Electrochemically Fabricated Surface-Mesostructured CuNi Bimetallic Catalysts for Hydrogen Production in Alkaline Media
Abstract
:1. Introduction
2. Materials and Methods
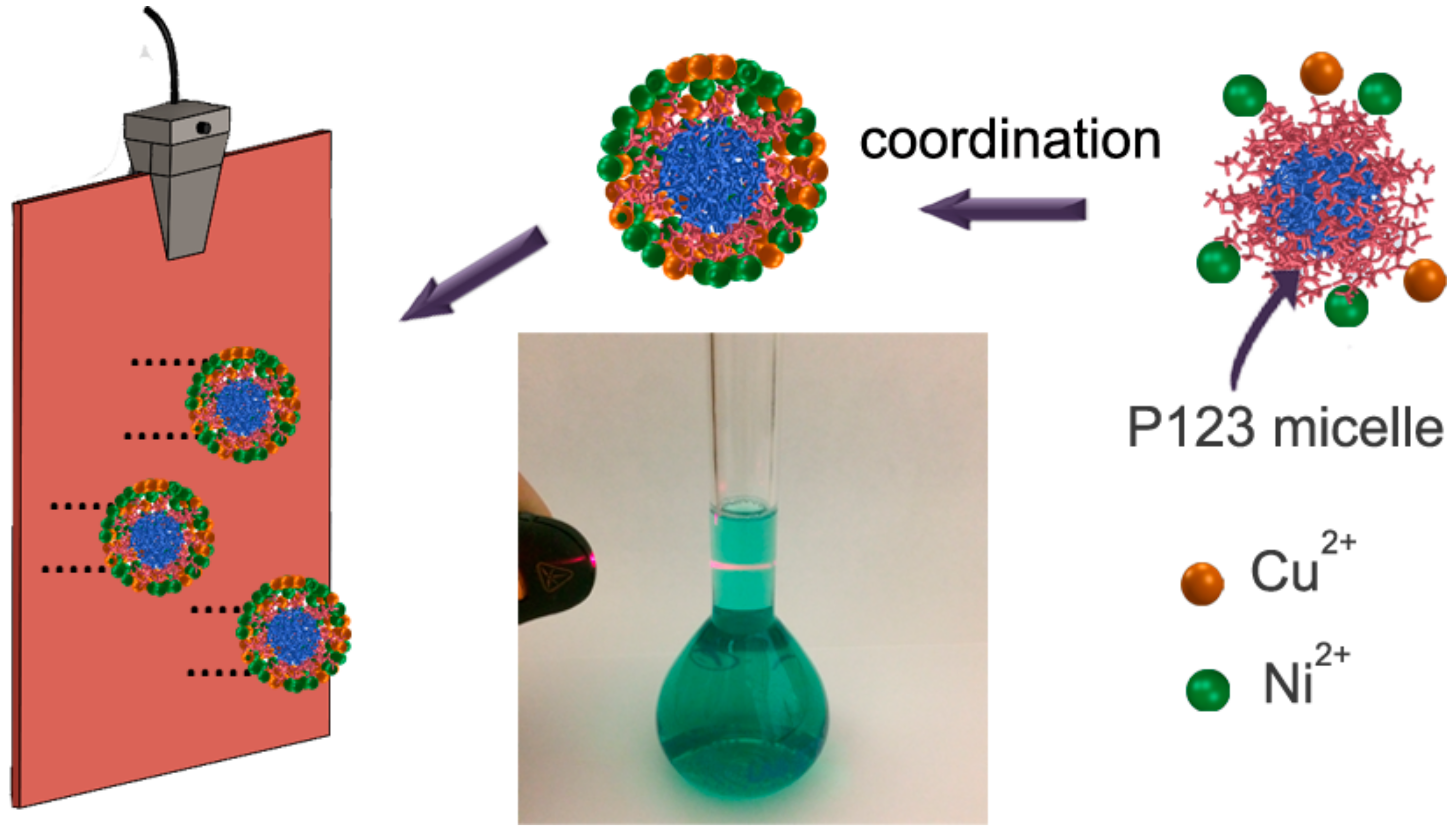
2.1. Synthesis of Cu20Ni80 and Cu45Ni55 Films
2.2. Electrocatalytic Activity toward HER
2.3. Structural Characterization
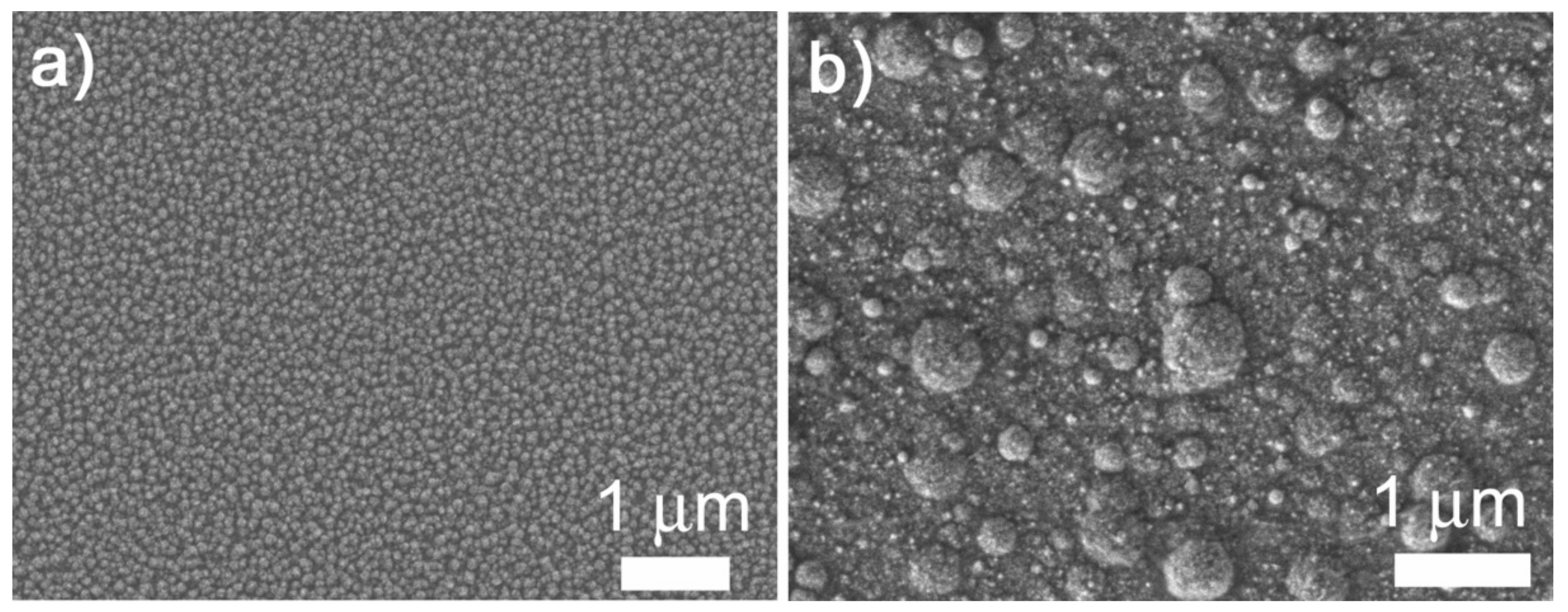
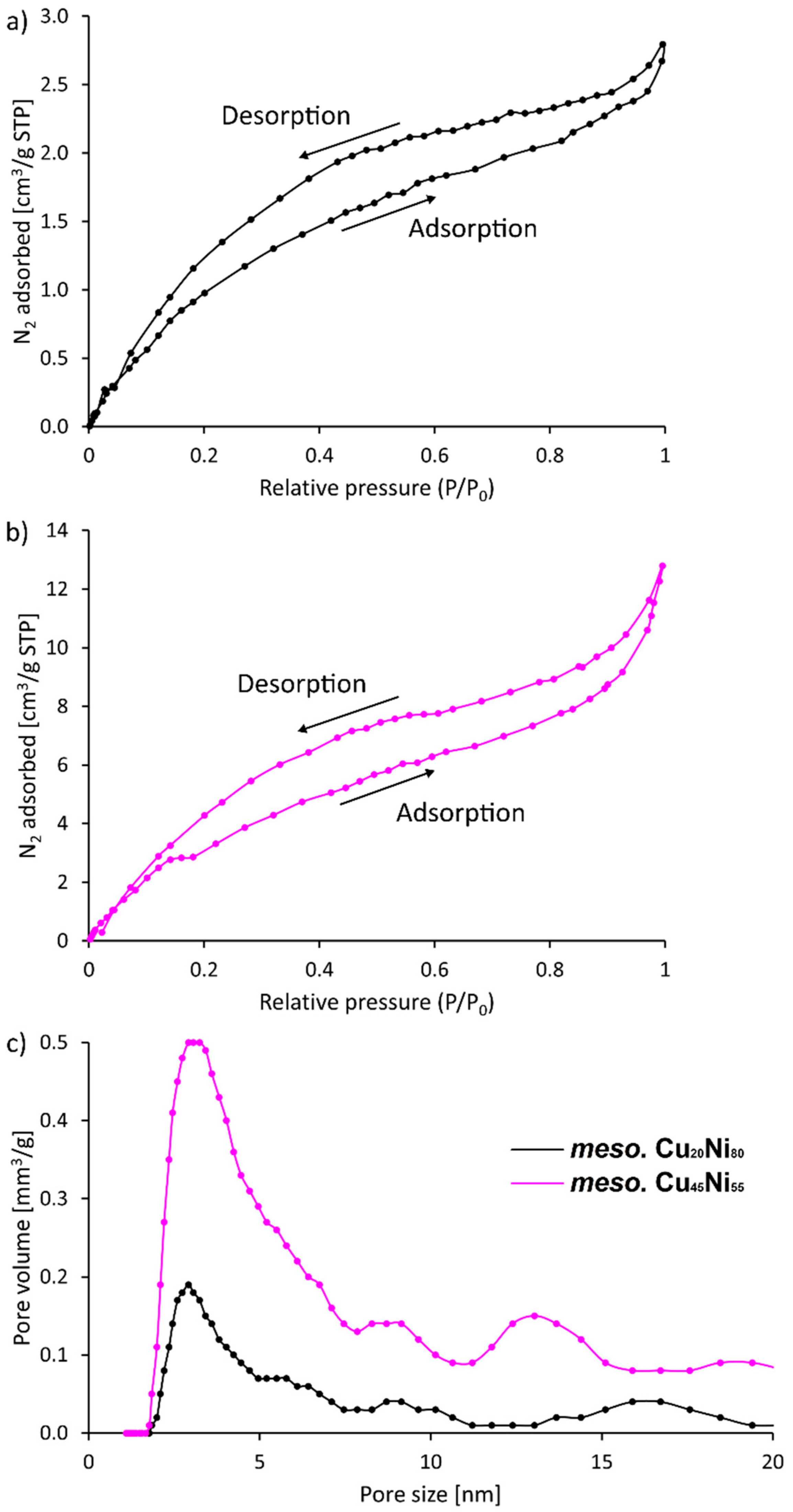
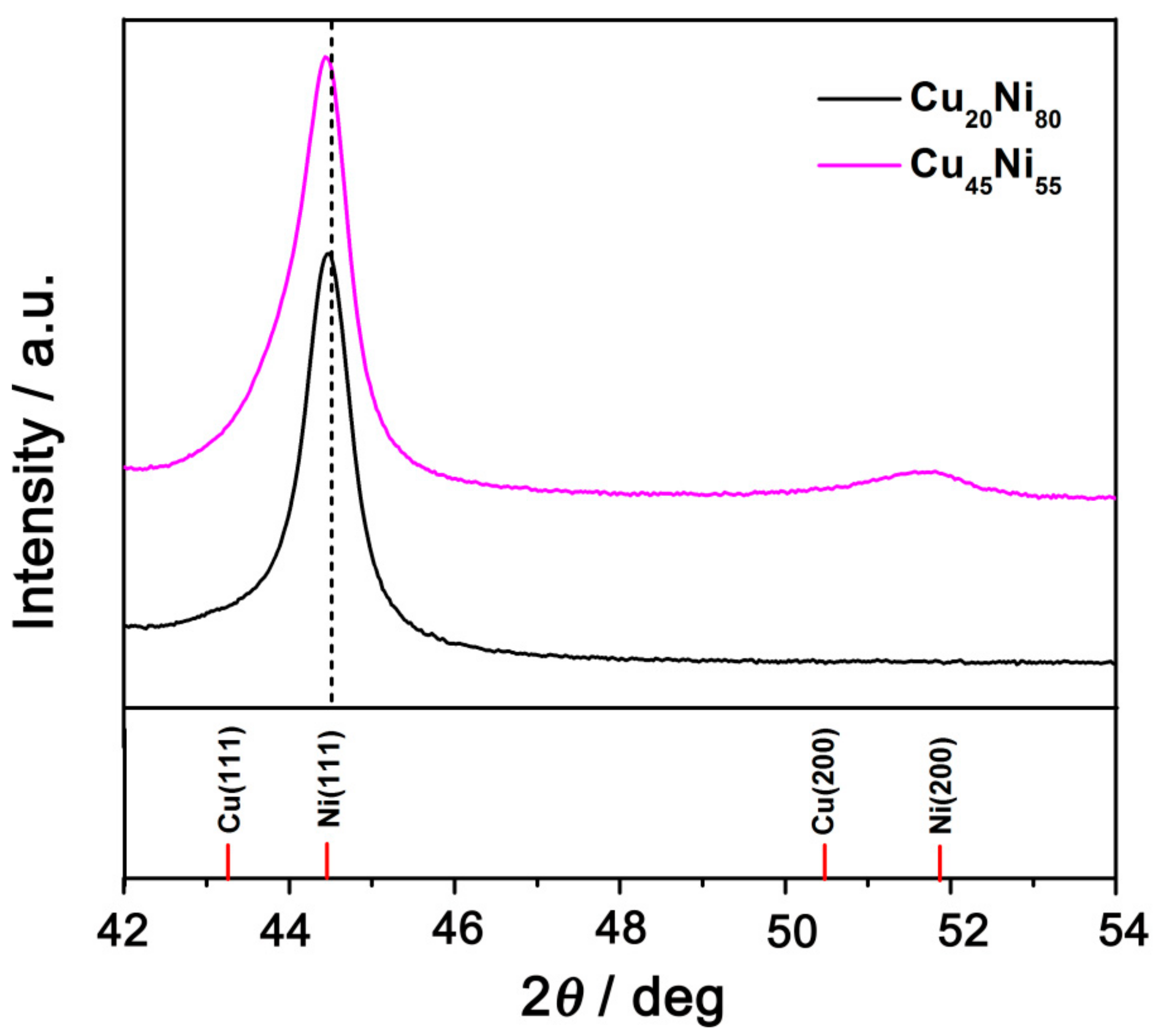
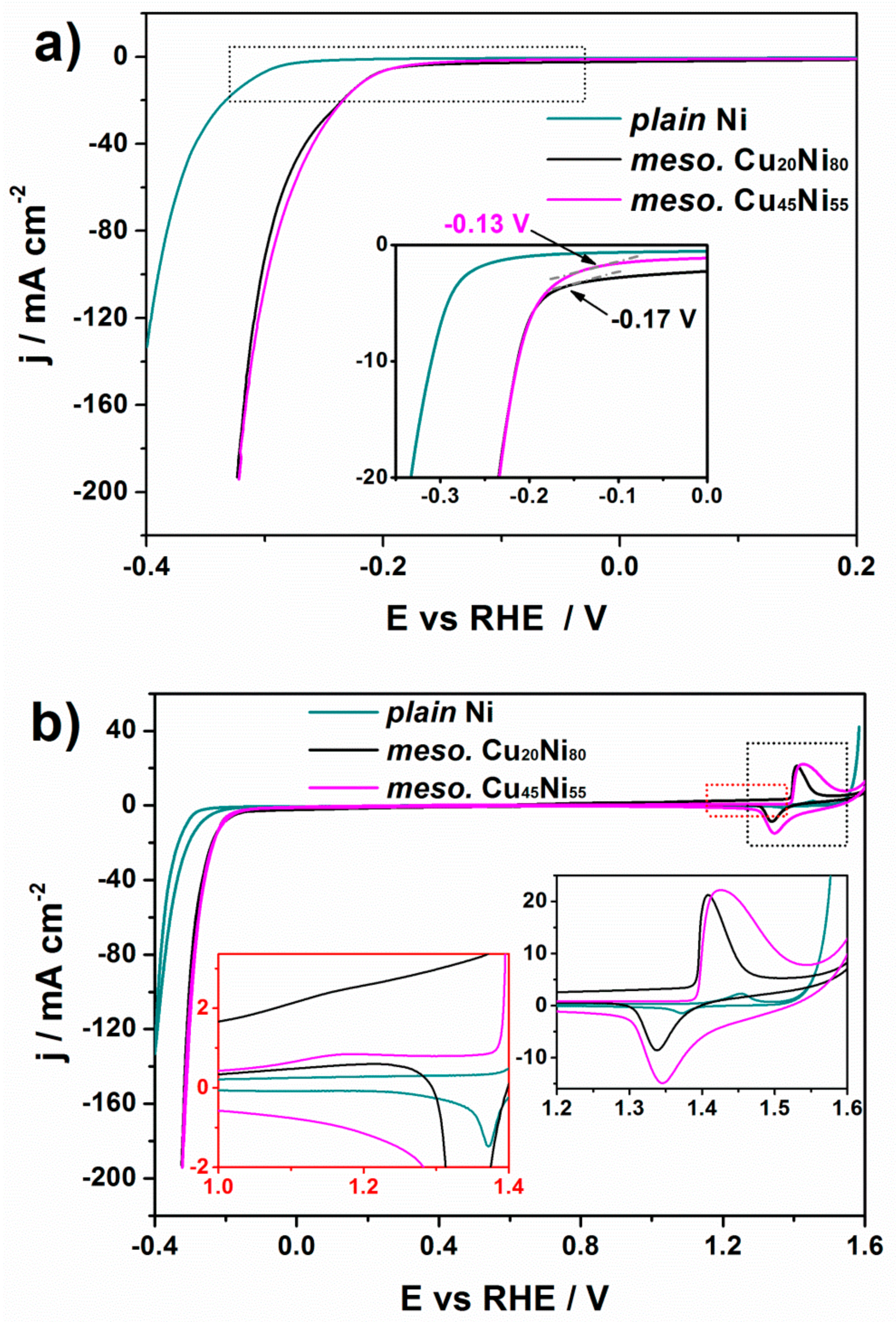
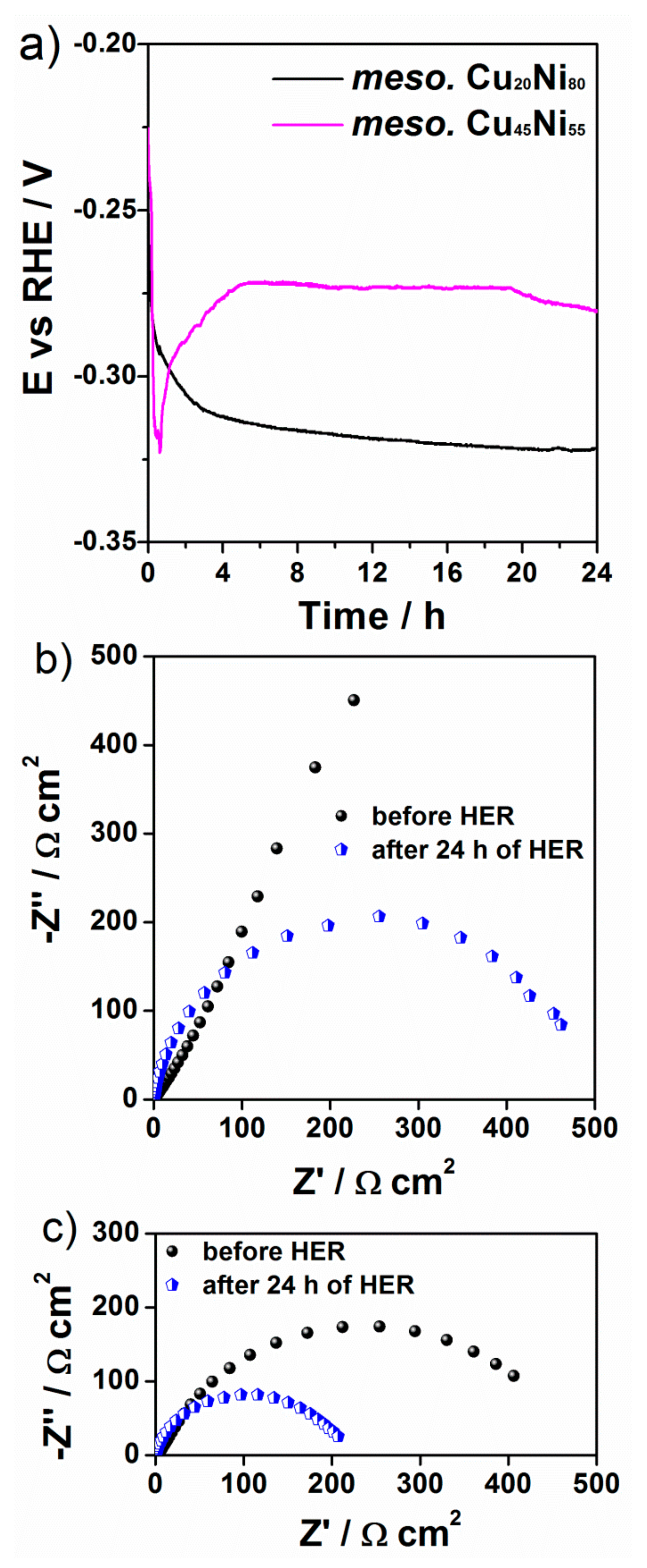
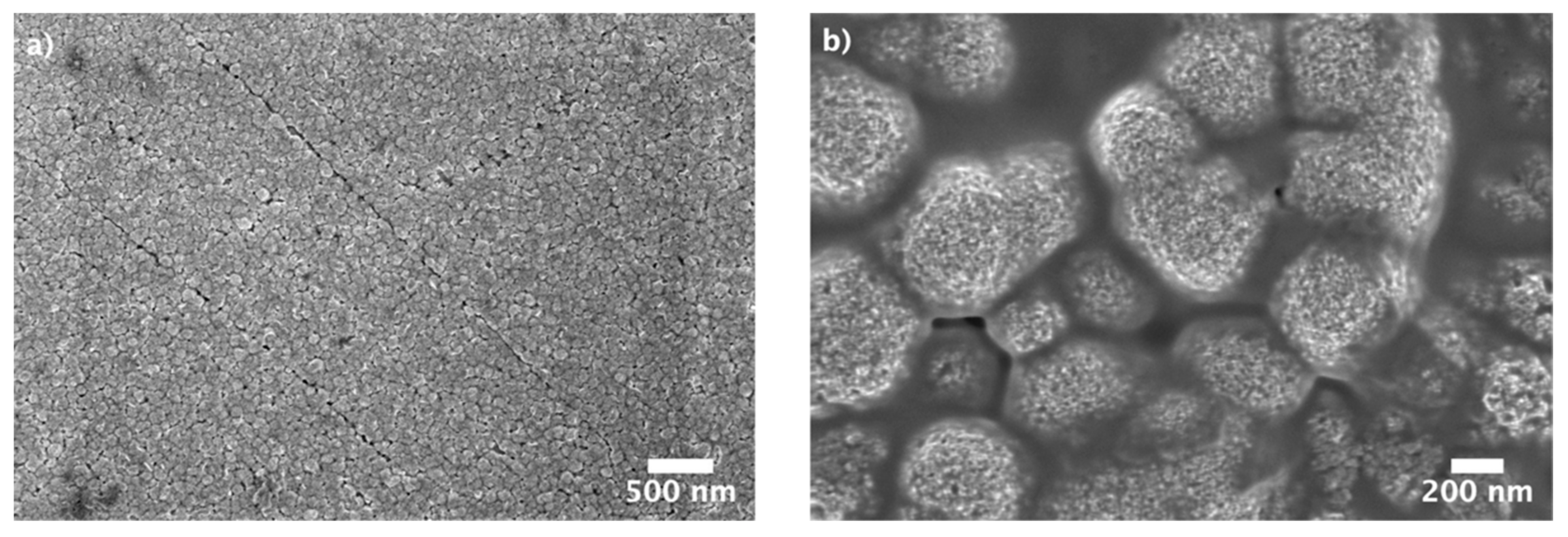
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Liu, J.; Nag, N.; Crozier, P.A. In situ preparation of Ni–Cu/TiO2 bimetallic catalysts. J. Catal. 2009, 262, 73–82. [Google Scholar] [CrossRef]
- Best, R.J.; Russel, W.W. Nickel, copper and some of their alloys as catalysts for ethylene hydrogenation. J. Am. Chem. Soc. 1959, 81, 4132–4137. [Google Scholar]
- Quintana, A.; Zhang, J.; Isarain-Chavez, E.; Menendez, E.; Cuadrado, R.; Robles, R.; Baro, M.D.; Guerrero, M.; Pane, S.; Nelson, B.J.; et al. Voltage-induced coercivity reduction in nanoporous alloy films: A boost toward energy-efficient magnetic actuation. Adv. Funct. Mater. 2017, 27, 1701904. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D. Study of bimetallic Cu–Ni/γ-Al2O3 catalysts for carbon dioxide hydrogenation. Int. J. Hydrogen Energy 1999, 24, 351–354. [Google Scholar] [CrossRef]
