Insights into Sorption–Mineralization Mechanism for Sustainable Granular Composite of MgO-CaO-Al2O3-SiO2-CO2 Based on Nanosized Adsorption Centers and Its Effect on Aqueous Cu(II) Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sorbent
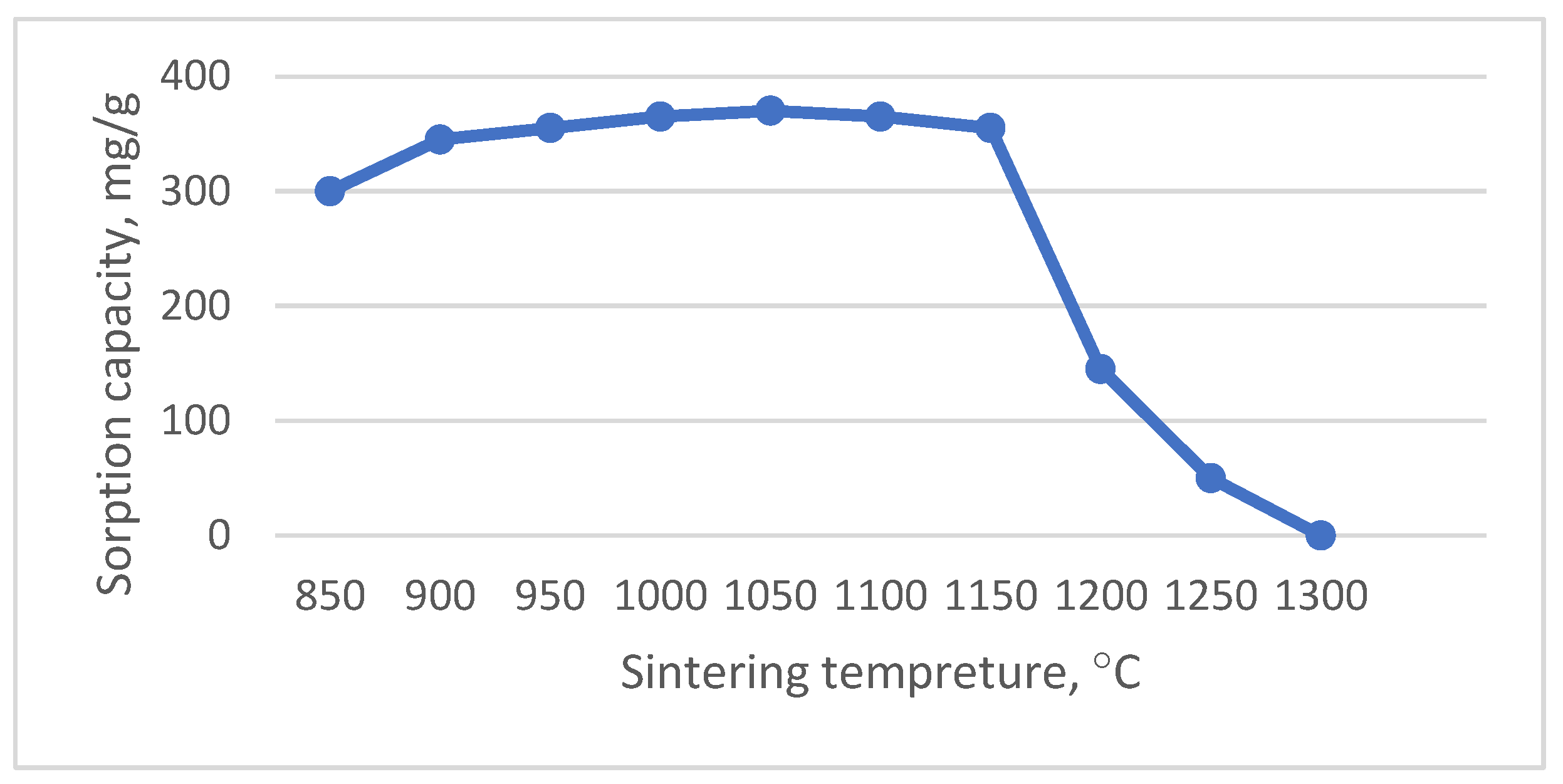
2.2. Sintering
2.3. Experimental Procedure
2.3.1. Sorption
2.3.2. Desorption
2.4. Sorbent Characterization
3. Results and Discussion
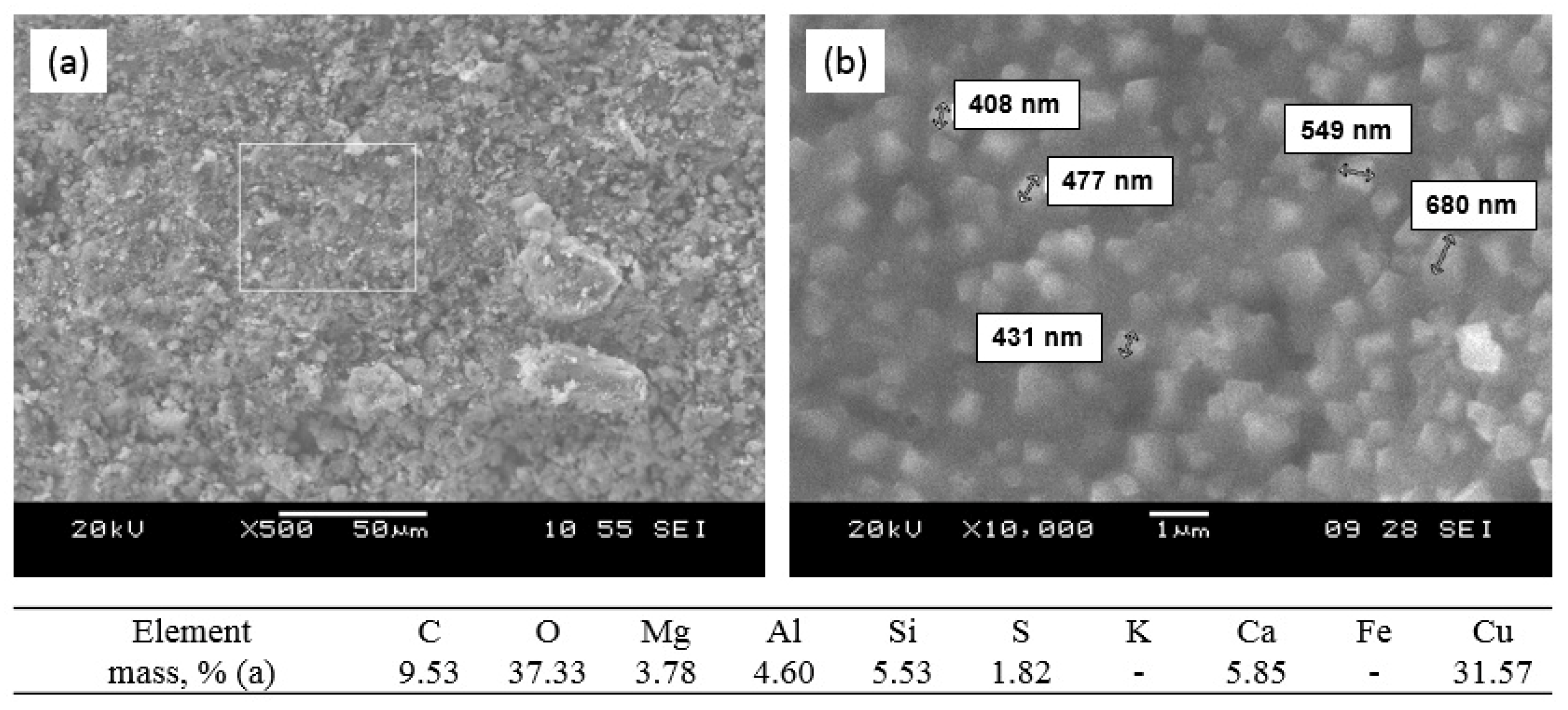
3.1. Sorption of Cu2+ Ions
3.2. Desorption in Distilled Water
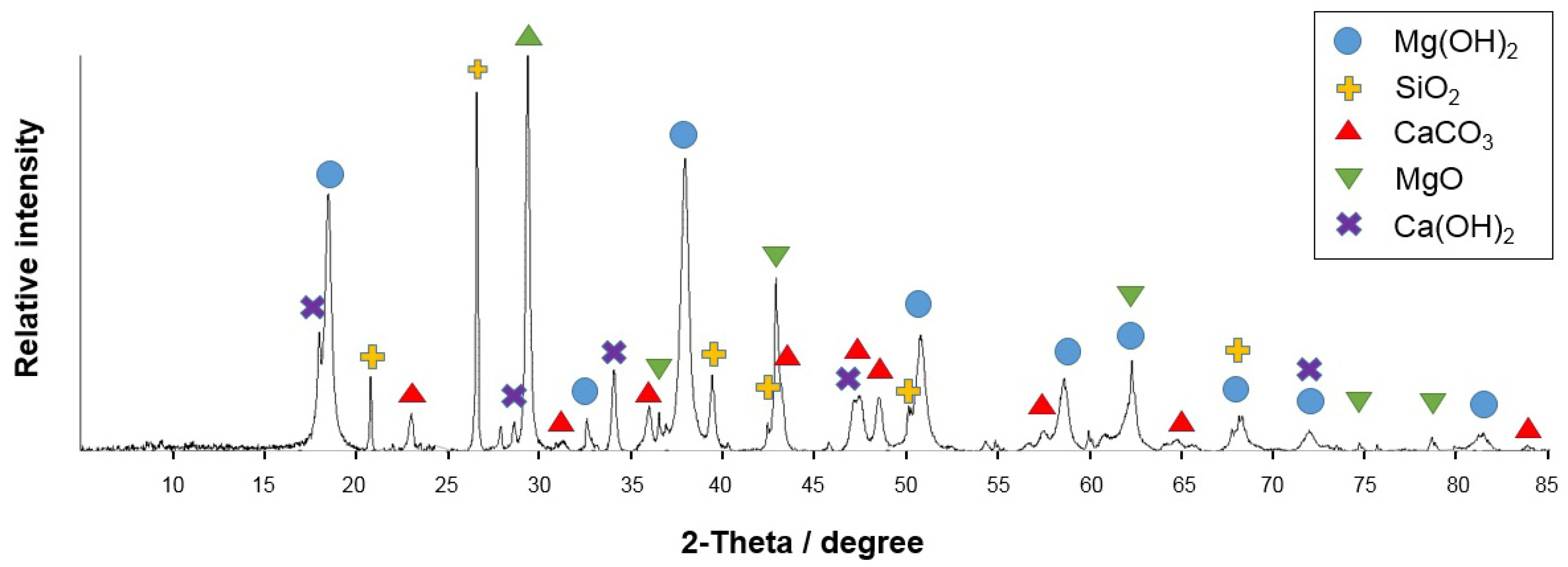
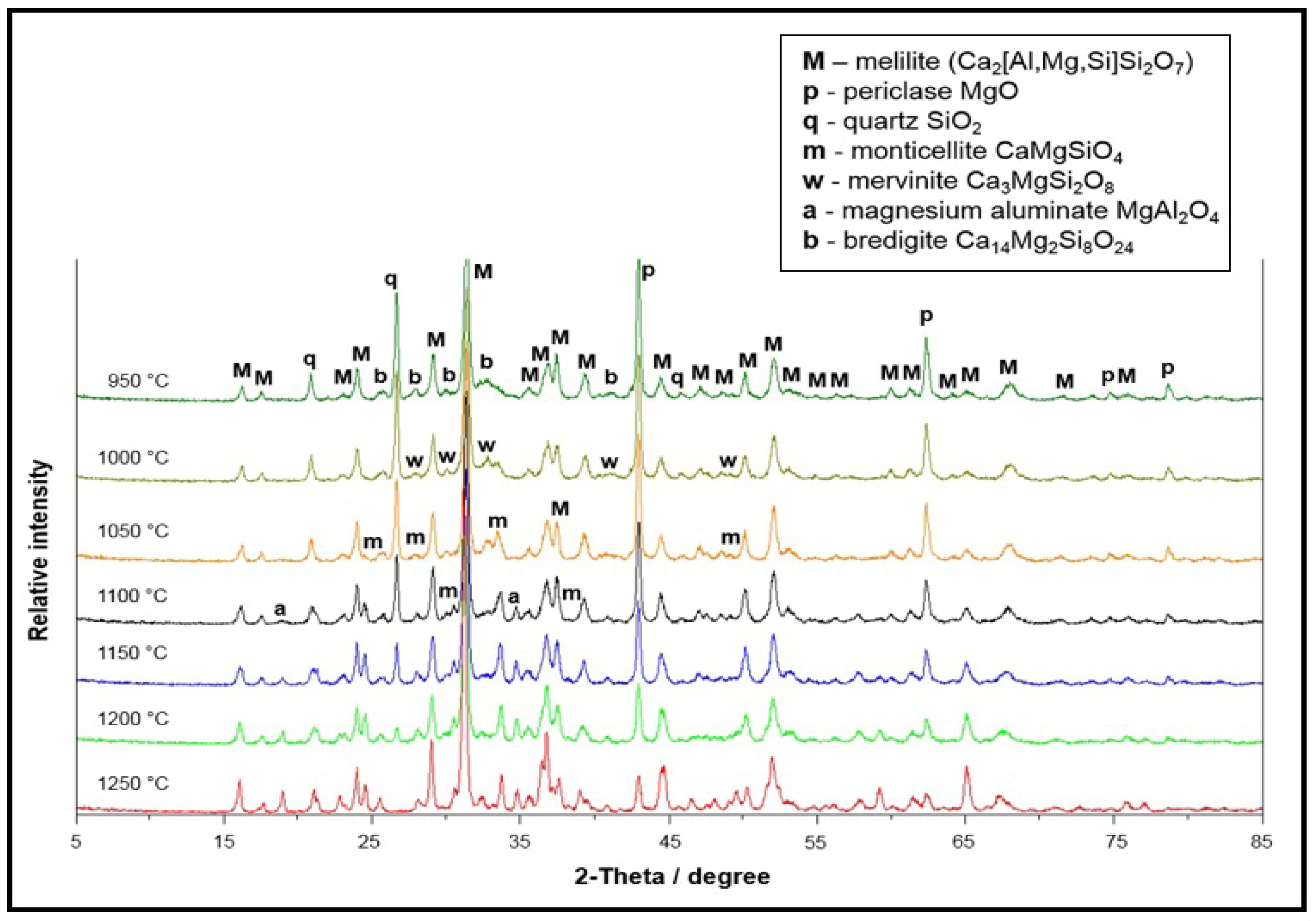
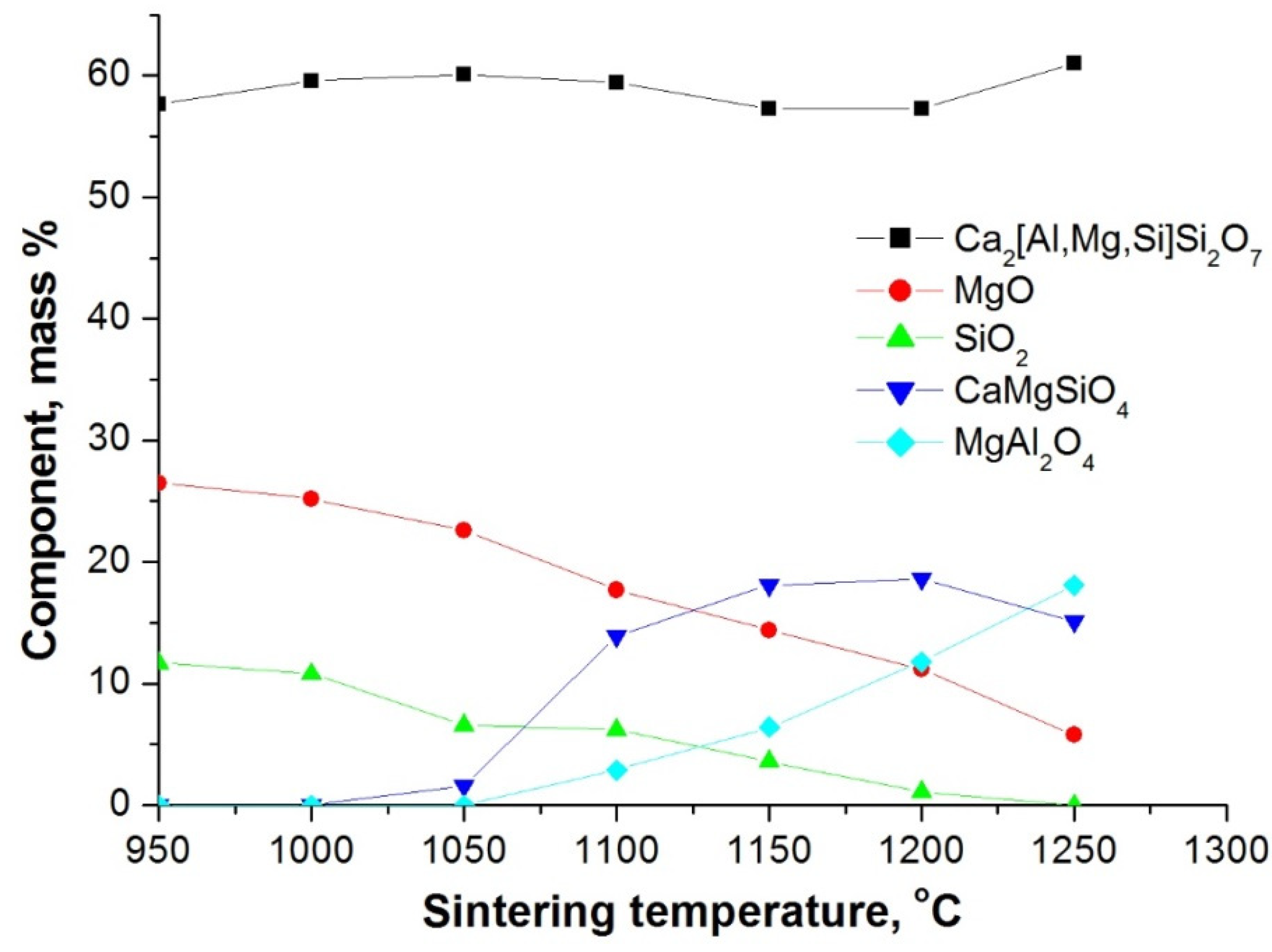
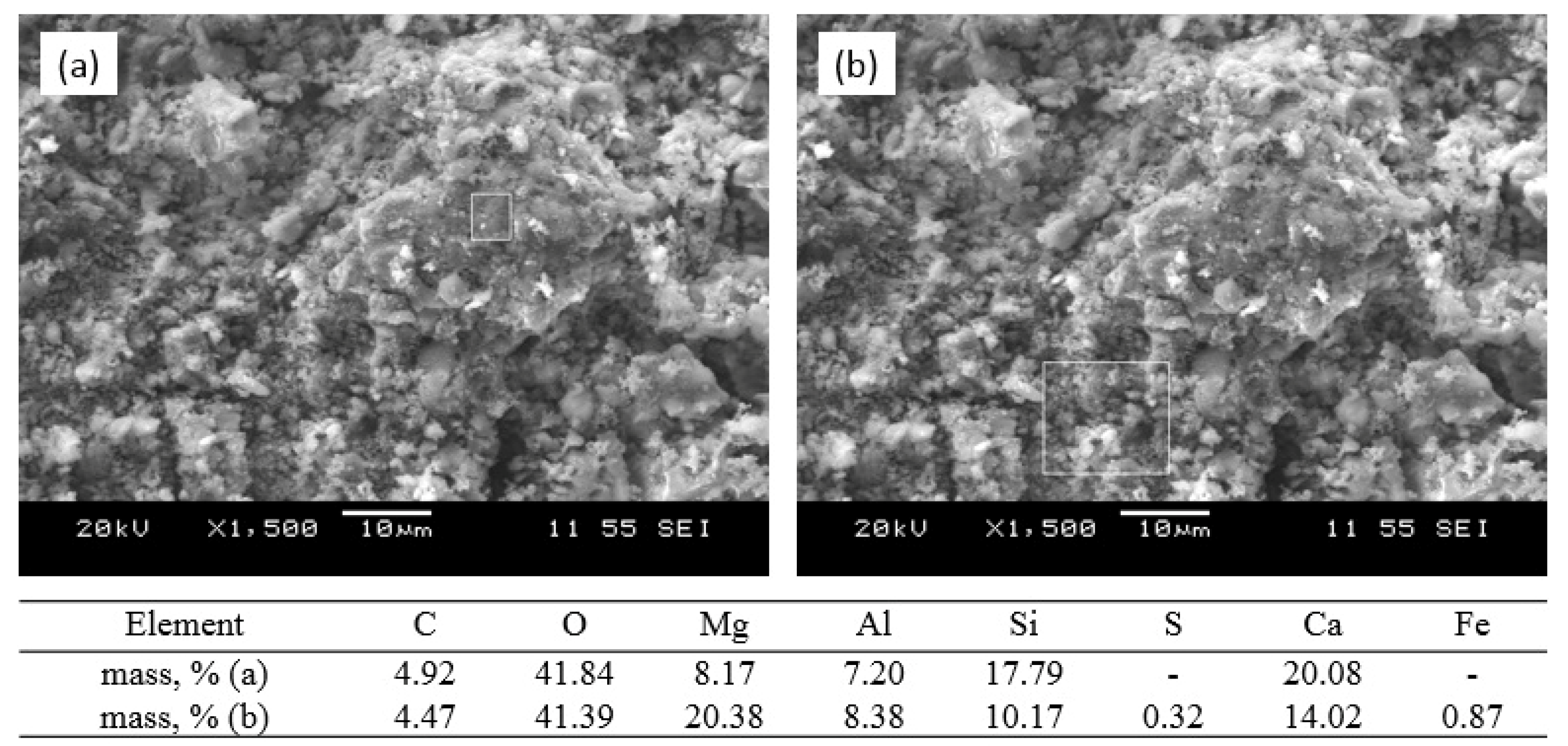
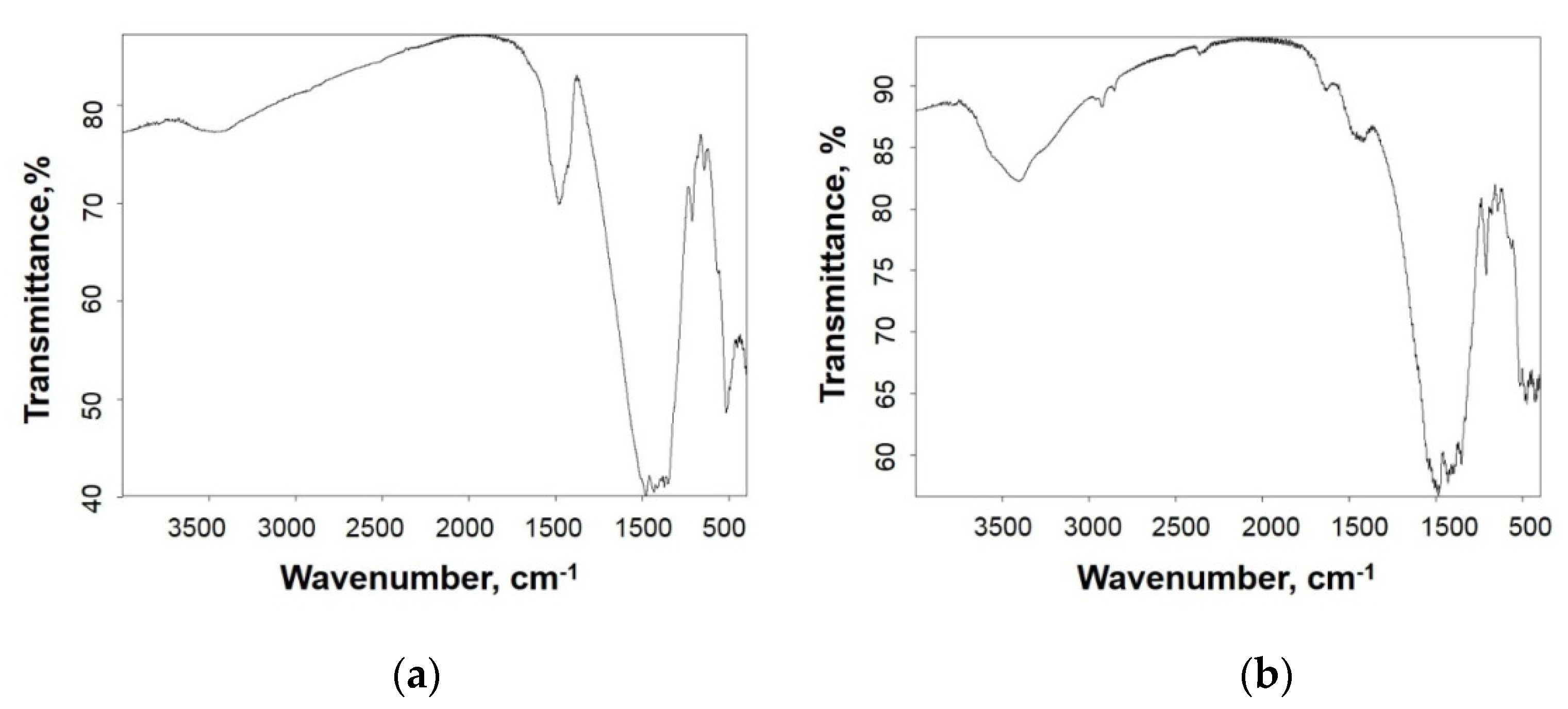
3.3. Phase Composition
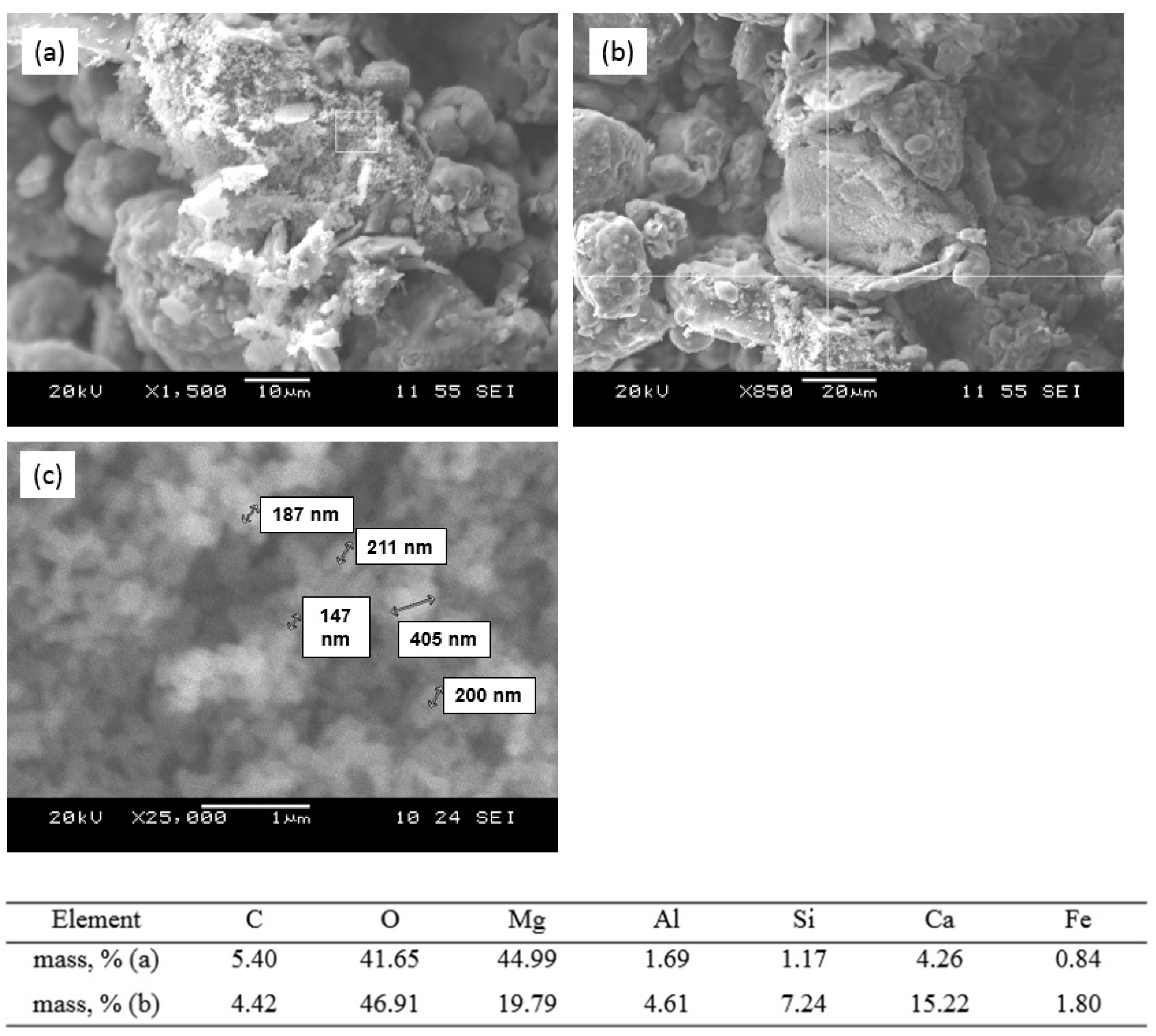
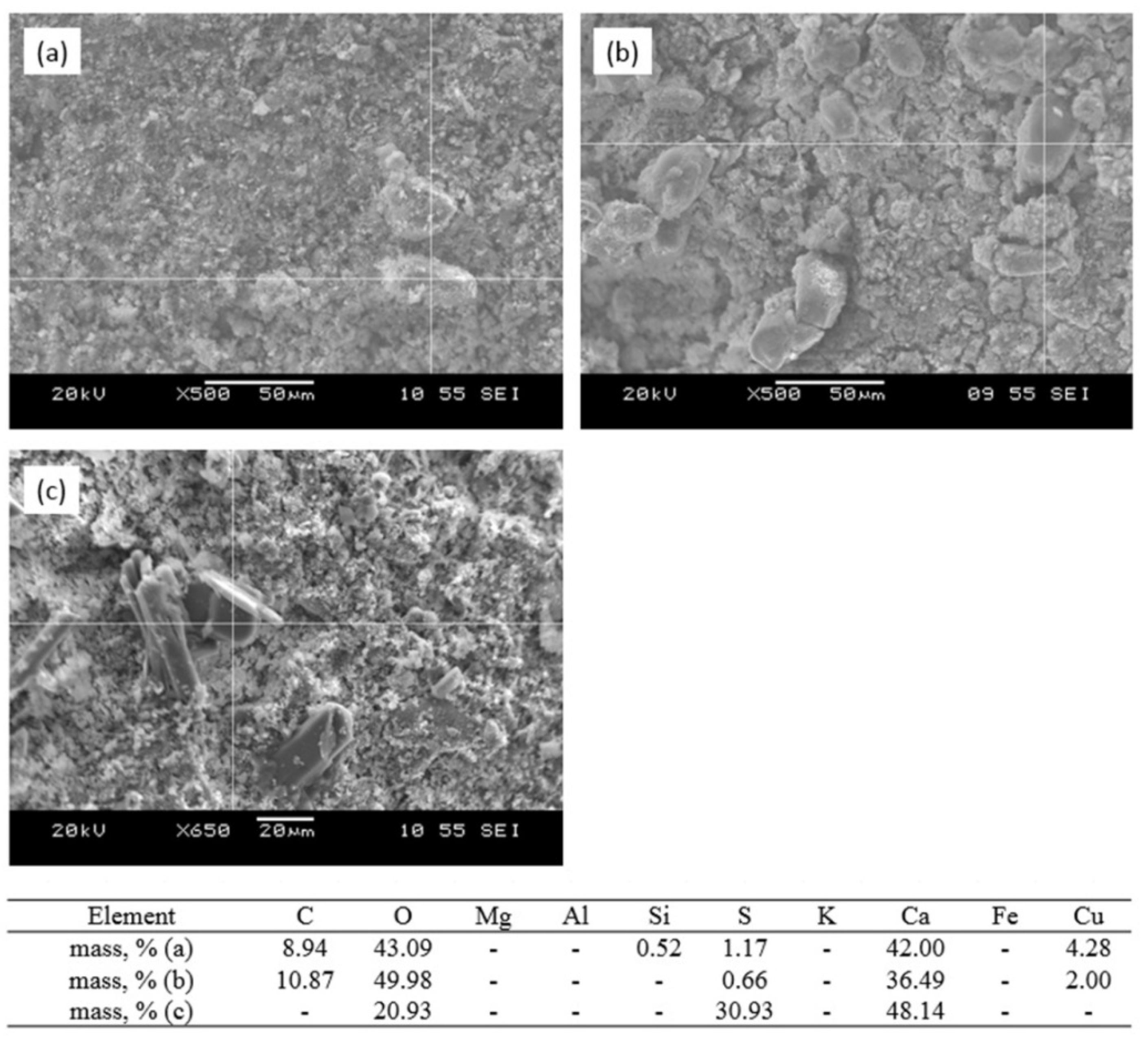
3.4. Sorption–Mineralization Mechanism
- sorption of Cu2+ ions by melilite as a result of ion exchange reactions with formation of the new nanosized formations of the mixed aluminosilicates (Figure 10a,b);
- extraction of the ion exchange products;
3.5. Comparison of Various Cu2+ Sorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliva, S.R.; Espinosa, A.F. Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem. J. 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Elzwayie, A.; Afan, H.A.; Allawi, M.F.; El-Shafie, A. Heavy metal monitoring, analysis and prediction in lakes and rivers: State of the art. Environ. Sci. Pollut. Res. 2017, 24, 12104–12117. [Google Scholar] [CrossRef]
