Bioavailability and toxicity of nanoscale/bulk rare earth oxides in soil: Physiological and ultrastructural alterations in Eisenia fetida. Environ. Sci. Nano 2021, 8, 1654–1666. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Atuanya, E.I.; Udochukwu, U.; Dave-Omoregie, A.O. Bioavailability and Toxicity of Plastic Contaminants to Soil and Soil Bacteria. Br. Microbiol. Res. J. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Ng, K.; Obbard, J. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761–767. [Google Scholar] [CrossRef]
- Yu, H.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Decrease in bioavailability of soil heavy metals caused by the presence of microplastics varies across aggregate levels. J. Hazard. Mater. 2020, 395, 122690. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xi, B.; Tan, W. Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci. Total Environ. 2021, 752, 141956. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Adams, C.A.; Sun, Y. Effects of Co-Contamination of Microplastics and Cd on Plant Growth and Cd Accumulation. Toxics 2020, 8, 36. [Google Scholar] [CrossRef]
- Adeel, M.; Zain, M.; Shafi, J.; Ahmad, M.; Rizwan, M.; Shafiq, M.; Rui, Y. Conservation agriculture, a way to conserve soil carbon for sustainable agriculture productivity and mitigating climate change: A review. Fresenius Environ. Bull. 2018, 27, 6297–6308. [Google Scholar]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021, 211, 111899. [Google Scholar] [CrossRef]
- Adeel, M.; Farooq, T.; Shakoor, N.; Ahmar, S.; Fiaz, S.; White, J.; Gardea-Torresdey, J.; Mora-Poblete, F.; Rui, Y. COVID-19 and Nanoscience in the Developing World: Rapid Detection and Remediation in Wastewater. Nanomaterials 2021, 11, 991. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M. Absorption of Carbon-13 Labelled Fullerene (C60) on Rice Seedlings and Effect of Phytohormones on Growth. J. Nanosci. Nanotechnol. 2021, 21, 3197–3202. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [Green Version]
- Munn, S.; Goumenou, M. Thresholds for Endocrine Disrupters and Related Uncertainties. Report of the Endocrine Disrupters Expert Advisory Group. 2013. Available online: https://www.researchgate.net/profile/Marina-Goumenou-2/publication/335820043_Thresholds_for_Endocrine_Disrupters_and_Related_Uncertainties_Report_of_the_Endocrine_Disrupters_Expert_Advisory_Group/links/5d7cff464585155f1e4d8a20/Thresholds-for-Endocrine-Disrupters-and-Related-Uncertainties-Report-of-the-Endocrine-Disrupters-Expert-Advisory-Group.pdf (accessed on 2 August 2021).
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Zhu, D.; Xia, T.; Qi, Y.; Yao, Y.; Guo, X.; Ji, R.; Chen, W. Polystyrene Nanoplastics-Enhanced Contaminant Transport: Role of Irreversible Adsorption in Glassy Polymeric Domain. Environ. Sci. Technol. 2018, 52, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.-P.; Pei, D.-S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef]
- Godoy, V.; Martínez-Férez, A.; Martín-Lara, M.; Vellido-Pérez, J.; Calero, M.; Blázquez, G. Microplastics as Vectors of Chromium and Lead during Dynamic Simulation of the Human Gastrointestinal Tract. Sustainability 2020, 12, 4792. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption properties of cadmium on microplastics: The common practice experiment and A two-dimensional correlation spectroscopic study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef]
- Lin, L.; Tang, S.; Wang, X.; Sun, X.; Yu, A. Hexabromocyclododecane alters malachite green and lead(II) adsorption behaviors onto polystyrene microplastics: Interaction mechanism and competitive effect. Chemosphere 2021, 265, 129079. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, Y.; Yuan, Y.; Hu, B.; Wang, B. Comparative analysis of kinetics and mechanisms for Pb(II) sorption onto three kinds of microplastics. Ecotoxicol. Environ. Saf. 2021, 208, 111451. [Google Scholar] [CrossRef]