- Kang, M.; Song, M.W.; Kim, T.W.; Kim, K.L. γ-alumina supported Cu-Ni bimetallic catalysts: Characterization and of 1,3-Butadiene. Can. J. Chem. Eng. 2002, 80, 63–70. [Google Scholar] [CrossRef]
- Solmaz, R.; Doner, A.; Kardas, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrogen Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, L.; Chien, C.L.; Searson, P.C. Formation of a core/shell microstructure in Cu–Ni thin films. J. Electrochem. Soc. 2008, 155, D569–D574. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, G.; Zhu, F.; Kim, S.; Markovic, N.; Chien, C.; Searson, P.C. Exploiting finite size effects in a novel core/shell microstructure. J. Appl. Phys. 2008, 103, 064313. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Baker, R.T.K. Decomposition of methane over a Ni-Cu-MgO catalyst to produce hydrogen and carbon nanofibers. J. Phys. Chem. B 2004, 108, 20273–20277. [Google Scholar] [CrossRef]
- Bergwerff, J.A.; Visser, T.; Leliveld, B.R.; Rossenaar, B.D.; de Jong, K.P.; Weckhuysen, B.M. Envisaging the physicochemical processes during the preparation of supported catalysts: Raman microscopy on the impregnation of Mo onto Al2O3 extrudates. J. Am. Chem. Soc. 2004, 126, 14548–14556. [Google Scholar] [CrossRef] [Green Version]