- Elbagermi, M.A.; Edwards, H.G.M.; Alajtal, A.I. Monitoring of Heavy Metals Content in Soil Collected from City Centre and Industrial Areas of Misurata, Libya. Int. J. Anal. Chem. 2013, 2013, 312581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lito, P.F.; Aniceto, J.P.S.; Silva, C.M. Removal of Anionic Pollutants from Waters and Wastewaters and Materials Perspective for Their Selective Sorption. Water Air Soil Pollut. 2012, 223, 6133–6155. [Google Scholar] [CrossRef]
- Skorobogatov, G.A.; Kalinin, A.I. Natural mineral sorbents for chemical and microbiological purification of potable water. Russ. J. Gen. Chem. 2011, 81, 2641–2652. [Google Scholar] [CrossRef]
- Bochkarev, G.R.; Pushkareva, G.A. Mineral dressing strontium removal from aqueous media by natural and modified sorbents. J. Min. Sci. 2009, 45, 104–109. [Google Scholar] [CrossRef]
- Vanore, P.; Coppola, E.; Iovino, P.; Leone, V.; Salvestrini, S.; Capasso, S. Sorption thermodynamics of organic pollutants onto zeolitic tuff: Isosteric and standard enthalpy. J. Water Chem. Technol. 2017, 39, 228–232. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Luckey, T.D.; Venugopal, B. Physiologic and chemical basis for metal toxicity. In Metal Toxicity in Mammals; Plenum Press: New York, NY, USA, 1977; Volume 1. [Google Scholar]
- Ennigrou, D.J.; Ali, M.B.S.; Dhahbi, M. Copper and Zinc removal from aqueous solutions by polyacrylic acid assisted-ultrafiltration. Desalination 2014, 343, 82–87. [Google Scholar] [CrossRef]
