Effects of Annealing Temperature on the Oxygen Evolution Reaction Activity of Copper–Cobalt Oxide Nanosheets
Abstract
1. Introduction
2. Materials and Methods
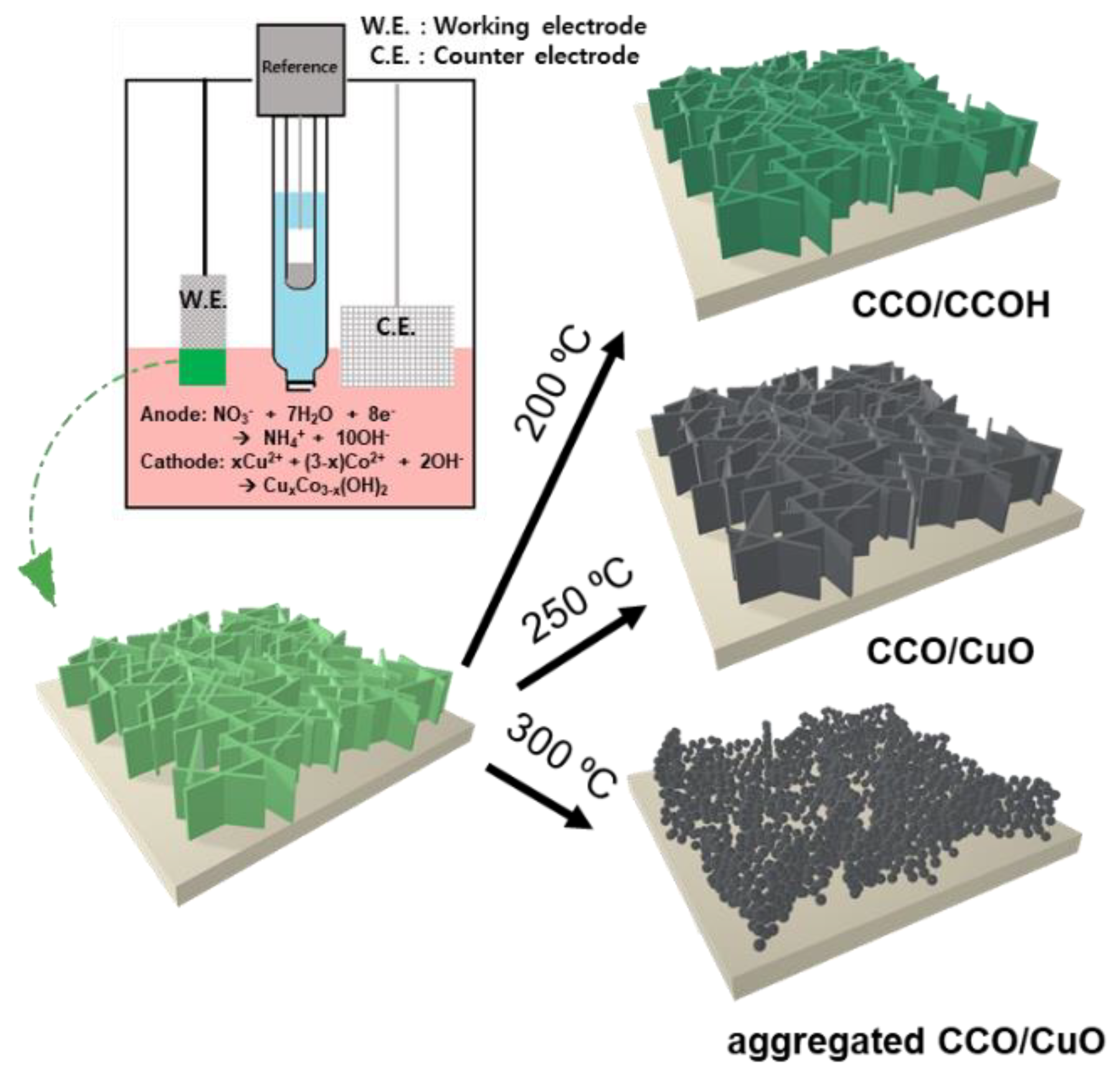
2.1. Preparation of Copper–Cobalt Oxide Electrodes by Cathodic Electrodeposition
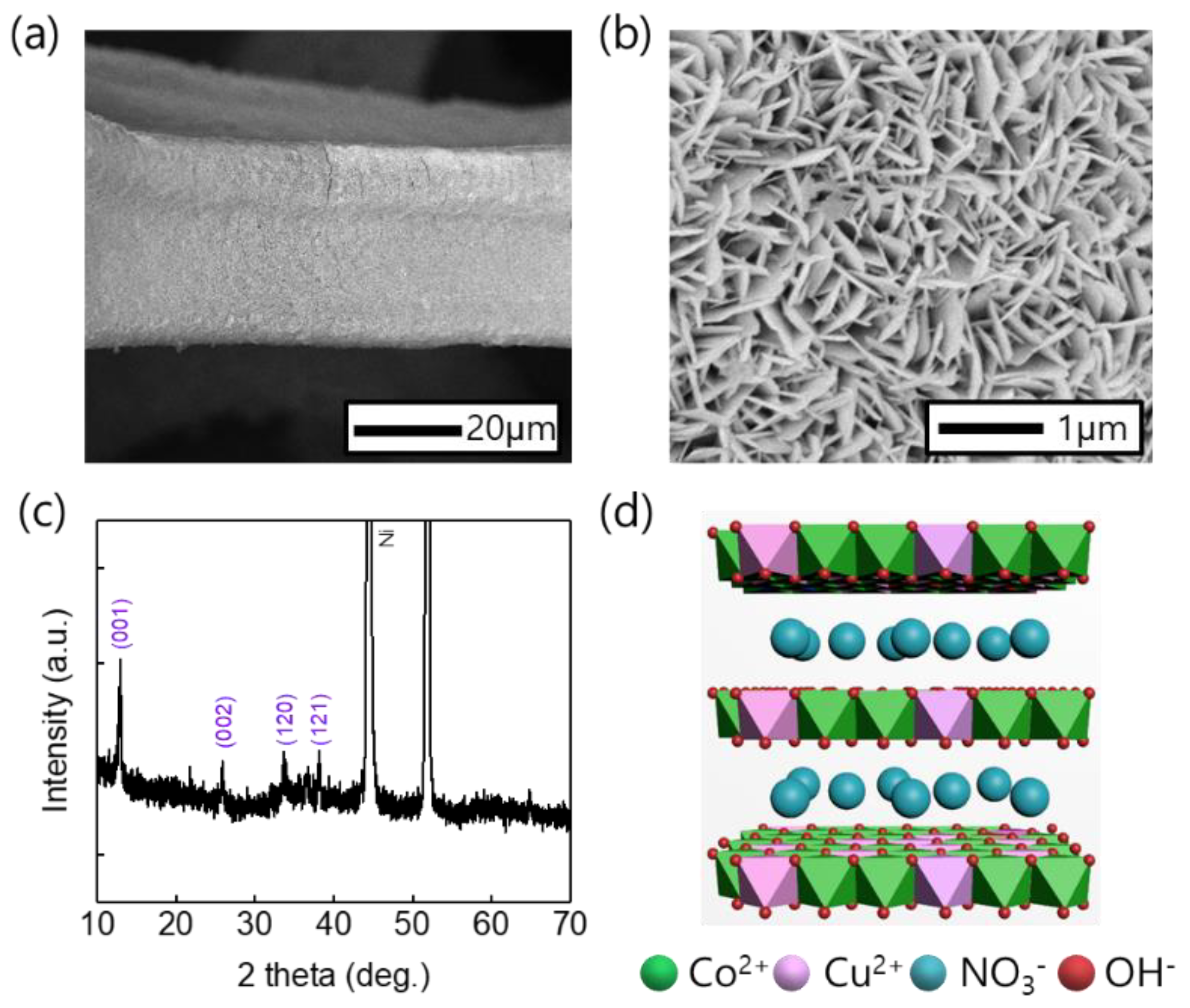
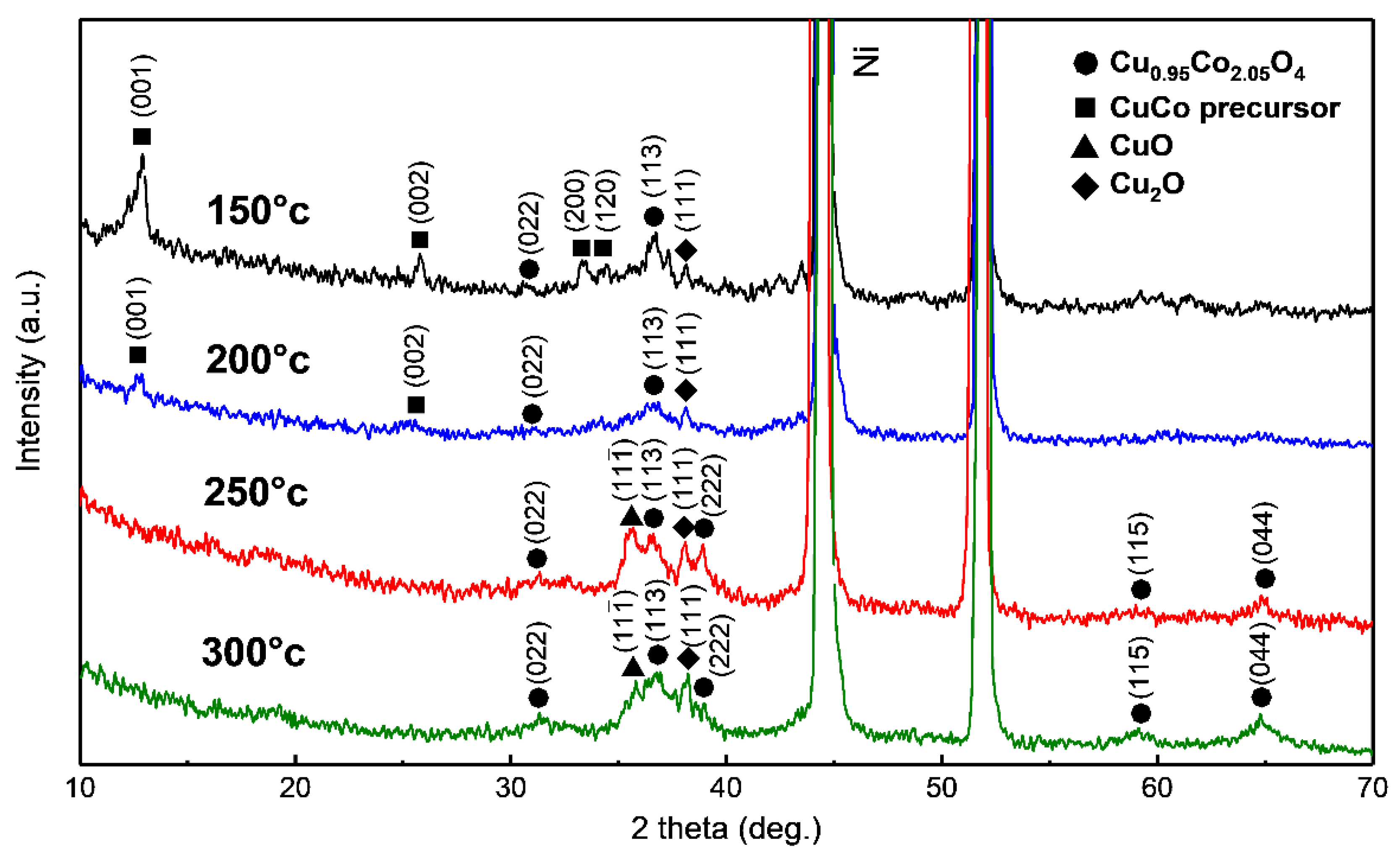
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
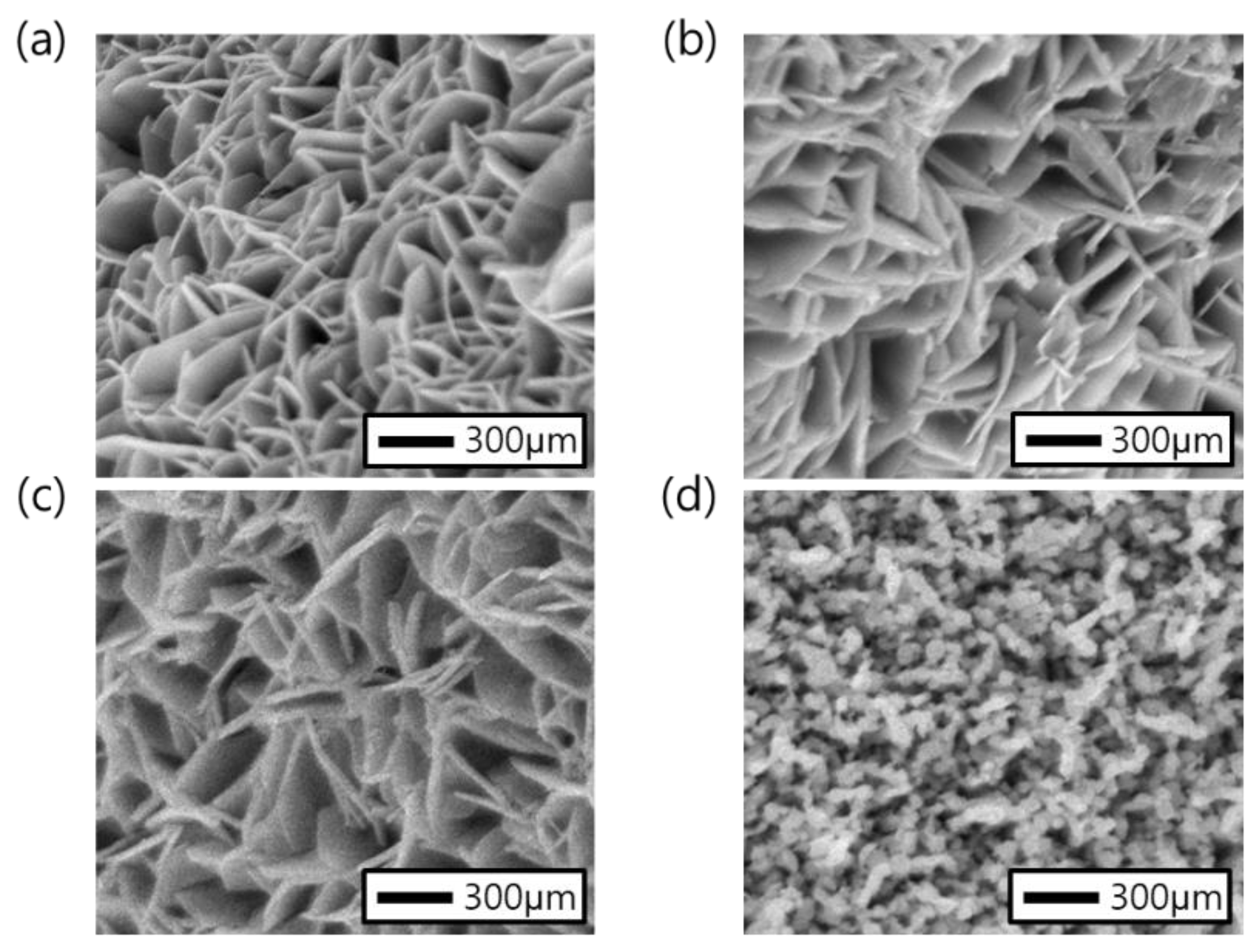
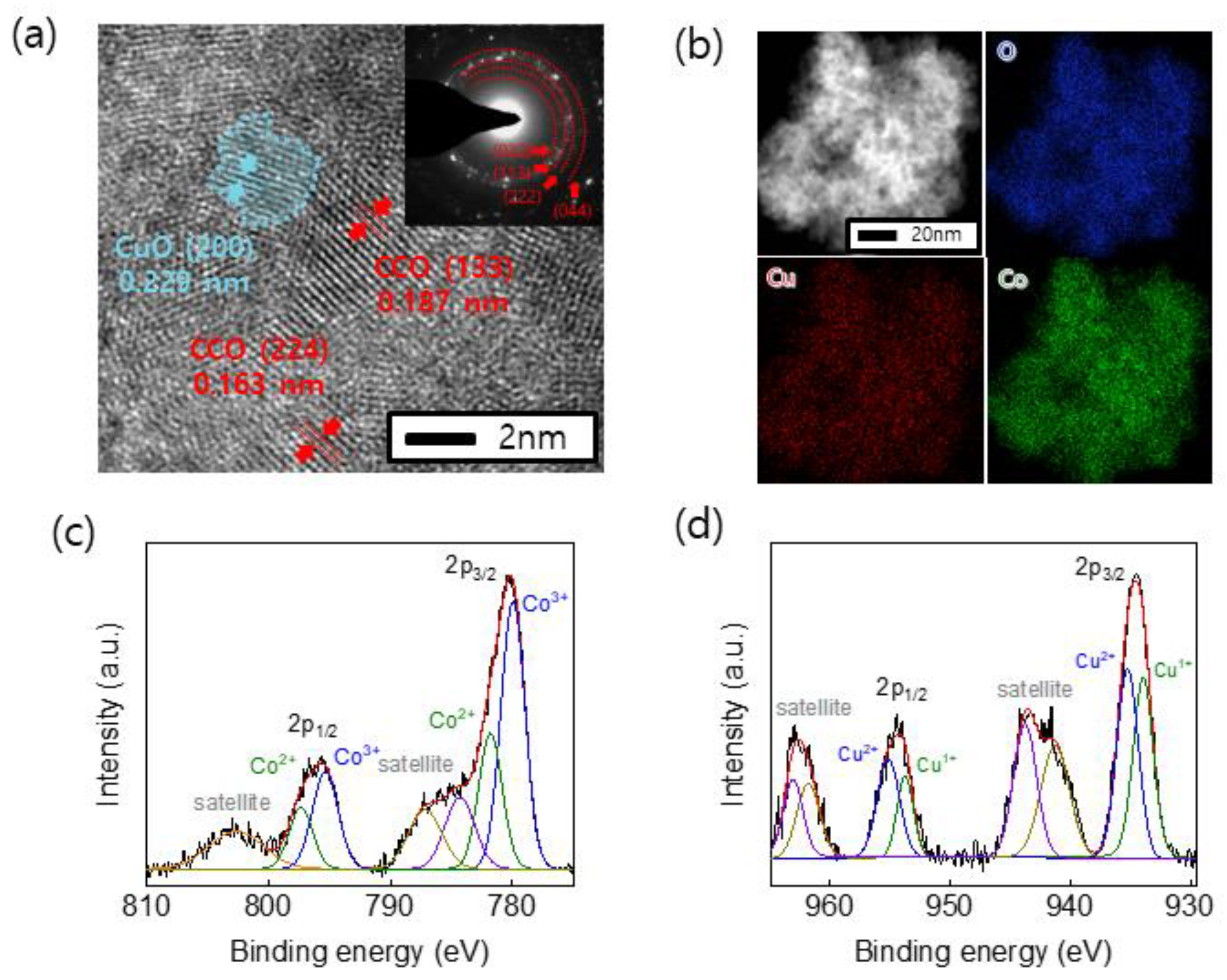
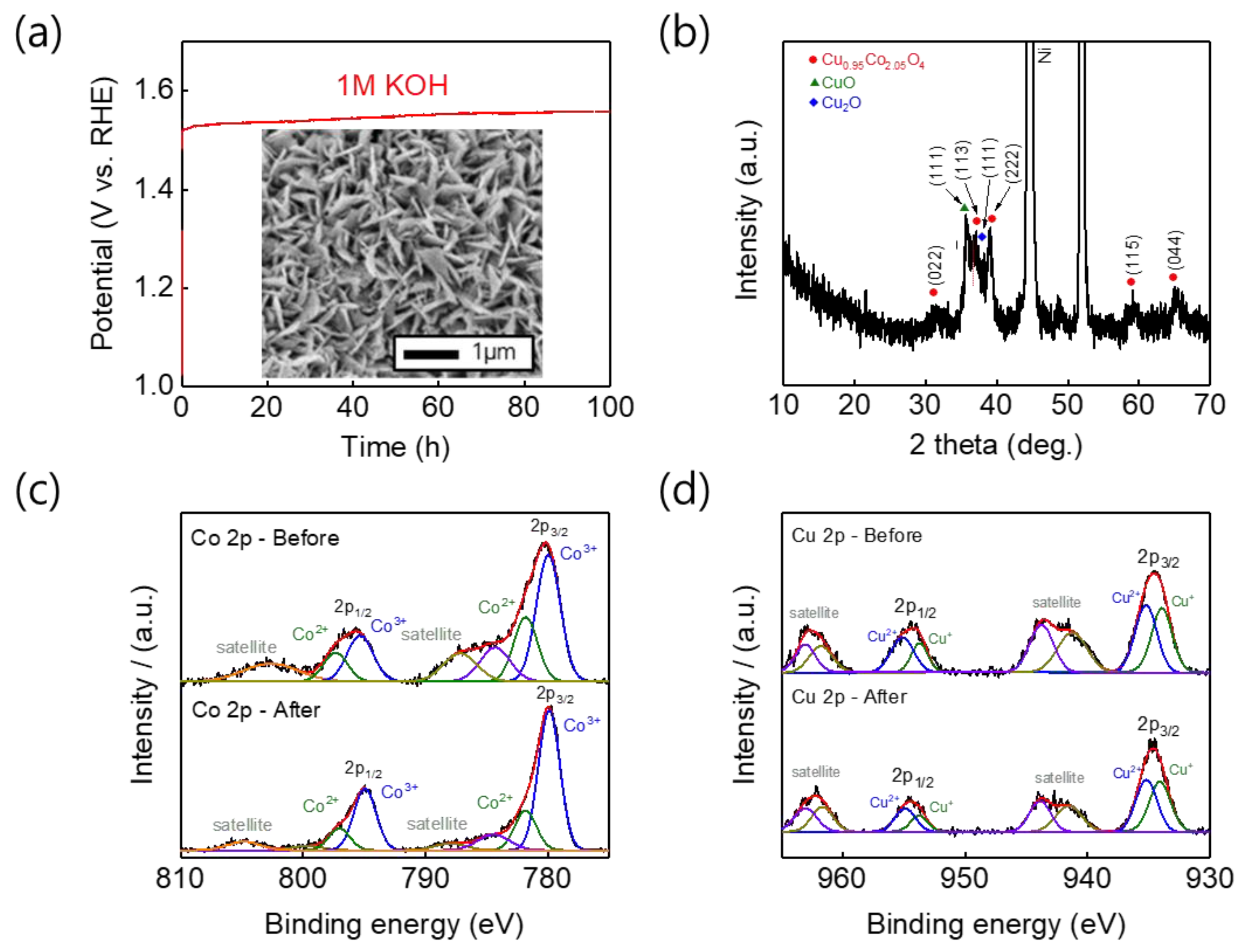
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, Z.; Luo, Y.; Asiri, A.M.; Sun, X. Efficient Electrochemical Water Splitting Catalyzed by Electrodeposited Nickel Diselenide Nanoparticles Based Film. ACS Appl. Mater. Interfaces 2016, 8, 4718–4723. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Geng, J.; Chen, C.; Kan, E.; Liu, Y.; Wang, Q.; Geng, B. A Reliable Aerosol-Spray-Assisted Approach to Produce and Optimize Amorphous Metal Oxide Catalysts for Electrochemical Water Splitting. Angew. Chem. Int. Ed. 2014, 53, 7547–7551. [Google Scholar] [CrossRef]
- Liu, G.; Ye, S.; Yan, P.; Xiong, F.; Fu, P.; Wang, Z.; Chen, Z.; Shi, J.; Li, C. Enabling an integrated tantalum nitride photoanode to approach the theoretical photocurrent limit for solar water splitting. Energy Environ. Sci. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.S. Recent theoretical ptrogress in the development of photoanode materials for solar water splitting photoelectrochemical cells. J. Mater. Chem. A 2015, 3, 10632–10659. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, C.; Wang, L.; Guo, S.; Zhang, Y.; Li, H.; Huang, H.; Liu, Y.; Tang, J.; Kang, Z. Control Strategy on Two-/Four-Electron Pathway of Water Splitting by Multidoped Carbon Based Catalysts. ACS Catal. 