- Pieta, I.S.; Rathi, A.; Pieta, P.; Nowakowski, R.; Holdynski, M.; Pisarek, M.; Kaminska, A.; Gawande, M.B.; Aboril, R. Electrocatalytic methanol oxidation over Cu, Ni and bimetallic Cu-Ni nanoparticles supported on graphitic carbon nitride. Appl. Catal. B-Environ. 2019, 244, 272–283. [Google Scholar] [CrossRef]
- Eugenio, S.; Silva, T.M.; Carmezim, M.J. Electrodeposition and characterization of nickel–copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Stokes, K.; Walsh, F.C. Electrochemical Corrosion Behaviour of 90–10 Cu–Ni Alloy in Chloride-based Electrolytes. J. Appl. Electrochem. 2004, 34, 659–669. [Google Scholar] [CrossRef]
- Ahn, S.H.; Park, H.Y.; Choi, I.; Yoo, S.J.; Hwang, S.J.; Kim, H.J.; Cho, E.; Yoon, C.W.; Park, H.; Son, H.; et al. Electrochemically fabricated NiCu alloy catalysts for hydrogen production in alkaline water electrolysis. Int. J. Hydrogen Energy 2013, 38, 13493–13501. [Google Scholar] [CrossRef]
- Poulopoulos, P.; Lindner, J.; Farle, M.; Baberschke, K. Change of magnetic anisotropy due to roughness: A quantitative scanning tunneling microscopy study on Ni/Cu (00l). Surf. Sci. 1999, 437, 277–284. [Google Scholar] [CrossRef]