- Banerjee, S.; Sarkar, R.R.; Chattopadhyay, J. Effect of copper contamination on zooplankton epidemics. J. Theor. Biol. 2019, 469, 61–74. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Copper(II) from Aqueous Solution by Peat. Water Air Soil Pollut. 2004, 158, 77–97. [Google Scholar] [CrossRef]
- Kaksonen, A.; Morris, C.; Rea, S.; Li, J.; Usher, K.M.; McDonald, R.; Hilario, F.; Hosken, T.; Jackson, M.; du Plessis, C. Biohydrometallurgical iron oxidation and precipitation: Part II—Jarosite precipitate characterisation and acid recovery by conversion to hematite. Hydrometallurgy 2014, 147–148, 264–272. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Ren, X.; Mei, H.; Shao, D.; Wang, X. Effect of Silicate on the Formation and Stability of Ni–Al LDH at the γ-Al2O3 Surface. Environ. Sci. Technol. 2014, 48, 13138–13145. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Pei, Z.; Li, C.; Shan, X.-Q.; Wen, B.; Zhang, S.; Zheng, L.; Zhang, J.; Xie, Y.; et al. Effects of humic acid on copper adsorption onto few-layer reduced graphene oxide and few-layer graphene oxide. Carbon 2014, 75, 227–235. [Google Scholar] [CrossRef]
- Wen, Y.; Ma, J.; Chen, J.; Shen, C.; Li, H.; Liu, W. Carbonaceous sulfur-containing chitosan–Fe(III): A novel adsorbent for efficient removal of copper (II) from water. Chem. Eng. J. 2015, 259, 372–380. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, W.; Mao, Y.; Zhang, L.; Cheng, J.; Gong, M.; Zhao, H.; Dai, L.; Zhang, S.; Zhao, Q. Adsorption behavior of copper ions from aqueous solution onto graphene oxide–CdS composite. Chem. Eng. J. 2015, 259, 603–610. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qu, Z.; Yan, N.; Zhao, Y.; Xu, X.; Li, L. Size-dependent nanocrystal sorbent for copper removal from water. Chem. Eng. J. 2016, 284, 565–570. [Google Scholar] [CrossRef]
- Chouyyok, W.; Shin, Y.; Davidson, J.; Samuels, W.D.; LaFemina, N.H.; Rutledge, R.D.; Fryxell, G.E.; Sangvanich, T.; Yantasee, W. Selective Removal of Copper(II) from Natural Waters by Nanoporous Sorbents Functionalized with Chelating Diamines. Environ. Sci. Technol. 2010, 44, 6390–6395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zhou, Q.; Zhou, S.; Wang, W.; Jin, J.; Xie, J.; Li, A.; Shuang, C. A bifunctional adsorbent with high surface area and cation exchange property for synergistic removal of tetracycline and Cu2+. Chem. Eng. J. 2014, 258, 26–33. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Kouznetsova, T.; Sauka, J.; Ivanets, A. Template synthesis and gas adsorption properties of ordered mesoporous aluminosilicates. Appl. Nanosci. 2021, 11, 1903–1915. [Google Scholar] [CrossRef]
- Ivanets, A.; Shashkova, I.; Kitikova, N.; Radkevich, A.; Venhlinskaya, E.; Dzikaya, A.; Trukhanov, A.V.; Sillanpää, M. Facile synthesis of calcium magnesium zirconium phosphate adsorbents transformed into MZr4P6O24 (M: Ca, Mg) ceramic matrix for radionuclides immobilization. Sep. Purif. Technol. 2021, 272, 118912. [Google Scholar] [CrossRef]