- Purwiyanto, A.I.S.; Suteja, Y.T.; Trisno; Ningrum, P.S.; Putri, W.A.E.; Rozirwan; Agustriani, F.; Fauziyah, F.; Cordova, M.R.; Koropitan, A.F. Concentration and adsorption of Pb and Cu in microplastics: Case study in aquatic environment. Mar. Pollut. Bull. 2020, 158, 111380. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yu, A. Pb(II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Song, L.; Fang, H.; Wang, D.-G. Aquatic toxicity of iron-oxide-doped microplastics to Chlorella pyrenoidosa and Daphnia magna. Environ. Pollut. 2020, 257, 113451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.-F.; Wang, S.-C.; Huang, T.-Y.; Li, M.-R.; Zhu, Z.-L.; Liu, G.-Z. Sorption of fluoroquinolones to nanoplastics as affected by surface functionalization and solution chemistry. Environ. Pollut. 2020, 262, 114347. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Ma, J.; Li, B. The structure of agricultural microplastics (PT, PU and UF) and their sorption capacities for PAHs and PHE derivates under various salinity and oxidation treatments. Environ. Pollut. 2020, 257, 113525. [Google Scholar] [CrossRef]
- Deshiikan, S.R.; Eschenazi, E.; Papadopoulos, K.D. Colloids and Surfaces A: Physicochemical and Engineering Aspects; Elsevier: Amsterdam, The Netherlands, 1998; Volume 145, p. 93. [Google Scholar]
- Wang, F.; Yang, W.; Cheng, P.; Zhang, S.; Zhang, S.; Jiao, W.; Sun, Y. Adsorption characteristics of cadmium onto microplastics from aqueous solutions. Chemosphere 2019, 235, 1073–1080. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci. Total Environ. 2020, 744, 140984. [Google Scholar] [CrossRef] [PubMed]
- Hester, E.R.; Harpenslager, S.F.; van Diggelen, J.M.H.; Lamers, L.L.; Jetten, M.S.M.; Lüke, C.; Lücker, S.; Welte, C.U. Linking Nitrogen Load to the Structure and Function of Wetland Soil and Rhizosphere Microbial Communities. mSystems 2018, 3, e00214-17. [Google Scholar] [CrossRef] [Green Version]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Janous, D.; Formanek, P. Methods of collection of plant root exudates in relation to plant metabolism and purpose: A review. J. Plant Nutr. Soil Sci. 2013, 176, 175–199. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.D.S.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, S.; Zhu, L.; Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 2018, 624, 48–54. [Google Scholar] [CrossRef]
- Abraham, J.; Ghosh, E.; Mukherjee, P.; Gajendiran, A. Microbial degradation of low density polyethylene. Environ. Prog. Sustain. Energy 2017, 36, 147–154. [Google Scholar] [CrossRef]
- Bauer, M.; Kube, M.; Teeling, H.; Richter, M.; Lombardot, T.; Allers, E.; Würdemann, C.A.; Quast, C.; Kuhl, H.; Knaust, F.; et al. Whole genome analysis of the marine Bacteroidetes ’Gramella forsetii’ reveals adaptations to degradation of polymeric organic matter. Environ. Microbiol. 2006, 8, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Williams, M.; Gregg, A.; Oliver, D.; Kumar, A.; Kookana, R.; Kirby, J.K. Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ. Pollut. 2019, 252, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Liu, X.; Qiu, W.; Song, Z. The mechanism of polystyrene microplastics to affect arsenic volatilization in arsenic-contaminated paddy soils. J. Hazard. Mater. 2020, 398, 122896. [Google Scholar] [CrossRef]
- Maity, S.; Pramanick, K. Perspectives and challenges of micro/nanoplastics-induced toxicity with special reference to phytotoxicity. Glob. Chang. Biol. 2020, 26, 3241–3250. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Bruno, V.; Nissen, L.; Vicari, A.; Bellaloui, N.; Little, N.S.; Shier, W.T. Persistence in soil of microplastic films from ultra-thin compostable plastic bags and implications on soil Aspergillus flavus population. Waste Manag. 2020, 113, 312–318. [Google Scholar] [CrossRef]