- Sinfelt, J.H. Catalysis by alloys and bimetallic clusters. Acc. Chem. Res. 1977, 10, 28. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, C.; Huang, W. Properties of polymer supported Ni-Cu bimetallic catalysts prepared by solvated metal atom impregnation. Chin. J. Polym. Sci. 1996, 14, 217–224. [Google Scholar]
- Zhang, J.; Baró, M.D.; Pellicer, E.; Sort, J. Electrodeposition of magnetic, superhydrophobic, non-stick, two-phase Cu–Ni Foam films and their enhanced performance for hydrogen evolution reaction in alkaline water media. Nanoscale 2014, 6, 12490–12499. [Google Scholar] [CrossRef]
- Rao, S.; Zou, X.; Wang, S.; Lu, Y.; Shi, T.; Hsu, H.; Xu, Q.; Lu, X. Electrodeposition of Ni-Cu alloy films from nickel matte in deep eutectic solvent. Mater. Chem. Phys. 2019, 232, 6–15. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Z.; Meng, L.; Shu, B.; Zhu, Y.; Zhu, X. Effect of strain rate on mechanical properties of Cu/Ni multilayered composites processed by electrodeposition. Mat. Sci. Eng. A 2018, 726, 154–159. [Google Scholar] [CrossRef]
- Vesali, N.; Erfanifam, S.; Jamilpanah, L.; Hasheminejad, M.; Rahmani, Y.; Mohseni, S.M. Growth behavior of Cu, Ni and Cu/Ni electrodeposited microwires within porous Si. Surf. Coat. Technol. 2019, 364, 16–21. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.S.