- Vesentsev, A.I.; Goldovskaya-Peristaya, L.F.; Sidnina, N.A.; Dobrodomova, E.V.; Zelentsova, E.S. Definition of kinetic dependences of sorption by ions of copper and lead by rocks of the Belgorod area. Sci. News Belgorod State Univ./Ser. Nat. Sci. 2007, 5, 105–108. [Google Scholar]
- Kononova, O.N.; Fyodorova, N.V.; Kachin, S.V.; Kholmogorov, A.G. Sorption of copper (II) from aqueous solutions on complexing ion exchangers and determination of copper by diffuse reflectance spectroscopy. J. Sib. Fed. Univ. Chem. 2009, 3, 195–209. [Google Scholar]
- Nikiforova, T.E.; Kozlov, V.A. Sorption of copper (II) cations from aqueous media by a cellulose-containing sorbent. Prot. Met. Phys. Chem. Surf. 2012, 48, 310–314. [Google Scholar] [CrossRef]
- Lakiza, N.V.; Neudachina, L.K.; Vshivkov, A.A. Kinetics of sorption of copper (II) ions by hybrid sorbents based on mixed oxides of silicon, aluminum, zirconium, and titanium. Sorpt. Chromatogr. Processes 2006, 6, 1001–1005. [Google Scholar]
- Marchenkova, T.F.; Kunilova, I.V. Research of sorption of copper, nickel, zink and silver on modified Sibai zeolite. Min. Inf. Anal. Bull. 2004, 11, 298–301. [Google Scholar]
- Morozova, A.G.; Lonzinger, T.M.; Skotnikov, V.A.; Sahu, J.N.; Mikhailov, G.G.; Schenk, J.L.; Bhattacharyya, A.; Kapelyushin, Y. Utilization of metallurgical slag with presence of novel CaO-MgO-SiO2-Al2O3 as a composite sorbent for wastewater treatment contaminated by cerium. J. Clean. Prod. 2020, 255, 120286. [Google Scholar] [CrossRef]
