Structural and Electrochemical Properties of Physically and Chemically Activated Carbon Nanoparticles for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis and Activation
2.2. Materials Characterization
2.3. Electrodes Testing
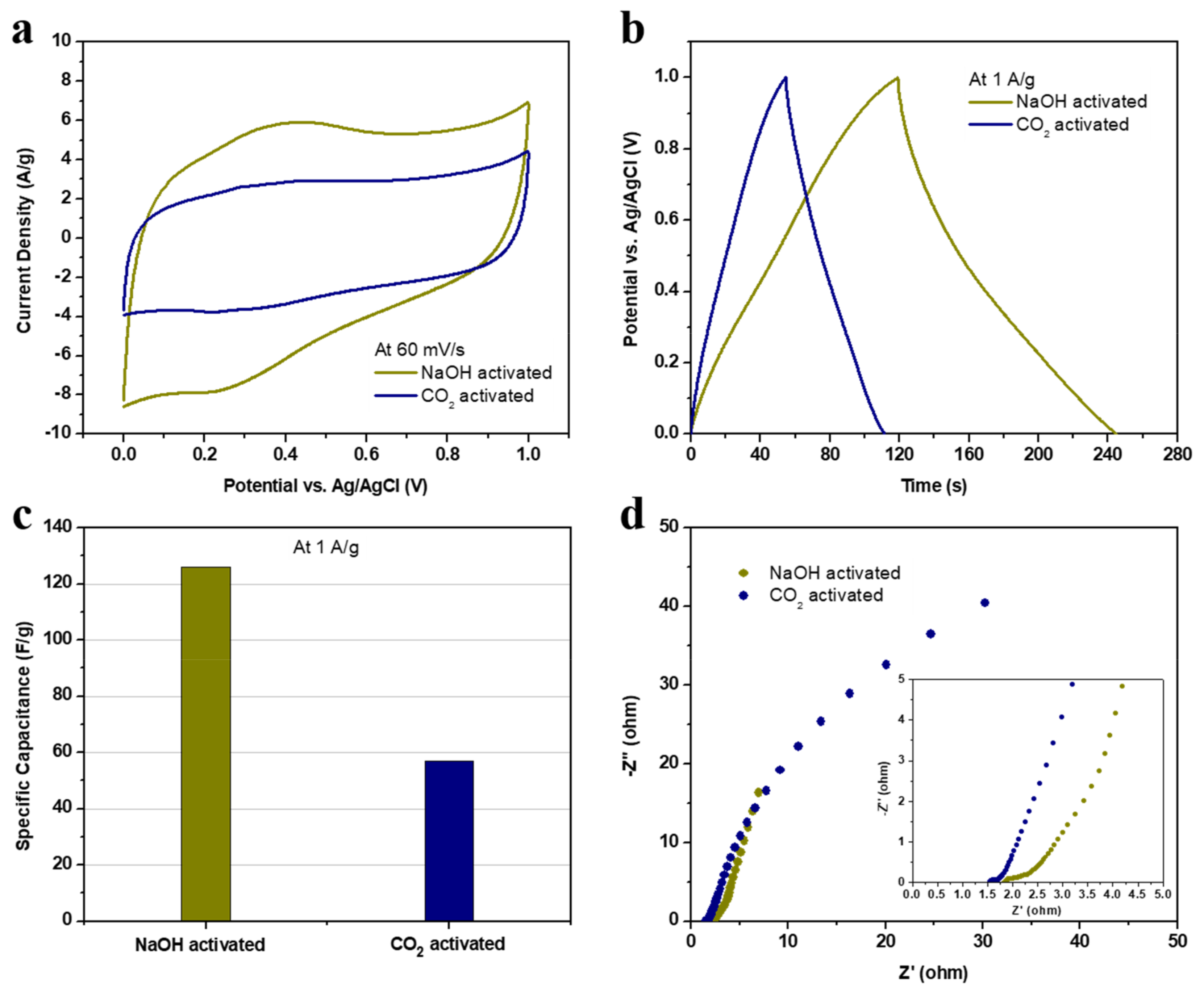
3. Results and Discussion
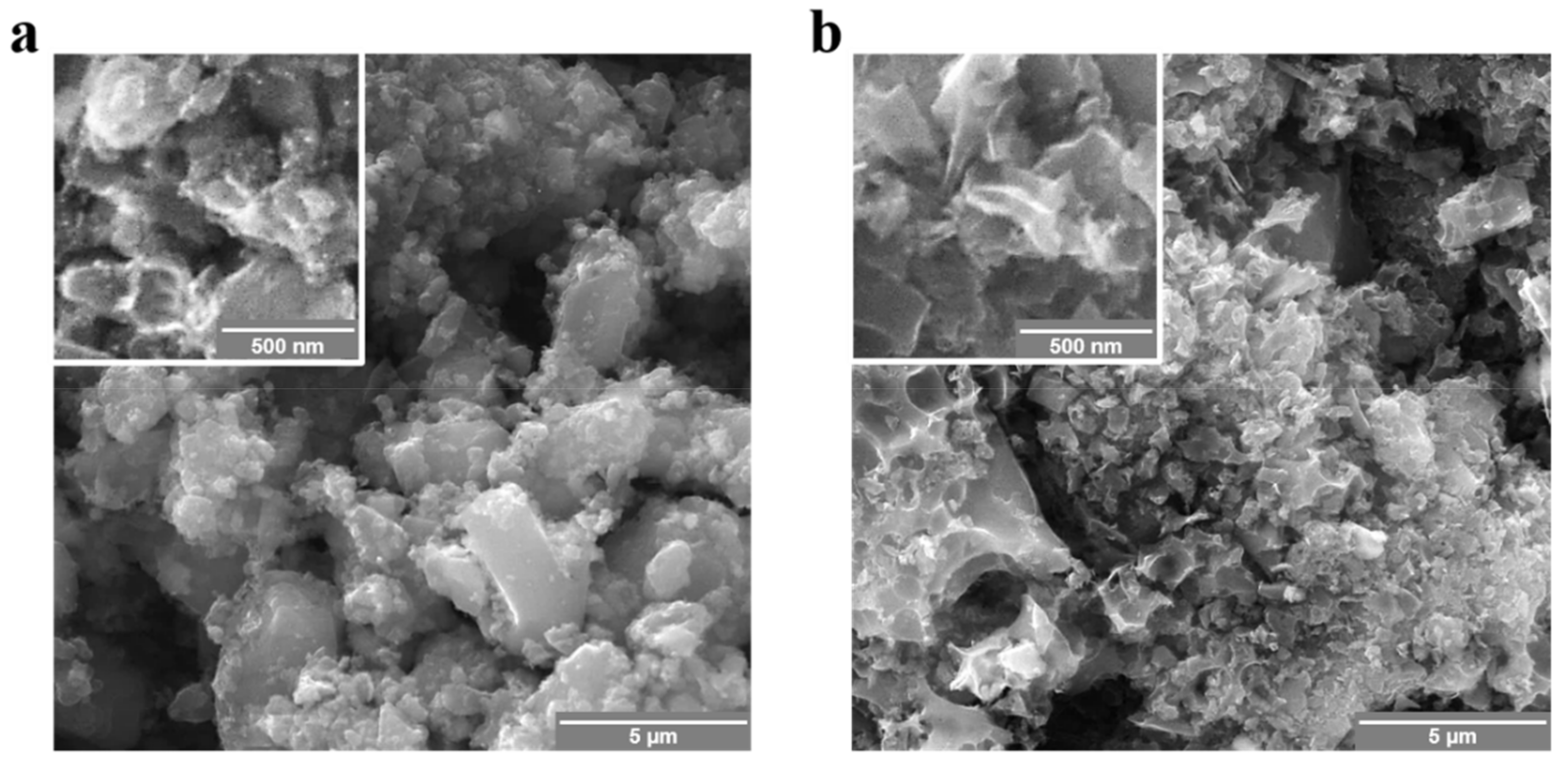
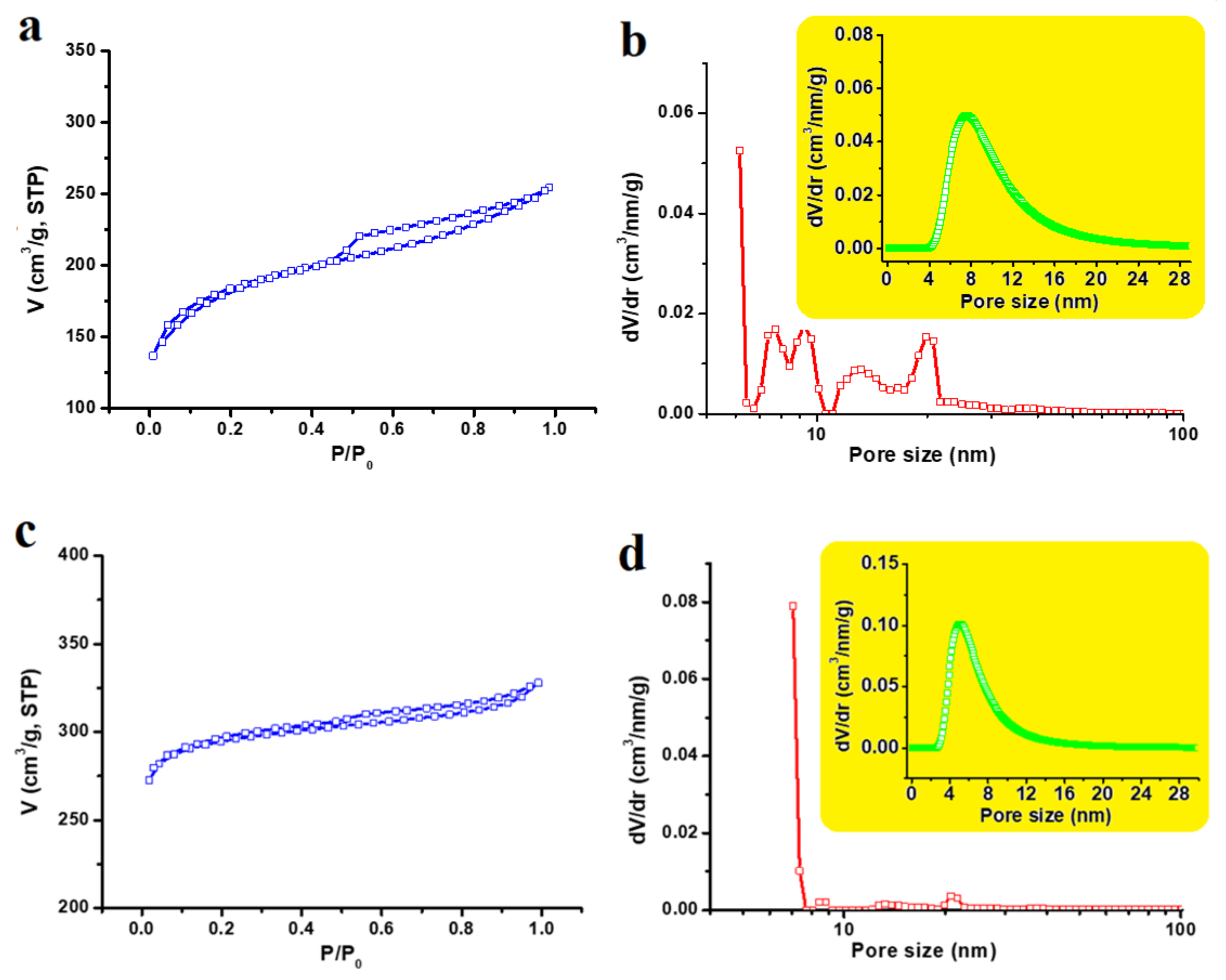
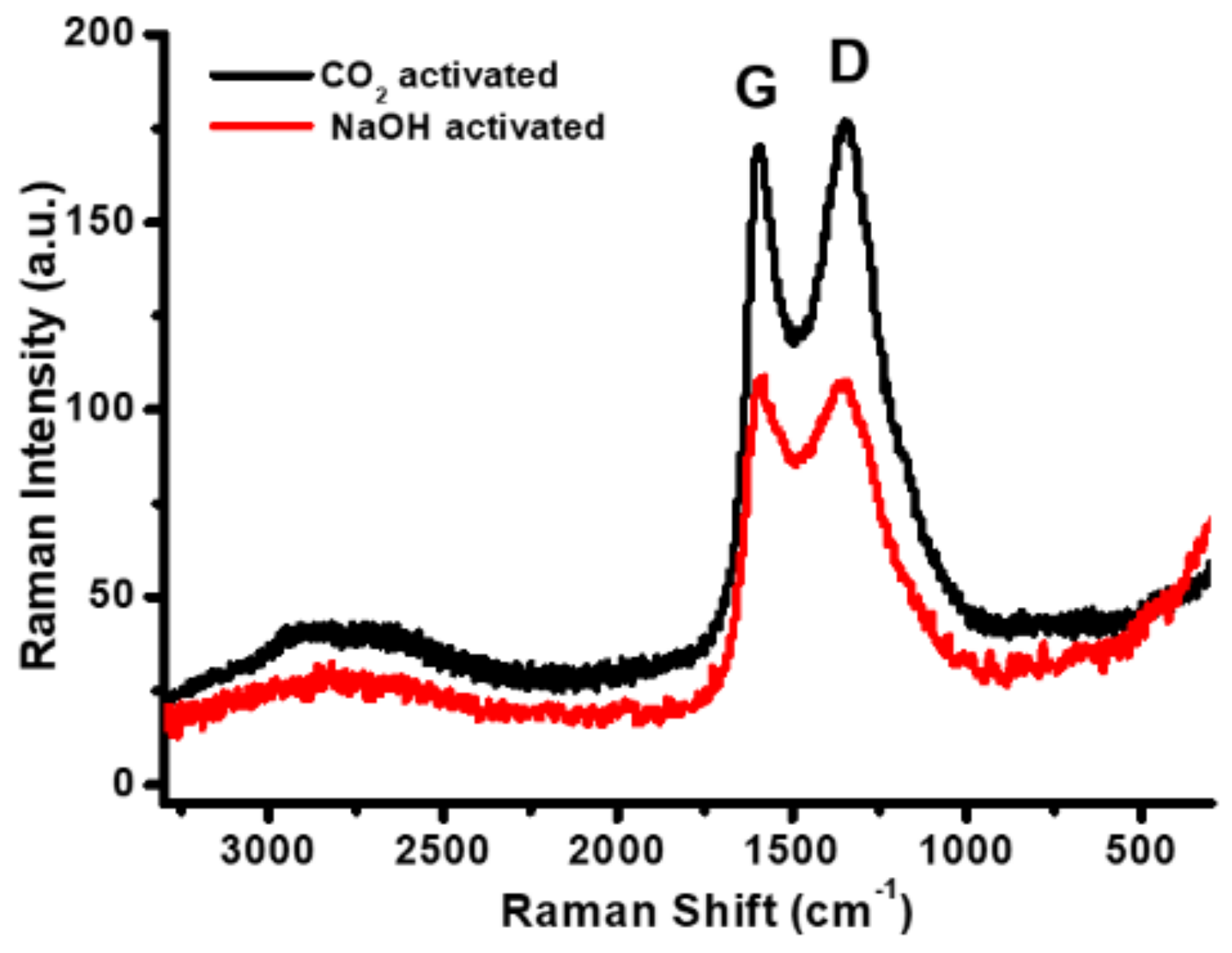
3.1. Materials Properties
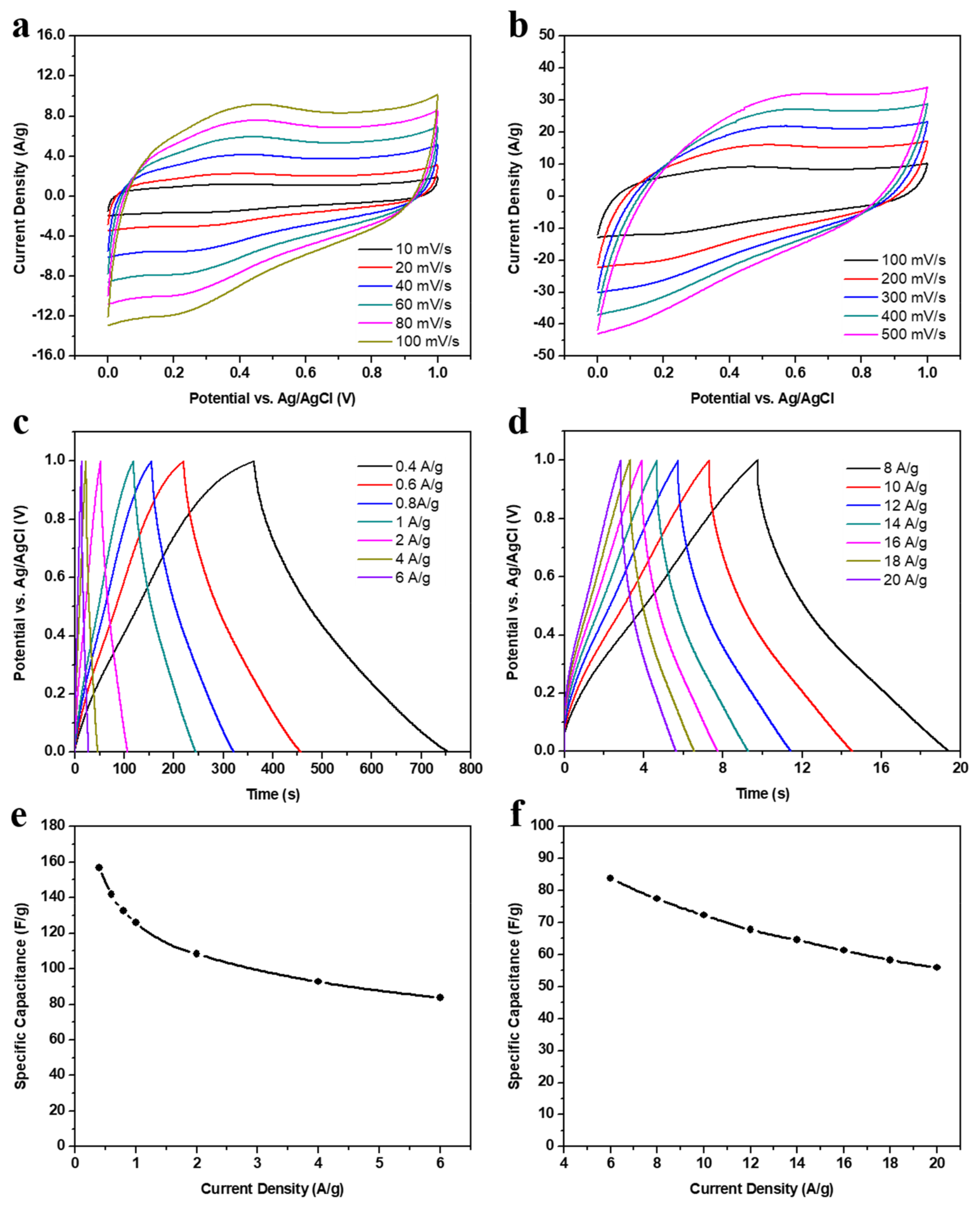
3.2. Electrodes Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Koohi-Kamali, S.; Tyagi, V.V.; Rahim, N.A.; Panwar, N.L.; Mokhlis, H. Emergence of energy storage technologies as the solution for reliable operation of smart power systems: A review. Renew. Sustain. Energy Rev. 2013, 25, 135–165. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Ho, P.; Park, S.; Id, O. Perspective on high-energy carbon-based supercapacitors. Energy Environ. Mater. 2020, 3, 286–305. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Chavan, U.S.; Pandey, A. Materials and Fabrication Methods for Electrochemical Supercapacitors: Overview. Electrochem. Energy Rev. 2020, 3, 155–186. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-Derived Hierarchical Porous Carbon with Controlled Porosity for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [Green Version]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.W.; Hou, C.H. A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Lu, S.Y.; Jin, M.; Zhang, Y.; Niu, Y.B.; Gao, J.C.; Li, C.M. Chemically Exfoliating Biomass into a Graphene-like Porous Active Carbon with Rational Pore Structure, Good Conductivity, and Large Surface Area for High-Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1702545. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Lei, C.H.; Lekakou, C. Activated carbon-carbon nanotube nanocomposite coatings for supercapacitor applications. Surf. Coat. Tech. 2013, 232, 326–330. [Google Scholar] [CrossRef]
- Fields, R.; Lei, C.H.; Markoulidis, F.; Lekakou, C. The Composite Supercapacitor. Energy Technol. 2016, 4, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.; Markoulidis, F.; Lekakou, C.; Laudone, G.M. Design of Porous Carbons for Supercapacitor Applications for Different Organic Solvent-Electrolytes. C-J. Carbon Res. 2021, 7, 15. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Rajendran, J.; Sundramoorthy, A.K. Promising nature-based activated carbon derived from flowers of Borassus flabellifer for supercapacitor applications. Carbon Lett. 2021, 31, 1145–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Yuan, Y.; Wang, J.; Lu, J.; Wang, S. Recent Progress in Biomass-Derived Electrode Materials for High Volumetric Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801007. [Google Scholar] [CrossRef]
- Zou, K.; Guan, Z.; Deng, Y.; Chen, G. Nitrogen-rich porous carbon in ultra-high yield derived from activation of biomass waste by a novel eutectic salt for high performance Li-ion capacitors. Carbon 2020, 161, 25–35. [Google Scholar] [CrossRef]
- Niu, J.; Guan, J.; Dou, M.; Zhang, Z.; Kong, J.; Wang, F. Sustainable Synthesis of Biomass-Derived Carbon Electrodes with Hybrid Energy-Storage Behaviors for Use in High-Performance Na-Ion Capacitors. ACS Appl. Energy Mater. 