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B-Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Abbas, S.A.; Kim, S.H.; Iabal, M.I.; Muhammad, S.; Yoon, W.S.; Jung, K.D. Synergistic effect of nano-Pt and Ni spine for HER in alkaline solution: Hydrogen spillover from Nano-Pt to Ni spine. Sci. Rep. 2018, 8, 2986. [Google Scholar] [CrossRef] [Green Version]
- Rosalbino, F.; Scavino, G.; Grande, M.A. Electrocatalytic activity of Ni–Fe–M (M = Cr, Mn, Cu) sintered electrodes for hydrogen evolution reaction in alkaline solution. J. Electroanal. Chem. 2013, 694, 114–121. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Elliott, J.M.; Owen, J.R.; Attard, G.S. Electrodeposition of Mesoporous Tin Films. Chem. Commun. 1999, 331–332. [Google Scholar] [CrossRef]
- Zhang, J.; Quintana, A.; Menendez, E.; Coll, M.; Pelliver, E.; Sort, J. Electrodeposited Ni-based magnetic mesoporous films as smart surfaces for atomic layer deposition: An “all-chemical” deposition approach toward 3D Nanoengineered composite layers. ACS Appl. Mater. Interfaces 2018, 10, 14877–14885. [Google Scholar] [CrossRef] [Green Version]
- Eiler, K.; Suriñach, S.; Sort, J.; Pellicer, E. Mesoporous Ni-rich Ni–Pt thin films: Electrodeposition, characterization and performance toward hydrogen evolution reaction in acidic media. Appl. Catal. B Environ. 2020, 265, 118597. [Google Scholar] [CrossRef]