2017, 7, 1637–1645. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 6225. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Audichon, T.; Mayousse, E.; Morisset, S.; Morais, C.; Comminges, C.; Napporn, T.W.; Kokoh, K.B. Electroactivity of RuO2-IrO2 mixed nanocatalysts toward the oxygen evolution reaction in a water electrolyzer supplied by a solar profile. Int. J. Hydrogen Energy 2014, 39, 16785–16796. [Google Scholar] [CrossRef]
- Sahoo, S.; Rout, C.S. Facile Electrochemical Synthesis of Porous Manganese-Cobalt-Sulfide Based Ternary Transition Metal Sulfide Nanosheets Architectures for High Performance Energy Storage Applications. Electrochim. Acta 2016, 220, 57–66. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Rădiţoiu, A.; Raduly, F.M.; Manea, R.; Frone, A.; Anastasescu, M.; Ispas, G.C.; Căprărescu, S. Bilayer coationgs based on silica materials and iron (III) phthalocyanine—Sensitized TiO2 photocatalyst. Mater. Res. Bull. 2021, 138, 111222. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z. Nikel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Khodabakhshi, M.; Chen, S.; Ye, T.; Wu, H.; Yang, L.; Zhang, W.; Chang, H. Hierarchical High Wrinkled Trimetallic NiFeCu Phosphide Nanosheets on Nanodendrite Ni3S2/Ni Foam as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 36268–36276. [Google Scholar] [CrossRef]
- Sabatini, M.T.; Boulton, L.T.; Sheppard, T.D. Borate ester: Simple catalysts for the sustainable synthesis of complex amides. Sci. Adv. 2017, 3, e1701028. [Google Scholar] [CrossRef]