- Morozova, A.G.; Lonzinger, T.M.; Mihajlov, G.G.; Skotnikov, V.A.; Berkovich, L.I. Granular Composite Sorbent Based on Calcium Silicates. Russian Patent 2575044, 2014. [Google Scholar]
- Morozova, A.G.; Lonzinger, T.M. Calcium Silicate Composite Sorbent. Russian Patent 2481153, 17 June 2011. [Google Scholar]
- Meyer, K. Physical and chemical crystallography. Moscow 1972. (In Russian) [Google Scholar]
- Garn, P.D.; Habash, T.S. Solid state reactions. A rigorous test of the Hedvall effect. J. Phys. Chem. 1979, 83, 229–231. [Google Scholar] [CrossRef]
- Tretyakov, Y. Solid-phase reactions. Chemistry 1978. (In Russian) [Google Scholar]
- Eitel, W. Physical Chemistry of the Silicates; University of Chicago Press: Chicago, IL, USA, 1954. [Google Scholar]
- Xu, Y.; Luo, C.; Zheng, Y.; Ding, H.; Wang, Q.; Shen, Q.; Li, X.; Zhang, L. Characteristics and performance of CaO-based high temperature CO2 sorbents derived from a sol–gel process with different supports. RSC Adv. 2016, 6, 79285–79296. [Google Scholar] [CrossRef]
- Ivanova, V.P.; Kasatov, B.K.; Krasavina, T.N.; Rozinova, E.L. Termicheskii analiz mineralov i gornykh porod. Nedra 1974, 399. [Google Scholar]
- Grigor’ev, A.I. Introduction to Vibrational Spectroscopy of Inorganic Compounds; MGU: Moscow, Russia, 1977. [Google Scholar]
- Yukhnevich, G.V. Infrared Spectroscopy of Water; Nauka: Moscow, Russia, 1973. [Google Scholar]