- Iqbal, M.; Kaneti, Y.V.; Kashimura, K.; Yoshino, M.; Jiang, B.; Li, C.; Yuliarto, B.; Bando, Y.; Sugahara, Y.; Yamauchi, Y. Continuous mesoporous Pd films with tunable pore sizes through polymeric micelle-assisted assembly. Nanoscale Horiz. 2019, 4, 960–968. [Google Scholar] [CrossRef]
- Pellicer, E.; Varea, A.; Pané, S.; Nelson, B.J.; Menendez, E.; Estrader, M.; Suriñach, S.; Baró, M.D.; Nogués, J.; Sort, J. Nanocrystalline electroplated Cu–Ni: Metallic thin films with enhanced mechanical properties and tunable magnetic behavior. Adv. Funct. Mater. 2010, 20, 983–991. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.E.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Machado, S.A.S.; Avaca, L.A. The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 1994, 39, 1385–1391. [Google Scholar] [CrossRef]
- Van Drunen, J.; Kinkead, B.; Wang, M.C.P.; Sourty, E.; Gates, B.D.; Jerkiewicz, G. Comprehensive Structural, surface-chemical and electrochemical characterization of nickel-based metallic foams. ACS Appl. Mater. Interfaces 2013, 5, 6712–6722. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-D.; Chen, H.-Y.; Xu, Y.F.; Liao, J.-F.; Chen, B.-X.; Rao, H.-S.; Kuang, D.-B.; Su, C.-Y. Self-supported NiMoP2 nanowires on carbon cloth as an efficient and durable electrocatalyst for overall water splitting. J. Mater. Chem. A 2017, 5, 7191–7199. [Google Scholar] [CrossRef]