- Jiang, W.J.; Niu, S.; Tang, T.; Zhang, Q.H.; Liu, X.Z.; Zhang, Y.; Chen, Y.Y.; Li, J.H.; Gu, L.; Wan, L.J.; et al. Crystallinity-Modulated Electrocatalytic Activity of a Nickell(II) Borate Thin Layer on Ni3B for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2017, 56, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Ma, C.; Wang, D.; Xue, W.; Dou, B.; Wang, H.; Hao, Z. Investigation of Formaldehyde Oxidation over Co3O4-CeO2 and Au/Co3O4-CeO2 Catalysts at Room Temperature: Effective Removal and Determination of Reaction Mechanism. Environ. Sci. Technol. 2011, 45, 3628–3634. [Google Scholar] [CrossRef]
- Dăncilă, A.M.; Căprărescu, S.; Bobirică, C.; Purcar, V.; Gărleanu, G.; Vasile, E.; Modrogan, C.; Borda, C.; Dobrotă, D. Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas. Processes 2020, 8, 562. [Google Scholar] [CrossRef]
- Purcar, V.; Răditoiu, V.; Nicolae, C.A.; Frone, A.N.; Răditoiu, A.; Raduly, F.M.; Somoghi, R.; Gabor, R.A.; Căprărescu, S. Synthesis and morpho-structural properties of TiO2-based materials. J. Optoelectron. Adv. Mater. 2019, 21, 281–286. [Google Scholar]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via Sol-Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef]
- Purcar, V.; Cinteza, O.; Donescu, D.; Bala, D.; Ghiurea, M.; Petcu, C.; Caprarescu, S. Surface modification of silica particles assisted by CO2. J. Supercrit. Fluids 2014, 87, 34–39. [Google Scholar] [CrossRef]
- Choi, W.S.; Jang, M.J.; Park, Y.S.; Lee, K.H.; Lee, J.Y.; Seo, M.H.; Choi, S.M. Three-Dimensional Honeycomb-Like Cu0.81Co2.19O4 Nanosheet Arrays Supported by Ni Foam and Their High Efficiency as Oxygen Evolution Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 38663–38668. [Google Scholar] [CrossRef]