- Ren, Y.; Li, N.; Feng, J.; Luan, T.; Wen, Q.; Li, Z.; Zhang, M. Adsorption of Pb(II) and Cu(II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J. Colloid Interface Sci. 2012, 367, 415–421. [Google Scholar] [CrossRef]


| Compound | Content, Mass % | Compound | Content, Mass % |
|---|---|---|---|
| Al2O3 | 14.70 | MnO | 0.03 |
| CaO | 28.20 | NiO | 0.04 |
| Cr2O3 | 0.05 | P2O5 | 0.10 |
| CuO | 0.06 | SiO2 | 25.38 |
| Fe2O3 | 1.49 | TiO2 | 0.86 |
| K2O | 0.12 | ZnO | 0.01 |
| MgO | 23.36 | LOI | 0.20 |
| Element | Content before Exposure, mg/L | Content after 30 Days Exposure Time, mg/L |
|---|---|---|
| Al | 0 | 0 |
| Ca | 0 | 27.2840 |
| Si | 0 | 0 |
| Na | 0 | 0 |
| K | 0 | 0 |
| Cu | 0 | 0 |
| pH | 8.31 |
| Type of Sorbent | Initial Concentration, mg/L | Intake of Cu2+, mg/g | Contact Time (Test Type), h | |
|---|---|---|---|---|
| MgO-CaO-Al2O3-SiO2-CO2 system | 500 | 16.7 | 168 (static) | This study |
| Graphene oxide | 15 | 75 | 24 (dynamic) | [20] |
| Multiwalled carbon | 15 | 2 | 24 (dynamic) | [20] |
| nanotubes | ||||
| Activated carbon | 15 | 14.7 | 24 (dynamic) | [20] |
| Magnetic porous ferrospinel MnFe2O4 | – | 60.5 | 3 (dynamic) | [43] |
| Carbonaceous sulfur-containing chitosan–Fe(III) | 500 | 413.2 | 0.25 (dynamic) | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, A.G.; Lonzinger, T.M.; Skotnikov, V.A.; Mikhailov, G.G.; Kapelyushin, Y.; Khandaker, M.U.; Alqahtani, A.; Bradley, D.A.; Sayyed, M.I.; Tishkevich, D.I.; et al. Insights into Sorption–Mineralization Mechanism for Sustainable Granular Composite of MgO-CaO-Al2O3-SiO2-CO2 Based on Nanosized Adsorption Centers and Its Effect on Aqueous Cu(II) Removal. Nanomaterials 2022, 12, 116. https://doi.org/10.3390/nano12010116