2020, 3, 2478–2489. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, N.; Liu, D.; Xu, J.; Sha, J.; Yin, J.; Tan, X.; Yang, H.; Lu, H.; Lin, H. Direct carbonization of rice husk to prepare porous carbon for supercapacitor applications. Energy 2017, 128, 618–625. [Google Scholar] [CrossRef]
- Makkawi, Y.; El Sayed, Y.; Salih; Nancarrow, M.P.; Banks, S.; Bridgwater, T. Fast pyrolysis of date palm (Phoenix dactylifera) waste in a bubbling fluidized bed reactor. Renew. Energy 2019, 143, 719–730. [Google Scholar] [CrossRef]
- Haghbin, M.R.; Shahrak, M.N. Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technol. 2021, 377, 890–899. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Alhamed, Y.A.; Bamufleh, H.S. Sulfur removal from model diesel fuel using granular activated carbon from dates’ stones activated by ZnCl2. Fuel 2009, 88, 87–94. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Al Shoaibi, A.; Srinivasakannan, C. Activated carbon from date palm seed: Process optimization using response surface methodology. Waste Biomass Valorization 2012, 3, 149–156. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Al Shoaibi, A.; Srinivasakannan, C. A comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. New Carbon Mater. 2012, 27, 344–351. [Google Scholar] [CrossRef]
- Merzougui, Z.; Addoun, F. Effect of oxidant treatment of date pit activated carbons application to the treatment of waters. Desalination 2008, 222, 394–403. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus physical activation of coconut shell: A comparative study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Kim, D.W.; Kil, H.S.; Nakabayashi, K.; Yoon, S.H.; Miyawaki, J. Structural elucidation of physical and chemical activation mechanisms based on the microdomain structure model. Carbon 2017, 114, 98–105. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Díez, N.; Fuertes, A.B. More Sustainable Chemical Activation Strategies for the Production of Porous Carbons. ChemSusChem. 2020, 14, 94–117. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organization. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Rodenas, M.; Amoros, D.; Solano, A. Understanding chemical reactions between carbons and NaOH and KOH An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Maciá-Agulló, J.A.; Moore, B.C.; Cazorla-Amorós, D.; Linares-Solano, A. Activation of coal tar pitch carbon fibres: Physical activation vs. chemical activation. Carbon 2004, 42, 1367–1370. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Jose, R. Conversion of Oil Palm Kernel Shell Biomass to Activated Carbon for Supercapacitor Electrode Application. Waste Biomass Valorization 2019, 10, 1731–1740. [Google Scholar] [CrossRef]
- Ruiz, V.; Blanco, C.; Raymundo-Pinero, E.; Khomenko, V.; Beguin, F.; Santamaria, R. Effects of thermal treatment of activated carbon on the electrochemical behaviour in supercapacitors. Electrochim. Acta 2007, 52, 4969–4973. [Google Scholar] [CrossRef] [Green Version]
- Salah, N.; Salah, Y.; Alshahrie, A.S. Methods of Producing Carbon Nanoparticles. US Patent 10,906,812 B1, 2 February 2021. Available online: https://patents.google.com/patent/US10906812B1 (accessed on 6 November 2021).