- Park, H.; Park, B.H.; Choi, J.; Kim, S.; Kim, T.; Youn, Y.S.; Son, N.; Kim, J.H.; Kang, M. Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure. Nanomaterials 2020, 10, 1727. [Google Scholar] [CrossRef]
- Ursúa, A.; Barrios, E.L.; Pascual, J.; Martin, I.S.; Sanchis, P. Integration of commercial alkaline water electrolysers with renewable energies: Limitations and improvements. Int. J. Hydrogen Energy 2016, 41, 12852–12861. [Google Scholar] [CrossRef]
- Schalenbach, M.; Kasian, O.; Mayrhofer, K.J.J. An alkaline water electrolyzer with nickel electrodes enables efficient high current density operation. Int. J. Hydrogen Energy 2018, 43, 11932–11938. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Andronescu, C.; Seisel, S.; Wilde, P.; Barwe, S.; Masa, J.; Chen, Y.T.; Ventosa, E.; Schuhmann, W. Influence of Temperature and Electrolyte Concentration on the Structure and Catalytic Oxygen Evolution Activity of Nickel-Iron Layered Double Hydroxide. Chem. Eur. J. 2018, 24, 13773–13777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, X.; Gu, L.; Zhang, Y.; Li, G.D.; Zou, X.; Chen, J.S. Corrosion engineering towards efficient oxygen evolution electrodes with stable catalytic activity for over 6000 hours. Nat. Commun. 2018, 9, 2609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Lim, B. Chemical transformation of iron alkoxide nanosheets to FeOOH nanoparticles for highly active and stable oxygen evolution electrocatalysts. J. Ind. Eng. Chem. 2018, 58, 100–104. [Google Scholar] [CrossRef]
- Guo, C.X.; Li, C.M. Room temperature-formed iron-doped nickel hydroxide on nickel foam as a 3D electrode for low polarized and high-current-density oxygen evolution. Chem. Commun. 2018, 54, 3262–3265. [Google Scholar] [CrossRef]