Morozova AG, Lonzinger TM, Skotnikov VA, Mikhailov GG, Kapelyushin Y, Khandaker MU, Alqahtani A, Bradley DA, Sayyed MI, Tishkevich DI, et al. Insights into Sorption–Mineralization Mechanism for Sustainable Granular Composite of MgO-CaO-Al2O3-SiO2-CO2 Based on Nanosized Adsorption Centers and Its Effect on Aqueous Cu(II) Removal. Nanomaterials. 2022; 12(1):116. https://doi.org/10.3390/nano12010116
Chicago/Turabian StyleMorozova, Alla G., Tatiana M. Lonzinger, Vadim A. Skotnikov, Gennady G. Mikhailov, Yury Kapelyushin, Mayeen Uddin Khandaker, Amal Alqahtani, D. A. Bradley, M. I. Sayyed, Daria I. Tishkevich, and et al. 2022. "Insights into Sorption–Mineralization Mechanism for Sustainable Granular Composite of MgO-CaO-Al2O3-SiO2-CO2 Based on Nanosized Adsorption Centers and Its Effect on Aqueous Cu(II) Removal" Nanomaterials 12, no. 1: 116. https://doi.org/10.3390/nano12010116
APA StyleMorozova, A. G., Lonzinger, T. M., Skotnikov, V. A., Mikhailov, G. G., Kapelyushin, Y., Khandaker, M. U., Alqahtani, A., Bradley, D. A., Sayyed, M. I., Tishkevich, D. I., Vinnik, D. A., & Trukhanov, A. V. (2022). Insights into Sorption–Mineralization Mechanism for Sustainable Granular Composite of MgO-CaO-Al2O3-SiO2-CO2 Based on Nanosized Adsorption Centers and Its Effect on Aqueous Cu(II) Removal. Nanomaterials, 12(1), 116. https://doi.org/10.3390/nano12010116