- NIST X-ray Photoelectron Spectroscopy Database. Available online: http://dx.doi.org/10.18434/T4T88K (accessed on 6 November 2021).
- Moulder, J.F. Standard XPS Spectra for Elements. In Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, 1st ed.; Chastain, J., Ed.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Ko, T.H.; Kuo, W.S.; Hu, C.H. Raman spectroscopic study of effect of steam and carbon dioxide activation on microstructure of polyacrylonitrile-based activated carbon fabrics. J. Appl. Polym. Sci. 2001, 81, 1090–1099. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Zheng, S.; Guo, X.; Xue, H.; Pang, H. Recent Progress in Some Amorphous Materials for Supercapacitors. Small 2018, 14, 1800426. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.A.; Taer, E.; Basri, N.H.; Manjunatha, J.G.; Ishak, M.M.; Dollah, B.N.M.; Hashmi, S.A. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Chen, W.M.; Hong, S.; Pan, M.Z.; Jiang, M.; Wu, Q.L.; Mei, C.T. Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors. Nanomaterials 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]

| CO2-Activated | NaOH-Activated | |
|---|---|---|
| BET Specific Surface Area | 603.5 m2/g | 1011 m2/g |
| DFT method summary: | ||
| Pore volume | 0.401 cm3/g | 0.452 cm3/g |
| Surface area | 611.337 m2/g | 1047.874 m2/g |
| Lower confidence limit | 1.232 nm | 0.705 nm |
| Fitting error | 0.229% | 0.039% |
| Pore width (mode) | 1.232 nm | 1.41 nm |
| DA method summary: | ||
| Best E | 4.602 kJ/mol | 17.211 kJ/mol |
| Best n | 1.000 | 1.000 |
| DA Micropore Volume | 0.416 cm3/g | 0.486 cm3/g |
| Pore Diameter (mode) | 1.56 nm | 1.2 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhebshi, N.A.; Salah, N.; Hussain, H.; Salah, Y.N.; Yin, J. Structural and Electrochemical Properties of Physically and Chemically Activated Carbon Nanoparticles for Supercapacitors. Nanomaterials 2022, 12, 122. https://doi.org/10.3390/nano12010122
Alhebshi NA, Salah N, Hussain H, Salah YN, Yin J. Structural and Electrochemical Properties of Physically and Chemically Activated Carbon Nanoparticles for Supercapacitors. Nanomaterials. 2022; 12(1):122. https://doi.org/10.3390/nano12010122
Chicago/Turabian StyleAlhebshi, Nuha A., Numan Salah, Humair Hussain, Yousef N. Salah, and Jian Yin. 2022. "Structural and Electrochemical Properties of Physically and Chemically Activated Carbon Nanoparticles for Supercapacitors" Nanomaterials 12, no. 1: 122. https://doi.org/10.3390/nano12010122
APA StyleAlhebshi, N. A., Salah, N., Hussain, H., Salah, Y. N., & Yin, J. (2022). Structural and Electrochemical Properties of Physically and Chemically Activated Carbon Nanoparticles for Supercapacitors. Nanomaterials, 12(1), 122. https://doi.org/10.3390/nano12010122