- Maiti, U.N.; Lim, J.; Lee, K.E.; Lee, W.J.; Kim, S.O. Three-Dimensional Shape Engineered, Interfacial Gelation of Reduced Graphene Oxide for High Rate, Large Capacity Supercapacitors. Adv. Mater. 2014, 26, 615–619. [Google Scholar] [CrossRef]
- Yu, L.; Ren, Z. Systematic study of the influence of iR compensation on water electrolysis. Mater. Today Phys. 2020, 14, 100253. [Google Scholar] [CrossRef]
- Jalal, M.R.; Hojjati, H.; Jalal, J.R.; Ebrahimi, S.; Bighashi, M.R.Z. Synthesis of Copper Hydroxide Nitrate (Cu2(OH)3NO3) micro-sheets by plasma electrolysis of Cu(NO3)2 aqueous solution in atmospheric air. J. Interfaces Thin Films Low Dimens. Syst. JITL 2018, 1, 109–112. [Google Scholar]
- Wang, X.; Huang, L. A novel one-step method to synthesize copper nitrate hydroxide nanorings. Trans. Nonferrous Met. Soc. China 2009, 19, s480–s484. [Google Scholar] [CrossRef]
- Kalyani, M.; Emerson, R.N. Electgrodeposition of nano crystalline cobalt oxide on porous copper electrode for supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 1214–1226. [Google Scholar] [CrossRef]
- Xia, H.; Peng, Z.; Cuncail, V.; Zhao, Y.; Hao, J.; Huang, Z. Self-supported porous Cobalt Oxide Nanowires with enhanced Electrocatalytic performance toward Oxygen evolution reaction. J. Chem. Sci. 2016, 128, 1879–1885. [Google Scholar] [CrossRef]
- Chi, B.; Lin, H.; Li, J. Cations distribution of CuxCo3−xO4 and its electrocatalytic activities for oxygen evolution reaction. Int. J. Hydrog. Energy 2008, 33, 4763–4768. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, K.; Wang, H.; Hu, S. Fluoride-Induced Dynamic Surface Self-Reconstruction Produces Unexpectedly Efficient Oxygen-Evolution Catalyst. Nano Lett. 2019, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, C.; Sun, Y.; Zhang, G.; Shen, X.; Zou, F.; Zhang, H.; Wu, Z.; Wegener, E.C.; Taubert, C.J.; et al. High-Performance Transition Metal Phosphide Alloy Catalyst for Oxygen Evolution Reaction. ACS Nano 2018, 12, 158–167. [Google Scholar] [CrossRef]