- Bender, F.; Mankelow, R.F.; Hibbert, D.B.; Gooding, J.J. Lyotropic liquid crystal templating of groups 11 and 12 metal films. Electroanalysis 2006, 16, 1558–1563. [Google Scholar] [CrossRef]
- Ren, B.; Li, D.; Jin, Q.; Cui, H.; Wang, C. Novel Porous Tungsten Carbide Hybrid Nanowires on Carbon Cloth for High-performance Hydrogen Evolution. J. Mater. Chem. A 2017, 5, 13196–13203. [Google Scholar] [CrossRef]
- Hahn, F.; Beden, B.; Croissant, M.J.; Lamy, C. In situ UV visible reflectance spectroscopic investigation of the nickel electrode-alkaline solution interface. Electrochim. Acta 1986, 31, 335–342. [Google Scholar] [CrossRef]
- Elhaleem, S.; Ateya, B.G. Cyclic voltammetry of copper in sodium hydroxide solutions. J. Electroanal. Chem. 1981, 117, 309–319. [Google Scholar]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano plot in hydrogen electrocatalysis—Use and abuse. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, J.; Li, F.; Jung, S.M.; Okyay, M.S.; Ahmad, I.; Kim, S.J.; Park, N.; Jeong, H.Y.; Baek, J.B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef] [PubMed]




| Cu20Ni80 | Cu45Ni55 | |
|---|---|---|
| ECSA (Cu) | 0.3 ± 0.05 m2·g−1 | 0.4 ± 0.1 m2·g−1 |
| ECSA (Ni) | 7.4 ± 0.4 m2·g−1 | 12.5 ± 0.3 m2·g−1 |
| η10 | 210 (220) mV | 210 (230) mV |
| b | 65 (115) mV | 68 (97) mV |