- Munoz, K.; Schmidt-Heydt, M.; Stoll, D.; Diehl, D.; Ziegler, J.; Geisen, R.; Schaumann, G.E. Effect of plastic mulching on mycotoxin occurrence and mycobiome abundance in soil samples from asparagus crops. Mycotoxin Res. 2015, 31, 191–201. [Google Scholar] [CrossRef]
- Kamiya, M.; Asakawa, S.; Kimura, M. Molecular analysis of fungal communities of biodegradable plastics in two Japanese soils. Soil Sci. Plant Nutr. 2007, 53, 568–574. [Google Scholar] [CrossRef]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; He, D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Miyazaki, J.; Kuriyama, Y.; Tokumoto, H.; Konishi, Y.; Nomura, T. Cytotoxicity and behavior of polystyrene latex nanoparticles to budding yeast. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 287–293. [Google Scholar] [CrossRef]
- Nomura, T.; Tani, S.; Yamamoto, M.; Nakagawa, T.; Toyoda, S.; Fujisawa, E.; Yasui, A.; Konishi, Y. Cytotoxicity and colloidal behavior of polystyrene latex nanoparticles toward filamentous fungi in isotonic solutions. Chemosphere 2016, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.D.S.; Horton, A.A.; Davis, T.; Maaß, S. Microplastics and Their Effects on Soil Function as a Life-Supporting System. In Microplastics in Terrestrial Environments; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–222. [Google Scholar]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Horton, H. Whale Found Dying Off Coast of Norway With 30 Plastic Bags in Its Stomach. 2017. Available online: http://www. telegraph. co. uk/news/2017/02/03/whale-found-dying-coastnorway-30-plastic-bags-stomach (accessed on 3 February 2017).
- Bandmann, V.; Müller, J.D.; Köhler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef] [Green Version]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle Uptake in Plants: Gold Nanomaterial Localized in Roots of Arabidopsis thaliana by X-ray Computed Nanotomography and Hyperspectral Imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef] [Green Version]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt, F.N.A.; O′Halloran, J.; Jansen, M.A.K. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef]
- Adeel, M.; Yang, Y.; Wang, Y.; Song, X.; Ahmad, M.A.; Rogers, H.J. Uptake and transformation of steroid estrogens as emerging contaminants influence plant development. Environ. Pollut. 2018, 243, 1487–1497. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.-J.; Adeel, M.; Shakoor, N.; Guo, K.-R.; Ma, D.-F.; Ahmad, M.A.; Lu, G.-Q.; Zhao, M.-H.; Li, S.-E.; Rui, Y.-K. Engineered Nanomaterials Suppress the Soft Rot Disease (Rhizopus stolonifer) and Slow Down the Loss of Nutrient in Sweet Potato. Nanomaterials 2021, 11, 2572. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Liu, X.; Wang, J. New Perspective on the Nanoplastics Disrupting the Reproduction of an Endangered Fern in Artificial Freshwater. Environ. Sci. Technol. 2019, 53, 12715–12724. [Google Scholar] [CrossRef]
- Kalčíková, G. Aquatic vascular plants—A forgotten piece of nature in microplastic research. Environ. Pollut. 2020, 262, 114354. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Dovidat, L.C.; Brinkmann, B.W.; Vijver, M.G.; Bosker, T. Plastic particles adsorb to the roots of freshwater vascular plant Spirodela polyrhiza but do not impair growth. Limnol. Oceanogr. Lett. 2019, 5, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.-J.; Sun, X.-D.; Zhu, F.-P.; Feng, Y.; Duan, J.-L.; Xiao, F.; Li, X.-Y.; Shi, Y.; Wang, Q.; Sun, J.-W.; et al. Nanoplastics Promote Microcystin Synthesis and Release from Cyanobacterial Microcystis aeruginosa. Environ. Sci. Technol. 2020, 54, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Shakoor, N.; Hussain, T.; Azeem, I.; Zhou, P.; Zhang, P.; Hao, Y.; Rinklebe, J.; Rui, Y. Bio-interaction of nano and bulk lanthanum and ytterbium oxides in soil system: Biochemical, genetic, and histopathological effects on Eisenia fetida. J. Hazard. Mater. 2021, 415, 125574. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geisler-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]