- Hai, G.; Jia, X.; Zhang, K.; Liu, X.; Wu, Z.; Wang, G. High-performance oxygen evolution catalyst using two-dimensional ultrathin metal-organic frameworks nanosheets. Nano Energy 2018, 44, 345–352. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Shan, C.; Xi, P.; Liu, W.; Tang, Y. Ce-Doped NiFe-Layered Double Hydroxide Ultrathin Nanosheets/Nanocarbon Hierarchical Nanocomposite as an Efficient Oxygen Evolution Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 6336–6345. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liu, D.; Zhou, L.; Du, R.; Li, T.T.; Miao, T.; Qian, J.; Hu, Y.; Huang, S. Normal-pulse-voltage-assisted in situ fabrication of graphene-wrapped MOF-derived CuO nanoflowers for water oxidation. Chem. Commun. 2020, 56, 8750–8753. [Google Scholar] [CrossRef]
- Bai, L.; Hsu, C.S.; Alexander, D.T.L.; Chen, H.M.; Hu, X. A Cobalt-Iron Double-Atom Catalyst for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 14190–14199. [Google Scholar] [CrossRef]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy. Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Babar, P.T.; Lokhande, A.C.; Pawar, B.S.; Gang, M.G.; Jo, E.; Go, C.; Suryawanshi, M.P.; Pawar, S.M.; Kim, J.H. Electrocatalytic performance evaluation of cobalt hydroxide and cobalt oxide thin films for oxygen evolution reaction. Appl. Surf. Sci. 2018, 427, 253–259. [Google Scholar] [CrossRef]