| TOF | 0.5 s−1 | 0.3 s−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Zhang, J.; Eiler, K.; Yang, Z.; Fan, L.; Yang, D.; Zhang, M.; Hou, Y.; Guan, R.; Sort, J.; et al. Electrochemically Fabricated Surface-Mesostructured CuNi Bimetallic Catalysts for Hydrogen Production in Alkaline Media. Nanomaterials 2022, 12, 118. https://doi.org/10.3390/nano12010118
Bai J, Zhang J, Eiler K, Yang Z, Fan L, Yang D, Zhang M, Hou Y, Guan R, Sort J, et al. Electrochemically Fabricated Surface-Mesostructured CuNi Bimetallic Catalysts for Hydrogen Production in Alkaline Media. Nanomaterials. 2022; 12(1):118. https://doi.org/10.3390/nano12010118
Chicago/Turabian StyleBai, Jingyuan, Jin Zhang, Konrad Eiler, Zhou Yang, Longyi Fan, Dalong Yang, Meilin Zhang, Yupu Hou, Renguo Guan, Jordi Sort, and et al. 2022. "Electrochemically Fabricated Surface-Mesostructured CuNi Bimetallic Catalysts for Hydrogen Production in Alkaline Media" Nanomaterials 12, no. 1: 118. https://doi.org/10.3390/nano12010118
APA StyleBai, J., Zhang, J., Eiler, K., Yang, Z., Fan, L., Yang, D., Zhang, M., Hou, Y., Guan, R., Sort, J., & Pellicer, E. (2022). Electrochemically Fabricated Surface-Mesostructured CuNi Bimetallic Catalysts for Hydrogen Production in Alkaline Media. Nanomaterials, 12(1), 118. https://doi.org/10.3390/nano12010118