| Plastics | Level (%) | Effects on Microorganism | References |
|---|---|---|---|
| PE | 1, 5, 10, 20 | Decreased xylosidase and β-glucosidase activity by 16–43% and MPs increased the soil microbial biomass (+43.6%). | [163] |
| PVC | 1, 5 | Shown positive effects on acidobacteria, bacteriodietes, and hydrolase and urease enzymes while negative effects are shown on Sphingomonadaceae and the Fluorescein diacetate enzyme. | [25,174] |
| PE | NA | MPs provided habitat to actinobacteria, bacteroidetes, Proteobacteria, gemmatimonadetes and Acidobacteria. Additionally, colonies of bacteria significantly varied in structure from those in the surrounding soil. | [157] |
| PE | 5 | In the fertilized soil, MPs significantly enhanced the bacterial and fungal community. MPs seem to indicate the selective impact on microbes and cause a serious hazard to biogeochemical cycles and microbes ecology. | [158] |
| PE | 0.076 g kg−1 | Increased Bacteriodietes, Acidobacteria, Nitrospirae, Gemmatimonadetes and diminished effect on nutrient cycling as well as positive effect on catalase urease enzymes. | [25] |
| PVC | 0.1 | Gut bacterial diversity increased and negative impact on soil macro- and micro-organisms. | [102] |
| PS | 0.2, 0.4, 0.8 | Positive and negative effects of numerous Pro Firmicutes, teobacteria, and Bacteroidetes in various MP concentrations. | [165] |
| PVC | NA | PVC increases Desulfobulbaceae, and Desulfobacteraceae and decreases Sedimenticolaceae and Chromatiaceae. | [164] |
| PE, PLA | 0.1, 1 and 10 | MPs change the AMF diversity and structure that depend on their concentration level and type. Enriched with Ambispora (10% of PLA and PE), Archaeosporaceae (PLA 10%), and PLA have a negative impact on plant physiology i-e fresh/dry Biomass and Chlorophyll content. | [155] |
| Plants | Plastic Types | Time (Days) | Media | Accumulation | Reference |
|---|---|---|---|---|---|
| Z. mays | PS (NH2, COOH) | 28 | Soil | Both types of plastic accumulate in leaves and further transport to the roots. | [46] |
| L. sativa | PS NPs (0.1 and 1 mg L−1) | 35 | Soil | NP uptake by plant leaves by stomata and translocation downwards to the plant. | [47] |
| C. sativus | PS (100, 300, 500, 700 nm) | 65 | Hydroponics | PS uptake by the root and future transport to the leaves, flower, and fruit through the stem. | [42] |
| A. cepa | PS (0.01, 0.1, 1 g L−1) | 72 h | Hydroponics | PS enters different cellular compartments. | [44] |
| A. thaliana | PS (SO3H, NH2) | 35 | Soil | Positive-charge NPs have a great effect on the root and their uptake and internalization as compared to negative-charge particles. | [40] |
| T. aestivum, L. sativa | NPs 0.2 and 2 μm | 20 10 | Sandysoil, hydroponics | PS enters the root through crack-entry mode and through transpiration, which pulls PS transport from root to shoot. | [41] |
| L. sativum | MPs and NPs (103–107 P mL−1) | 72 h | Filter paper | Microplastic accumulates in the pores of the L. sativum seed and delays germination. | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. https://doi.org/10.3390/nano11112935
Azeem I, Adeel M, Ahmad MA, Shakoor N, Jiangcuo GD, Azeem K, Ishfaq M, Shakoor A, Ayaz M, Xu M, et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials. 2021; 11(11):2935. https://doi.org/10.3390/nano11112935
Chicago/Turabian StyleAzeem, Imran, Muhammad Adeel, Muhammad Arslan Ahmad, Noman Shakoor, Gama Dingba Jiangcuo, Kamran Azeem, Muhammad Ishfaq, Awais Shakoor, Muhammad Ayaz, Ming Xu, and et al. 2021. "Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review" Nanomaterials 11, no. 11: 2935. https://doi.org/10.3390/nano11112935
APA StyleAzeem, I., Adeel, M., Ahmad, M. A., Shakoor, N., Jiangcuo, G. D., Azeem, K., Ishfaq, M., Shakoor, A., Ayaz, M., Xu, M., & Rui, Y. (2021). Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials, 11(11), 2935. https://doi.org/10.3390/nano11112935