- Dang, W.J.; Shen, Y.Q.; Lin, M.; Jiao, H.; Xu, L.; Wang, Z.L. Noble-metal-free electrocatalyst based on a mixed CoNi metal-organic framework for oxygen evolution reaction. J. Alloys Compd. 2019, 792, 69–76. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Liu, X.; Zhu, B.; Li, G.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C. Coupling pentlandite nanoparticles and dual-doped carbon networks to yield efficient and stable electrocatalysts for acid water oxidation. J. Mater. Chem. A 2019, 7, 461–468. [Google Scholar] [CrossRef]
- Wang, F.; Xue, H.; Tian, Z.; Xing, W.; Feng, L. Fe2P as a novel efficient catalyst promoter in Pd/C system for formic acid electro-oxidation in fuel cells reaction. J. Power Source 2018, 375, 37–42. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, J.; Liu, K.; Cao, R. A Nickel-Based Integrated Electrode from an Autologous Growth Strategy for Highly Efficient Water Oxidation. Adv. Energy Mater. 2016, 6, 1502489. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jing, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α–Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef]
- Andronescu, C.; Barwe, S.; Ventosa, E.; Masa, J.; Vasile, E.; Konkena, B.; Möller, S.; Schuhmann, W. Powder Catalyst Fixation for Post-Electrolysis Structural Characterization of NiFe Layered Double Hydroxide Based Oxygen Evolution Reaction Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 11258–11262. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Rousse, G.; Svane, K.L.; Pearce, P.E.; Abakumov, A.M.; Deschamps, M.; Cibin, G.; Chadwich, A.V.; Corte, D.A.; Hansen, H.A.; et al. Cation insertion to break the activity/stability relationship for highly active oxygen evolution reaction catalyst. Nat. Commun. 2020, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [PubMed]


| Sample | Temperature | Electrolyte | Current Density (mA/cm2) | Overpotential (mV) | Ref. |
|---|---|---|---|---|---|
| CCO | Room temp. | 1 M KOH | 10 | 240 | Our work |
| NiFe-OH-F | 243 | [38] | |||
| Fe-Co-P | 252 | [39] | |||
| NiFe-UMNs | 260 | [40] | |||
| 1% Ce-NiFe-LDH/CNT | 270 | [41] | |||
| NiFe-LDH/CNT | 299 | [41] | |||
| CuO NF@G/CF | 320 | [42] | |||
| Co-N-C | 321 | [43] | |||
| Ni(OH)2 | 330 | [44] | |||
| Co3O4 | 340 | [45] | |||
| Ni-NDC/PANI-NF | 361 | [46] | |||
| Ni | 365 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.H.; Park, Y.S.; Yang, J.; Jang, M.J.; Jeong, J.; Lee, J.-H.; Park, H.-S.; Park, Y.H.; Choi, S.M.; Lee, J. Effects of Annealing Temperature on the Oxygen Evolution Reaction Activity of Copper–Cobalt Oxide Nanosheets. Nanomaterials 2021, 11, 657. https://doi.org/10.3390/nano11030657
Kim GH, Park YS, Yang J, Jang MJ, Jeong J, Lee J-H, Park H-S, Park YH, Choi SM, Lee J. Effects of Annealing Temperature on the Oxygen Evolution Reaction Activity of Copper–Cobalt Oxide Nanosheets. Nanomaterials. 2021; 11(3):657. https://doi.org/10.3390/nano11030657
Chicago/Turabian StyleKim, Geul Han, Yoo Sei Park, Juchan Yang, Myeong Je Jang, Jaehoon Jeong, Ji-Hoon Lee, Han-Saem Park, Yong Ho Park, Sung Mook Choi, and Jooyoung Lee. 2021. "Effects of Annealing Temperature on the Oxygen Evolution Reaction Activity of Copper–Cobalt Oxide Nanosheets" Nanomaterials 11, no. 3: 657. https://doi.org/10.3390/nano11030657
APA StyleKim, G. H., Park, Y. S., Yang, J., Jang, M. J., Jeong, J., Lee, J.-H., Park, H.-S., Park, Y. H., Choi, S. M., & Lee, J. (2021). Effects of Annealing Temperature on the Oxygen Evolution Reaction Activity of Copper–Cobalt Oxide Nanosheets. Nanomaterials, 11(3), 657. https://doi.org/10.3390/nano11030657





